Abstract

2-Ethoxyethaneseleninic acid reacts with electron rich aromatic substrates to deliver, by way of the selenoxides, the (2-ethoxyethyl) seleno ethers, which can in turn be transformed into a diverse set of aryl selenylated products. Among these, a family of 5-uridinyl derivatives shows sub-micromolar inhibition of human and malarial orotate phosphoribosyltransferase.
A wide assortment of selenium based reagents allows the introduction of Se into organic structures by both nucleophilic and electrophilic pathways, and the resulting organoselenium products can be oxidized, reduced, or otherwise converted to useful targets that may or may not retain Se.1 Despite the toxic nature of organoselenium derivatives in general, many of these have shown marked biological and enzyme inhibitory activities that may find important applications.2 Electrophilic introduction of Se is commonly performed by using selenenyl chlorides and their relatives, and is mostly limited to ArSe–X examples. We recently demonstrated that alkaneseleninic acids (RSeO2H) react as electrophiles toward electron rich aromatic rings such as phenols and indoles.3,4 We have now modified this reaction to allow the incorporation of the versatile 2-ethoxyethaneselenenyl substituent, and show that tranformations of the latter can, in the case of 5-selenylated uridine, produce products that are inhibitory to malarial and human orotate phosphoribosyltransferase.
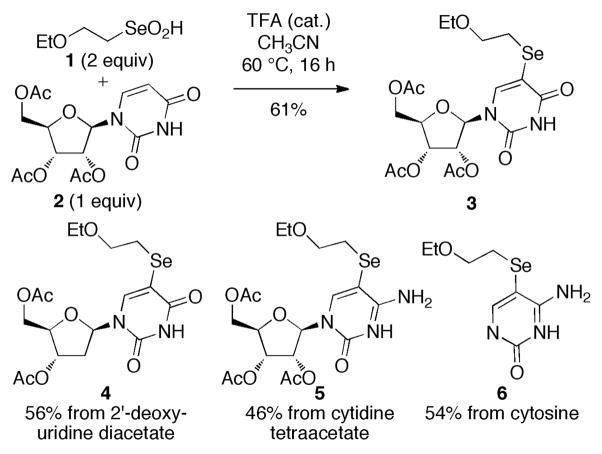
2-Ethoxyethaneseleninic acid (1, Scheme 1), prepared from bromoethyl ethyl ether, reacts with uridine triacetate 2 under acidic conditions (catalytic trifluoroacetic acid) to give as the major product the 5-selenylated nucleoside 3.5 The 5-selenylated pyrimidines 4–6 were prepared analogously.
Scheme 1.
Electrophilic Selenylation with EtOCH2CH2SeO2H
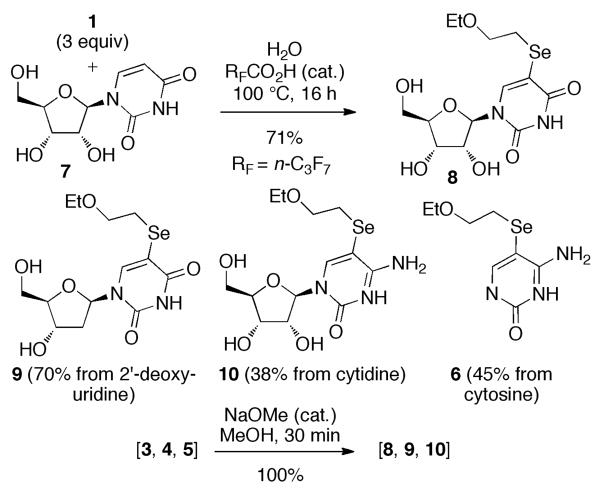
Would this selenylation reaction work in aqueous solution? Water soluble nucleosides did indeed give the 5-selenylated products 8, 9, and 10, and cytosine gave 6, when the reaction was performed in the presence of heptafluorobutanoic acid (bp 120 °C), Scheme 2. Deacetylation of the nucleoside triacetates from Scheme 1 confirmed their structures.
Scheme 2.
Selenylation in Aqueous Solution
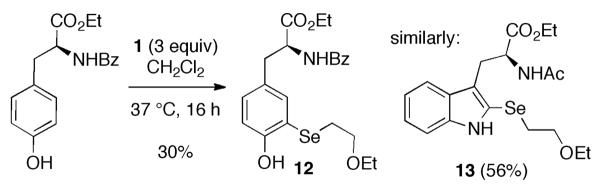
More reactive aromatic rings, such as those contained in tyrosine and tryptophan, selenylated more easily, even without added acid catalyst (Scheme 3). Less reactive rings, such as those in phenylalanine derivatives, did not selenylate.
Scheme 3.
Selenylation of tyrosine and tryptophan derivatives
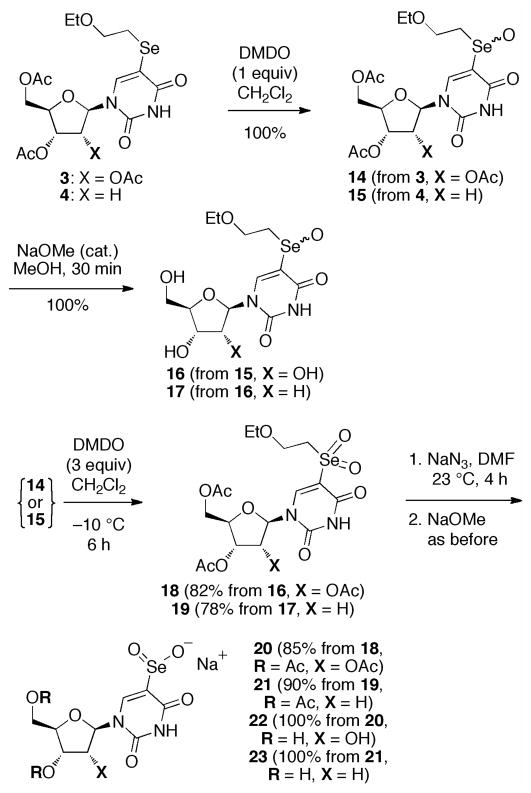
By altering the oxidation state and substitution at Se, selenoethers can be transformed to a variety of related organoselenium species. Thus, DMDO oxidation of 3 (Scheme 4) led cleanly to the stable selenoxide 14 (two diasteriomers at Se) or, with additional reagent, the selenone 18. Retro-ene elimination of ArSeOH,6 normally spontaneous at 23 °C, is suppressed by the beta heteroatom in the ethoxyethyl chain.7 Nucleophilic dealkylation of 18 with sodium azide8 gave the uridine 5-seleninic acid 20. Respective deacetylation of 14 and 20 gave the triols 16 and 22, and, in the analogous 2′-deoxy series, 15 and 21 gave diols 17 and 23.
Scheme 4.
Oxidation of 5-Selenylated Nucleosides
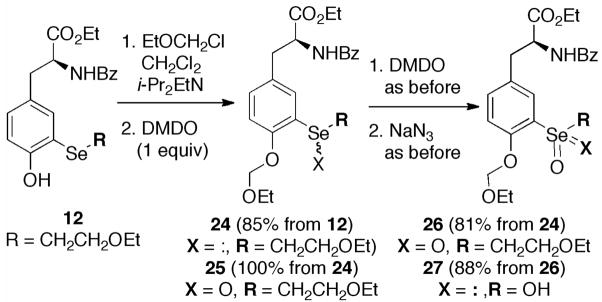
Because of the susceptibility of phenols to oxidation, comparable transformations of 12 could only be accomplished following protection of the phenolic –OH (Scheme 5). The selenoxide 25 and selenone 26 were prepared as before, and dealkylation gave the seleninate 27. Analogous oxidation of 13 was unsuccessful.
Scheme 5.
Oxidation of Selenylated Tyrosine Derivative
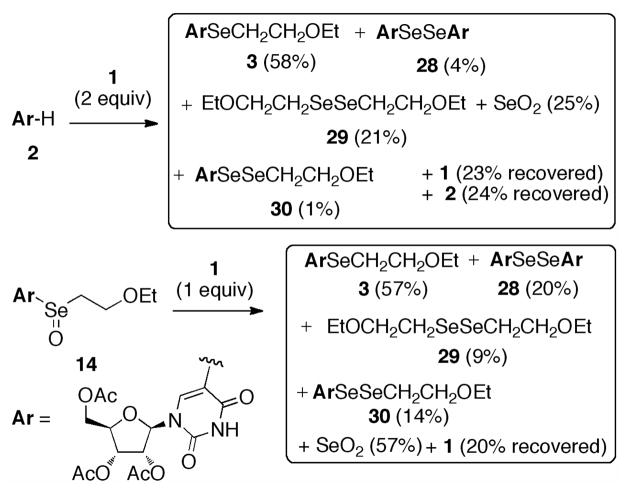
Careful purification of product mixtures and identification of minor products allowed some insight into the mechanism of selenylation (Scheme 6). Reaction of 2 gave, in addition to 3, the diselenides 28, 29, and 30. By subjecting selenoxide 14 to the same conditions, we were able to isolate selenoether 3 and a different mix of 28, 29, and 30. Diselenide 28 results from reductive coupling9 of ArSeOH, the product of retro-ene elimination from 14, and 29 and 30 result from reductive coupling of 1 and diselenide scrambling,10 respectively. These results strongly implicate selenoxide 14 as an intermediate in the selenylation of 2. Formation of 14 could arise from initial addition of electrophilic EtOCH2CH2Se(OH)2 +, followed by loss of water. Reduction of 14 to 3 evidently occurs in part by co-oxidation of seleninate 1 to 2-ethoxyethaneselenonic acid, which then decomposes to 2-ethoxyethanol and SeO2. The latter was isolated in both reactions, and identified unambiguously by 77Se NMR.
Scheme 6.
Full Product Analysis of Selenylation Reactions
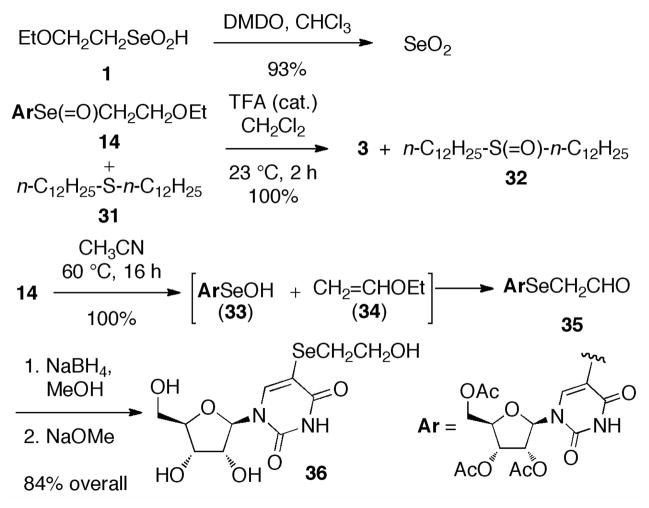
Several control reactions (Scheme 7) provide further support for the intermediacy of 14. Purposeful oxidation of seleninate 1 with DMDO gave SeO2, as expected. Redox reaction of 14 with didodecyl disulfide (31) led to sulfoxide 32 along with 3 (catalytic TFA was required for this reaction), illustrating the ease with which O may be transferred from the selenoxide. However, adding 31 to the reaction of 1 and 2 did not improve the yield, but rather blocked the selenylation by reducing 1. Finally, thermolysis of 14 in the absence of acid or a reducing agent gave the aldehyde 35, presumably by way of the retro-ene reaction, followed by efficient re-addition of the selenenic acid 33 to alkene 34.11 Reduction of 35 and then deacetylation gave the tetrol 36.
Scheme 7.
Control Reactions for Selenylation Mechanism
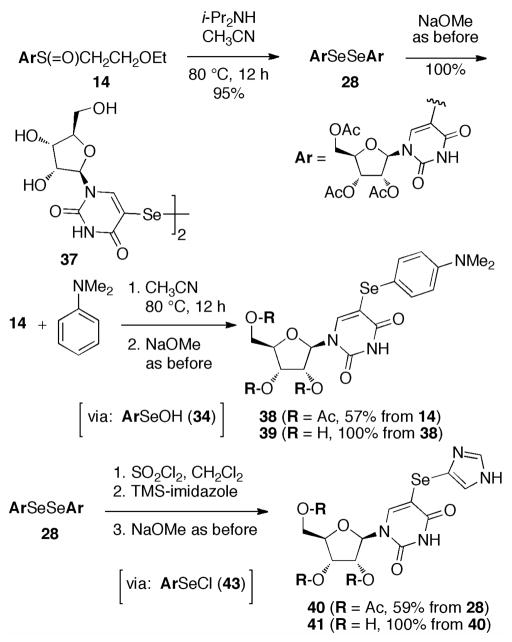
The thermolytic formation of 33 under mild conditions allowed its interception by i-Pr2NH (Scheme 8), providing not the selenenamide,12 but rather the diselenide 28. Alternatively, 33 was trapped by dimethylaniline13 to give the (mixed) diarylselenide 38, and 28 was oxidatively cleaved and then coupled to N-trimethylsilylimidazole14 to provide 38. Respective deacetylation gave 5-selenylated uridines 37, 29, and 41.
Scheme 8.
Transformations of ArSeOH
Orotate phosphoribosyltransferase (OPRT) is an essential enzyme for the de novo biosynthesis of pyrimidine nucleotides, promoting the attachment of orotic acid to phosphoribosylpyrophosphate.15 This is the only pathway for pyrimidine nucleotide production in Plasmodium falciparum, the major causative agent of malaria.16 Furthermore, new ways of interrupting nucleotide synthesis in rapidly proliferating human cancer cells may offer alternative therapy options for this disease. The 5-selenated nucleosides were evaluated as competitive inhibitors of malarial and human OPRTs. Six of the relatively non-polar uridine derivatives (8, 10, 36, 37, 39, and 41) reached sub-micromolar Ki values for human OPRT (HsOPRT), and three of these (37, 39, and 41) were also sub-micromolar inhibitors of P. falciparum OPRT (PfOPRT) (Table 1). The most active nucleoside, diselenide 37, is particularly interesting as it may represent a prototype for OPRT inhibitiors that can bind to both subunits of a homodimeric active site. Synthetic access to the selenylated nucleosides also provides new opportunities for investigating the inhibition of additional nucleoside processing enzymes, including pyrimidine nucleoside kinases, thymidine kinase, thymidylate synthetase, thymidine phosphorylase, and orotidine monophosphate decarboxylase.
Table 1.
Inhibition of OPRTs by Selenylated Nucleosides
| compound | Ki, μM (PfOPRT) | Ki, μM (HsOPRT) |
|---|---|---|
| 6 | 2.15 ± 0.75 | 1.14 ± 0.48 |
| 8 | 1.30 ± 0.33 | 0.26 ± 0.03 |
| 10 | 1.44 ± 0.44 | 0.44 ± 0.10 |
| 16 | 6.10 ± 1.60 | 2.40 ± 0.39 |
| 22 | 10.82 ± 1.98 | 5.89 ± 1.31 |
| 36 | 1.54 ± 0.47 | 0.72 ± 0.21 |
| 37 | 0.16 ± 0.02 | 0.16 ± 0.03 |
| 39 | 0.75 ± 0.21 | 0.24 ± 0.10 |
| 41 | 0.92 ± 0.28 | 0.91 ± 0.27 |
Supplementary Material
Acknowledgments
The authors are grateful to the Prusoff Foundation for financial support at Rutgers, and to the Rohm and Haas Company for a graduate assistantship to MA. Support at the Albert Einstein College of Medicine was provided by NIH research grants GM41916 and AI049512.
Footnotes
Supporting Information Available: Details of the enzymatic evaluation, and the preparation and spectroscopic characterization for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Iwaoka M, Tomoda S. Top Curr Chem. 2000;208:55–80. [Google Scholar]; Wirth T. Top Curr Chem. 2000;208:1–5. [Google Scholar]; Back TG. Organoselenium Chemistry – A Practical Approach. Oxford Press; New York: 2000. [Google Scholar]
- 2.Mugesh G, du Mont WW, Sies H. Chem Rev. 2001;101:2125–2179. doi: 10.1021/cr000426w. [DOI] [PubMed] [Google Scholar]; Soriano-Garcia M. Curr Med Chem. 2001;11:1657–1669. doi: 10.2174/0929867043365053. [DOI] [PubMed] [Google Scholar]; Armishaw CJ, Daly NL, Nevin ST, Adams DJ, Craik DJ, Alewood PF. J Biol Chem. 2006;281:14136–14143. doi: 10.1074/jbc.M512419200. [DOI] [PubMed] [Google Scholar]; Abdo M, Liu S, Zhou B, Walls CD, Knapp S, Zhang ZY. J Am Chem Soc. 2008;130:13196–13197. doi: 10.1021/ja804489m. [DOI] [PubMed] [Google Scholar]; Desai D, Madhunapantula SV, Gowdahalli K, Sharma A, Chandagaludoreswamy R, El-Bayoumy K, Robertson GP, Amin S. Bioorg Med Chem Lett. 2010;20:2038–2043. doi: 10.1016/j.bmcl.2009.09.071. and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdo M, Knapp S. J Am Chem Soc. 2008;130:9234–9235. doi: 10.1021/ja8034448. [DOI] [PubMed] [Google Scholar]
- 4.Electrophilic aryl selenylations: (phenols) Oddershede J, Henriksen L, Larsen S. Org Biomol Chem. 2003;1:1053–1060. doi: 10.1039/b211130f.and references cited therein. (indoles) Crich D, Davies JW. Tetrahedron Lett. 1989;30:4307–4308.and references cited therein.
- 5.Other uridine selenylations: Choi S, Kalman TI, Bardos TJ. J Med Chem. 1979;22:618–621. doi: 10.1021/jm00192a004.Schinazi R, Arbiser J, Lee J, Kalman T, Prusoff W. J Med Chem. 1986;29:1293–1295. doi: 10.1021/jm00157a031.Lee CH, Kim YH. Tetrahedron Lett. 1991;32:2401–2404.Goudgaon NM, Naguib FNM, el Kouni MH, Schinazi RF. J Med Chem. 1993;36:4250–4254. doi: 10.1021/jm00078a015.Roh KR, Chang HK, Kim YH. Heterocycles. 1998;48:437–4441.Hassan AEA, Sheng J, Jiang J, Zhang W, Huang Z. Org Lett. 2009;11:2503–2506. doi: 10.1021/ol9004867.
- 6.Sharpless KB, Lauer RF. J Am Chem Soc. 1973;95:2697–2699. [Google Scholar]
- 7.Tiecco M, Testaferri L, Tingoli M, Marini F. J Org Chem. 1993;58:1349–1354. and references cited therein. [Google Scholar]
- 8.Tiecco M, Testaferri L, Bagnoli L, Scarponi C, Temperini A, Marini F, Santi C. Tetrahedron: Asymmetry. 2007;18:2758–2767. [Google Scholar]
- 9.Reich HJ, Wollowitz S, Trend JE, Chow F, Wendelborn DF. J Org Chem. 1978;43:1697–1705. [Google Scholar]
- 10.Pleasants JC, Guo W, Rabenstein DL. J Am Chem Soc. 1989;111:6553–6558. [Google Scholar]
- 11.Reich HJ, Jasperse CP. J Org Chem. 1888;53:2390–2392. [Google Scholar]
- 12.Reich HJ, Renga JM. J Org Chem. 1975;40:3313–3314. [Google Scholar]
- 13.Gassman PG, Miura A, Miura T. J Org Chem. 1982;47:951–954. [Google Scholar]
- 14.Back TG, Kerr RG. Can J Chem. 1986;64:308–310. [Google Scholar]
- 15.Zhang Y, Luo M, Schramm VL. J Am Chem Soc. 2009;131:4685–4694. doi: 10.1021/ja808346y. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jones ME. Ann Rev Biochem. 1980;49:253–279. doi: 10.1146/annurev.bi.49.070180.001345. [DOI] [PubMed] [Google Scholar]; Gero AM, O’Sullivan WJ. Bood Cells. 1990;16:467–484. [PubMed] [Google Scholar]
- 16.Krungkrai SR, Aoki S, Palacpac NMQ, Sato D, Mitamura T, Krungkrai J, Horii T. Mol Biochem Parasitol. 2004;134:245–255. doi: 10.1016/j.molbiopara.2003.12.006. [DOI] [PubMed] [Google Scholar]; Bello AM, Poduch E, Liu Y, Wei L, Crandall I, Wang X, Dyanand C, Kain KC, Pai EF, Kotra LP. J Med Chem. 2008;51:439–448. doi: 10.1021/jm7010673. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.