Abstract
The anterior cingulate cortex (ACC) has been implicated in alcohol and drug addiction. We recently identified the small G protein K-ras as an alcohol-regulated gene in the ACC by gene expression analysis. We show here that the adiponectin receptor 2 (AdipoR2) was differentially regulated by alcohol in the ACC in a K-ras-dependent manner. Additionally, withdrawal-associated increased drinking was attenuated in AdipoR2 null mice. Intracellular recordings revealed that adiponectin increased the excitability of ACC neurons and that this effect was more pronounced during alcohol withdrawal, suggesting that AdipoR2 signaling may contribute to increased ACC activity. Altogether, the data implicate K-ras-regulated pathways involving AdipoR2 in the cellular and behavioral actions of alcohol that may contribute to overactivity of the ACC during withdrawal and excessive alcohol drinking.
Keywords: Neural plasticity, PI3K, Jak/Stat, ERK, MAPK, adipokines, Acrp30, apM1
Introduction
Human and animal studies implicate circuits involving the anterior cingulate cortex (ACC) in discriminating among possible rewards (Cardinal et al., 2003) and avoiding previously experienced noxious stimuli (Johansen and Fields, 2004). The ACC, together with the insula and orbitofrontal cortices is also associated with integrated autonomic control and is sensitive to interoceptive signals (Gray and Critchley, 2007). Adaptations in the ACC have been implicated in alcohol and drug addiction (Kalivas and Volkow, 2005; Koob and Volkow, 2009) and increased ACC activity correlates with subjective craving ratings in alcohol abusers (Acheson et al., 2009; Lingford-Hughes et al., 2006; Myrick et al., 2004).
Earlier studies from this and other groups have investigated the effects of alcohol on gene expression in the prelimbic and infralimbic subdivisions of the PFC (Bjork et al., 2006; Hashimoto and Wiren, 2008; Kerns et al., 2005; Repunte-Canonigo et al., 2007), but the ACC has received less attention. We recently identified the small G protein K-ras as an alcohol-regulated gene in the ACC by microarray analyses (Repunte-Canonigo et al., submitted). Additionally, K-ras heterozygous null mice (K-ras+/−) did not increased their drinking after withdrawal from intermittent ethanol vapor exposure unlike their wild-type littermates, suggesting a role for K-ras regulated genes in excessive alcohol drinking (Repunte-Canonigo et al., 2007). Pathways analysis with the GSEA Molecular Signature Database identified the adipocytokine signaling pathway, the Jak/Stat pathway, and the insulin/PI3K pathway among the pathways differentially regulated by alcohol in a K-ras-depedent manner (Repunte-Canonigo et al., Submitted). As these signaling pathways are involved in adipokine signaling and since the gene for the adiponectin receptor 2 (AdipoR2) was among the differentially expressed genes, we decided to investigate the role of the AdipoR2 in alcohol actions. Here we show that AdipoR2 was differentially regulated by alcohol in the ACC in a K-ras-dependent manner and that withdrawal-associated increased drinking was attenuated in AdipoR2 homozygous null mice (AdipoR2−/−). Additionally, intracellular recordings revealed that adiponectin increased the excitability of ACC neurons and that this effect was more pronounced during alcohol withdrawal. Lastly, AdipoR2 was expressed in multiple brain regions suggesting that it may be a broad regulator of neuronal excitability and of alcohol actions in the brain.
Results
As shown in Fig. 1A both acute and repeated alcohol administration of an intoxicating dose of alcohol significantly increased AdipoR2 mRNA content in the rat ACC. To determine the K-ras-dependence of alcohol regulation of AdipoR2, we used mutant mice with bidirectional manipulations of K-ras function: K-ras+/− mice, which have reduced K-ras function, and Nf1+/−, which have increased K-ras function, as NF1 is a negative K-ras regulator (Costa et al., 2002). To this end, K-ras+/−, Nf1+/− mice and their respective wild type littermates received repeated injections of an intoxicating dose of alcohol or saline. RT-PCR analysis of the ACC of K-ras and Nf1+/− mice showed a significantly greater increase in AdipoR2 gene expression in alcohol-treated Nf1+/− mice than in K-ras+/− mice, suggesting that K-ras activity contributes to the induction of AdipoR2 by alcohol (Fig. 1B).
Fig. 1. Alcohol induces AdipoR2 expression in the ACC in a K-ras-dependent manner.
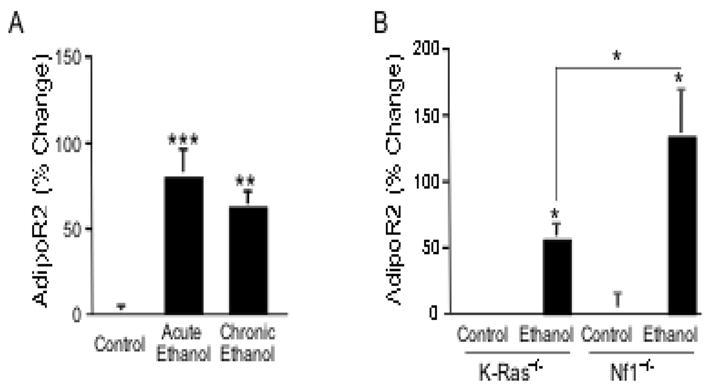
A) Significant increase of AdipoR2 gene expression was seen in the rat ACC by RT-PCR 12 hours after either acute or repeated administration of an intoxicating dose of alcohol (1-way ANOVA: F(2,12)= 15.67, p=0.0008, **p<0.01, ***p<0.001 from control). B) RT-PCR analysis of the ACC of K-ras+/− and Nf1+/− mice showed a significantly greater induction of AdipoR2 mRNA in the ACC of alcohol-treated Nf1+/− mice than in K-ras+/− mice after repeated administration of an intoxicating dose of alcohol (*p<0.05, **p<0.01 from respective controls or as indicated by the bracket. Pairwise Bonferroni-corrected comparisons). AdipoR2 was significantly increased in the ACC of both mouse lines (F(1,14)=19.47, P<0.001).
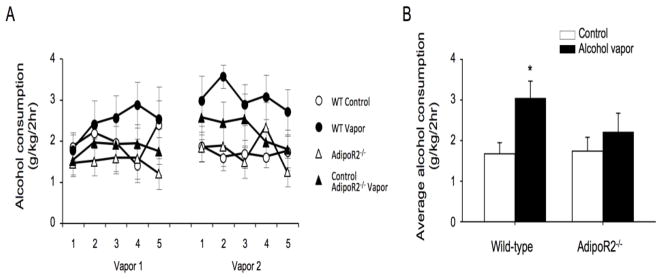
We next investigated alcohol drinking by AdipoR2−/− mice (Liu et al., 2007) in a behavioral paradigm in which intermittent exposure to alcohol vapors is associated with increased alcohol intake during subsequent withdrawal (Finn et al., 2007; Lopez and Becker, 2005). We observed that AdipoR2−/− mice did not show increased drinking during withdrawal from intermittent alcohol vapor exposure, in contrast to their wild-type littermates (Fig. 2), reminiscent of previous results with K-ras+/− mice (Repunte-Canonigo et al., Submitted). Alcohol vapor-exposure did not differentially affect body weights of AdipoR2−/− mice vs. their wild type littermates, suggesting that the effect of AdipoR2 deficiency to reduce alcohol intake was not due to alterations of body weight homeostasis in AdipoR2 deficient mice.
Fig. 2. Alcohol vapor exposure significantly increased alcohol consumption in wild-type (WT), but not in AdipoR2−/− mice.
A) Exposure to chronic intermittent alcohol vapor did not increase alcohol consumption over air-exposed in AdipoR2−/− mice, but did so in their wild-type littermate controls. Following the second vapor exposure there was a significant effect of condition in WT mice (F(1,11) = 6.6, p < 0.05), but not in AdipoR2 −/− mice (F(1,11) = 0.73, n.s.). B) Average consumptions across the final 5 days of the two bottle choice paradigm are shown. Alcohol vapor exposure significantly increased alcohol consumption in wild-type mice, but not in AdipoR2−/− mice (*p<0.05, by Bonferroni-corrected t-test).
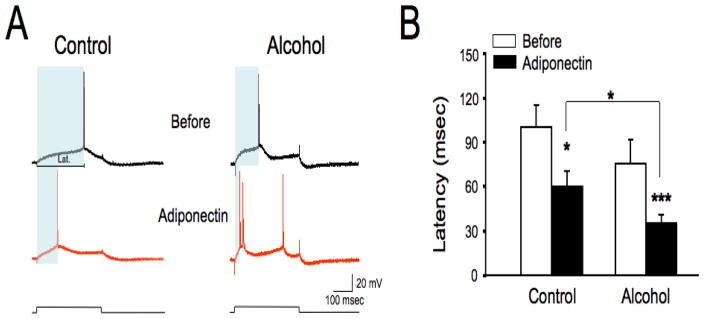
Since adiponectin has been previously shown to directly affect neurons of hypothalamus (Ahima and Lazar, 2008) and area postrema (Fry et al., 2006), but not in other cerebrum areas, we performed intracellular recordings with sharp electrodes to investigate whether the AdipoR2 ligand adiponectin affects the intrinsic membrane properties of ACC neurons. Recordings were carried in acute coronal brain slices of the rostral cerebrum in layer V/VI neurons of the perigenual AAC, which projects to the nucleus accumbens (NAc) core among other regions (Cardinal et al., 2003). A 15 min bath application of adiponectin (10 nM) did not significantly affect resting membrane potentials of ACC neurons neither in saline-injected control rats nor in rats treated repeatedly as outlined above with an intoxicating dose of alcohol (not shown). However, as shown in Fig. 3, adiponectin significantly decrease action potential latency in ACC neurons of both saline-injected controls and chronic alcohol-treated rats (in msec: control= 109.41±15.41, n=10; chronic alcohol= 84.98±11.37, n=9; control+adiponectin= 68.08±12.34, n=10; chronic alcohol+adiponectin= 38.78±6.09, n=9; Fig. 3B). Additionally, the action potential latency was significantly briefer in ACC neurons from adiponectin-treated slices obtained from chronic-alcohol treated animals than in ACC neurons from saline-injected controls, suggesting that chronic alcohol treatment sensitized ACC neurons to the latency-reducing effect of adiponectin (Fig. 3B). The effect of adiponectin to reduce action potential latency was mediated by AdipoR2 as adiponectin had no effects on the latency of ACC neurons from AdipoR2−/− mice (adiponectin × genotype interaction: F(1,15)= 6.75, p=0.02, in msec: wild-type = 62.16±22.60, n=11; AdipoR2−/− = 86.76±21.03, n=6; wild-type + adiponectin=28.11±6.70; AdipoR2−/− + adiponectin = 86.31±19.13). Together, these results suggest that AdipoR2 is a K-ras regulated gene involved in the cellular and behavioral actions of alcohol that may contribute to overactivity of the ACC during withdrawal.
Fig. 3. Adiponectin increases intrinsic excitability in layer V/VI ACC neurons.
A) Intracellular recordings from ACC layer V/VI neurons with sharp electrodes in current clamp mode in saline-injected control rats and in rats chronically treated with an intoxicating dose of alcohol. Depolarizing current pulses were injected to elicit a single action potential. Following application of adiponectin to the bath (10 nM for 15 min), a protracted decrease of the action potential latency was observable even after washout and for the whole duration of the recording session. The latency, i.e. the time from the onset of the stimulus to the time of occurrence of the first action potential is shaded in the four traces shown and indicated by the horizontal line marked ‘Lat.” in the control trace before addition of adiponectin. B) Quatification of the effect of adiponectin on the latency of layer V/VI ACC neurons. Adiponectin decreased action potential latency, as demonstrated by a main effect [F(1,17)= 37.88, p<0.0001]. Pairwise Bonferroni-corrected comparisons showed that adiponectin decreased latency both in ACC neurons from saline-injected controls and from alcohol-treated rats. Moreover, ACC neurons from adiponectin-treated slices from the alcohol-injected group also showed significantly briefer latencies than those from the adiponectin-treated slices from saline-injected controls. The resting membrane potential of the sample neurons shown was −67.8 mV for the control neurons and −63.67 mV for the neurons from the alcohol-treated rat. Data represent the Mean ± SEM. Because of unequal variance and non-Gaussian distribution of values, latency data are indicated as back-transformed Means ± SEM after logarithmic transformation. *p<0.05, ***p<0.001 from corresponding basal value or as indicated by the bracket, Bonferroni-corrected t-test).
Lastly, to map the distribution of AdipoR2 mRNA we performed in situ hybridization in the rat CNS. Results showed abundant AdipoR2 mRNA expression in various cortical and subcortical regions in addition to the ACC (Fig. 4). The broad distribution of AdipoR2 in multiple brain regions and its regulation by alcohol suggests that it may be a broad regulator of neuronal excitability and that its role in the action of alcohol on the brain may not be limited to the ACC.
Fig. 4. Adiponectin receptors are widely expressed in the CNS.
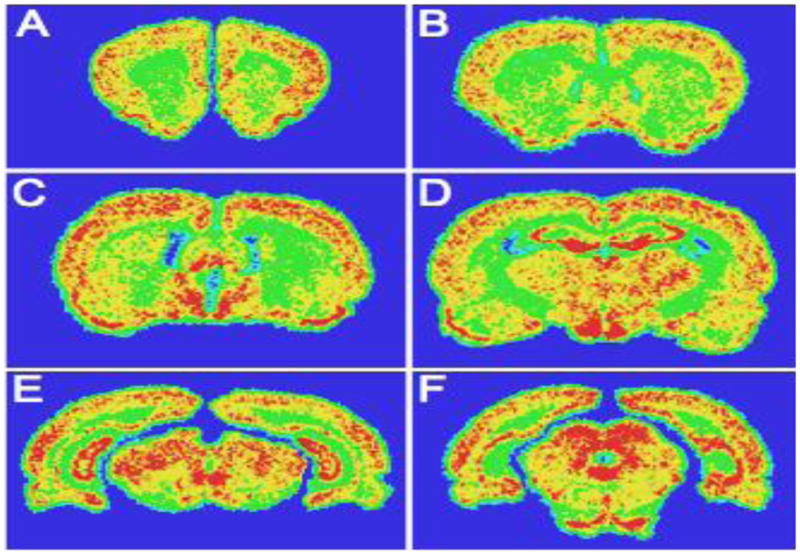
A, B) Hybridization signal at the level of the rostral forebrain showing moderate to strong adiponectin receptor expression in the cortex and low to moderate expression in the striatum, nucleus accumbens and septum. C, D) Prominent adiponectin receptor expression was observed in the hypothalamus in the medial preoptic area, ventromedial hypothalamus and arcuate nucleus and in the thalamus. Moderate signal was seen over the BNST and amygdala; Moderate to strong expression was also observed over the hypothalamus and thalamus, with the medial preoptic area, ventromedial hypothalamic and arcuate nuclei, paraventricular thalamic nucleus, anterodorsal, and habenula showing more robust signal. Strong signal was seen over the CA3 and dentate gyrus of the hippocampus, while the CA1 field displayed signal at a moderate level (D). E) Moderate to strong expression was seen in the mesencephalon with moderate signal over the substantia nigra and the ventral tegmental area and moderate to strong signal over the periacqueductal grey and pontine nuclei. F) Adiponectin receptor expression was also seen over the periacqueductal grey, superior colliculi and pontine nuclei. Intensity: red>yellow>green>blue
Discussion
Molecular pathways relevant to adipokine regulation and signaling were among the pathways differentially regulated by alcohol in mice with bidirectional manipulations of K-ras (Repunte-Canonigo et al., Submitted). Here we showed that the adiponectin receptor AdipoR2 was regulated by alcohol in a K-ras-dependent manner. Additionally, withdrawal-associated increased drinking was attenuated in AdipoR2 null mice paralleling results in K-ras+/− mice (Repunte-Canonigo et al., submitted). The effect of AdipoR2 deletion was quantitatively smaller than that of K-ras+/− mice, consistent with the view that, as K-ras can affect a broad range of genes, multiple K-ras-regulated genes may contribute to its actions in regulating alcohol consumption in animals with a history of dependence.
Adiponectin differentially affected the excitability of ACC neurons from rats with a history of alcohol exposure. In particular, we observed that adiponectin shortened the latency for action potential generation in ACC neurons and that this effect was significantly greater in animals with a history of chronic alcohol exposure. Shorter action potential latency can affect the way neural networks process information (Henze and Buzsaki, 2001). In particular, increased excitability of ACC neurons may contribute to increased prefrontal drive to the NAc, which has been implicated in drug craving and seeking (Kalivas and Volkow, 2005).
Adiponectin is present in the cerebrospinal fluid (CSF), although at much lower concentrations than in plasma (Pan et al., 2006; Qi et al., 2004; Spranger et al., 2006). The source of CSF adiponectin remains unclear (Pan et al., 2006; Qi et al., 2004; Spranger et al., 2006). Previous studies showed electrophysiological effects of adiponectin in the hypothalamus and area postrema neurons (Fry et al., 2006; Qi et al., 2004). However, a role of adiponectin in regulating neuronal function beyond these regions had not been described. In situ hybridization showed that, in addition to the ACC, AdipoR2 was widely distributed in the brain in both cortical and subcortical regions, suggesting the possibility that AdipoR2 signaling may play general regulatory roles in neuronal excitability as well as specific roles in the effects of alcohol exposure on other alcohol target regions.
Mounting evidence also implicates other orexigenic and anorectic peptides such as leptin, insulin and ghrelin in the regulation of alcohol drinking (Addolorato et al., 2009; Jerlhag et al., 2009). Leptin, an adipokine whose receptors occur in various brain regions (Fulton et al., 2006), has also been shown to have a role in neuronal plasticity (Pinto et al., 2004). While functional effects of adiponectin signaling had, to our knowledge, not previously been observed in the ACC, leptin replacement in leptin-deficient individuals increased gray matter in the ACC in patients with congenital leptin deficiency (Matochik et al., 2005). Additionally, central ghrelin administration increased alcohol intake in a 2-bottle choice limited access paradigm (Jerlhag et al., 2009).
Together, the present results suggest that AdipoR2 is a K-ras regulated gene involved in the cellular actions of alcohol and in the regulation of alcohol intake. AdipoR2 broad distribution in the brain suggests that its actions may not be limited to the ACC.
Experimental procedures
Animals
All animals were group housed and kept in a 12-h reverse light cycle. Male Wistar rats (Charles Rivers, Reighley, NC; 250g at the beginning of the experiment) were used. We also used three to four month old male K-ras+/−, Nf1+/− mice (Costa et al., 2002), AdipoR2−/− and their respective male littermates (Liu et al., 2007) in the C57BL/6J background.
Passive alcohol exposure and dissections
Rats were treated with one or five daily intraperitoneal (IP) injections of an intoxicating dose (3g/kg) of 16% w/v alcohol in saline (Bloom et al., 1982) or isovolemic saline after habituation with three isovolemic injections of saline at the beginning of the dark cyle. Mice were treated IP paralleling the rats: they were habituated to the IP injections with three isovolemic injections of saline, followed by five daily IP injections of an intoxicating dose of alcohol (4g/kg) producing comparable blood alcohol levels to the rats or isovolemic saline. Animals were sacrificed under deep halothane anesthesia 12 hours after the last alcohol administration. For the purpose of this study, the ACC was defined according to Robbins, Everitt and associates as cingulate area Cg2 together with overlying Cg1 encompassing pre-/perigenual and postgenual regions (Cardinal et al., 2003) and dissected as described (Repunte-Canonigo et al., Submitted).
RT-PCR
RT-PCR was carried out as previously described (Ahmed et al., 2005; Repunte-Canonigo et al., 2007) in the ACC from individual rats (n=4–5) and mice (n=4–6) using an iQ5 Real-Time PCR Detection System (BioRad, Hercules, CA). The relative amounts of target mRNA were normalized to β-actin, which was determined to be invariant by RT-PCR, using the standard curve method (Livak, 1997).
Voluntary alcohol drinking paradigm
Male AdipoR2−/− mice (Liu et al., 2007) and their respective male wild-type littermates were used for these experiments. Food and water were available ad libitum and mice were group housed except during alcohol drinking sessions. Previous studies have shown increased drinking in mice after repeated bouts of intermittent alcohol vapor exposure (Finn et al., 2007; Lopez and Becker, 2005). This paradigm was carried out according to the version of Finn et al. (Finn et al., 2007). Mutant and wild-type (C57BL/6J) littermate mice had access to two bottles, one containing water and the other containing 10% (w/v) alcohol, for 2 hours starting 3 hours into the dark phase for 10 days. Mice were then assigned to two groups matched for baseline alcohol and water consumption. The alcohol vapor group was placed into alcohol vapor chambers (La Jolla Alcohol Research, CA) for 16 hours per day for 3 days. The control group was placed in identical chambers receiving air. Tail blood sampling for blood alcohol level (BAL) determination was carried out daily. Target BAL were 150–200 mg%. Seventy-two hours following removal from the chambers, mice received access to water vs. 10% alcohol for 2 hours, and again over the next 4 days. The following week, mice were re-exposed to the alcohol vapor/control conditions and again tested for two-bottle choice drinking for 5 days. Mice were weighed every 4–6 days throughout the experiment.
Electrophysiological techniques and intracellular recordings
Brain slices (350μm) were obtained from the rat or mouse cerebrum with a Campden Instruments (Loughborough, UK). Sharp intracellular recordings were conducted as previously described (Berton et al., 1999; Sanna et al., 2002) from ACC layer V/VI neurons in the perigenual portion of the ACC region (approximately between the rostral and the middle thirds of the area dissected for the gene expression studies). Briefly, slices were collected in oxygenated sucrose-based artificial CSF (ACSF) (in mM: Sucrose 248, KCl 2.5, CaCl2 1, MgCl2 2, NaHCO3 26, NaH2PO4 1,25, Glucose 1). Slices were then preincubated for at least 1 hour in ACSF containing HEPES (in mM: NaCl 120, HEPES 10, KCl 3.5, CaCl2 2.2, MgSO4 2, NaHCO3 24, NaH2PO4 1,25, Glucose 10). For electrophysiological recording, slices were transferred to a submerged recording chamber, perfused with oxygenated ACSF at 28 ± 1°C (in mM: 130 NaCl, 3.5 KCl, 24 NaHCO3, 1.25 NaH2PO4, 2.2 CaCl2, 10 glucose, and 2 MgSO4, pH 7.4 (oxygenated by bubbling a mixture of 95% O 2–5%CO2). The perfusion flow was maintained at 1.2 ml/min in a chamber volume of 1.5 ml. Intracellular recordings were carried out with sharp micropipettes filled with 2 M potassium acetate (MΩ 150–200). Records were obtained in current clamp mode using an Axoclamp-2B amplifier (Axon Instruments, Union City, CA, USA). The action potential latency was measured from the onset of the depolarizing current pulse to the time of occurrence of the first action potential (Armano et al., 2000). To this aim, 10–20 intracellular depolarizing current pulse (350 msec) were injected every 10 sec into the neuron, starting from the resting membrane potential of the cell. The intensity of depolarizing current pulse tests was determined for every cell to evoke a single action potential. The bridge balance was monitored and adjusted as needed during the recording time. Repeated application of this test pulse did not alter the number of evoked action potentials or the resting membrane potential of the neuron. Data acquisition and analysis was performed with Axon and Labview (National Instruments, Austin, TX) software. Human recombinant globular adiponectin (Cat. # ADI-01B) was obtained from Phoenix Pharmaceuticals, Inc. (Belmont CA).
In situ hybridization was carried out as previously described on rat brain slices (Morales et al., 1998) using as a template for riboprobe synthesis the open reading frame of the rat AdipoR2 (NM_001037979) that displays 66% homology with AdipoR1. An AdipoR1-specific probe showed a weaker signal in a comparable distribution (not shown), suggesting that the signal observed is likely to be due primarily to AdipoR2 mRNA.
Acknowledgments
We are grateful to Dr. Tyler Jacks of the Massachusetts Institute of Technology and Alcino Silva of the University of California at Los Angeles for the K-ras and Nf1 heterozygous null mice. This work was supported by NIH grants AA01319, AA017371 (PS), DA027129-0109 (VRC), AA013523 (Integrated Neuroscience Initiative on Alcoholism to AJR), DA023680 (PC) and by the San Diego Alcohol Research Center (AA006420).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acheson A, Robinson JL, Glahn DC, Lovallo WR, Fox PT. Differential activation of the anterior cingulate cortex and caudate nucleus during a gambling simulation in persons with a family history of alcoholism: studies from the Oklahoma Family Health Patterns Project. Drug Alcohol Depend. 2009;100:17–23. doi: 10.1016/j.drugalcdep.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addolorato G, Leggio L, Hillemacher T, Kraus T, Jerlhag E, Bleich S. Hormones and drinking behaviour: new findings on ghrelin, insulin, leptin and volume-regulating hormones. An ESBRA Symposium report. Drug Alcohol Rev. 2009;28:160–5. doi: 10.1111/j.1465-3362.2008.00023.x. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Lazar MA. Adipokines and the peripheral and neural control of energy balance. Mol Endocrinol. 2008;22:1023–31. doi: 10.1210/me.2007-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SH, Lutjens R, van der Stap LD, Lekic D, Romano-Spica V, Morales M, Koob GF, Repunte-Canonigo V, Sanna PP. Gene expression evidence for remodeling of lateral hypothalamic circuitry in cocaine addiction. Proc Natl Acad Sci U S A. 2005;102:11533–8. doi: 10.1073/pnas.0504438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armano S, Rossi P, Taglietti V, D’Angelo E. Long-term potentiation of intrinsic excitability at the mossy fiber-granule cell synapse of rat cerebellum. J Neurosci. 2000;20:5208–16. doi: 10.1523/JNEUROSCI.20-14-05208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton F, Brancucci A, Beghe F, Cammalleri M, Demuro A, Francesconi W, Gessa GL. Gamma-Hydroxybutyrate inhibits excitatory postsynaptic potentials in rat hippocampal slices. Eur J Pharmacol. 1999;380:109–16. doi: 10.1016/s0014-2999(99)00515-4. [DOI] [PubMed] [Google Scholar]
- Bjork K, Saarikoski ST, Arlinde C, Kovanen L, Osei-Hyiaman D, Ubaldi M, Reimers M, Hyytia P, Heilig M, Sommer WH. Glutathione-S-transferase expression in the brain: possible role in ethanol preference and longevity. Faseb J. 2006;20:1826–35. doi: 10.1096/fj.06-5896com. [DOI] [PubMed] [Google Scholar]
- Bloom F, Lad P, Pittman Q, Rogers J. Blood alcohol levels in rats: non-uniform yields from intraperitoneal doses based on body weight. Br J Pharmacol. 1982;75:251–4. doi: 10.1111/j.1476-5381.1982.tb08780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Marbini HD, Toner AJ, Bussey TJ, Robbins TW, Everitt BJ. Role of the anterior cingulate cortex in the control over behavior by Pavlovian conditioned stimuli in rats. Behav Neurosci. 2003;117:566–87. doi: 10.1037/0735-7044.117.3.566. [DOI] [PubMed] [Google Scholar]
- Costa RM, Federov NB, Kogan JH, Murphy GG, Stern J, Ohno M, Kucherlapati R, Jacks T, Silva AJ. Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature. 2002;415:526–30. doi: 10.1038/nature711. [DOI] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12–41) Alcohol Clin Exp Res. 2007;31:939–49. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Fry M, Smith PM, Hoyda TD, Duncan M, Ahima RS, Sharkey KA, Ferguson AV. Area postrema neurons are modulated by the adipocyte hormone adiponectin. J Neurosci. 2006;26:9695–702. doi: 10.1523/JNEUROSCI.2014-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–22. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Gray MA, Critchley HD. Interoceptive basis to craving. Neuron. 2007;54:183–6. doi: 10.1016/j.neuron.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto JG, Wiren KM. Neurotoxic consequences of chronic alcohol withdrawal: expression profiling reveals importance of gender over withdrawal severity. Neuropsychopharmacology. 2008;33:1084–96. doi: 10.1038/sj.npp.1301494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze DA, Buzsaki G. Action potential threshold of hippocampal pyramidal cells in vivo is increased by recent spiking activity. Neuroscience. 2001;105:121–30. doi: 10.1016/s0306-4522(01)00167-1. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Landgren S, Salome N, Heilig M, Moechars D, Datta R, Perrissoud D, Dickson SL, Engel JA. Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci U S A. 2009;106:11318–23. doi: 10.1073/pnas.0812809106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Fields HL. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nat Neurosci. 2004;7:398–403. doi: 10.1038/nn1207. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kerns RT, Ravindranathan A, Hassan S, Cage MP, York T, Sikela JM, Williams RW, Miles MF. Ethanol-responsive brain region expression networks: implications for behavioral responses to acute ethanol in DBA/2J versus C57BL/6J mice. J Neurosci. 2005;25:2255–66. doi: 10.1523/JNEUROSCI.4372-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of Addiction. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingford-Hughes AR, Daglish MR, Stevenson BJ, Feeney A, Pandit SA, Wilson SJ, Myles J, Grasby PM, Nutt DJ. Imaging alcohol cue exposure in alcohol dependence using a PET 15O-H2O paradigm: results from a pilot study. Addict Biol. 2006;11:107–15. doi: 10.1111/j.1369-1600.2006.00001.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Michael MD, Kash S, Bensch WR, Monia BP, Murray SF, Otto KA, Syed SK, Bhanot S, Sloop KW, Sullivan JM, Reifel-Miller A. Deficiency of adiponectin receptor 2 reduces diet-induced insulin resistance but promotes type 2 diabetes. Endocrinology. 2007;148:683–92. doi: 10.1210/en.2006-0708. [DOI] [PubMed] [Google Scholar]
- Livak K. Applied Biosystems. PE. 1997. ABI Prism 7700 Sequence Detection System, User Bulletin 2. [Google Scholar]
- Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl) 2005;181:688–96. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Matochik JA, London ED, Yildiz BO, Ozata M, Caglayan S, DePaoli AM, Wong ML, Licinio J. Effect of leptin replacement on brain structure in genetically leptin-deficient adults. J Clin Endocrinol Metab. 2005;90:2851–4. doi: 10.1210/jc.2004-1979. [DOI] [PubMed] [Google Scholar]
- Morales M, Criado JR, Sanna PP, Henriksen SJ, Bloom FE. Acute ethanol induces c-fos immunoreactivity in GABAergic neurons of the central nucleus of the amygdala. Brain Res. 1998;798:333–6. doi: 10.1016/s0006-8993(98)00457-0. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, George MS. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- Pan W, Tu H, Kastin AJ. Differential BBB interactions of three ingestive peptides: obestatin, ghrelin, and adiponectin. Peptides. 2006;27:911–6. doi: 10.1016/j.peptides.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–5. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10:524–9. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- Repunte-Canonigo V, Lutjens R, van der Stap LD, Sanna PP. Increased expression of protein kinase A inhibitor alpha (PKI-alpha) and decreased PKA-regulated genes in chronic intermittent alcohol exposure. Brain Res. 2007;1138:48–56. doi: 10.1016/j.brainres.2006.09.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repunte-Canonigo V, Berton F, Cottone P, Reifel-Miller A, Roberts A, Moreales M, Francesconi W, Sanna P. Genome-wide gene expression analysis identifies K-ras as a regulator of alcohol intake Submitted. [Google Scholar]
- Sanna PP, Cammalleri M, Berton F, Simpson C, Lutjens R, Bloom FE, Francesconi W. Phosphatidylinositol 3-kinase is required for the expression but not for the induction or the maintenance of long-term potentiation in the hippocampal CA1 region. J Neurosci. 2002;22:3359–65. doi: 10.1523/JNEUROSCI.22-09-03359.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger J, Verma S, Gohring I, Bobbert T, Seifert J, Sindler AL, Pfeiffer A, Hileman SM, Tschop M, Banks WA. Adiponectin does not cross the blood-brain barrier but modifies cytokine expression of brain endothelial cells. Diabetes. 2006;55:141–7. [PubMed] [Google Scholar]