Abstract
ATP-binding cassette (ABC) transporters are able to efflux their substrate drugs from the cells. We compared expression of efflux proteins in normal human corneal epithelial tissue, primary human corneal epithelial cells (HCEpiC), and corneal epithelial cell culture model (HCE model) based on human immortal cell line. Expression of multidrug resistance protein 1 (MDR1), multidrug resistance-associated protein 1–6 (MRP1–6) and breast cancer resistance protein (BCRP) was studied using quantitative RT-PCR, Western blot, and immunohistochemistry. Only MRP1, MRP5, and BCRP were expressed in the freshly excised human corneal epithelial tissue. Expression of MRP1 and MRP5 was localized predominantly in the basal cells of the central cornea and limbus. Functional efflux activity was shown in the cell models, but they showed over-expression of most efflux transporters compared to that of normal corneal epithelium. In conclusion, MRP1, MRP5, and BCRP are expressed in the corneal epithelium, but MDR1, MRP2, MRP3, MRP4, and MRP6 are not significantly expressed. HCE cell model and commercially available primary cells deviate from this expression profile.
Keywords: corneal epithelium, cell model, primary cells, HCE, ABC transporters, multidrug resistance transporters, efflux pumps, cell culture, multidrug resistance-associated proteins, P-glycoprotein
INTRODUCTION
The cornea is the major route of drug absorption into the intraocular tissues after instillation of eye drops.1 The outermost corneal tissue, the corneal epithelium, forms a tight barrier for drug permeation, because the most apical epithelial cells are interconnected by tight junctions.1 Small and lipophilic molecules (optimal log DpH74 2–3) can diffuse through the corneal epithelium by partitioning first into the cell membranes, and then diffusing further from the epithelium into the stroma and the endothelium.2 Corneal permeability of hydrophilic drugs is low due to the rate-limiting poor partitioning into the surface layers of the corneal epithelium. It is important to realize that the deeper layers of the corneal epithelium, the stroma, and the endothelium, allow intercellular diffusion of hydrophilic, and even large molecules, such as proteins.3,4 On the contrary, very lipophilic drugs (log DpH7.4>3) partition easily into the corneal epithelium, but their overall corneal permeation is rate-limited by their slow transfer from the epithelium to the hydrophilic stroma.5 Therefore, they have lower passive permeability than the molecules with intermediate lipophilicity (log P 2–3).6,7 In general, the ocular bioavailability of the topically administered drugs is low, usually less than 5%.3,8
Efflux proteins restrict the intracellular accumulation of drugs by transporting them from the intracellular to the extracellular space. ATP-binding cassette (ABC) transporters are among the most important efflux transporters. ABC subclasses B, C, and G include at least 10 efflux transporters that may be relevant in pharmacokinetics. It has been estimated that 25% of clinically used drugs are substrates of efflux transporters. ABC transporters are expressed in several epithelial and endothelial tissue barriers that limit drug permeation between compartments of the body, for example, epithelium of small intestine, blood–brain barrier (BBB), kidney tubuli, and blood–retina barrier.9,10
Expression profile of the efflux transporters in the human corneal epithelium is still poorly known, because most studies in the field have been done with whole cornea specimens, animal tissues, or cell lines. Conflicting results on expression of multidrug resistance protein 1 (MDR1) and multidrug resistance-associated protein 1 (MRP1) have been published, whereas expression levels of breast cancer resistance protein (BCRP) and MRP2 have been insignificant or low.11–13 Discrepancies emphasize the need for further studies on these transporters, particularly by using methods that allow reliable comparison of the expression within the same study. Interestingly, expression of several other ABC transporters such as MRP3, MRP4, MRP5, and MRP6 has not been studied in the normal isolated human corneal epithelium.
Cultured cell models are important alternatives to animal studies in pharmacology. Previously, our research group introduced a cell culture model of immortalized human corneal epithelial cells (HCE model) for drug studies.14 The morphology of the HCE model resembles the normal cornea and the permeability barrier of the HCE model is comparable with the isolated rabbit corneas in diffusion chambers.14,15 Thus, this model can be useful in permeability studies of ocular drug candidates. However, the active transporters of the HCE model are poorly characterized.
The aim of this study was to characterize the overall expression profile of effluxing ABC transporters in the normal human corneal epithelium. The profile was compared to the expression pattern of the HCE model and commercially available human primary corneal epithelial cells (HCEpiC cells). Expression profiles of MDR1 (ABCB1), MRP1-6 (ABCC1-6), and BCRP (ABCG2) were studied at the mRNA and protein levels, and the efflux activity of the cell models was investigated.
MATERIALS AND METHODS
Materials
Calcein-AM and cyclosporin A (CsA) were purchased from Calbiochem (La Jolla, CA). 5(6)-Carboxy-2′, 7′-dichlorofluorescein (CDCF) and 5(6)-carboxy-2′,7′-dichlorofluorescein diacetate (CDCFDA) were from Fluka (Buchs, Switzerland), MK571 was from Cayman Chemicals (Ann Arbor, MI) and verapamil from ICN Biomedicals (Irvine, CA). Probenecid and progesterone were from Sigma (St Louis, MO). MRP1 (MRPr1) and MRP3 (M3II-9) antibodies were purchased from Alexis Biochemicals (San Diego, CA), MRP2 (M2 III-6), MRP4 (M4I-10), MRP5 (M5I-1), and BCRP (BXP-21) were from Abcam (Cambridge, UK) and β-actin (C4) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Horseradish peroxidase-conjugated goat secondary antibodies were from Zymed Laboratories, Inc. (San Francisco, CA). ECL™ Western Blotting Detection Reagents were purchased from GE Healthcare (Little Chalfont, Buckinghamshire, UK).
Cell Culture
Immortalized human corneal epithelial cells (HCE cells) (passages 23–41) were grown as described by Toropainen et al.15 For experiments where nonconfluent, dividing state of cells was studied cells were cultured in flasks and harvested at 80% confluency. For HCE cell culture model cells were cultured on collagen-coated polyester filters (Transwell-Clear™, Costar, Cambridge, MA) at the seeding density of 90,000 cells/cm2. After 7–10 days, HCE cells were exposed to a liquid-air interface by culturing cells without the apical medium for 2–3 weeks. The cell culture medium was supplemented with 40 µg/mL L(+)-ascorbic acid. The tightness of the HCE culture on filter was followed by measuring transepithelial electrical resistance (TER) (Endohm, World Precision Instruments, Sarasota, FL). Stratified and polarized HCE cell layers with TER value greater than 380 Ω cm2 were used in the experiments.
Primary HCEpiC cell line was purchased from ScienCell Research Laboratories (San Diego, CA) and cells were cultured according to supplier’s instructions, and cells with passage numbers 5–12 were used in the experiments.
Human Corneal Epithelial Tissue
Human corneal epithelial tissue samples were obtained from patients, who underwent photorefractive keratectomy (PRK) eye surgery for the correction of their refractive error. Patients with manifest ocular pathology or ocular topical drug therapy were excluded. The epithelial cells were scraped off with a Beaver blade before the photoablation (Silmäkeskus Laser Oy, Oper Oy, Helsingin yksityinen silmasäiraala Oy, Finland). Tissue samples were immediately frozen in dry ice and were maintained in −70°C till RNA and protein extraction. This study was approved by the Northern Savo regional committee for research ethics (Kuopio) and written permission was obtained from patients before collecting tissue samples. The research was carried out in accordance with the Helsinki Declaration.
For the immunohistochemical analysis, whole human corneas were obtained from donor eyes provided by the National Disease Research Interchange (NDRI, Philadelphia, PA) and processed within 24–48 h from death.
Real-Time RT-PCR
The mRNA expression levels of MDR1, MRP1–6, and BCRP were measured with quantitative realtime RT-PCR. Primers and probes for MDR1, MRP2, and BCRP were custom-made as described by Korjamo et al.16 FAM-labeled Assay on Demand TaqMan® Gene Expression assay (Applied Biosystems, Foster City, CA) was used to study MRP1 (Hs00219905_m1), MRP3 (Hs00358656_m1), MRP4 (Hs00195260), MRP5 (Hs00981071), and MRP6 (Hs00184566_m1).
Total RNA was extracted by using TRI Reagent® (Sigma) and treated with DNase (DNA free, Ambion, Austin, TX). RNA concentration was measured using RiboGreen quantification assay (Molecular Probes, Leiden, The Netherlands). Two micrograms of RNA was transcribed using M-Mulv reverse transcriptase and random primers (Fermentas, Hanover, MD), and cDNA corresponding to 40 ng RNA was amplified with ABI Prism 7500 (Applied Biosystems, UK) using TaqMan universal master mix (Applied Biosystems, Foster City, CA) and transporter specific primer/probe sets.
The amounts of mRNA-transcripts were calculated from standard curves derived from DNAfragments carrying the same nucleotide sequence. The DNA-standards were achieved from expression plasmids of MDR1,17 MRP1,18 MRP2,19 MRP3,20 MRP4,21 MRP5,22 MRP6,23 and BCRP.24 Briefly, the DNA-fragment containing certain efflux protein sequence was cut with appropriate restriction enzymes and purified from agarose gel after electrophoresis. Concentration of DNA was quantified with PicoGreen reagent (Molecular Probes).
Western Blot
Western blot was used to estimate the expression of MRP1–5 and BCRP at the protein level. Cells were lysed in a buffer containing 1% Triton X-100, 20 mM Tris–HCl (pH 6.8), 150 mM NaCl, 1mM EDTA, and 1:200 protease inhibitor cocktail for 30 min on ice. After centrifugation the cell extracts (50 or 75 µg protein) were separated on 7.5% or 10% SDS–PAGE gel and electroblotted onto a nitrocellulose membrane (Amersham Biosciences, Freiburg, Germany). Used primary antibody dilutions were 1:5000 for MRP1, 1:4000 for MRP2, 1:200 for MRP3, 1:5000 for MRP4, 1:500 for MRP5, 1:2000 for BCRP, and 1:2000 for β-actin. Primary antibodies were detected with HRP-conjugated goat secondary antibodies diluted 1:2000 and ECL detection system.
Immunohistochemistry
Localization of the MRP1 and MRP5 proteins in human corneal tissue sections was detected with immunohistochemistry. Freshly frozen human corneal cryosections (5–8 µm thick) were prepared as previously described.25–27 After fixation with ice-cold methanol at −20°C for 10 min, the tissue sections were blocked with 2% BSA (w/v) in PBS for 30 min and then incubated with 1:50 dilution of primary antibody, 1% BSA (w/v) in PBS at 4°C overnight. Following several washes in PBS, the sections were labeled with a 1:500 dilution of a FITC-labeled goat anti-rat secondary antibody (Sigma) 1 h at room temperature. The slides were rinsed three times with PBS and covered with antifade medium (Vectashield; Vector Laboratories, Burlingame, CA). Fluorescence was visualized on a fluorescence microscope (Axiophot2; Carl Zeiss Meditec, Inc., Thornwood, NY). To check the specificity of fluorescent labeling, the sections were solely reacted with the secondary antibody.
Calcein Efflux Assay
Functionality of efflux proteins in primary HCE-piC and immortalized HCE cells was assessed by the calcein efflux assay.28,29 Cells were incubated with calcein acetoxymethyl ester (calcein-AM) which is hydrolyzed to fluorescent calcein inside the cells. Calcein-AM is a substrate for MDR1 and MRP1, whereas calcein is transported by MRP1 and MRP2.30,31 Inhibition of efflux transport leads to calcein retention that can be quantified by measuring the intracellular fluorescence. Inhibitors used were 15 µM CsA, 200 µM progesterone, 500 µM verapamil, and 100 µM MK571.
Briefly, the cells were cultured on 96-well plates for 4 days with the seeding density of 50,000 cells/well and incubated thereafter for 15 min at 37°C in a reaction buffer (25 mM Hepes, HBSS, pH 7.4) containing efflux inhibitors or in reaction buffer only (control). Test compounds were dissolved in DMSO (final concentration, 1%). Calcein-AM (2 µM) was added to the cells, and incubation was continued for 20 min at 37°C. Test solutions were replaced with ice-cold reaction buffer and the fluorescence was measured with Victor 1420 Multilabel Counter (Wallac, Finland) using 480 nm for excitation and 535 nm for emission. The results are presented as percentage of fluorescence compared to the control; where the control is uninhibited transport (100%). The experiments were repeated 4–6 times and each measurement had three replicates.
CDCF Efflux Assay
Efflux protein activity was also assessed by measuring the transport of CDCF in HCEpiC and HCE cells. Cells were loaded with CDCFDA, the diacetate form of CDCF, which passively permeates the cell membranes. The hydrolyzed form CDCF can pass the cell membrane by active efflux mediated by MRP2, MRP3, MRP5, and perhaps also by MRP1.32–35 Probenecid was used as nonspecific inhibitor of MRP proteins.36
HCEpiC and HCE cells were grown on 96-well plates for 4 days with the seeding density of 50,000 cells/well. First, the cells were washed and preincubated with reaction buffer (25 mM Hepes, HBSS, pH 7.4) for 30 min at 37°C. Then, 5 µM CDCFDA in the reaction buffer was added to the wells and incubated for 20 min. After CDCFDA loading, the cells were washed two times with the reaction buffer. Fresh buffer with or without 1 mM probenecid was added to the wells and samples were taken at 30, 60, 90, and 120 min. Concentration was determined by the measuring fluorescence with Victor 1420 Multilabel Counter using 480 nm for excitation and 535 nm for emission. Experiments were performed using horizontal plate mixer (Titramax 1000 and Incubator 1000, Heidolph-Instruments, Schwabach, Germany) at 150 rpm and 37°C. The CDCF efflux assays were repeated three times each measurement having four replicates.
Statistical Testing
Kruskal–Wallis analysis was used to compare multiple experimental groups of calcein efflux assay. If the differences between groups were statistically significant (p < 0.05), analysis was continued with comparisons versus control group using Dunn’s method. Statistical analyses were calculated with SigmaPlot 11.0 (Systat Software, Inc., San Jose, CA).
RESULTS
Efflux Protein Expression at mRNA Level
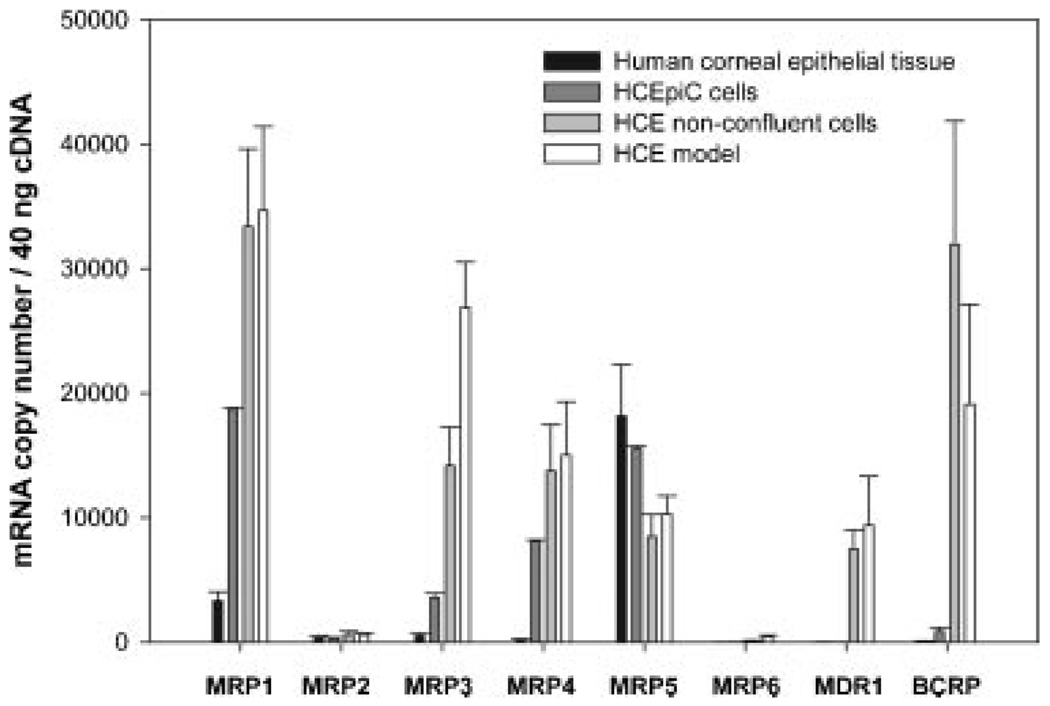
The expression of eight efflux transporters, namely MDR1, MRP1-MRP6, and BCRP was studied at the mRNA level in human corneal epithelial tissue, primary HCEpiC cells, in non-confluent HCE cells and in HCE model using realtime RT-PCR with gene-specific DNA-standards (Fig. 1). Interestingly, only MRP1 and MRP5 mRNA were clearly present in the human corneal epithelium. MRP5 was expressed at fivefold higher level than MRP1. Importantly, the data shows that there is no or very low mRNA expression of MDR1, MRP2, MRP3, MRP4, MRP6, or BCRP in the normal human cornea. In the cell lines several efflux transporters were upregulated. The expression of MRP1, MRP3, and MRP4 were 6-, 7-, and 46-fold higher in the HCEpiC cells and 10-, 52-, and 85-fold higher in the HCE model than in human corneal epithelium. In addition, in HCE model moderate MDR1 and high BCRP expression was detected. The polarization and stratification seems to have only a slight effect on the efflux protein expression in HCE cells, since only small differences were detected between the HCE model and nonconfluent HCE cells.
Figure 1.
mRNA expression levels of efflux proteins in human corneal epithelial tissue, in primary HCEpiC cells, in nonconfluent HCE cells and in stratified, polarized HCE cells grown on filters (HCE model). Data are expressed as mean ± SD (n = 2–4).
Expression at Protein Level
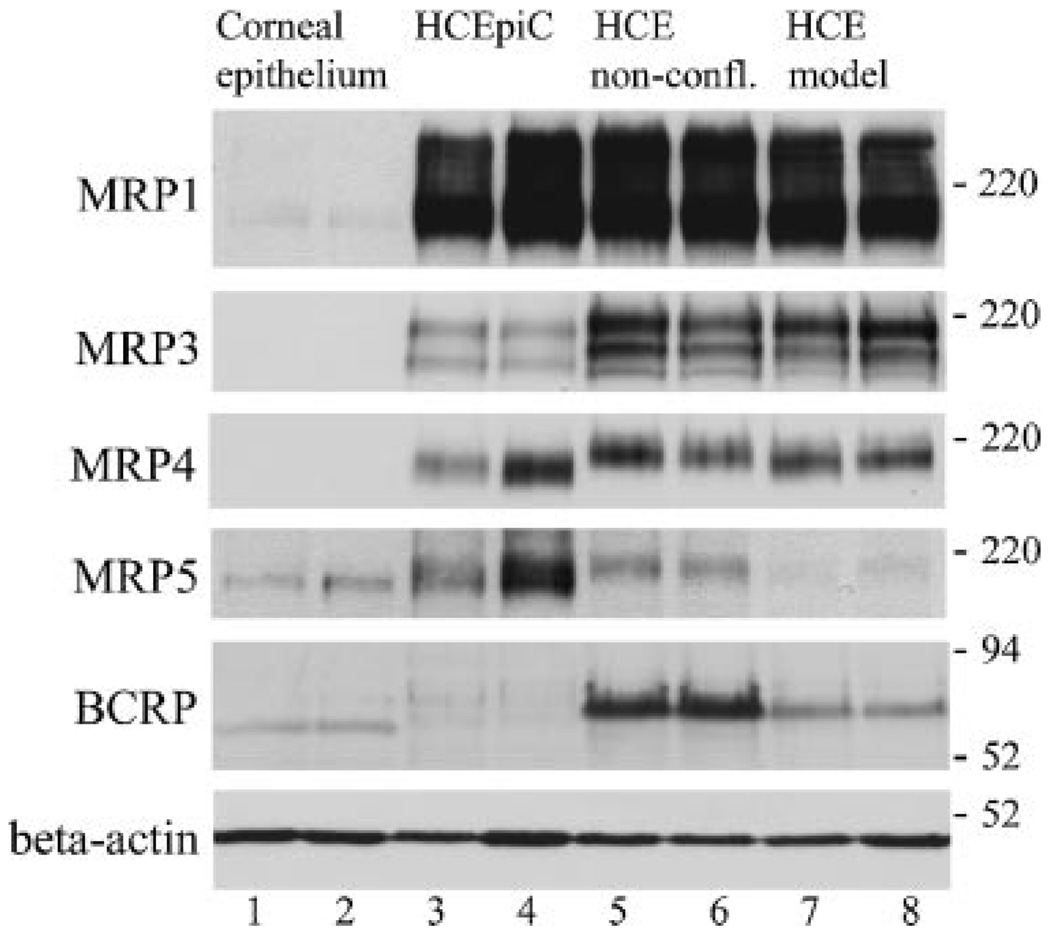
MRP1 protein was most abundantly expressed at the protein level in HCEpiC and HCE cells (Fig. 2, lanes 3–8). However, only faint MRP1 expression was observed in the epithelial tissue (lanes 1 and 2). Expression of MRP2 protein was not observed in the human corneal epithelial tissue or cell samples (data not shown). MRP3 and MRP4 were moderately expressed in HCEpiC and HCE cell lines, but not in the epithelial tissue. MRP5 expression was detected in all examined samples with the highest expression levels in the HCEpiC primary cell line. Expression of BCRP was evident in the HCE cell line (lanes 5–8). However, in the corneal epithelium, the expressed BCRP protein (lanes 1 and 2) was migrating slightly faster in SDS–PAGE than the BCRP protein expressed in the HCE cell line. Only minor BCRP expression was seen in the HCEpiC cells (lanes 3 and 4). Different samples contained similar amounts of protein as detected by β-actin. These Western blot results of efflux proteins correlated well with the results gained from real-time RT-PCR studies.
Figure 2.
Efflux protein expression in human corneal epithelial tissue (lanes 1 and 2), HCEpiC (lanes 3 and 4), nonconfluent HCE cells grown in flasks (lanes 5 and 6), and stratified HCE cells grown on filters (HCE model) (lanes 7 and 8) each detected from two separate samples by Western blot. Each sample contains 50 µg protein except in MRP1- and MRP5-protein immuno-detection 75 µg protein was used (excluding lane 4, in which 50 µg protein extract was used). Immunodetection of various efflux proteins was performed as described in Materials and Methods Section.
Localization of Efflux Proteins in Human Corneal Epithelium
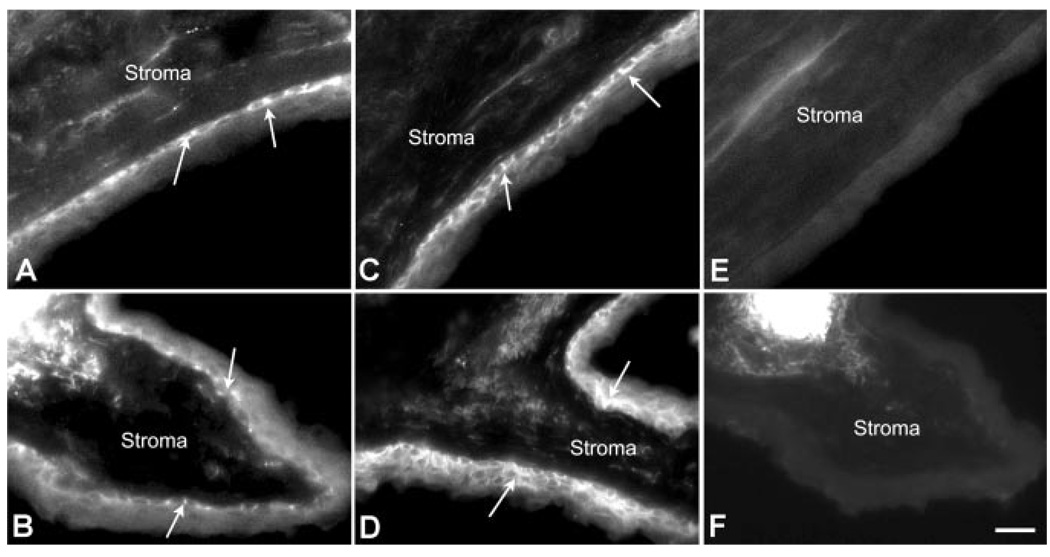
Since MRP1 and MRP5 expression in human corneal tissue was observed by real-time RT-PCR and Western blot, the cellular localization of MRP1 and MRP5 was examined by immunohis-tochemistry (Fig. 3). MRP1 immunostaining was predominantly detected in the basal cell layer (a.k.a. basolateral membrane) of the central and limbal human corneal epithelium (Fig. 3A and B). MRP5 protein appears to be strongly expressed in the basal cells of the central cornea (Fig. 3C) and in the basal and wing cells of the limbal region (Fig. 3D). The labeling of MRP1 and MRP5 seems to be distributed all over the plasma membrane of these basal cells facing the stroma. There was no apparent labeling of either protein in the apical membrane. No fluorescence was observed in the negative controls, the corneal and limbal sections (Fig. 3E and F, respectively) that were solely incubated with the FITC-conjugated secondary antibody.
Figure 3.
Immunolocalization of MRP1 and MRP5 in the human corneal epithelium. Cryosections from human donor tissue (5–8 µm thick) fixed in ice-cold methanol were probed with anti-MRP1 or anti-MRP5 antibodies. Positive MRP1 and MRP5 fluorescent labeling was restricted to the basolateral surfaces in both the central (A and C) and limbal (B and D) corneal regions, respectively. No fluorescent staining was observed when the central (E) and limbal (F) corneal epithelial sections were incubated with the FITC-conjugated secondary antibody alone. Arrows indicate epithelial labeling. The stromal surface is labeled to indicate the basal side of the epithelial membrane. Bar represents 20 µm.
Efflux Protein Activity
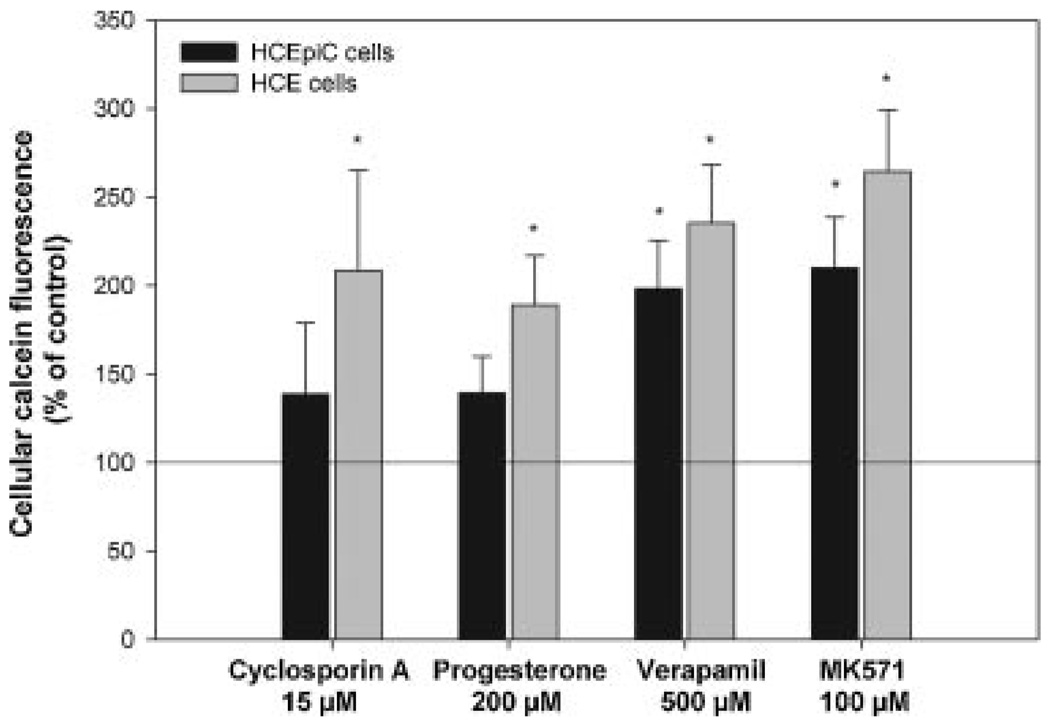
The functionality of efflux proteins was assessed only in the cultured corneal epithelial cells, since studying the efflux protein activity in the normal human corneal epithelium is very difficult due to the small number of cells isolated. Overall efflux protein activity in HCEpiC and HCE cell lines was examined by measuring intracellular calcein retention in the presence of various efflux protein inhibitors. Inhibition of calcein efflux activity was detected in both cell lines (Fig. 4). In the primary HCEpiC cells, nonspecific efflux protein inhibitors MK571 and verapamil, increased calcein retention approximately by twofold, when compared to control cells. MK571 has been earlier considered as MRP-specific inhibitor, but recently found to inhibit also MDR1 and BCRP.37 In HCE cells, the inhibitory effect of MK571 and verapamil on efflux transport was ~2.5-fold. Also CsA and progesterone increased the calcein retention by 2.0-fold in the HCE model, but in the primary cell line (HCEpiC) the calcein retention was only slightly elevated.
Figure 4.
Calcein retention into HCEpiC and HCE cells after incubating with calcein-AM in the presence of efflux protein inhibitors including 15 µM cyclosporine A, 200 µM progesterone, 500 µM verapamil, and 100 µM MK571. The results are expressed as a percentage of fluorescence relative to control (100%). The data are means ± SEM (n = 4–6), *p < 0.05.
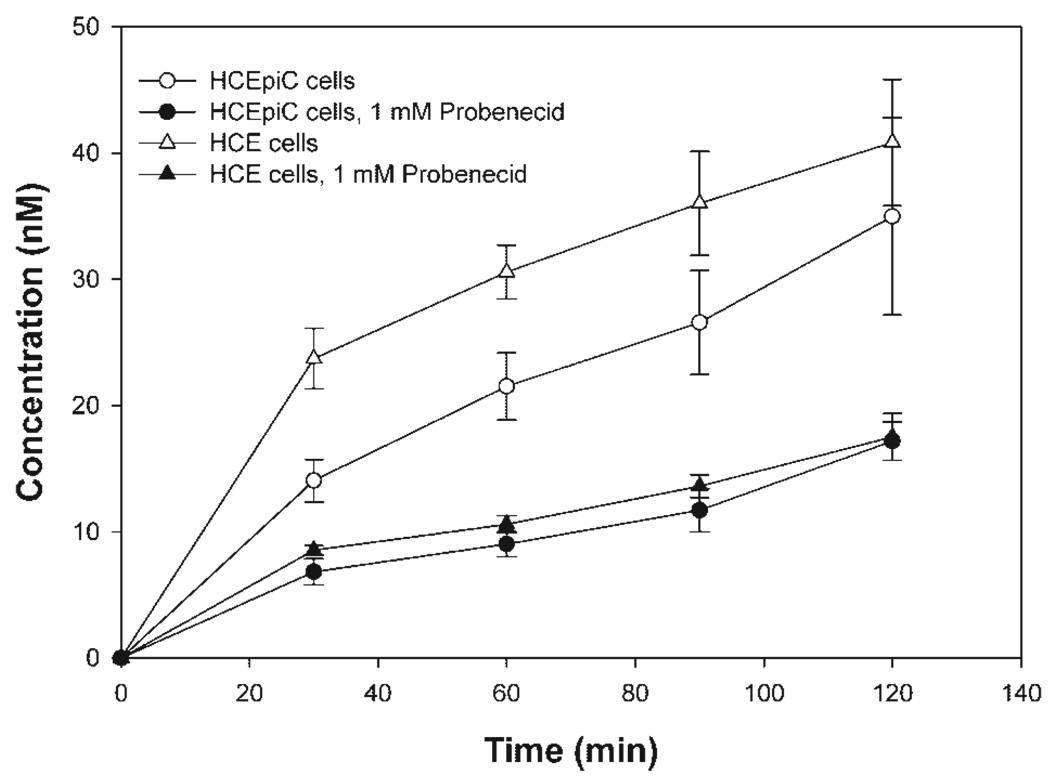
MRP-dependent efflux activity was also examined by measuring CDCF transport in HCEpiC and HCE cells. CDCF efflux was detected in both cell lines and probenecid inhibited appearance of CDCF with similar efficacy (Fig. 5). Since CDCF is high affinity substrate of MRP5,34 the efflux of CDCF in the primary HCEpiC and immortal HCE cell lines may suggest some MRP5-mediated efflux, but also other transporters, including MRP3 and MRP1, may also play role in CDCF efflux.
Figure 5.
Time-dependent CDCF efflux from HCEpiC and HCE cells in the absence and presence of inhibitor, 1 mM probenecid. The data are expressed as means ± SD (n = 3).
DISCUSSION
Apically localized efflux proteins in the corneal epithelium may limit the drug absorption by pumping drugs from the cells into the lacrimal fluid. Conversely, basolaterally localized efflux proteins may improve drug absorption by facilitating the drug transfer from the epithelial cells to the stroma. At least 21 ophthalmic drugs (e.g., antibiotics, antiviral, antifungal, and anti-inflammatory drugs) have been suggested to interact with efflux proteins.38 Therefore, it is important to investigate the expression and function of efflux proteins in the human corneal epithelium. In this study, we examined the presence of efflux proteins in the human corneal epithelial tissue and the validity of commercially available human primary cells and HCE model in ocular drug efflux studies. This is the first quantitative and systematic study on efflux transport expression in the normal corneal epithelium and the available cell models. The quantitative RT-PCR method with plasmid standards allows comparison of the efflux transporter expression at mRNA level. Previously, only individual efflux proteins have been studied in cornea epithelium derived cell lines or in the whole corneas (human, rabbit, mouse) that include several different cell types. Our data reveals that the cell lines (also commercial primary cells) deviate substantially from freshly isolated human corneal epithelium in terms of efflux transporter expression. In the cell lines several efflux proteins were over-expressed. We show that MRP1, MRP5, and BCRP are present in the normal freshly isolated human corneal epithelial tissue, whereas MDR1, MRP2, MRP3, MRP4, and MRP6 are absent or expressed at very low levels.
In human corneal epithelium MRP1 expression was observed both at mRNA and protein levels. Immunolocalization of MRP1 revealed expression in the basal layer of human corneal and limbal epithelium. However, our data are in contrast to the results of Becker et al.,12 who did not detect MRP1 expression in the human cornea, possibly due to the less sensitive regular RT-PCR that they used. Here, we demonstrate the expression of MRP1 in corneal epithelium by using real-time quantitative RT-PCR, immunohistochemistry, and Western blot, which enables us to reliably compare expression level between tissue and cell lines. In addition, Becker et al. used whole transplantation corneas (including also keratocytes and endothelial cells) that were stored considerable times before the experiments. In this study, we used pure corneal epithelial tissues without other contaminating cell types. The tissue was obtained fresh to avoid degradation of mRNA.
MRP1 has a wide substrate specificity including both neutral hydrophobic and anionic compounds and glutathione, glucuronide, and sulfate conjugates.39 In addition, topically used ocular drugs, such as antibiotic agents ofloxacin and erythromycin,40 antifungal clotrimazole,41 and immunomodulator CsA42 are suggested to interact with MRP1.38 The clinical significance of these interactions on the corneal drug permeability has not been studied yet.
Very low mRNA levels of MRP2, MRP3, and MRP4 detected in human cornea epithelium do not seem to be translated into detectable protein amounts. Both corneal epithelial cell lines, HCEpiC, and HCE, over-expressed several efflux proteins as compared to expression pattern in the human corneal epithelial tissue. In the primary cells and in HCE model the expression of MRP1, MRP3, and MRP4 were clearly upregulated. In addition in HCE model, the expression of MDR1 and BCRP were clearly present. Although HCEpiC cells are isolated from human tissue and they should represent corneal epithelium, culturing of commercial HCEpiC cells in the laboratory conditions, out of natural environment seem to influence the efflux protein expression. This may lead to erroneous conclusions regarding the in vivo relevance of the findings in the cell studies. In HCE cells, the immortalization of human epithelial cells with T-antigen gene of simian virus (SV) 40 may alter the genomic content and gene expression43 and, furthermore, the large T antigen may inhibit the function of p53 and retinoblastoma protein 1 (RB-1) and thereby affect expression of MDR1 and MRP1.44
Noteworthy, our study reports the expression of MRP5 in the corneal epithelium at mRNA and protein level. MRP5 is strongly expressed in the basal layer of human corneal epithelium and limbus. The physiological function ofMRP5 is still unclear but it has been shown to transport important second messengers cAMP and cGMP.45,46 By efflux mechanism, MRP5 may confer resistance to antiviral and anticancer compounds such as anti-metabolite drugs.47,48 The relevance of this mechanism in the treatment of cornea by anti-metabolites such as 5-fluorouracil49,50 and in corneal transport of antiviral and anticancer drugs requires further studies.
Different roles for transporters are possible depending on their location. In the corneal epithelium, the functional effects of efflux proteins depend on their membrane localization (apical or basolateral) and protein expression levels. The apical location might reduce drug absorption, but the basolateral expression could enhance permeation into the anterior chamber. Since partitioning from the corneal epithelium to the stroma is rate-limiting step for lipophilic compounds2 which easily permeate trough corneal epithelium, efflux pumps may increase the rate of drug absorption by increasing the transfer rate of their substrates from the epithelium to the corneal stroma. In this study basolateral location of both MRP1 and MRP5 was detected. Although both MRP1 and MRP5 are expressed in the apical side in the BBB,51 MRP1 is mainly localized in basolateral membranes52 and also ectopically expressed MRP5 is directed to the basolateral membrane in MDCK cells.53 Obviously, the affinity and the capacity of the efflux protein for each drug determine the quantitative impact of the transporter on the corneal absorption.
In this study, expression of MRP6 and MDR1 mRNA was not found in the human corneal epithelial tissue even though expression of MRP6 and MDR1 has been reported in mouse and in rabbit corneal epithelium, respectively.11,54–56 In addition, MRP2 expression has been detected in primary rabbit corneal epithelial cells,57 but it is not present in the normal human corneal epithelium. These differences from other species and cell models may lead to erroneous conclusions regarding the role of the efflux proteins in the human corneal epithelium. Thus, it is important to know that MDR1, MRP2, MRP3, MRP4, and MRP6 are not expressed in the human corneal epithelium.
Western blots of efflux proteins revealed the presence of BCRP protein expression in the human corneal epithelium, although the mRNA of BCRP was expressed at very low levels (Figs. 1 and 2). The discrepancy between low mRNA levels and detectable protein levels may be due to the slow turnover rate of BCRP protein in the corneal epithelial cells or posttranscriptional modifications which may delay mRNA degradation and enhance translation. The molecular mass of BCRP protein in corneal epithelial tissue samples was slightly smaller than observed in cell lines. This might be due to differential posttranslational modification of BCRP in the corneal epithelium. Several reports have described localization of BCRP in the subset of limbal basal cells and lack of BCRP expression in the central human corneal epithelial tissue.58–62 However, recently Chang et al.63 showed by immunohistochemistry BCRP expression in the layers of central epithelium during healing process of wounded corneas, although at lower levels than in the limbal region. BCRP is localized apically in many normal tissues52 and its substrate specificity is wide including ocular drugs such as fluoroquinolone antibiotics ciprofloxacin, ofloxacin, and norfloxacin.64 BCRP inhibitors include also topically used CsA and dexamethasone.65,66
It is impossible to study efflux protein function in human corneal epithelium in vivo, and the cell phenotype may change during their culture after isolation. Furthermore, it is difficult to obtain adequate quantity of epithelial cells from the human corneas to the transport studies. Therefore, the functionality of efflux proteins was confirmed only in primary cells and HCE cell line. These results suggest, in accordance with the efflux protein over-expression, significant MRP-mediated efflux in both cell lines. CDCF efflux was not, however, completely inhibited in the presence of probenecid. This may be due to the passive leakage of CDCF from the cells even though it is almost completely in the ionized form at pH 7.4 (CDCF pKa 5.1). It is also possible that some other unknown efflux proteins may transport CDCF and probenecid may not be able to block that efflux transport. The role of individual efflux proteins cannot be determined due to their wide and overlapping substrate specificity and expression of multiple MRP transporters in these cells. However, neither primary HCEpiC nor immortalized HCE cells properly describe the efflux protein function of the human corneal epithelium.
CONCLUSION
In conclusion, we demonstrate mRNA level expression of MRP1, MRP5, and BCRP in the freshly isolated pure human corneal epithelium, and the insignificant levels of MRP2, MRP3, MRP4, MRP6, and MDR1 expression. The expression of MRP1, MRP5, and BCRP was shown at protein level in the corneal epithelium, and we localized the MRP1 and MRP5 to the basal corneal epithelium. Primary HCEpiC cells and immortalized HCE model express efflux proteins more abundantly, thereby limiting the usefulness of these models in the efflux studies. MRP1, MRP5, and BCRP may have influence on ocular pharma-cokinetics and physiology.
ACKNOWLEDGMENTS
This work was financially supported by the Academy of Finland, the Finnish Cultural Foundation, the South Savo Regional Fund, and the USA National Institutes of Health (EY-14878). We are grateful to Dr. Branimir Sikic (Stanford University School of Medicine, USA), Dr. Susan Cole (Cancer Research Institute, Queen’s University, Kingston, Canada), Dr. Piet Borst (Netherlands Cancer Institute, The Netherlands), Dr. John Schuetz (St. Jude Children’s Research Hospital, Memphis, USA), Astellas Pharma, Inc. (former Yamanouchi Pharmaceutical Co., Ltd), Dr. Andras Varadi and Dr. Attila Iliás (Hungarian Academy of Sciences, Hungary), Dr. Douglas Ross (University of Maryland, Marlene and Stewart Greenebaum Cancer Center, Baltimore, USA), Dr. Jan Taipalensuu and Lucia Lazorova (Uppsala University, Sweden) for providing the plasmids for the realtime RT-PCR measurements. We thank M.Sc. Mika Reinisalo for his advices regarding qRT-PCR standards, M.Sc. Sanna Siissalo for her help in CDCF studies, M. Sc. Tuomas Ryhänen for his advices and help in Western blot studies, Dr. M. Suhonen for providing compounds for calcein efflux assay and Leena Pietilä for technical assistance. Pirjo Karppinen (Silmäkeskus Laser), Dr. Juha Lehtosalo (Helsingin Yksityinen Silmäsairaala Oy) and Heikki Karinen (Oper Oy) are acknowledged for their kind co-operation with corneal epithelial tissue samples.
Abbreviations
- ABC
ATP-binding cassette
- BCRP
breast cancer resistance protein
- CsA
cyclosporin A
- CDCF
5(6)-carboxy-2′,7′-dichlorofluorescein
- MDR1
multidrug resistance protein 1
- MRP
multidrug resistance-associated protein
REFERENCES
- 1.Maurice DM, Mishima S. Ocular pharmacokinetics. In: Sears ML, editor. Pharmacology of the eye. Berlin: Springer-Verlag; 1984. pp. 19–116. [Google Scholar]
- 2.Huang HS, Schoenwald RD, Lach JL. Corneal penetration behavior of beta-blocking agents II: Assessment of barrier contributions. J Pharm Sci. 1983;72:1272–1279. doi: 10.1002/jps.2600721109. [DOI] [PubMed] [Google Scholar]
- 3.Järvinen K, Järvinen T, Urtti A. Ocular absorption following topical delivery. Adv Drug Deliv Rev. 1995;16:3–19. [Google Scholar]
- 4.Toropainen E, Hornof M, Kaarniranta K, Johansson P, Urtti A. Corneal epithelium as a platform for secretion of transgene products after transfection with liposomal gene eyedrops. J Gene Med. 2007;9:208–216. doi: 10.1002/jgm.1011. [DOI] [PubMed] [Google Scholar]
- 5.Suhonen P, Jarvinen T, Rytkonen P, Peura P, Urtti A. Improved corneal pilocarpine permeability with O,O’-(1,4-xylylene) bispilocarpic acid ester double prodrugs. Pharm Res. 1991;8:1539–1542. doi: 10.1023/a:1015806802973. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed I, Gokhale RD, Shah MV, Patton TF. Physicochemical determinants of drug diffusion across the conjunctiva, sclera, and cornea. J Pharm Sci. 1987;76:583–586. doi: 10.1002/jps.2600760802. [DOI] [PubMed] [Google Scholar]
- 7.Chien DS, Sasaki H, Bundgaard H, Buur A, Lee VH. Role of enzymatic lability in the corneal and conjunctival penetration of timolol ester prodrugs in the pigmented rabbit. Pharm Res. 1991;8:728–733. doi: 10.1023/a:1015845916293. [DOI] [PubMed] [Google Scholar]
- 8.Urtti A, Pipkin JD, Rork G, Sendo T, Finne U, Repta AJ. Controlled drug delivery devices for experimental ocular studies with timolol 2. Ocular and systemic absorption in rabbits. Int J Pharm. 1990;61:241–249. [Google Scholar]
- 9.Yu XQ, Xue CC, Wang G, Zhou SF. Multidrug resistance associated proteins as determining factors of pharmacokinetics and pharmacodynamics of drugs. Curr Drug Metab. 2007;8:787–802. doi: 10.2174/138920007782798171. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy BG, Mangini NJ. P-glycoprotein expression in human retinal pigment epithelium. Mol Vis. 2002;8:422–430. [PubMed] [Google Scholar]
- 11.Dey S, Patel J, Anand BS, Jain-Vakkalagadda B, Kaliki P, Pal D, Ganapathy V, Mitra AK. Molecular evidence and functional expression of P-glycoprotein (MDR1) in human and rabbit cornea and corneal epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44:2909–2918. doi: 10.1167/iovs.02-1142. [DOI] [PubMed] [Google Scholar]
- 12.Becker U, Ehrhardt C, Daum N, Baldes C, Schaefer UF, Ruprecht KW, Kim KJ, Lehr CM. Expression of ABC-transporters in human corneal tissue and the transformed cell line, HCE-T. J Ocul Pharmacol Ther. 2007;23:172–181. doi: 10.1089/jop.2006.0095. [DOI] [PubMed] [Google Scholar]
- 13.Zhang T, Xiang CD, Gale D, Carreiro S, Wu EY, Zhang EY. Drug transporter and cytochrome P450 mRNA expression in human ocular barriers: Implications for ocular drug disposition. Drug Metab Dispos. 2008;36:1300–1307. doi: 10.1124/dmd.108.021121. [DOI] [PubMed] [Google Scholar]
- 14.Toropainen E, Ranta VP, Talvitie A, Suhonen P, Urtti A. Culture model of human corneal epithelium for prediction of ocular drug absorption. Invest Ophthalmol Vis Sci. 2001;42:2942–2948. [PubMed] [Google Scholar]
- 15.Toropainen E, Ranta VP, Vellonen KS, Palmgren J, Talvitie A, Laavola M, Suhonen P, Hamalainen KM, Auriola S, Urtti A. Paracellular and passive transcellular permeability in immortalized human corneal epithelial cell culture model. Eur J Pharm Sci. 2003;20:99–106. doi: 10.1016/s0928-0987(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 16.Korjamo T, Honkakoski P, Toppinen MR, Niva S, Reinisalo M, Palmgren JJ, Monkkonen J. Absorption properties and P-glycoprotein activity of modified Caco-2 cell lines. Eur J Pharm Sci. 2005;26:266–279. doi: 10.1016/j.ejps.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Chen G, Duran GE, Steger KA, Lacayo NJ, Jaffre-zou JP, Dumontet C, Sikic BI. Multidrug-resistant human sarcoma cells with a mutant P-glycoprotein, altered phenotype, and resistance to cyclosporins. J Biol Chem. 1997;272:5974–5982. doi: 10.1074/jbc.272.9.5974. [DOI] [PubMed] [Google Scholar]
- 18.Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- 19.Kool M, de Haas M, Scheffer GL, Scheper RJ, van Eijk MJ, Juijn JA, Baas F, Borst P. Analysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologues of the multidrug resistance-associated protein gene (MRP1), in human cancer cell lines. Cancer Res. 1997;57:3537–3547. [PubMed] [Google Scholar]
- 20.Kool M, van der Linden M, de Haas M, Scheffer GL, de Vree JM, Smith AJ, Jansen G, Peters GJ, Ponne N, Scheper RJ, Elferink RP, Baas F, Borst P. MRP3, an organic anion transporter able to transport anti-cancer drugs. Proc Natl Acad Sci USA. 1999;96:6914–6919. doi: 10.1073/pnas.96.12.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adachi M, Sampath J, Lan LB, Sun D, Hargrove P, Flatley R, Tatum A, Edwards MZ, Wezeman M, Matherly L, Drake R, Schuetz J. Expression of MRP4 confers resistance to ganciclovir and compromises bystander cell killing. J Biol Chem. 2002;277:38998–39004. doi: 10.1074/jbc.M203262200. [DOI] [PubMed] [Google Scholar]
- 22.McAleer MA, Breen MA, White NL, Matthews N. pABC11 (also known as MOAT-C and MRP5), a member of the ABC family of proteins, has anion transporter activity but does not confer multidrug resistance when overexpressed in human embryonic kidney 293 cells. J Biol Chem. 1999;274:23541–23548. doi: 10.1074/jbc.274.33.23541. [DOI] [PubMed] [Google Scholar]
- 23.Ilias A, Urban Z, Seidl TL, Le Saux O, Sinko E, Boyd CD, Sarkadi B, Varadi A. Loss of ATP-dependent transport activity in pseudoxanthoma elasticum-associated mutants of human ABCC6 (MRP6) J Biol Chem. 2002;277:16860–16867. doi: 10.1074/jbc.M110918200. [DOI] [PubMed] [Google Scholar]
- 24.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner HC, Bernstein A, Candia OA. Presence of CFTR in the conjunctival epithelium. Curr Eye Res. 2002;24:182–187. doi: 10.1076/ceyr.24.3.182.8297. [DOI] [PubMed] [Google Scholar]
- 26.Turner HC, Alvarez LJ, Candia OA, Bernstein AM. Characterization of serotonergic receptors in rabbit, porcine and human conjunctivae. Curr Eye Res. 2003;27:205–215. doi: 10.1076/ceyr.27.4.205.16600. [DOI] [PubMed] [Google Scholar]
- 27.Oen H, Cheng P, Turner HC, Alvarez LJ, Candia OA. Identification and localization of aquaporin 5 in the mammalian conjunctival epithelium. Exp Eye Res. 2006;83:995–998. doi: 10.1016/j.exer.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Eneroth A, Astrom E, Hoogstraate J, Schrenk D, Conrad S, Kauffmann HM, Gjellan K. Evaluation of a vincristine resistant Caco-2 cell line for use in a calcein AM extrusion screening assay for P-glycoprotein interaction. Eur J Pharm Sci. 2001;12:205–214. doi: 10.1016/s0928-0987(00)00117-2. [DOI] [PubMed] [Google Scholar]
- 29.Vellonen KS, Honkakoski P, Urtti A. Substrates and inhibitors of efflux proteins interfere with the MTT assay in cells and may lead to underestimation of drug toxicity. Eur J Pharm Sci. 2004;23:181–188. doi: 10.1016/j.ejps.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Essodaigui M, Broxterman HJ, Garnier-Suillerot A. Kinetic analysis of calcein and calcein-acetoxymethylester efflux mediated by the multidrug resistance protein and P-glycoprotein. Biochemistry. 1998;37:2243–2250. doi: 10.1021/bi9718043. [DOI] [PubMed] [Google Scholar]
- 31.Prime-Chapman HM, Fearn RA, Cooper AE, Moore V, Hirst BH. Differential multidrug resistance-associated protein 1 through 6 isoform expression and function in human intestinal epithelial Caco-2 cells. J Pharmacol Exp Ther. 2004;311:476–484. doi: 10.1124/jpet.104.068775. [DOI] [PubMed] [Google Scholar]
- 32.Breeuwer P, Drocourt JL, Bunschoten N, Zwietering MH, Rombouts FM, Abee T. Characterization of uptake and hydrolysis of fluorescein diacetate and carboxyfluorescein diacetate by intra-cellular esterases in Saccharomyces cerevisiae, which result in accumulation of fluorescent product. Appl Environ Microbiol. 1995;61:1614–1619. doi: 10.1128/aem.61.4.1614-1619.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chandra P, Zhang P, Brouwer KL. Short-term regulation of multidrug resistance-associated protein 3 in rat and human hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1252–G1258. doi: 10.1152/ajpgi.00362.2004. [DOI] [PubMed] [Google Scholar]
- 34.Pratt S, Chen V, Perry WI, III, Starling JJ, Dantzig AH. Kinetic validation of the use of carboxydichlorofluorescein as a drug surrogate for MRP5-mediated transport. Eur J Pharm Sci. 2006;27:524–532. doi: 10.1016/j.ejps.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 35.Zamek-Gliszczynski MJ, Xiong H, Patel NJ, Turn-cliff RZ, Pollack GM, Brouwer KL. Pharmacokinetics of 5 (and 6)-carboxy-2′,7′-dichlorofluorescein and its diacetate promoiety in the liver. J Pharmacol Exp Ther. 2003;304:801–809. doi: 10.1124/jpet.102.044107. [DOI] [PubMed] [Google Scholar]
- 36.Haimeur A, Conseil G, Deeley RG, Cole SP. The MRP-related and BCRP/ABCG2 multidrug resistance proteins: Biology, substrate specificity and regulation. Curr Drug Metab. 2004;5:21–53. doi: 10.2174/1389200043489199. [DOI] [PubMed] [Google Scholar]
- 37.Matsson P, Pedersen JM, Norinder U, Bergstrom CA, Artursson P. Identification of novel specific and general inhibitors of the three major human ATP-binding cassette transporters P-gp, BCRP and MRP2 among registered drugs. Pharm Res. 2009 doi: 10.1007/s11095-009-9896-0. [Epub ahead of print, published online: May 07, 2009] [DOI] [PubMed] [Google Scholar]
- 38.Mannermaa E, Vellonen KS, Urtti A. Drug transport in corneal epithelium and blood-retina barrier: Emerging role of transporters in ocular pharmacokinetics. Adv Drug Deliv Rev. 2006;58:1136–1163. doi: 10.1016/j.addr.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 39.Bakos E, Homolya L. Portrait of multifaceted transporter, the multidrug resistance-associated protein 1 (MRP1/ABCC1) Pflugers Arch. 2007;453:621–641. doi: 10.1007/s00424-006-0160-8. [DOI] [PubMed] [Google Scholar]
- 40.Terashi K, Oka M, Soda H, Fukuda M, Kawabata S, Nakatomi K, Shiozawa K, Nakamura T, Tsukamoto K, Noguchi Y, Suenaga M, Tei C, Kohno S. Interactions of ofloxacin and erythromycin with the multidrug resistance protein (MRP) in MRP-overexpressing human leukemia cells. Antimicrob Agents Chemother. 2000;44:1697–1700. doi: 10.1128/aac.44.6.1697-1700.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klokouzas A, Barrand MA, Hladky SB. Effects of clotrimazole on transport mediated by multidrug resistance associated protein 1 (MRP1) in human erythrocytes and tumour cells. Eur J Biochem. 2001;268:6569–6577. doi: 10.1046/j.0014-2956.2001.02611.x. [DOI] [PubMed] [Google Scholar]
- 42.Leier I, Hummel-Eisenbeiss J, Cui Y, Keppler D. ATP-dependent para-aminohippurate transport by apical multidrug resistance protein MRP2. Kidney Int. 2000;57:1636–1642. doi: 10.1046/j.1523-1755.2000.00007.x. [DOI] [PubMed] [Google Scholar]
- 43.Yamasaki K, Kawasaki S, Young RD, Fukuoka H, Tanioka H, Nakatsukasa M, Quantock AJ, Kinoshita S. Genomic aberrations and cellular heterogeneity in SV40-immortalized human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2009;50:604–613. doi: 10.1167/iovs.08-2239. [DOI] [PubMed] [Google Scholar]
- 44.Bush JA, Li G. Cancer chemoresistance: The relationship between p53 and multidrug transporters. Int J Cancer. 2002;98:323–330. doi: 10.1002/ijc.10226. [DOI] [PubMed] [Google Scholar]
- 45.Jedlitschky G, Burchell B, Keppler D. The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. J Biol Chem. 2000;275:30069–30074. doi: 10.1074/jbc.M005463200. [DOI] [PubMed] [Google Scholar]
- 46.Wielinga PR, van der Heijden I, Reid G, Beijnen JH, Wijnholds J, Borst P. Characterization of the MRP4- and MRP5-mediated transport of cyclic nucleotides from intact cells. J Biol Chem. 2003;278:17664–17671. doi: 10.1074/jbc.M212723200. [DOI] [PubMed] [Google Scholar]
- 47.Ritter CA, Jedlitschky G, Meyer zu Schwabedissen H, Grube M, Kock K, Kroemer HK. Cellular export of drugs and signaling molecules by the ATP-binding cassette transporters MRP4 (ABCC4) and MRP5 (ABCC5) Drug Metab Rev. 2005;37:253–278. doi: 10.1081/dmr-200047984. [DOI] [PubMed] [Google Scholar]
- 48.Borst P, de Wolf C, van de Wetering K. Multidrug resistance-associated proteins 3, 4 and 5. Pflugers Arch. 2007;453:661–673. doi: 10.1007/s00424-006-0054-9. [DOI] [PubMed] [Google Scholar]
- 49.Pratt S, Shepard RL, Kandasamy RA, Johnston PA, Perry W, III, Dantzig AH. The multidrug resistance protein 5 (ABCC5) confers resistance to 5-fluorouracil and transports its monophos-phorylated metabolites. Mol Cancer Ther. 2005;4:855–863. doi: 10.1158/1535-7163.MCT-04-0291. [DOI] [PubMed] [Google Scholar]
- 50.Poothullil AM, Colby KA. Topical medical therapies for ocular surface tumors. Semin Ophthalmol. 2006;21:161–169. doi: 10.1080/08820530500351694. [DOI] [PubMed] [Google Scholar]
- 51.Nies AT, Jedlitschky G, Konig J, Herold-Mende C, Steiner HH, Schmitt HP, Keppler D. Expression and immunolocalization of the multidrug resistance proteins, MRP1-MRP6 (ABCC1-ABCC6), in human brain. Neuroscience. 2004;129:349–360. doi: 10.1016/j.neuroscience.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 52.Leslie EM, Deeley RG, Cole SP. Multidrug resistance proteins: Role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol. 2005;204:216–237. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 53.Wijnholds J, Mol CA, van Deemter L, de Haas M, Scheffer GL, Baas F, Beijnen JH, Scheper RJ, Hatse S, De Clercq E, Balzarini J, Borst P. Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proc Natl Acad Sci USA. 2000;97:7476–7481. doi: 10.1073/pnas.120159197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawazu K, Yamada K, Nakamura M, Ota A. Characterization of cyclosporin A transport in cultured rabbit corneal epithelial cells: P-glycoprotein transport activity and binding to cyclophilin. Invest Ophthalmol Vis Sci. 1999;40:1738–1744. [PubMed] [Google Scholar]
- 55.Dey S, Gunda S, Mitra AK. Pharmacokinetics of erythromycin in rabbit corneas after single-dose infusion: Role of P-glycoprotein as a barrier to in vivo ocular drug absorption. J Pharmacol Exp Ther. 2004;311:246–255. doi: 10.1124/jpet.104.069583. [DOI] [PubMed] [Google Scholar]
- 56.Beck K, Hayashi K, Nishiguchi B, Le Saux O, Hayashi M, Boyd CD. The distribution of Abcc6 in normal mouse tissues suggests multiple functions for this ABC transporter. J Histochem Cytochem. 2003;51:887–902. doi: 10.1177/002215540305100704. [DOI] [PubMed] [Google Scholar]
- 57.Karla PK, Pal D, Mitra AK. Molecular evidence and functional expression of multidrug resistance associated protein (MRP) in rabbit corneal epithelial cells. Exp Eye Res. 2007;84:53–60. doi: 10.1016/j.exer.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 58.Watanabe K, Nishida K, Yamato M, Umemoto T, Sumide T, Yamamoto K, Maeda N, Watanabe H, Okano T, Tano Y. Human limbal epithelium contains side population cells expressing the ATP-binding cassette transporter ABCG2. FEBS Lett. 2004;565:6–10. doi: 10.1016/j.febslet.2004.03.064. [DOI] [PubMed] [Google Scholar]
- 59.Chen Z, de Paiva CS, Luo L, Kretzer FL, Pflugfelder SC, Li DQ. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22:355–366. doi: 10.1634/stemcells.22-3-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolosin JM, Budak MT, Akinci MA. Ocular surface epithelial and stem cell development. Int J Dev Biol. 2004;48:981–991. doi: 10.1387/ijdb.041876jw. [DOI] [PubMed] [Google Scholar]
- 61.de Paiva CS, Chen Z, Corrales RM, Pflugfelder SC, Li DQ. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells. 2005;23:63–73. doi: 10.1634/stemcells.2004-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Budak MT, Alpdogan OS, Zhou M, Lavker RM, Akinci MA, Wolosin JM. Ocular surface epithelia contain ABCG2-dependent side population cells exhibiting features associated with stem cells. J Cell Sci. 2005;118:1715–1724. doi: 10.1242/jcs.02279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang CY, Green CR, McGhee CN, Sherwin T. Acute wound healing in the human central corneal epithelium appears to be independent of limbal stem cell influence. Invest Ophthalmol Vis Sci. 2008;49:5279–5286. doi: 10.1167/iovs.07-1260. [DOI] [PubMed] [Google Scholar]
- 64.Merino G, Alvarez AI, Pulido MM, Molina AJ, Schinkel AH, Prieto JG. Breast cancer resistance protein (BCRP/ABCG2) transports fluoroquinolone antibiotics and affects their oral availability, pharmacokinetics, and milk secretion. Drug Metab Dispos. 2006;34:690–695. doi: 10.1124/dmd.105.008219. [DOI] [PubMed] [Google Scholar]
- 65.Ozvegy C, Litman T, Szakacs G, Nagy Z, Bates S, Varadi A, Sarkadi B. Functional characterization of the human multidrug transporter, ABCG2, expressed in insect cells. Biochem Biophys Res Commun. 2001;285:111–117. doi: 10.1006/bbrc.2001.5130. [DOI] [PubMed] [Google Scholar]
- 66.Pavek P, Merino G, Wagenaar E, Bolscher E, Novotna M, Jonker JW, Schinkel AH. Human breast cancer resistance protein: Interactions with steroid drugs, hormones, the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine, and transport of cimetidine. J Pharmacol Exp Ther. 2005;312:144–152. doi: 10.1124/jpet.104.073916. [DOI] [PubMed] [Google Scholar]