Abstract
A full account of an asymmetric synthesis of reblastatin (1), the first total synthesis of autolytimycin (2) and related structural compounds is described. The syntheses expand the utility of a highly regio-and diastereoselective hydrometalation aldehyde addition sequence to assemble the fully functionalized ansa chain of the natural products. Also documented is an intramolecular copper-mediated amidation reaction to close the 19-membered macrolactams. The amidation reaction was also employed for the generation of structural derivatives (6–9) of phenolic ansamycins. Ansamycin natural products and selected structural analogs were evaluated in a competitive binding assay to breast cancer cell lysate and a cytotoxicity assay. Both reblastatin (1) and autolytimycin (2) were shown to bind the Hsp90 protein with enhanced binding activity (~25 nM) than 17-allylamino-17-demethoxygeldanamycin (17-AAG, 4), a geldanamycin (3) derivative currently under evaluation for treatment of cancer (~100 nM).
Introduction
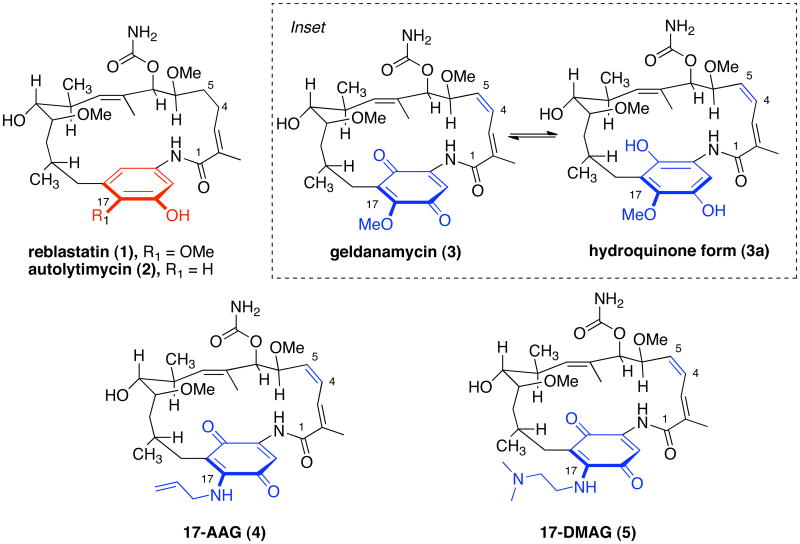
Heat shock protein 90 (Hsp90) is a protein chaperone responsible for regulation of proteins in cell signaling, proliferation and survival processes, including client proteins involved in multiple oncogenic signaling (signal transduction and transcription) pathways.1,2 As a result, Hsp90 has gained attention as an important therapeutic target for cancer treatment. Hsp90 is effectively inhibited by geldanamycin (3) and many other benzoquinone ansamycin derivatives,3a which bind to the ATP binding site of the N-terminal domain.3b The ability of geldanamycin (3)4 and structurally related agents to affect multiple oncogenic pathways simultaneously is a unique and therapeutically attractive feature of this class of natural products.5 Despite the cellular potency of geldanamycin (3), the development of this natural product as a clinical agent has been halted due to liver toxicity, insolubility and cellular instability.6 The benzoquinone moiety of geldanamycin (3) is proposed to be the cause of the observed hepatotoxicity.7 To stabilize the quinone and increase water solubility of the compound, various 17-aminated semi-synthetic derivatives of geldanamycin (3) were prepared (Figure 1).8,9 In turn, less hepatotoxic and more soluble compounds, tanespimycin (4) (17-allylamino-17-demethoxygeldanamycin, 17-AAG) and alvespimycin (5) (17-(dimethylaminoethylamino)-17-demethoxygeldanamycin, 17-DMAG) are currently in clinical trials for the treatment of cancer. 10,11 However, 17-AAG has been difficult to formulate because of its insolubility characteristics of the quinone and hydroquinone.
Figure 1.
Structure of Ansamycin Antibiotics.
Reduction of the quinone to hydroquinone moiety in ansamycin natural products was shown to increase the binding affinity towards Hsp90.12,14 A biosynthetic engineering approach has been applied to create nonbenzoquinoid compounds (i.e. phenolic) as Hsp-90 inhibitors.13 Given the close structural resemblance of reblastatin (1)14 and autolytimycin (2)15,16 to geldanamycin (3) and its hydroquinone form 3a (that exhibits enhanced binding affinity over the quinone form; see inset Figure 1), the phenol-containing natural products should bind and inhibit the chaperone activity of Hsp90.17 The following discussion details the total syntheses of phenol-containing ansamycins 1 and 2, as well as their structural derivatives 6–9, and their biological evaluation as effective binders and inhibitors of Hsp90 protein.
Reblastatin (1) and autolytimycin (2) are polyketide antibiotics that exhibit promising antitumor activity, acting as inhibitors of Hsp90. Reblastatin (1) was isolated in 2000 by Takatsu and co-workers during screening experiments intended to identify novel compounds that inhibit phosphorylation of the retinoblastoma protein (Rb).14 This material was isolated as a minor component from the culture of Streptomyces hygroscopicus subsp. hygroscopicus SANK 61995, which also produces the known Hsp90 disruptor geldanamycin (3). Like many of the ansamycins, reblastatin’s chemical structure is comprised of a 19-membered lactam joined at the meta positions of a phenol ring (Figure 1). The ansa chain of this natural product contains six stereogenic centers, two (E)-trisubstituted double bonds, and a C7-carbamate functionality. In the initial report, reblastatin (1) was shown to inhibit proliferation of cell lines against human histiocytic lymphoma U-937 with an IC50 value of 0.43 μg/mL.14 Additionally, the natural product was reported to exhibit potent inhibitory activity in the cell-based oncostatin M signaling assay with an IC50 value of 0.16 μM.16 In 2005, we reported the first total synthesis of reblastatin (1), whereby confirming its absolute stereochemistry.18 The related phenolic natural product autolytimycin (2) was isolated in 2001 from a strain of Streptomyces autolyticus JX-47 and was shown to exhibit activity in a cell based oncostatin M signaling assay.15,16 Autolytimycin (2) differs structurally from reblastatin (1) at the C17 position of the aromatic region, as one has a methoxy group and the other does not. The architectural similarities to geldanamycin (3) and other members of the ansamycin family provided incentive to explore these molecules as potential Hsp90 inhibitors. Further, by replacement of the para-quinone with a phenol ring should attenuate (or possibly remove) the hepatotoxic effects ascribed to the quinone (as in geldanamycin and macbecin) while retaining the over all topology of the ansamycin framework and binding affinity for Hsp90.
RESUTLS AND DISCUSSION
Synthesis of reblastatin (1), autolytimycin (2), and structural analogs (6–9)
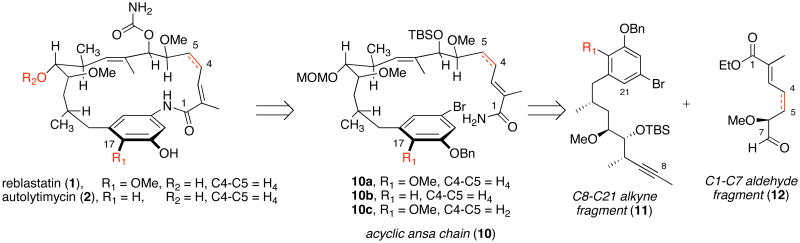
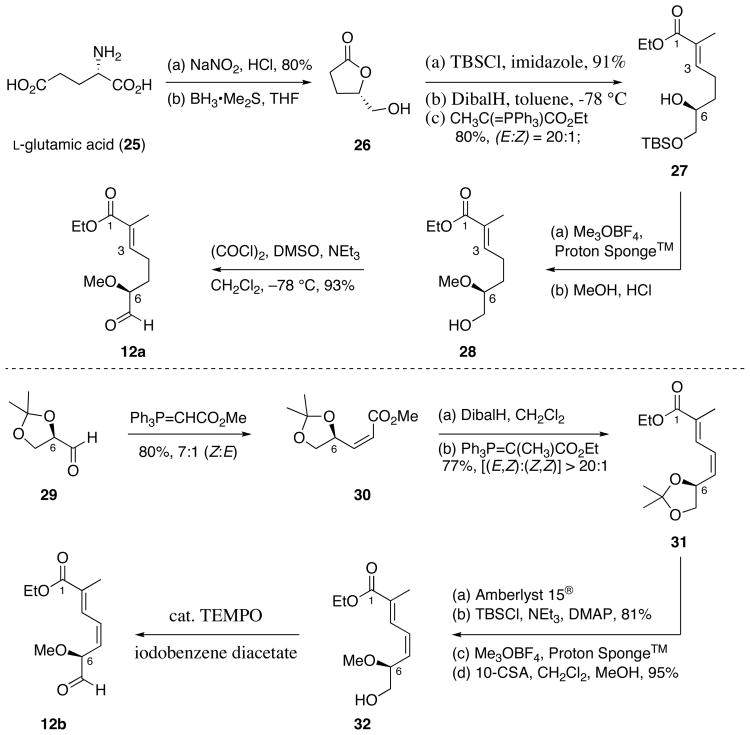
In earlier investigations concerning the chemical synthesis of the ansamycins, macbecin and herbimycin, we took a rather linear approach focusing on the use of crotylsilane reagents in the context of acyclic stereocontrol as reliable means to establish the stereochemical relationships in the ansa-chain. In that context, we sought to introduce and eventually establish the chiral organosilane reagents bearing C-centered chirality, as carbon nucleophiles that would complement the more established chiral enolate-based bond construction methodology (and aldol surrogates). 19 We had anticipated that these reagents would be useful for the asymmetric synthesis of polyproprionate derived natural products. Our synthetic strategy to access reblastatin (1), autolytimycin (2), and structural analogs (6–9) explores a more convergent approach (Schemes 1 and 2).18 The 19-membered macrocycles were envisioned to be formed from their acyclic frameworks 10 through an intramolecular copper (I)-mediated amidation reaction,20 thereby expanding the scope and utility of the Buchwald aryl amidation methodology.21 Preparation of the acyclic skeletons 10 would arise from tandem hydrozirconation-transmetalation-nucleophilic addition sequence.22 The hydrozirconation protocol developed by our laboratories for regioselective preparation of (E)-vinyl zirconium species would establish the configuration of C8–C9 trisubstituted double bond.23 Introduction of the C7-alcohol would arise from the addition of the in situ derived (E)-vinyl metal species (from alkyne 11) to aldehydes 12. Structural analogs of the core macrocycles could arise from unsaturation at the C4–C5 positions of aldehyde 12, functionalization of the aromatic ring, and modification of the secondary C11-alcohol of the ansa chain. These minor synthetic changes could provide lend insight into the necessary chemical modifications needed to produce more potent inhibitors of Hsp90 with less toxicity and higher solubility.
Scheme 1.
Retrosynthesis of Reblastatin (1), Autolytimycin (2), and Structural Derivatives (6–9)
Scheme 2.
Retrosynthetic Analysis of Alkyne Fragments 11.
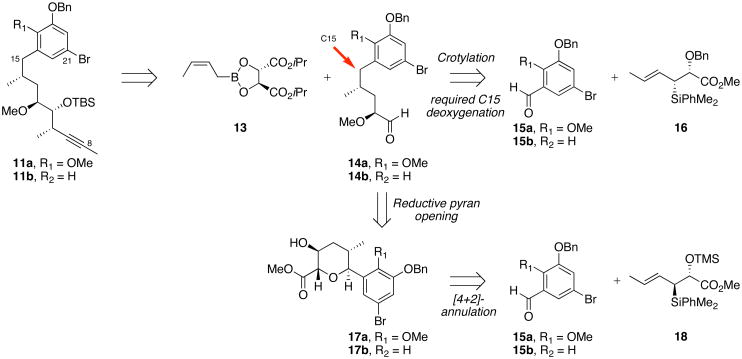
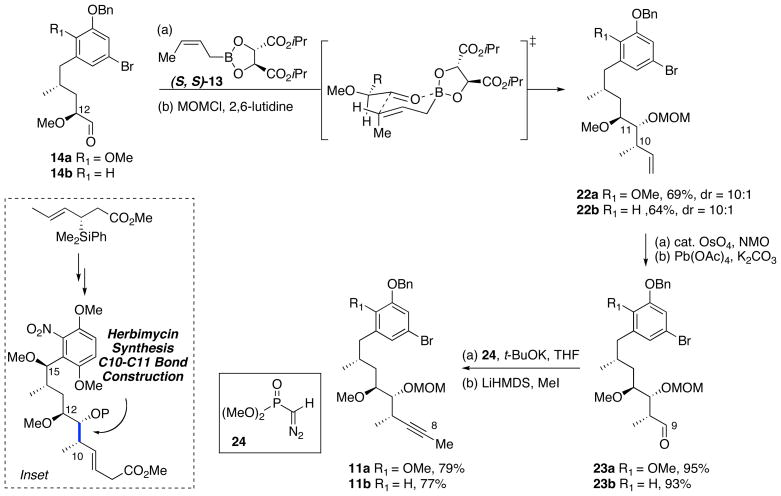
To aid in the synthesis of structural analogs of the phenolic ansamycins, a new approach toward the synthesis of C8–C21 alkyne fragment was designed (Scheme 2).24 Advanced alkyne intermediates 11a–b would now be derived from crotylation of aldehydes 14a–b with (Z)-crotylboron reagent 13. Our initial synthetic strategy for preparing of these intermediate aldehydes 14a–b involved crotylation reaction of aldehydes 15a–b with syn-(E)-crotylsilane 16.18 Following the crotylation with aryl aldehydes 15a–b, the resulting C15 benzylic oxygen would have to be removed to prepare reblastatin (1) or autolytimycin (2). In order to reduce the number of synthetic steps and improve the overall efficiency of this approach, a reductive pyran ring opening was developed (17 → 14; Scheme 2).24 Tetrahydropyrans 17a–b can be accessed from a Lewis acid promoted condensation of aromatic aldehydes 15a–b with anti-(E)-crotylsilane reagent 18, allowing for a more direct route toward intermediate aldehydes of type 14.
Preparation of the C11–C21 Aldehydes 14
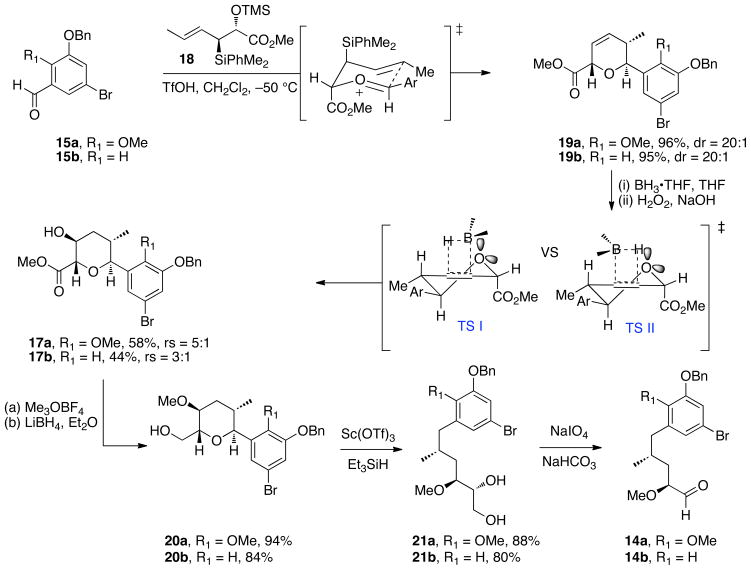
The synthesis of phenolic ansamycins began with the preparation of the key intermediate aldehydes 14a–b as depicted in Scheme 3. Exposure of aromatic aldehydes 15a25 and 15b26 and (E)-anti crotylsilane 18 to TfOH at −50 °C afforded 2,6-trans dihydropyrans 19a–b in greater than 95% yields and excellent selectivities.27 Hydroboration of the dihydropyran with BH3•THF and subsequent oxidation with alkaline hydrogen peroxide yielded secondary alcohols 17a–b in moderate yields and regioselectivity; the major isomer is proposed to emerge from an electrostatic and sterically preferred TS I vs TS II.28 Methylation of alcohols 17a–b with Meerwein’s reagent was followed by reduction of methyl ester with LiBH4 to give pyran intermediates 20a–b. Reductive opening of aryl pyranosides 20a–b was performed in the presence of Sc(OTf)3 and Et3SiH to give the C11–C21 aromatic diol fragments 21a–b in good yields.24 Notably, the less reactive C17-H aryl pyranoside 20b required slightly elevated temperature (40 °C vs 25 °C) and longer reaction time for nucleophilic ring opening. The intermediate diols were then subjected to oxidative cleavage with NaIO4 providing chiral aldehydes 14a and 14b in six steps and 46% and 28% yield, respectively.
Scheme 3.
Synthesis of the C11–C21 Aldehyde Portions (14a–b) of Alkyne Fragments.
Upon completion of the α-methoxy aldehyde fragments 14a–b, preparation of the necessary C8–C21 subunits required installation of the C10–C11 syn-homoallylic alcohols and formation of the C8-methyl alkyne.18 Although our crotylsilane addition was previously utilized in the synthesis of macbecin I and herbimycin29 (see inset), for atom economical reasons we elected to use the Roush’s (Z)-crotylboronate reagent (S, S)-1330 to simultaneously install the terminal olefin and set the required C10–C11-syn stereochemistry. Accordingly, the “matched crotylation” of α-methoxy aldehydes 14a–b with (S,S)-crotylboronate 13 afforded the desired homoallylic alcohols in moderate diastereoselectivity (dr = 10:1).31 The derived homoallylic alcohols were converted to their MOM ethers 22a–b, followed by dihydroxylation of the terminal alkenes with catalytic osmium tetraoxide. Subsequent oxidative cleavage of the resulting diols, exposed the chiral α-methyl aldehydes 23a–b in 95% and 93% yield, respectively. Treatment with Gilbert–Seyferth reagent 2432 provided the terminal alkynes in high yields, and these materials were subjected to methylation using LiHMDS and MeI to furnish the desired internal alkynes 11a–b in 79% and 77% yield, respectively, over the two steps. The fully functionalized C9–C21 aromatic fragments 11a–b were prepared in 12 steps and 24% and 13% overall yields, respectively, starting from aromatic aldehydes 15a–b.
Synthesis of the C1–C7 Aldehyde Fragments 12
The synthesis of C1–C7 fragments 12a–b utilized starting materials available from the chiral pool (Scheme 5).33 The α,β-unsaturated aldehyde 12a was obtained from readily accessible γ-lactone 26.34 Following the reported protocol by Herdeis,34 L-glutamic acid (25) was converted to chiral γ-lactone 26 via a two-step sequence (84% yield). Making use of an analogous strategy employed earlier by Forsyth for the synthesis of the C ring of thyrsiferol,35 γ-lactone 26 was converted to the (E)-α,β-unsaturated ester 27 via a three-step sequence that began with protection of the primary hydroxy group of 26 as its TBS ether, followed by partial reduction of the γ-lactone with DibalH and treatment of the resulting lactol with (carboethoxyethylidene)triphenylphosphorane, thereby providing α,β-unsaturated ester 27 as the (E)-isomer in 73% yield (3 steps). Methylation of the secondary alcohol followed by TBS deprotection with dilute HCl and subsequent Swern oxidation36 of the resulting primary alcohol 28 provided the desired intermediate chiral aldehyde 12a in 93% yield (3 steps).
Scheme 5.
Synthesis of α,β-Unsaturated (12a) and the C1–C7 (Z,E)-Diene Fragment (12b)
The preparation of the C1–C7 aldehyde fragment 12b required successful installation of the C4–C5 (Z)-olefin (Scheme 5). For this reason, we utilized a phosphorous-based olefination approach as a convenient method to install the necessary olefin. Accordingly, 2,3-O-isopropylidene-D-glyceraldehyde37 29 was treated with (carbomethoxymethylene)triphenylphosphorane in methanol at 0 °C to afford the desired (Z)-olefin 30 in 80% yield (Z:E = 7:1).38 Reduction of the methyl ester 30 with DibalH and subsequent exposure of the resulting aldehyde to (carboethoxyethylidene)triphenylphosphorane in refluxing benzene proceeded to give (E,Z)-diene 31 in 77% yield and excellent selectivity ([(E,Z):(Z,Z)] = 20:1). Deprotection of the acetonide with Amberlyst 15® yielded an unstable diol that was used without further purification; the primary alcohol was selectively protected as a TBS-ether in 81% over the two steps. Methylation of the secondary alcohol followed by TBS deprotection with 10-camphorsulfonic acid and subsequent TEMPO-catalyzed oxidation39 of the resulting primary alcohol 32 afforded the desired conjugated Z, E-aldehyde 12b in 94% yield (3 steps).
Fragment Coupling via Hydrozirconation-Transmetalation Aldehyde Addition Protocol
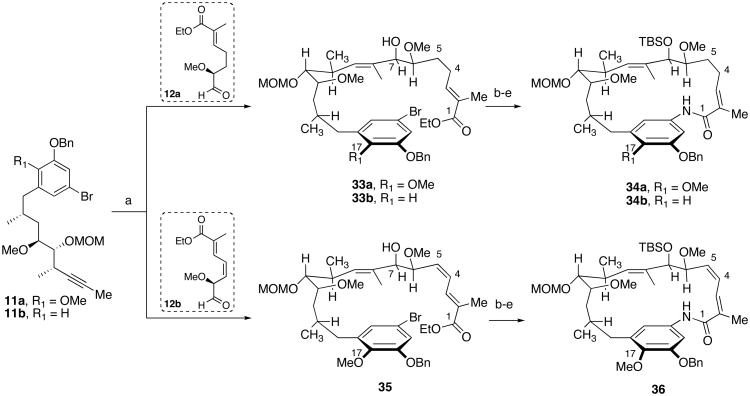
The structural complexity of the advanced coupling partners, methyl-terminated alkynes 11a–b and α-methoxy aldehydes 12a–b, represented an interesting synthetic challenge and an opportunity to evaluate a hydrozirconation-transmetalation-aldehyde addition sequence. In this one-pot process, several transformations occur in a predictable fashion: beginning with the regioselective reduction of the internal alkyne via cis-addition of zirconium and hydride, transmetalation to an organozinc species with retention of the newly formed alkene configuration, and subsequent addition to the aldehyde to afford the desired (E)-allylic alcohol. With the execution of a highly convergent and flexible route to access reblastatin (1) and autolytimycin (2), we sought to use this synthetic approach to prepare structurally related derivatives to further study the biological activity and structure-activity relationships of this class of ansamycins. This idea could be realized because of the success of the tandem hydrozirconation-transmetalation-nucleophilic addition sequence developed during our synthesis of reblastatin (1).18 The highly regioselective hydrozirconation-transmetalation sequence of C8–C21 alkyne fragments 11a and 11b and diastereoselective addition to C1–C7 aldehyde 12a or 12b proceeded to give the desired allylic alcohols as single diastereomers in moderate yields (53–70% for 33a, 40–89% for 33b, 51–62% for 35). The stereochemical course of the reaction for the in situ addition of organozinc species to α-methoxy aldehyde is consistent with a Cram-chelate transition state, providing the desired syn isomer in 20:1 selectivity.40 These examples highlight the synthetic utility of the hydrozirconation-transmetalation-nucleophilic addition sequence and ultimately allowed access to a variety of acyclic ansamycins.
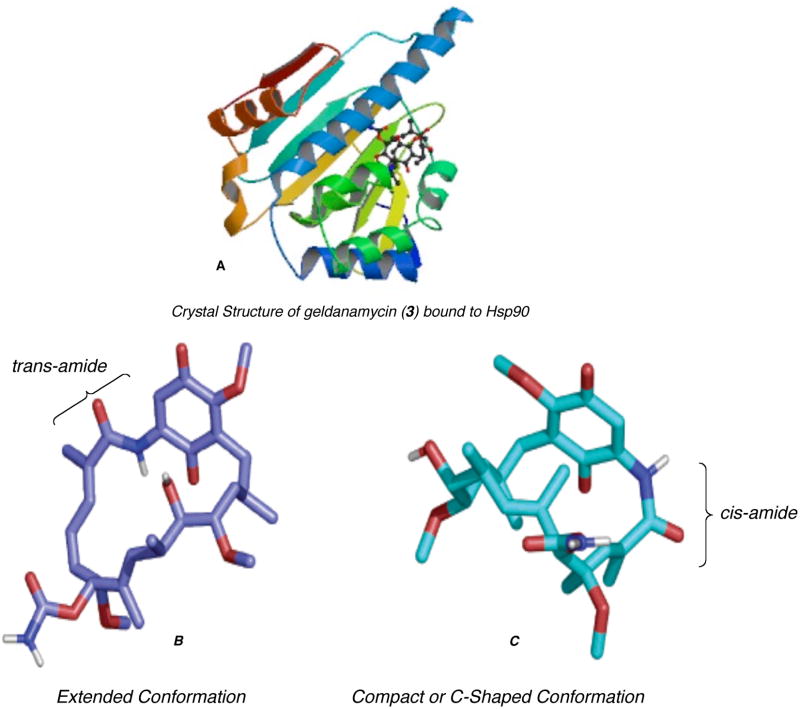
The highly regio- and diastereoselective coupling reaction of C1–C7 and C8–C21 fragments afforded the complete acyclic carbon framework of the ansamycins. The synthetic plan for structural analogs is based on the successful assembly of the macrolactam core of reblastatin (1).18 In particular, we wanted to prepare derivatives of reblastatin (1) with (Z, E)-conjugated diene and modified aromatic functionality at the C17 position. Despite structural studies on complexes of ansamycin derivatives with the ATPase domains of Hsp90, many aspects of their inhibitory mechanism remain unresolved. For instance, it is known that in solution geldanamycin (3) exists in an extended conformation A with the trans geometry about the amide bond.41 However, it binds Hsp90 in a much more compact conformation (C-shaped) B with a cis-amide bond (Figure 2).6 More specifically geldanamycin is orientated with the macrocycle and C7 carbamate directed toward the bottom of the binding pocket and the benzoquinone ring directed toward the top of the pocket as it opens to the surface of the binding domain. In contrast to the extended structure adopted by unbound geldanamycin, the protein bound antibiotic is nearly folded over, so that the benzoquinone ring and of the macrocycle are positioned above and below each other (Figure 2).
Figure 2.
(A) Crystal structure of geldanamycin (3) bound to Hsp90; solid-state trans-amide (B) and protein-bound cis-amide (C) conformations of geldanamycin (3) (see ref 6b).
Also, the presence of a C17-appendage (excluding a methoxy group) does not significantly affect binding of geldanamycin (3) to the protein. In addition to replacing the para-quinone ring with a phenol group, our synthetic plan for analog synthesis was based on examination of the contact points in the crystal structure of geldanamycin (3) bound to Hsp90. The analogs incorporated subtle changes within the ansamycin macrocycle to study the effect on binding to Hsp90; incorporation of C4–C5 olefin in reblastatin (1), loss of C17-methoxy ether in autolytimycin (2), and functionalization of C11-alcohol in reblastatin (1). We anticipated that these structural changes could provide additional insight into the necessary structural modifications needed to design more potent ansamycin derived Hsp90 inhibitors.
The newly formed secondary alcohols were thus converted to the macrocyclization precursors 10a–c (refer to Scheme 1) in a short three-step sequence (Scheme 6). Protection of C7-alcohols 33a–b and 35 as TBS ethers, followed by saponification of the ethyl esters with LiOH and conversion of the resulting acids to the unsaturated amides via a mixed anhydride proceeded in good overall yield (53% for 10a, 55% for 10b, 53% for 10c). It is important to note that the efficiency of the intramolecular amidation reaction was not affected by subtle changes within the ansa chain of the ansamycin derivatives and the product macrolactams 34a–b, 36 were accessed in greater than 80% yield for the macrocyclization step. Since our initial publication, the CuI/1,2-diamine protocol was employed in the total synthesis of geldanamycin (3)24b as well as other 8 to 14-membered lactam-containing natural products.20,42
Scheme 6.
Synthesis of the Macrocyclic Cores of Phenolic Derivativesa
a Reagents and conditions: (a) (i) 2 equiv. Cp2ZrHCl, 11a or 11b, toluene, 50 °C, (ii) ZnMe2, toluene, −65 °C, (iii) 12a or 12b, 0 °C, 53–70% for 33a, 40–89% for 33b, 51–62% for 35, dr = 20:1; (b) TBSOTf, 2,6-lutidine, CH2Cl2, 0 °C; (c) LiOH, THF/MeOH/H2O; (d) (i) (CH3)2CHCH2OCOCl, NEt3, CH2Cl2, −20 °C, (ii) NH3 (l); (e) CuI, N,N′-dimethylethylenediamine, K2CO3, toluene, 100 °C, 36 h, >80% for 34a, 80% yield for 34b, 82% yield for 36.
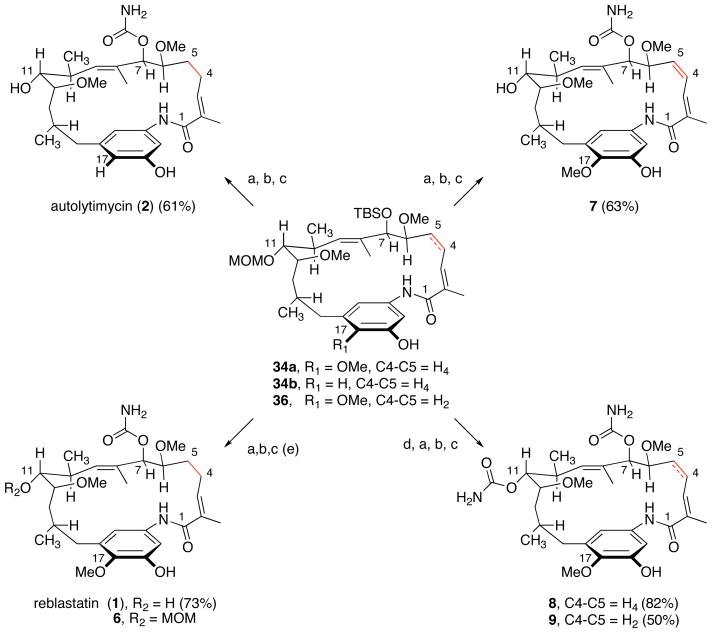
Conversion of the desired macrocyclic cores 34a–b and 36 en route to the ansamycin derivatives is depicted in Scheme 7. Towards that end, a three-step sequence was employed to convert the C7-silylethers to the required carbamate functionality. Deprotection of the initial silyl-ethers 34a–b, 36 was achieved using buffered HF•pyridine. The resulting C7-secondary alcohols were converted to their carbamates using reaction conditions reported by Kocovsky43 and provided the desired materials in greater than 80% yield. Initial attempts to selectively remove the MOM ether under mild conditions44 resulted in decomposition of the macrolactam. Ultimately, deprotection of the MOM and benzyl ethers was accomplished using AlCl3 and anisole45 to reveal reblastatin (1), autolytimycin (2) and diene derivative 7 in 75%, 61% and 63% yield, respectively. Similarly, exposure of the intermediate carbamate derived from 34a to BCl3 at low temperatures provided reblastatin (1) in 53% isolated yield, with recovered MOM-protected reblastatin 6 in 15% yield. Both synthetic reblastatin (1) and autolytimycin (2) exhibited physical, spectroscopic and spectrometric characteristics (1H, 13C NMR, IR, [α]D, and HRMS) identical to those reported for the natural products.46
Scheme 7.
Synthesis of Reblastatin (1), Autolytimycin (2) and Structural Analogs (6 – 9)a
aReagents and conditions: (a) HF•pyridine, pyridine, THF; (b) (i) Cl3CCONCO, CH2Cl2, (ii) MeOH, K2CO3; (c) AlCl3, anisole, CH2Cl2, −78 °C to rt; (d) MgBr2, EtSH, Et2O, rt; (e) BCl3, CH2Cl2, −78 °C, 15% for 6.
Employing a similar strategy, we were able to functionalize the C11 secondary alcohols of the ansamycin derivatives as carbamates. Sequential treatment of macrolactams 34a and 36 with a solution of MgBr2 and HF•Pyr afforded the respective C7, C11-diols which were treated with excess trichloroisocyanate reagent to afford the bis-carbamates. The resulting C7, C11 bis-carbamate derivatives bearing benzyl ether protected C18 phenolic oxygens were then treated with AlCl3 to provide the desired phenolic ansamycins 8 and 9 in 82% and 50% yields, respectively, over the four steps.
The convergent route to reblastatin (1), autolytimycin (2) and related structures (6–9) required an average of 23 steps with overall yields ranging between 2.79 – 5.67%, which underscores the critical role that chemical synthesis plays in the production of new chemical entities, and in the present case, compounds possessing enhanced binding affinity for Hsp90.
Biological Evaluation for Hsp90 Inhibition
The synthesis of natural product-like libraries (and small molecule collections) comprised of sophisticated macrocycles rich in topological or stereochemical variation is an underdeveloped field with enormous potential in biomedical research.47 Many molecules in nature are produced through convergent biosynthesis to afford natural products (often called hybrids) whose biological properties come about through their enhanced interactions with proteins. 48
Having designed and executed a convergent and flexible approach to access the macrocyclic lactams reblastatin (1) and autolytimycin (2) and selected related structures, we sought to explore their effectiveness as inhibitors of Hsp90. In that regard, reblastatin (1), autolytomycin (2) and four structural derivatives 6–9 were evaluated in a competitive binding assay from MDA-MB-468 breast cancer lysate.18 We anticipated that reblastatin (1), bearing a phenolic hydroxyl, would bind to the molecule chaperone Hsp90 in a similar manner to that of the natural product geldanamycin (3).17 Additionally, synthetic derivatives devoid of the benzoquinone moiety and C-17 methoxy group could potentially provide new agents with improved toxicity profiles over geldanamycin (3), 17-AAG (4) and 17-DMAG (5). All evidence to date confirms that the reduction of the quinone moiety in geldanamycin derivatives increases the drug’s affinity for Hsp90.49 Growing experimental and clinical evidence of the potential of these phenolic molecules as anticancer agents support the need to develop chemical syntheses of this class of natural products like reblastatin (1) and its structurally related agents.6
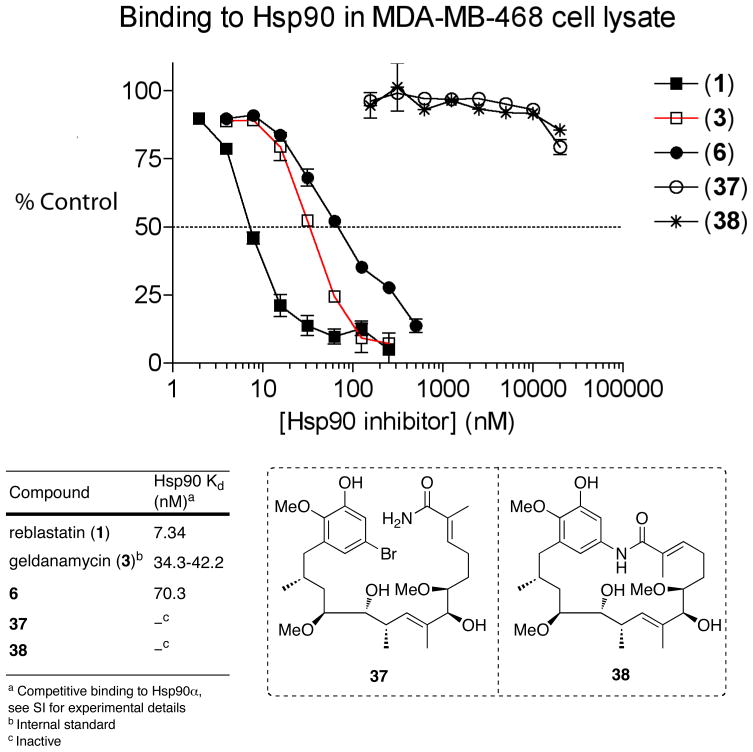
Soon after completion of the total synthesis of reblastatin (1), the natural product and its select analogs 6, 37–38 (Figure 3) were evaluated for their binding affinity for Hsp90. Reblastatin (1) was shown to exhibit 4-fold enhanced nanomolar activity (7.34 nM) over geldanamycin 3 (34–42 nM) in a competitive binding assay to Hsp90 derived from MDA-MB-468 breast caner cell lysate (Figure 3).50 Further, the MOM-ether derivative 6 also displayed Hsp90 inhibition (70.3 nM), whereas removal of C7-urethane 38 or an acyclic framework derivative 37 were ineffective as Hsp90 inhibitors; findings are consistent with previous SAR studies on geldanamycin (3).8,9
Figure 3.
Competitive Binding Assay for Reblastatin (1) and Structural Analogs 6, 37–38 to Hsp90 obtained from MDA-MB-468 Breast Cancer Cell Lysate. Reblastatin (1) binds to Hsp90 with slightly better nanomolar activity (7.34 nM) than geldanamycin (3, 34–42 nM). Derivative 6 also showed good inhibitory activity (70.3 nM). Compounds 37 and 38 were inactive in the binding assay.
Figure 4.
Cytotoxicity and Hsp90 Binding Affinity of Reblastatin (1), Autolytimycin (2), 17-AAG (4) and Analogs (7–9).
The structure of the geldanamycin-binding domain of Hsp90 reveals a pronounced pocket that is highly conserved across species. Geldanamycin binds inside this pocket, adopting a compact structure similar to that of a polypeptide chain in a turn conformation. Geldanamycin is orientated with the macrocycle and C7 carbamate directed toward the bottom of the binding pocket and the benzoquinone ring directed toward the top of the pocket as it opens to the surface of the binding domain. In contrast to the extended structure adopted by unbound geldanamycin, the protein bound antibiotic is nearly folded over, so that the benzoquinone ring and of the macrocycle are positioned above and below each other (Figure 2).13c The preliminary competitive binding assays of reblastatin (1) and related structural analogs revealed several important structural interactions between Hsp90 and phenol containing ansamycins. The removal of the C21-hydroxyl (of the reduced hydroquinone of geldanamycin) and its associated polar contact in with the protein resulted in higher binding affinity for the molecular chaperone. Suggesting that binding to Hsp90 is not dependent on the presence of the quinone moiety and/or loss of C4–C5 olefin (e.g. geldanamycin), thus inhibition of the molecular chaperone can be extended to phenol containing ansamycins.17
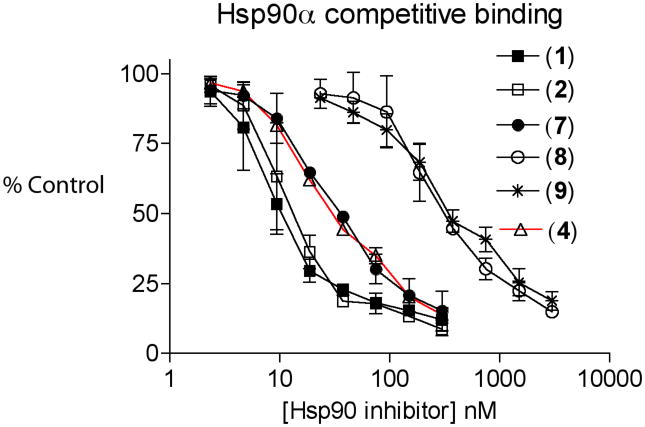
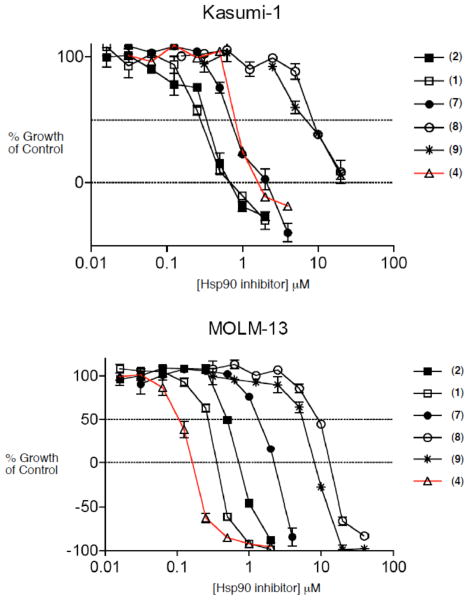
Having achieved a successful total synthesis of autolytimycin (2) and four structurally similar derivatives (7–9), we were positioned to examine these structures as inhibitors of the bioactivity of Hsp90 in cancer cells. Along with reblastatin (1), the ansamycin derivatives 2, 7–9 were evaluated for cytotoxicity in two acute myeloid leukemia cell lines and for binding affinity to Hsp90. The Kasumi-1 and MOLM-13 cell growth inhibition (IC50) assays and the apparent binding affinities (EC50) to recombinant human Hsp90 were measured using previously reported protocols (Figure 4).51 The results for the phenolic analogues are shown in Table 1. The affinity of Hsp90 to reblastatin 1 (26 nM) and autolytimycin 2 (36 nM) natural products is about 4 fold higher than for the corresponding control, 17-AAG 4 (110 nM). Interestingly, the C4–C5 olefin containing derivative 7 showed a Kd value (86 nM) similar to that of 17-AAG (4), while the two bis-urethane analogues (8 and 9) exhibited weak binding affinity (about 10-fold lower) altogether. The natural products 1 and 2 were both more potent (IC50 280 nM) at inhibition of Kasumi-1 cell growth than 17-AAG 4 (IC50 480 nM). Similar to the Hsp90 binding data, the C4–C5 olefin analogue 7 was slightly less potent (IC50 760 nM) in the Kasumi-1 assay. Interestingly, the phenolic ansamycins were less potent against the MOLM-13 cell line with reblastatin (1) being 3-fold less active (Kd ~ 270 nM) than 17-AAG 4 (Kd ~ 90 nM), while autolytimycin (2) was about 6-fold less active (Kd ~ 510 nM). The binding assay of the phenolic ansamycins revealed that modification to the aromatic moiety and the C4–C5 olefin are well tolerated for Hsp90 binding affinity, while modifications at the C-11 alcohol (addition of carbamate functional group) resulted in substantial loss of activity. The analogs were also screened against two acute myeloid leukemia cell lines (Kasumi-I and MOLM-13). Reblastatin (1) and autolytimycin (2) were more potent than 17-AAG (4) in inhibiting cell growth in Kasumi-I, but were 3- and 5-fold, respectively, less active in the MOLM-13 cell line. The binding affinity observed for these natural product derivatives confirm that in vitro binding to the molecular target Hsp90 is necessary for activity but not sufficient for cytotoxicity.52
Table 1.
Cytotoxicity and Hsp90 Binding Affinity of Reblastatin (1), Autolytimycin (2), 17-AAG (4) and Analogs (7–9).
| Compound | Hsp90 Kd (nM)a | Kasumi-1 IC50 (nM)b | MOLM-13 IC50 (nM)b |
|---|---|---|---|
| reblastatin (1) | 26 | 290 | 270 |
| autolytimcyin (2) | 36 | 280 | 510 |
| 7 | 84 | 760 | 1550 |
| 8 | 1475 | 7280 | 5930 |
| 9 | 1265 | 9030 | 9590 |
| 17-AAG (4)c | 110 | 480 | 90 |
Competitive binding to Hsp90α, see SI for experimental details
See SI for experimental details
Internal standard
Conclusions
In summary, an effective synthetic route for a second-generation synthesis of reblastatin (1), the first total synthesis of autolytimycin (2) and structurally related ansamycin analogs (6–9) were developed. The natural products were prepared in highly enantioenriched form, with a longest linear sequence of 26 steps. A regio- and diastereoselective hydrometalation-transmetalation-aldehyde addition reaction was utilized to assemble the fully functionalized ansa frameworks of the natural products and derivatives. Additionally, the copper-mediated amidation reaction tolerated subtle changes within the ansa chain and aromatic moiety to produce structurally similar macrocycles in high yields. The synthetic natural products were tested for Hsp90 binding affinities and cytotoxicity assays. Although, the natural products reblastatin (1) and autolytimycin (2) were shown to bind the Hsp90 protein with slightly better affinity (4-fold over 17-AAG 4), the synthetic derivatives were less potent in the growth inhibition assays. Further investigation of these synthetic derivatives is needed to determine the in vitro efficacy and pharmacological profiles of these compounds over 17-AAG (4).
Experimental Section
The following experimental information is representative and describes the complete details of the convergent synthesis of autolytimycin (2).
1-(benzyloxy)-3,5-dibromobenzene
To a solution of 3,5-dibromophenol (17.3 g, 68.7 mmol) in acetone (140 mL) at room temperature was added potassium carbonate (14.2 g, 103 mmol) followed by benzyl bromide (8.98 mL, 75.5 mmol). Reaction was allowed to run at room temperature for 16 hours. The heterogeneous mixture was filtered over Celite®, rinsed with ethyl acetate, and concentrated under reduced pressure to give a yellow oil. Purification by flash chromatography (silica, 10% EtOAc/hexanes) affords bis-bromo phenol as a colorless oil (23.0g, 67.2 mmol, 97.8%). 1H NMR (CDCl3, 400 MHz): δ 7.57-7.50 (m, 5H), 7.43 (dt, J = 1.6, 3.4 Hz, 1H), 7.24 (d, J = 1.6, 2H); 13C NMR (CDCl3, 100 MHz): δ 159.8, 135.7, 128.7, 128.3, 127.5, 126.6, 123.1, 117.2, 70.5; IR (neat) νmax: 3080, 3033, 2933, 2871, 1583, 1436, 1256, 1024, 830, 746, 696 cm−1; HRMS (CI, NH3) m/z calc’d for C13H10Br2O [M+23]+ 362.8996, found 362.8986.
3-(benzyloxy)-5-bromobenzaldehyde (15b)
To a solution of 1-(benzyloxy)-3,5-dibromobenzene (12.2 g, 35.7 mmol) in tetrahydrofuran (360 mL) at −78 °C was added n-BuLi in hexane (2.50 M, 15.7 mL) dropwise. Reaction stirred for 30 minutes and N,N-dimethylformamide (4.14 mL, 53.5 mmol) was added in one portion. Reaction was warmed to room temperature and stirred for additional 30 minutes. Reaction solution was poured into vigorously stirring solution of 10% aq. KH2PO4 and diethyl ether (310 mL:190 mL). The biphasic mixture stirred for additional 10 minutes. The organic layer was separated and the aqueous solution was extracted with diethyl ether (2x). The combined organic layers were dried with magnesium sulfate, filtered, and concentrated under reduced pressure. Purification by flash chromatography (silica, 5% EtOAc/hexanes) gives aldehyde 15b as a white solid (9.0 g, 30.9 mmol, 86.6%). Mp: 52 °C; 1H NMR (CDCl3, 400 MHz): δ 9.88 (s, 1H), 7.58 (t, J = 1.6, 1H), 7.42-7.33 (m, 7H), 5.09 (s, 2H); 13C NMR (CDCl3, 100 MHz): δ 190.5, 159.8, 138.6, 135.6, 128.7, 128.4, 127.5, 125.9, 124.6, 123.5, 113.0, 70.5; IR (neat) νmax: 3068, 3033, 2837, 2727, 1702, 1570, 1270, 1028, 847, 696 cm−1; HRMS (CI, NH3) m/z calc’d for C14H11BrO2 [M+23]+ 312.9840, found 312.9851.
(2S,5S,6S)-methyl 6-(3-(benzyloxy)-5-bromophenyl)-5-methyl-5,6-dihydro-2H-pyran-2-carboxylate (19b)
To a solution of benzaldehyde 15b (9.00 g, 30.9 mmol) and (E)-crotylsilane 18 (12.5 g, 35.5 mmol) in methylene chloride (600 mL) at −50 °C was added trifluoromethanesulfonic acid (2.74 mL, 30.9 mmol) dropwise. Reaction was allowed to stir for 12 hours at −50 °C before it was quenched by addition of saturated solution of sodium bicarbonate. The aqueous phase was extracted with methylene chloride (2x). The combined organic layers were dried with magnesium sulfate, filtered, and concentrated under reduced pressure. Purification by flash chromatography (silica, 10% EtOAc/hexanes) yields dihydropyran 19b as a clear oil (12.0 g, 28.8 mmol, 93.0%). (c 1.0, CHCl3); 1H NMR (CDCl3, 400 MHz): δ 7.41-7.30 (m, 5H), 7.16 (t, J = 1.6 Hz, 1H), 7.07 (t, J = 2.0 Hz, 1H), 6.95 (dt, J = 1.2, 2.4 Hz, 1H), 5.89 (tq, J = 2.0, 2.8, 9.8, 24.6, 2H), 5.03 (s, 2H), 4.86 (q, J = 2.8, 6.0 Hz, 1H), 4.32 (d, J = 9.6 Hz, 1H), 3.75 (s, 3H), 2.39 (m, 1H), 0.81 (d, J = 7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 171.2, 159.4, 143.3, 136.3, 133.6, 128.6, 128.1, 127.5, 123.3, 122.7, 122.4, 117.7, 113.3, 79.3, 73.1, 70.3, 52.1, 34.7, 16.4; IR (neat) νmax: 3037, 2955, 2875, 1753, 1570, 1443, 1272, 1148, 1109, 782 cm−1; HRMS (CI, NH3) m/z calc’d for C21H21BrO4 [M+23]+ 439.0521, found 439.0535.
(2S,3S,5S,6S)-methyl 6-(3-(benzyloxy)-5-bromophenyl)-3-hydroxy-5-methyltetrahydro-2H-pyran-2-carboxylate (17b)
To a solution of dihydropyran 19b (6.00 g, 14.4 mmol) in tetrahydrofuran (150 mL) at 0 °C was added BH3•THF complex in tetrahydrofuran (1.11 M, 22.0 mL) dropwise. Reaction was allowed to warm up to room temperature on its own and stirred for an additional 1 hour at room temperature (about 3 hours). The reaction was cooled to 0 °C and quenched by sequential addition of sodium hydroxide in water (2.00 M, 35.9 mL) and 30% H2O2 (14.7 mL). The quenched reaction was stirred for 1/2 hour at 0 °C and for 1/2 hour at room temperature. The mixture was diluted with water and the aqueous layer was extracted with diethyl ether (5x). The combined organic layers were dried with magnesium sulfate, filtered, and concentrated under reduced pressure. Purification by flash chromatography (silica, 30% EtOAc/hexanes) provides alcohol 17b as a white foam (3.00 g, 28.8 mmol, 47.0%). (c 1.0, CHCl3); 1H NMR (CDCl3, 400 MHz): δ 7.41-7.30 (m, 5H), 7.14 (t, J = 1.4 Hz, 1H), 7.06 (t, J = 2.2 Hz, 1H), 6.92 (dd, J = 1.4, 2.2 Hz, 1H), 5.03 (s, 2H), 4.49 (t, J = 1.8 Hz, 1H), 4.33 (m, 2H), 3.79 (s, 3H), 2.20 (d, J = 7.2 Hz, 1H), 2.10 (m, 1H), 1.94 (ddt, J = 1.6, 3.6, 14.4, 1H), 1.50 (ddd, J = 2.8, 3.6, 12.2, 14.4 Hz, 1H) 0.70 (d, J = 6.4 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 170.4, 159.3, 143.2, 136.2, 128.5, 128.1, 127.5, 123.1, 122.6, 117.4, 113.4, 81.5, 78.3, 70.2, 65.8, 52.2, 35.8, 29.6, 17.5; IR (neat) νmax: 3412, 2953, 2928, 2872, 1747, 1570, 1441, 1270, 1136, 1057, 696 cm−1; HRMS (CI, NH3) m/z calc’d for C21H23BrO5 [M+23]+ 457.0627, found 457.0672.
(2S,3S,5S,6S)-methyl 6-(3-(benzyloxy)-5-bromophenyl)-3-methoxy-5-methyltetrahydro-2H-pyran-2-carboxylate
To a solution of alcohol 17b (5.70 g, 13.1 mmol) in methylene chloride (6.50 mL) at room temperature was added sequentially 4 Å molecular sieves (8.50 g), N,N,N′,N′-tetramethyl-1,8-naphthalenediamine (8.42 g, 39.3 mmol), and trimethyloxonium tetrafluoroborate (4.84 g, 32.7 mmol). Reaction was allowed to stir for 16 hours at room temperature and was filtered over Celite® and washed with methylene chloride. The volatiles were concentrated under reduced pressure and the white residue was redissolved in ethyl acetate. The organic layer was washed with 1 M HCl solution (2x). The aqueous layers were extracted with ethyl acetate (2x). The combined organic layers were dried with magnesium sulfate, filtered, and concentrated under reduced pressure. Purification by flash chromatography (silica, 15% EtOAc/hexanes) affords product as a white foam (5.30 g, 11.8 mmol, 90.1%). (c 0.5, CHCl3); 1H NMR (CDCl3, 400 MHz): δ 7.41-7.31 (m, 5H), 7.15 (t, J = 1.4 Hz, 1H), 7.04 (t, J = 2.0 Hz, 1H), 6.93 (dd, J = 1.4, 2.0 Hz, 1H); 5.02 (s, 2H), 4.66 (br. s, 1H), 4.32 (d, J = 10.4 Hz, 1H), 3.79 (obs. m, 1H), 3.78 (s, 3H), 3.44 (s, 3H), 2.10-2.01 (m, 2H), 1.35 (dt, J = 2.6, 12.6, 15.2 Hz, 1H), 0.63 (d, J = 6.4 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 171.1, 159.3, 143.6, 136.3, 128.6, 128.1, 127.5, 123.3, 122.6, 117.5, 113.4, 81.7, 75.0, 74.8, 70.2, 56.6, 52.1, 33.3, 29.9, 17.6; IR (neat) νmax: 2952, 2928, 2873, 2826, 1748, 1570, 1441, 1141, 1095, 995, 696 cm−1; HRMS (CI, NH3) m/z calc’d for C22H25BrO5 [M+23]+ 471.0783, found 471.0774.
((2R,3S,5S,6S)-6-(3-(benzyloxy)-5-bromophenyl)-3-methoxy-5-methyltetrahydro-2H-pyran-2-yl)methanol (20b)
To a solution ester (6.00 g, 13.0 mmol) in diethyl ether (260 mL) at 0° C was added lithium tetrahydroborate (582 mg, 26.7 mmol). Reaction was stirred for 1/2 hour at 0 °C and 1 hour at room temperature. Water was carefully added and the reaction mixture was extracted with diethyl ether (3x). The combined organic layers were dried with magnesium sulfate, filtered, and concentrated under reduced pressure. Purification by flash chromatography (silica, 30% EtOAc/hexanes) gives alcohol 20b as a yellow foam (4.90 g, 11.6 mmol, 89%). (c 0.94, CHCl3); 1H NMR (CDCl3, 400 MHz): δ 7.41-7.31 (m, 5H), 7.12 (t, J = 1.4 Hz, 1H), 7.04 (t, J = 2.0 Hz, 1H), 6.92 (t, J = 1.6 Hz, 1H), 5.02 (s, 2H), 4.15 (d, J = 8.4 Hz, 1H), 3.94-3.84 (m, 2H), 3.60 (m, 1H), 3.37 (s, 3H), 3.31 (q, J = 3.6, 8.0 Hz, 1H), 2.18 (m, 1H), 1.90 (m, 2H), 1.53 (ddd, J = 3.6, 10.4, 14.0 Hz, 1H), 0.79 (d, J = 6.8 Hz, 3H); 13C NMR (CDCl3, 75.0 MHz): δ 159.4, 143.8, 136.2, 128.6, 128.1, 127.5, 122.9, 122.7, 117.2, 113.1, 78.8, 75.4, 73.7, 70.2, 60.7, 56.3, 32.2, 30.4, 18.0; IR (neat) νmax: 3433, 2929, 2873, 2825, 1570, 1440, 1270, 1046, 833, 696 cm−1; HRMS (CI, NH3) m/z calc’d for C21H25BrO4 [M+23]+ 443.0834, found 443.0838.
(2R,3S,5R)-6-(3-(benzyloxy)-5-bromophenyl)-3-methoxy-5-methylhexane-1,2-diol (21b)
To a suspension of aluminum trichloride (7.75 g, 58.1 mmol) in methylene chloride (500 mL) at −78 °C was added anisole (58.0 mL, 534 mmol) dropwise. The light yellow solution was stirred for an additional 5 minutes before alcohol 20b (4.90 g, 11.6 mmol) in methylene chloride (80.0 mL) was added dropwise. The reaction mixture was allowed to warm up to 0 °C over 3 hours (on its own) and stirred for an additional 1 hour at that temperature. Reaction was quenched by slow addition of 0.5 M HCl (80.0 ml). The biphasic mixture was diluted with ammonium chloride and extracted with ethyl acetate (2x). The combined organic layers were dried with magnesium sulfate, filtered, and concentrated under reduced pressure. Purification by flash chromatography (silica, 50% EtOAc/hexanes) yields phenol as a colorless oil (3.60 g, 10.9 mmol, 94.0%).
To a solution of the above phenol (1.35 g, 4.08 mmol) in methylene chloride (24.0 mL) at room temperature was added triethylsilane (5.21 mL, 32.6 mmol) followed by scandium(III) triflate (3.01 g, 6.11 mmol). The sealed tube was closed and the reaction was allowed to run at 42 °C for 48 hours. Upon completion, the mixture was cooled to room temperature and \was quenched by addition of water. The aqueous layer was extracted with methylene chloride (3x). The combined organic layers were dried with magnesium sulfate, filtered, and concentrated under reduced pressure. Purification by flash chromatography (silica, 80% EtOAc/hexanes) affords diol as a colorless oil (1.20 g, 3.60 mmol, 88.2%). To a solution of the above diol (2.90 g, 8.70 mmol) in acetone (50.0 mL) was added potassium carbonate (1.80 g, 13.0 mmol) followed by benzyl bromide (1.14 mL, 9.57 mmol). The heterogenous mixture stirred at room temperature for 48 hours before it was filtered over Celite® and washed with ethyl acetate. The solvent was concentrated under reduced pressure and the resulting residue was purified by flash chromatography (silica, 60% EtOAc/hexanes) to afford diol 21b as a yellow oil (3.40 g, 8.00 mmol, 92.0%). (c 1.2, CHCl3); 1H NMR (CDCl3, 400 MHz): δ 7.41-7.28 (m, 5H), 6.96 (t, J = 2.2 Hz, 1H), 6.90 (t, J = 1.4 Hz, 1H), 6.69 (t, J = 2.2 Hz, 1H), 5.00 (s, 2H), 3.73-3.62 (m, 3H), 3.40 (s, 3H), 3.38 (t, J = 3.6 Hz, 1H) 2.58 (A of ABq, J = 5.8, 13.2 Hz, 1H), 2.41 (d, J = 5.2 Hz, 1H), 2.31 (B of ABq, J = 8.8, 13.2 Hz, 1H), 2.11 (dd, J = 3.4, 8.0 Hz, 1H), 1.91 (m, 1H), 1.62 (dtd, J = 4.2, 9.6, 14.0 Hz, 1H), 1.16 (dtd, J = 3.6, 9.6, 14.0 Hz, 1H), 0.85 (d, J = 6.4 Hz, 3H); 13C NMR (CDCl3, 75.0 MHz): δ 159.2, 144.1, 136.4, 128.5, 128.0, 127.4, 124.8, 122.4, 115.3, 114.9, 80.7, 72.7, 70.1, 63.3, 58.4, 43.9, 37.4, 31.1, 19.0; IR (neat) νmax: 3405, 2930, 1603, 1566, 1447, 1269, 1156, 1096, 1028, 851, 736, 697 cm−1; HRMS (CI, NH3) m/z calc’d for C21H27BrO4 [M+23]+ 445.0990, found 445.0980.
(3S,4R,5S,7R)-8-(3-(benzyloxy)-5-bromophenyl)-5-methoxy-3,7-dimethyloct-1-en-4-ol
To a solution of diol 21b (0.270 g, 0.596 mmol) in acetone (6.00 mL) and water (6.00 mL) mixture was added sodium bicarbonate (0.150 g, 1.79 mmol) and sodium metaperiodate (0.191 g, 0.893 mmol). The resulting suspension was stirred at room temperature for 2 hours. The solution was diluted with water followed and ethyl acetate. The aqueous phase was saturated with solid sodium chloride and extracted with ethyl acetate (3x). The combined organic layers were dried with magnesium sulfate, filtered, and concentrated under reduced pressure. The crude aldehyde 14b was used in the next step without further purification.
To a flame dried round bottom flask was added 4 Å powdered molecular sieves (600 mesh, 0.330 g), toluene (4.00 mL) and 1.00 M of (4S,5S,Z)-diisopropyl 2-(but-2-enyl)-1,3,2-dioxaborolane-4,5-dicarboxylate 13 in toluene (1.66 mL) under an atmosphere of nitrogen. The reaction mixture was allowed to stir for 20 minutes at room temperature before it was cooled to −78 °C. A solution of aldehyde 14b (0.200 g, 0.475 mmol) in toluene (1.00 mL) was added dropwise via a cannula. The reaction mixture stirred at −78 °C for 16 hours before it was quenched with 1 N NaOH solution (5 mL). The two-phase mixture was allowed to warm up to room temperature and stirred for 1 hour. The solution mixture was filtered over a pad of Celite® and the aqueous phase was extracted with diethyl ether (3x). The combined organic layers were dried with magnesium sulfate, filtered, and concentrated under reduced pressure. The residue was purified by flash chromatography (silica, 10% EtOAc/hexanes) to yield homoallylic alcohol as a viscous clear oil, dr 20:1. (c 1.0, CHCl3); 1H NMR (CDCl3, 400 MHz): δ 7.41-7.30 (m, 5H), 6.95 (t, J = 2.0 Hz, 1H), 6.91 (t, J = 2.0 Hz, 1H), 6.70 (t, J = 2.0 Hz, 1H), 5.60 (m, 1H), 5.02 (m, 4H), 3.61 (dt, J = 2.4, 9.2 Hz, 1H), 3.33 (s, 3H), 3.25 (dt, J = 2.4, 10.8 Hz, 1H), 2.58 (A of ABx, J = 5.8, 13.2 Hz, 1H), 2.29 (B of ABx, J = 9.0, 13.2 Hz, 1H), 2.22 (obs. m, 1H), 2.05 (d, J = 2.4 Hz, 1H), 1.93 (m, 1H), 1.63 (ddd, J = 3.2, 10.6, 14.0 Hz, 1H), 1.18 (ddd, J = 2.4, 10.4, 14.4 Hz, 1H), 1.12 (d, J = 6.4 Hz, 3H), 0.77 (d, J = 6.8 Hz, 1H); 13C NMR (CDCl3, 75.0 MHz): δ 159.2, 144.5, 139.8, 136.4, 128.6, 128.0, 127.5, 124.8, 122.4, 115.3, 115.2, 114.9, 80.0, 73.1, 70.1, 57.0, 44.4, 40.4, 34.4, 30.8, 18.5, 17.5; IR (neat) νmax: 3451, 3067, 2928, 1603, 1567, 1448, 1269, 1087, 916, 697 cm−1; HRMS (CI, NH3) m/z calc’d for C24H31BrO3 [M+23]+ 469.1354, found 469.1335.
1-(benzyloxy)-3-bromo-5-((2R,4S,5R,6S)-4-methoxy-5-(methoxymethoxy)-2,6-dimethyloct-7-enyl)benzene (22b)
To a solution of alcohol (1.80 g, 4.02 mmol) in methylene chloride (20.0 mL) and N,N-diisopropylethylamine (4.20 mL, 24.1 mmol) at 0 °C was added DMAP (147 mg, 1.21 mmol) and chloromethyl methyl ether (1.22 mL, 16.1 mmol) dropwise. The reaction was allowed to warm up to room temperature overnight and was quenched by addition of saturated solution of sodium bicarbonate. The aqueous layer was extracted with methylene chloride (3x). The combined organic layers were dried with magnesium sulfate, filtered, and concentrated under reduced pressure. The crude product was purified by flash chromatography (silica, 5% EtOAc/hexanes) to yield MOM-ether 22b as a yellow oil (64%, three steps). (c 0.9, CHCl3); 1H NMR (CDCl3, 400 MHz): δ 7.41-7.30 (m, 5H), 6.94 (t, J = 2.0 Hz, 1H), 6.92 (t, J = 1.6 Hz, 1H), 6.71 (dd, J = 1.6, 2.0 Hz, 1H), 5.67 (m, 1H), 5.00 (m, 4H), 4.81 (A of ABq, J = 6.4 Hz, 1H), 4.62 (B of ABq, J = 6.8 Hz, 1H), 3.61 (dd, J = 2.0, 8.8 Hz, 1H), 3.39 (s, 3H), 3.32 (obst. m, 1H), 3.31 (s, 3H), 2.64 (A of ABx, J = 5.2, 13.6 Hz, 1H), 2.27 (obs. q, J = 8.8, 15.6 Hz, 1H), 2.24 (obs. B of ABx, J = 9.2, 13.6 Hz, 1H), 1.93 (m, 1H), 1.68 (ddd, J = 3.2, 10.6, 14.0 Hz, 1H), 1.21 (ddd, J = 2.0, 10.2, 14.0 Hz, 1H), 1.11 (d, J = 6.8 Hz, 3H), 0.75 (d, J = 6.4 Hz, 1H); 13C NMR (CDCl3, 75.0 MHz): δ 159.2, 144.6, 140.9, 136.5, 128.6, 128.0, 127.5, 124.9, 122.2, 115.2, 114.9, 114.8, 97.4, 80.7, 79.0, 70.0, 57.0, 56.0, 44.3, 40.6, 36.4, 31.0. 18.5, 17.6; IR (neat) νmax: 2957, 2926, 1604, 1448, 1154, 1097, 1032, 918, 696 cm−1; HRMS (CI, NH3) m/z calc’d for C26H35BrO4 [M+23]+ 513.1616, found 513.1606.
1-(benzyloxy)-3-bromo-5-((2R,4S,5R,6S)-4-methoxy-5-(methoxymethoxy)-2,6-dimethyloct-7-ynyl)benzene
To a solution of alkene 22b (0.420 g, 0.710 mmol) in a 4:1 mixture of acetone (6.40 mL) and water (1.60 mL) was added N-methylmorpholine-N-oxide (0.166 g, 1.42 mmol). Osmium tetraoxide (0.20 M in toluene, 530 μL) was added dropwise and the reaction flask was stoppered. The reaction mixture stirred at room temperature for 4 hours before it was quenched with saturated solution of sodium thiosulfate. The aqueous layer was saturated with solid NaCl and extracted with ethyl acetate (3x). The combined organic layers were dried with magnesium sulfate, filtered, and concentrated under reduced pressure. The crude diol was used without further purification in the next step.
To solution of crude diol (0.440 g, 0.703 mmol) in dry benzene (14.0 mL) was added potassium carbonate (0.292 g, 2.11 mmol) followed by lead tetraacetate (0.468 g, 1.05 mmol). The reaction stirred for 30 minutes before it was quenched with saturated solution of sodium bicarbonate. The aqueous phase was extracted with ethyl acetate (3x). The combined organic layers were dried with magnesium sulfate, filtered, and concentrated under reduced pressure. The crude aldehyde 23b was used directly in the next step.
To a suspension of potassium tert-butoxide (0.108 g, 0.967 mmol) in tetrahydrofuran (1.00 mL) under an atmosphere of Nitrogen at −78 °C was added dimethyl diazomethylphosphonate (0.166 g, 1.10 mmol) in tetrahydrofuran (1.00 mL) dropwise. The reaction mixture stirred for 15 minutes before a −78 °C solution of aldehyde 23b (0.164 g, 0.276 mmol) in tetrahydrofuran (1.00 mL) was added dropwise. The reaction mixture stirred for 15 minutes at −78 °C and was diluted with ethyl acetate and washed with brine. The aqueous layer was extracted with ethyl acetate (3x). The combined organic layers were dried with magnesium sulfate, filtered, and concentrated under reduced pressure. Purification by flash chromatography (silica, 2–5% EtOAc/hexanes) yielded terminal alkyne as a yellow oil (80%). (c 1.0, CHCl3); 1H NMR (CDCl3, 400 MHz): δ 7.41-7.31 (m, 5H), 6.94 (m, 2H), 6.72 (t, J = 1.8 Hz, 1H), 5.00 (s, 2H), 4.81 (A of ABq, J = 6.8 Hz, 1H), 4.65 (B of ABq, J = 6.8 Hz, 1H), 3.72 (dd, J = 2.0, 8.8 Hz, 1H), 3.67 (dt, J = 2.2, 8.0 Hz, 1H), 3.39 (s, 3H), 3.36 (s, 3H), 2.64 (A of ABx, J = 5.4, 13.2 Hz, 1H), 2.51 (m, 1H), 2.28 (B of ABx, J = 9.2, 13.6 Hz, 1H), 2.11 (d, J = 2.4 Hz, 1H), 1.97 (m, 1H), 1.69 (ddd, J = 3.6, 10.2, 14.0 Hz, 1H), 1.30 (d, J = 6.8 Hz, 3H), 1.25 (obst. m, 1H), 0.82 (d, J = 6.4 Hz, 1H); 13C NMR (CDCl3, 75.0 MHz): δ 159.3, 144.6, 136.5, 128.6, 128.1, 127.5, 124.9, 122.3, 115.2, 114.9, 97.4, 85.8, 80.7, 78.7, 70.7, 70.1, 57.1, 56.2, 44.2, 36.5, 31.1, 28.2, 18.6, 18.1; IR (neat) νmax: 3297, 2931, 2823, 1567, 1448, 1157, 1097, 1030 920, 697 cm−1; HRMS (CI, NH3) m/z calc’d for C26H33BrO4 [M+23]+ 511.1460, found 511.1450.
1-(benzyloxy)-3-bromo-5-((2R,4S,5R,6S)-4-methoxy-5-(methoxymethoxy)-2,6-dimethylnon-7-ynyl)benzene (11b)
To a solution of terminal alkyne (0.355 g, 0.602 mmol) in tetrahydrofuran (6.00 mL) at −78 °C under an atmosphere of Nitrogen was added lithium hexamethyldisilazide (1.0 M in THF, 1.3 mL) dropwise. The reaction mixture was allowed to stir for 30 minutes at −78 °C before methyl iodide (375 μL, 6.02 mmol) was added dropwise. The reaction mixture was stirred for one hour at −78 °C and one hour at room temperature. The mixture was quenched by addition of water. The aqueous phase was extracted with ethyl ether (3x). The combined organic layers were dried with magnesium sulfate, filtered, and concentrated under reduced pressure. Purification by flash chromatography (silica, 4% EtOAc/hexanes) provides methyl alkyne 11b as a clear oil (334 mg, 0.554 mmol, 92%). (c 1.7, CHCl3); 1H NMR (CDCl3, 400 MHz): δ 7.41-7.30 (m, 5H), 6.94 (m, 2H), 6.73 (br. s, 1H), 5.00 (s, 2H), 4.81 (A of ABq, J = 6.4 Hz, 1H), 4.64 (B of ABq, J = 6.8 Hz, 1H), 3.66 (d, J = 8.8 Hz, 1H), 3.38 (s, 3H), 3.35 (s, 3H), 2.66 (A of ABx, J = 5.2, 13.6 Hz, 1H), 2.43 (m, 1H), 2.26 (B of ABx, J = 9.6, 13.6 Hz, 1H), 1.96 (m, 1H), 1.76 (d, J = 2.4 Hz, 3H), 1.67 (ddd, J = 3.6, 10.4, 14.4 Hz, 1H), 1.27 (ovlp m, 1H), 1.24 (d, J = 6.8 Hz, 3H), 0.82 (d, J = 6.4 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 159.3, 144.7, 136.5, 128.6, 128.0, 127.5, 124.9, 122.3, 115.2, 114.9, 97.3, 80.9, 80.5, 79.2, 78.0, 70.1, 57.0, 56.1, 44.2, 36.5, 31.1, 28.5, 18.6, 18.5, 3.4; IR (neat) νmax: 3065, 3033, 2920, 2822, 1604, 1567, 1448, 1269, 1154, 1033, 920, 697 cm−1; HRMS (CI, NH3) m/z calc’d for C27H35BrO4 [M+23]+ 525.1616, found 525.1654.
(S)-5-oxotetrahydrofuran-2-carboxylic acid
To a solution of L-glutamic acid 25 (20.0 g, 136.0 mmol) in water (136 mL) and 2 N HCl (80 mL) at 0 °C was added dropwise solution of sodium nitrate (11.2 g, 163 mmol) in water (80 mL) over 2 hours. After addition was complete, the reaction mixture was allowed to warm up to room temperature and stirred for 16 hours. The aqueous phase was saturated with solid NaCl and was extracted with ethyl acetate (3x). The combined organic layers were dried with magnesium sulfate, filtered, and concentrated under reduced pressure. The crude yellow solid was used in the next step without further purification (12.0 g, 92.0 mmol, 80%). (c 1.1, EtOH); 1H NMR (400 MHz, DMSO): δ 4. 96 (dt, J = 1.2, 5.2 Hz, 2H), 3.35 (m, 1H), 2.16 (m, 2H), −0.16 (bs, 1H); 13C NMR (75 MHz, CDCl3): 175.8, 174.7, 75.1, 26.6, 25.8; IR (neat) νmax: 2999, 1760, 1419, 1180, 1067 cm−1; HRMS (CI, NH3) m/z calc’d for C5H6O4 [M+]+ 130.0266, found 130.0250.
(S)-5-(hydroxymethyl)-dihydrofuran-2(3H)-one (26)
To a solution of (S)-5-oxotetrahydrofuran-2-carboxylic acid (14.0 g, 108.0 mmol) in tetrahydrofuran (100 mL) was added BH3•Me2S (12.3 mL, 130 mmol) over one hour. The resulting mixture was stirred at room temperature for 4 hours. Reaction was quenched with methanol (63 mL) and the resulting mixture was concentrated in vacuo. The residue was purified by flash chromatography (silica, 10% MeOH/CH2Cl2) to afford lactone 26 as a colorless oil (10.0 g, 86.0 mmol, 80%). (c 1.02, EtOH); 1H NMR (400 MHz, CDCl3): δ 4.62 (m, 1H), 3.89 (dd, J = 2.8, 12.6 Hz, 1H), 3.64 (dd, J = 4.6, 12.6 Hz, 1H), 2.64-2.51 (m, 2H), 2.30-2.12 (m, 3H); 13C NMR (75 MHz, CDCl3): 177.8, 80.8, 64.0, 28.5, 23.1; IR (neat) νmax 3423, 2942, 1770, 1356, 1190, 1063, 937 cm−1; HRMS (CI, NH3) m/z calc’d for C5H8O3 [M+]+ 116.0473, found 116.0451.
(S)-5-((tert-butyldimethylsilyloxy)methyl)dihydrofuran-2(3H)-one
To a solution of alcohol 26 (10.0 g, 86.0 mmol) in N,N-dimethylformamide (45 mL) at 0 °C was added 1H-imidazole (14.6g, 215 mmol) and tert-butyldimethylsilyl chloride (16.9 g, 112 mmol). The reaction mixture was allowed to warm up to room temperature and was stirred for 12 hours. Water was added and the aqueous phase was extracted with hexanes (3x). The combined organic layers were dried with magnesium sulfate, filtered, and concentrated under reduced pressure. The residue was purified by flash chromatography (silica, 10% EtOAc/hexanes) to afford a colorless oil (19.8 g, 86.0 mmol, 99%). (c 1.0, CDCl3); 1H NMR (400 MHz, CDCl3): δ 4.56 (m, 1H), 3.84 (dd, J = 3.2, 11.2 Hz, 1H), 3.66 (dd, J = 2.8, 11.2 Hz, 1H), 2.63-2.55 (m, 1H), 2.48-2.40 (m, 1H), 2.29-2.11 (m, 2H), 0.93 (s, 9H), 0.04 (s, 6H); 13C NMR (75 MHz, CDCl3): 177.3, 80.1, 64.9, 28.4, 25.7, 23.5, 18.2, −5.5, −5.6; IR (neat) νmax: 2954, 2932, 2858, 1779, 1256, 1122, 838 cm−1; HRMS (CI, NH3) m/z calc’d for C11H22O3Si [M+1]+ 231.1416, found 231.1426.
(S,E)-ethyl 7-(tert-butyldimethylsilyloxy)-6-hydroxy-2-methylhept-2-enoate (27)
To a solution of TBS ether (19.8 g, 86.0 mmol) in toluene (287 mL) at −78 °C was added DibalH (1.0 M in toluene, 93.0 mL, 92.9 mmol). The reaction mixture was stirred for 2 hours at that temperature and was quenched by addition of methanol (30 mL). The mixture was then allowed to warm up to room temperature and diluted with ethyl acetate, washed with saturated solutions of NaKtartrate and NaCl. The organic layer was dried over MgSO4, filtered and concentrated in vacuo. The crude product was used in the next step without further purification.
To a solution of lactol (19.6 g, 86.0 mmol) in benzene (453 mL) added (carbethoxyethylidene)triphenyl phosphorane (37.4 g, 103 mmol). The reaction mixture was allowed to reflux for 2 hours. The mixture was cooled to room temperature and concentrated in vacuo. The residue was purified by flash chromatography (silica, 15% EtOAc/hexanes) to provide alcohol 27 as a colorless oil (19.8 g, 62.6 mmol, 73 %). (c 0.99, CDCl3); 1H NMR (400 MHz, CDCl3): δ 6.73 (t, J = 6.8 Hz, 1H), 4.16 (q, J = 6.8, 14.4 Hz, 2H), 3.61 (m, 2H), 3.38 (t, J = 8.4 Hz, 1H), 2.31 (m, 2H), 1.83 (s, 3H), 1.55 (m, 2H), 1.28 (t, J = 7.0 Hz, 3H), 0.88 (s, 9H), 0.05 (s, 6H); 13C NMR (75 MHz, CDCl3): 168.1, 141.4, 128.3, 71.2, 67.1, 60.3, 31.7, 25.9, 24.8, 18.3, 14.2, 12.3, −5.4; IR (neat) νmax: 3501, 2954, 2930, 2858, 1711, 1258, 1096, 838 cm−1; HRMS (CI, NH3) m/z calc’d for C16H32O4Si [M+1]+ 317.2148, found 317.2156.
(S,E)-ethyl 7-(tert-butyldimethylsilyloxy)-6-methoxy-2-methylhept-2-enoate
To a solution of alcohol 27 (18.0 g, 56.9 mmol) in dichloromethane (1140 mL) was added 4Å powdered molecular sieves (600 mesh, 37.0 g), Proton-Sponge™ (37.0 g, 171 mmol), and Me3OBF4 (21.0 g, 142 mmol). The resulting mixture was stirred at room temperature for 3 hours, filtered over Celite®, and concentrated in vacou. The residue was dissolved in ethyl acetate and washed with 1 M CuSO4 and NaCl solutions. The combined organic layers were dried with magnesium sulfate, filtered, and concentrated under reduced pressure. The residue was purified by flash chromatography (silica, 7% EtOAc/hexanes) to afford a colorless oil (17.8 g, 53.9 mmol, 95%). (c 0.95, CHCl3); 1H NMR (400 MHz, CDCl3): δ 6.75 (t, J = 7.2 Hz, 1H), 4.17 (q, J = 7.0, 14.3 Hz, 2H), 3.63 (dd, J = 5.5, 10.4 Hz, 1H), 3.53 (dd, J = 5.5, 10.4 Hz, 1H), 3.39 (s, 3H), 3.17 (m, 1H), 2.25 (q, J = 7.9, 15.2 Hz, 2H), 1.82 (s, 3H), 1.68-1.51 (m, 2H), 1.27 (t, J = 7.0 Hz, 3H), 0.87 (s, 9H), 0.04 (s, 6H); 13C NMR (75 MHz, CDCl3): 168.1,141.8, 128.1, 81.1, 64.8, 60.3, 57.8, 30.2, 25.9, 24.5, 18.2, 14.2, 12.2, −5.4; IR (neat) νmax: 2954, 2931, 2858, 1713, 1257, 1117, 838 cm−1; HRMS (CI, NH3) m/z calc’d for C17H34O4Si [M+1]+ 331.2305, found 331.2287.
(S,E)-ethyl 7-hydroxy-6-methoxy-2-methylhept-2-enoate (28)
To TBS ether (17.8 g, 53.9 mmol) was added 2 % solution of HCl in methanol (900 mL). The reaction mixture was stirred for 1 hour at room temperature and concentrated in vacou. The residue was purified by flash chromatography (silica, 30% EtOAc/hexanes) to afford primary alcohol 28 as a colorless oil (11.5 g, 53.2 mmol, 99%). (c 1.01, CHCl3); 1H NMR (400 MHz, CDCl3): δ 6.72 (dt, J = 1.5, 7.6 Hz, 1H), 4.17 (q, J = 7.3, 14.0 Hz, 2H), 3.70 (dd, J = 3.7, 11.6 Hz, 1H), 3.49 (dd, J = 5.5, 11.6 Hz, 1H), 3.39 (s, 3H), 3.26 (m, 1H), 2.22 (q, J = 7.3, 15.9 Hz, 2H), 1.82 (s, 3H), 1.71 (m, 1H), 1.59 (m, 1H), 1.27 (t, J = 7.0 Hz, 3H); 13C NMR (75 MHz, CDCl3): 167.9, 141.1, 128.2, 80.8, 63.4, 60.3, 57.0, 29.4, 24.3, 14.1, 12.1; IR (neat) νmax: 3448, 2980, 2933, 1709, 1273, 1095, 746 cm−1; HRMS (CI, NH3) m/z calc’d for C11H20O4 [M+1]+ 217.1440, found 217.1422.
(S,E)-ethyl 6-methoxy-2-methyl-7-oxohept-2-enoate (12a)
To oxalyl chloride (156 μL, 1.85 mmol) in methylene chloride (2.40 mL) at −78 °C was added dimethyl sulfoxide (262 μL, 3.70 mmol) dropwise. The reaction stirred for 15 minutes before alcohol 28 (200 mg, 0.925 mmol) in methylene chloride (5.00 mL) was added via a cannula. The mixture stirred for 1/2 hour before triethylamine (0.773 mL, 5.55 mmol) was added dropwise. The reaction was allowed to warm up to room temperature for 4 hours and water was added. The aqueous phase was extracted with dichloromethane (3x). The combined organic layers were washed with brine, dried over MgSO4, filtered, and concentrated. The residue was purified by flash chromatography (silica, 10% EtOAc/hexanes) to afford aldehyde 12a as a colorless oil (197 mg, 0.919 mmol, 99%). (c 1.75, CDCl3); 1H NMR (400 MHz, CDCl3): δ 9.62 (d, J = 2.0 Hz, 1H), 6.65 (dt, J = 1.2, 7.2 Hz, 1H), 4.13 (q, J = 7.4 14.2 Hz, 2H), 3.51 (m, 1H), 3.40 (s, 3H), 2.25 (m, 2H), 1.79 (s, 3H), 1.74 (m, 2H), 1.24 (t, J = 7.2 Hz, 3H); 13C NMR (75 MHz, CDCl3): 203.5, 168.0, 139.9, 128.5, 84.7, 60.5, 58.3, 28.5, 23.7, 14.2, 12.3; IR (neat) νmax: 2984, 2828, 1734, 1709, 1266, 1133 cm−1; HRMS (CI, NH3) m/z calc’d for C11H18O4 [M+]+ 214.1205, found 214.1203.
(2E,6S,7S,8E,10S,11R,12S)-ethyl 15-(3-(benzyloxy)-5-bromophenyl)-7-hydroxy-6,12-dimethoxy-11-(methoxymethoxy)-2,8,10-trimethylpentadeca-2,8-dienoate (33b)
To a sealed tube containing 11b (15.0 mg, 0.0299 mmol) was added toluene (300 μL). Bis(cyclopentadienyl)zirconium chloride hydride (15.2 mg, 0.0600 mmol) was added and the reaction mixture was heated to 50 °C. After 6 hours, the reaction tube was allowed to cool to room temperature and then it was cooled to −65 °C. 2.00 M of dimethylzinc in toluene (15.4 μL) was added dropwise and the solution mixture was warmed to 0 °C. aldehyde 12a (7.83 mg, 0.0366 mmol) was added dropwise to the reaction mixture dropwise. After 1 hour at 0 °C, the reaction mixture was quenched with saturated solution of ammonium chloride. The aqueous layer was extracted with diethyl ether (3x). The combined organic layers were dried with magnesium sulfate, filtered, and concentrated under reduced pressure. Purification by flash chromatography (silica, 30% EtOAc/hexanes) yields reproducible 40–89% of desired product 33b as a yellow oil. (c 1.1, CHCl3); 1H NMR (CDCl3, 400 MHz): δ 7.43-7.28 (m, 5H), 6.94 (s, 1H), 6.91 (s, 1H), 6.71 (s, 1H), 6.66 (t, J = 7.4 Hz, 1H), 5.29 (d, J = 10.0 Hz, 1H), 4.99 (s, 2H), 4.81 (A of ABq, J = 6.4 Hz, 1H), 4.60 (B of ABq, J = 6.4 Hz, 1H), 4.13 (q, J = 7.4, 14.6 Hz, 1H), 3.84 (dd, J = 3.2, 6.4 Hz, 1H), 3.60 (d, J = 9.2 Hz, 1H), 3.40 (s, 3H), 3.38 (s, 3H), 3.28 (s, 3H), 3.20 (m, 2H), 2.64 (dd, J = 5.2, 13.6 Hz, 1H), 2.54 (d, J = 2.8 Hz, 1H), 2.51 (m, 1H), 2.24 (m, 3H), 1.94 (m, 1H), 1.81 (s, 3H)1.72 (m, 1H), 1.61 (s, 3H), 1.54 (m, 1H), 1.28 (ovlp. m, 1H), 1.23 (t, J = 6.4 Hz, 1H), 1.14 (m, 1H), 1.06 (d, J = 6.4 Hz, 1H), 0.72 (d, J = 6.4 Hz, 1H); 13C NMR (CDCl3, 75.0 MHz): δ 167.8, 159.2, 144.3, 140.9, 136.4, 133.8, 131.4, 128.5, 128.2, 128.0, 127.4, 124.8, 122.3, 115.1, 114.9, 97.3, 81.2, 81.0, 79.4, 78.6, 70.0, 60.3, 58.4, 56.8, 56.0, 44.1, 36.4, 34.4, 30.8, 29.3, 23.9, 18.6, 17.7, 14.2, 12.3; IR (neat) νmax: 3457, 2928, 1708, 1448, 1269, 1097, 1030, 697 cm−1; HRMS (CI, NH3) m/z calc’d for C38H55BrO8 [M+23]+ 741.2978, found 741.3000.
(2E,6S,7S,8E,10S,12S,14R)-ethyl 15-(3-(benzyloxy)-5-bromophenyl)-7-(tert-butyldimethylsilyloxy)-6,12-dimethoxy-11-(methoxymethoxy)-2,8,10,14-tetramethylpentadeca-2,8-dienoate
To a solution of alcohol 33b (13.0 mg, 0.0173 mmol) in methylene chloride (200 μL) at 0 °C was added 2,6-lutidine (8.03 μL, 0.0694 mmol) and tert-butyldimethylsilyl trifluoromethanesulfonate (7.96 μL, 0.0347 mmol). Reaction was allowed to stir for 2 hours at 0 °C and was quenched by addition of saturated solution of sodium bicarbonate. The aqueous layer was extracted with methylene chloride (3x). The combined organic layers were dried with magnesium sulfate, filtered, and concentrated under reduced pressure. Purification by flash chromatography (silica, 20% EtOAc/hexanes) provided 95% of desired product as a yellow oil. (c 1.3, CHCl3); 1H NMR (CDCl3, 400 MHz): δ 7.41-7.29 (m, 5H), 6.93 (s, 1H), 6.91 (s, 1H), 6.71 (s, 1H), 6.64 (dt, J = 1.6, 7.4 Hz, 1H), 5.13 (d, J = 10.0 Hz, 1H), 4.99 (s, 1H), 4.83 (A of ABq, J = 6.4 Hz, 1H), 4.60 (B of ABq, J = 6.4 Hz, 1H), 4.11 (q, J = 7.0, 14.2 Hz, 1H), 3.94 (d, J = 6.8 Hz, 1H), 3.59 (d, J = 9.6 Hz, 1H), 3.44 (s, 3H), 3.38 (s, 3H), 3.26 (s, 3H), 3.18 (d, J = 10.8 Hz, 1H), 3.06 (ddd, J = 2.8, 7.0, 10.0 Hz, 1H), 2.64 (dd, J = 5.2, 13.6 Hz, 1H), 2.46 (m, 1H), 2.23 (m, 3H), 1.93 (m, 1H), 1.79 (s, 3H), 1.73 (m, 1H), 1.56 (s, 3H), 1.46 (m, 1H), 1.30 (m, 1H), 1.21 (t, J = 7.2 Hz, 3H), 1.12 (m, 1H), 1.04 (d, J = 6.8 Hz, 3H), 0.87 (s, 9H), 0.71 (d, J = 6.4 Hz, 1H), 0.05 (s, 3H), − 0.01 (s, 3H); 13C NMR (CDCl3, 75.0 MHz): δ 168.0, 159.3, 144.5, 141.5, 136.5, 135.0, 130.5, 128.6, 128.0, 127.5, 124.9, 122.3, 115.2, 114.9, 97.4, 83.4, 81.0, 80.6, 79.2, 70.1, 60.3, 59.8, 56.8, 56.1, 44.2, 36.4, 30.8, 30.1, 25.8, 24.9, 18.7, 18.1, 17.8, 14.2, 12.5, 12.4, −4.7, −4.9; IR (neat) νmax: 2955, 2928, 2856, 2823, 1710, 1448, 1258, 1111, 1031, 837, 776 cm−1; HRMS (CI, NH3) m/z calc’d for C44H69BrO8Si [M+23]+ 855.3843, found 855.3819.
(2E,6S,7S,8E,10S,12S,14R)-15-(3-(benzyloxy)-5-bromophenyl)-7-(tert-butyldimethylsilyloxy)-6,12-dimethoxy-11-(methoxymethoxy)-2,8,10,14-tetramethylpentadeca-2,8-dienamide
Into a 10mL round bottom flask containing ester (12.0 mg, 0.0139 mmol) was added a mixture of tetrahydrofuran (400 μL), methanol (400 μL), and water (200 μL). Lithium hydroxide, monohydrate (11.6 mg, 0.278 mmol) was added and reaction mixture was allowed to stir for 48 hours. The reaction mixture was concentrated under reduced pressure. The residue was diluted with pH 4.5 NaH2PO4 (10 mL) and extracted with methylene chloride (5x) with aqueous phase saturated with solid NaCl each time. The combined organic layers were dried with magnesium sulfate, filtered, and concentrated under reduced pressure. The crude acid was used in the next step without further purification.
To a solution of acid (17.0 mg, 0.0203 mmol) in methylene chloride (1.00 mL) at −20 °C, was added sequentially triethylamine (85.4 μL, 0.50 M solution in methylene chloride) and ethyl chloroformate (46.8 μL, 0.50 M solution in methylene chloride). The reaction mixture was allowed to stir for 30 minutes at −20 °C (checked by TLC) before anhydrous ammonia (l) was bubbled into the solution (until the disappearance of the mixed anhydride by TLC). The reaction was quenched with water and warmed to room temperature. The aqueous layer was extracted with methylene chloride (3x). The combined organic layers were dried with magnesium sulfate, filtered, and concentrated under reduced pressure. Purification by flash chromatography (silica, 50% EtOAc/hexanes) provided 60% (2 steps) of desired product as a yellow oil and recovered acid in 40%. (c 1.0, CHCl3); 1H NMR (CDCl3, 400 MHz): δ 7.41-7.29 (m, 5H), 6.94 (s, 1H), 6.91 (s, 1H), 6.71 (s, 1H), 6.32 (t, J = 6.6 Hz, 1H), 5.41 (br. s, 2H), 5.13 (d, J = 9.6 Hz, 1H), 4.99 (s, 2H), 4.82 (A of ABq, J = 6.8 Hz, 1H), 4.60 (B of ABq, J = 6.4 Hz, 1H), 3.94 (d, J = 6.8 Hz, 1H), 3.59 (d, J = 9.6 Hz, 1H), 3.43 (s, 3H), 3.38 (s, 3H), 3.27 (s, 3H), 3.18 (d, J = 10.8 Hz, 1H), 3.07 (ddd, J = 2.4, 6.8, 9.6 Hz, 1H), 2.65 (dd, J = 5.2, 13.6 Hz, 1H), 2.46 (m, 1H), 2.21 (m, 3H), 1.92 (m, 1H), 1.80 (s, 3H), 1.71 (ddd, J = 3.6, 10.8, 14.4 Hz, 1H), 1.56 (s, 3H), 1.46 (m, 1H), 1.27 (m, 1H), 1.13 (m, 1H), 1.03 (d, J = 6.4 Hz, 3H), 0.87 (s, 9H), 0.71 (d, J = 6.4 Hz, 1H), 0.05 (s, 3H), −0.01 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 171.2, 159.2, 144.5, 137.3, 136.4, 134.9, 130.5, 130.0, 128.6, 128.1, 127.5, 124.9, 122.3, 115.2, 115.0, 97.4, 83.6, 81.1, 80.3, 79.4, 70.1, 59.7, 56.8, 56.1, 44.2, 36.4, 34.3, 30.9, 30.2, 25.8, 24.8, 18.8, 18.1, 17.6, 12.64, 12.56, −4.7, −4.9; IR (neat) νmax: 3342, 2954, 2927, 1684, 1457, 1376, 1250, 1110, 1030, 837 cm−1; HRMS (CI, NH3) m/z calc’d for C42H66BrNO7Si [M+23]+ 826.3690, found 826.3662.
(4E,8S,9S,10E,12S,13R,14S,16R)-20-(Benzyloxy)-9-(tert-butyldimethylsilyloxy)-8,14-dimethoxy-13-(methoxymethoxy)-4,10,12,16-tetramethyl-2-aza-bicyclo[16.3.1]docosa-1(21),4,10,18(22),19-pentaen-3-one (34b)
To a solution of amide (10.0 mg, 0.0120 mmol) in toluene (0.800 mL) in a sealed tube was added potassium carbonate (4.96 mg, 0.0359 mmol). Copper(I) iodide (1.1 mg, 0.0060 mmol) and N,N′-dimethyl-1,2-ethanediamine (1.28 μL, 0.0120 mmol) were added sequentially and sealed tube was closed. The green suspension was heated to 100 °C for 36 hours. Contents were filtered over plug of silica and washed with ethyl acetate. Concentration of the solvent followed by flash chromatography (silica, 50% EtOAc/hexanes) yields product 34b in 82 % yield. (c 1.3, CHCl3); 1H NMR (CDCl3, 400 MHz): δ 7.41-7.30 (m, 5H), 7.13 (br. s, 1H), 6.69 (br. s, 1H), 6.55 (s, 1H), 6.36 (s, 1H), 5.85 (t, J = 6.0 Hz, 1H), 5.00 (s, 2H), 4.98 (obst. d, 1H), 4.81 (A of ABq, J = 6.4 Hz, 1H), 4.65 (B of ABq, J = 6.8 Hz, 1H), 3.77 (d, J = 7.6 Hz, 1H), 3.54 (d, J = 8.8 Hz, 1H), 3.45 (s, 3H), 3.41 (s, 3H), 3.31 (s, 3H), 3.12 (d, J = 10.0 Hz, 1H), 2.98 (dt, J = 3.0 Hz, 7.8 Hz, 1H), 2.78 (dd, J = 4.0, 13.2 Hz, 1H), 2.43 (m, 1H), 2.36 (dd, J = 6.4, 13.6 Hz, 1H), 2.20 (m, 1H), 2.11 (m, 1H), 1.91 (m, 1H), 1.74 (s, 3H), 1.62 (m, 1H), 1.53 (s, 1H), 1.25 (m, 1H), 1.17-1.08 (m, 2H), 1.04 (d, J = 6.8 Hz, 1H), 0.86 (s, 9H), 0.72 (d, J = 6.8 Hz, 1H), 0.04 (s, 3H), −0.03 (s, 3H); 13C NMR (CDCl3, 75.0 MHz): δ 173.0, 159.1, 142.1, 139.1, 136.6, 136.1, 134.6, 131.1, 131.0, 128.6, 128.0, 127.5, 118.0, 113.8, 105.7, 97.6, 83.3, 82.4, 82.0, 79.6, 70.0, 60.8, 57.0, 56.2, 42.9, 34.4, 33.5, 31.3, 30.7, 25.8, 23.7, 18.4, 18.1, 17.6, 13.7, 11.0, −4.7, − 4.9; IR (neat) νmax: 3300, 2927, 2856, 1664, 1594, 1461, 1111, 1032, 837 cm−1; HRMS (CI, NH3) m/z calc’d for C42H65NO7Si [M+23]+ 746.4428, found 746.4434.
(4E,8S,9S,10E,12S,13R,14S,16R)-Carbamic acid 20-(benzyloxy)-8,14-dimethoxy-13-(methoxymethoxy)-4,10,12,16-tetramethyl-3-oxo-2-aza-bicyclo[16.3.1]docosa-1(21),4,10,18(22),19-pentaen-9-yl ester
To a solution of TBS ether 34b (13.0 mg, 0.0172 mmol) in tetrahydrofuran (1.30 mL) in a nalgene vial, was added premixed solution of [pyridine hydrofluoride:pyridine:THF (1:1:2.5) by volume] (600 μL) dropwise. The reaction was allowed to run at room temperature for 24 hours before another 600 μL of HF mixture was added. The reaction stirred for another 12 hours and was quenched by addition of sodium bicarbonate. The aqueous layer was extracted with ethyl acetate (3x). The combined organic layers were dried with magnesium sulfate, filtered, and concentrated under reduced pressure. The crude product was purified by flash chromatography (silica, 50–100% EtOAc/hexanes) to yield secondary alcohol as a clear glass. This material was used immediately in the next step without further purification.
To a solution of the above alcohol (4.0 mg, 0.0063 mmol) in CH2Cl2 (800 μL) was added trichloroacetyl isocyanate (1.5 μL, 0.0126 mmol). The reaction was stirred for 15 minutes and methanol (1.0 mL) was added followed by potassium carbonate (4.0 mg). The reaction stirred for ½ hour (monitor by TLC) before solvent was evaporated in vacuo. Purification by flash chromatography (silica, 80% EtOAc/hexanes) provided 80% (2 steps) of the desired product as a clear glass. (c 0.8, CHCl3); 1H NMR (CDCl3, 400 MHz): δ 8.08 (br. s, 1H), 7.43-7.28 (m, 6H), 6.53 (s, 1H), 6.39 (s, 1H), 5.98 (t, J = 6.4 Hz, 1H), 5.35 (d, J = 10.0 Hz, 1H), 5.17 (d, J = 4.0 Hz, 1H), 5.02 (s, 2H), 4.83 (A of ABq, J = 6.4 Hz, 1H), 4.78 (br. s, 2H), 4.63 (B of ABq, J = 6.4 Hz, 1H), 3.59 (d, J = 9.2 Hz, 1H), 3.41 (s, 3H), 3.38 (s, 3H), 3.34 (m, 1H), 3.29 (s, 3H), 3.20 (m, 1H), 2.74 (d, J = 10.0 Hz, 1H), 2.52-2.38 (m, 2H), 2.18 (m, 2H), 1.83 (s, 3H), 1.61 (m, 2H), 1.52 (s, 3H), 1.44 (m, 2H), 1.28 (m,1H), 1.07 (d, J = 6.8 Hz, 3H), 0.79 (d, J = 6.4 Hz, 3H); 13C NMR (CDCl3, 75.0 MHz): δ 171.2, 159.1, 156.5, 143.4, 139.5, 136.8, 133.9, 131.1, 129.7, 128.5, 127.9, 127.6, 115.4, 113.1, 104.4, 97.3, 83.5, 80.6, 80.1, 79.9, 77.2, 70.0, 59.0, 56.6, 56.1, 42.9, 42.2, 35.5, 34.5, 29.4, 25.0, 19.7, 17.8, 13.3, 13.0; IR (neat) νmax: 3345, 2927, 1733, 1653, 1457, 1375, 1154, 1106, 1032, 837, 740 cm−1; HRMS (CI, NH3) m/z calc’d for C37H52N2O8 [M+23]+ 675.3621, found 675.3606.
Autolytimycin (2)
To a suspension of aluminum trichloride (11.7 mg, 0.0879 mmol) in methylene chloride (2.20 mL) at −78 °C was added anisole (2.90 mL) dropwise. The light yellow solution was stirred for an additional 5 minutes before carbamate (6.0 mg, 0.00879 mmol) in methylene chloride (0.700 mL) was added dropwise. The reaction mixture was allowed to warm up to 0 °C over 3 hours (on its own) and stirred for an additional 1 h at room temperature. Reaction was quenched by slow addition of 0.5 M HCl. The biphasic mixture was diluted with ammonium chloride and extracted with ethyl acetate (3x). The combined organic layers were dried with magnesium sulfate, filtered, and concentrated under reduced pressure. Purification by flash chromatography (silica, 1–5% MeOH/CH2Cl2) yields autolytimycin (2) as a white solid in 76% yield. (c 0.4, MeOH); Mp: 246 °C; 1H NMR (d6-DMSO, 50 °C, 300 MHz): δ 9.20 (s, 1H), 9.13 (s, 1H), 6.65 (s, 1H), 6.34 (br. s, 2H), 6.25 (s, 2H), 5.75 (br t, J = 6.2, 1H), 5.25 (d, J = 9.6 Hz, 1H), 4.86 (d, J= 7.2 Hz, 1H), 4.18 (d, J = 4.8 Hz, 1H), 3.38 (m, 1H), 3.32 (s, 3H), 3.24 (obst. m, 1H), 3.23 (s, 3H), 3.01 (m, 1H), 2.54 (dd, J = 5.1, 13.2 Hz, 1H), 2.35 (m, 1H), 2.28 (dd, J = 6.0, 12.9, Hz, 1H), 2.17 (m, 1H), 2.07 (m, 1H), 1.78 (m, 1H), 1.71 (s, 3H), 1.51 (m, 1H), 1.41 (s, 3H), 1.29 (m, 1H), 1.25 (m, 1H), 1.17 (m, 1H), 0.92 (d, J = 6.6 Hz, 3H), 0.75 (d, J = 6.9 Hz, 1H); 13C NMR (d6-DMSO, 50 °C, 75 MHz): δ 170.8, 157.4, 156.1, 141.2, 140.1, 134.2, 133.1, 131.8, 129.9, 115.0, 112.6, 105.8, 80.7, 80.65, 79.5, 73.4, 58.2, 56.3, 42.7, 34.0, 33.5, 30.5, 29.6, 23.3, 18.7, 16.6, 13.2, 11.7; IR (neat) νmax: 3332, 3272, 3197, 2913, 2877, 2824, 1711, 1653, 1617, 1593, 1399, 1383, 1109, 1039, 872 cm−1; HRMS (CI, NH3) m/z calc’d for C28H42N2O7 [M+23]+ 541.2890, found 541.2900.
Supplementary Material
Scheme 4.
Completion of the C9–C21 Aromatic Fragments (11)
Acknowledgments
Financial support for this research was obtained from NIH CA53604. JSP is grateful to Amgen, Johnson & Johnson, Merck Co., Novartis, Pfizer and GSK for financial support. IEW acknowledges a Novartis Graduate Fellowship. The authors are grateful to Dr. Ana Gabarda for early contributions to the reblastatin synthesis.
Footnotes
Experimental details and selected spectral data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For reviews on Hsp90 molecular chaperone see: Wegele H, Müller L, Buchner J. Rev Physiol Biochem Pharmacol. 2004;151:1. doi: 10.1007/s10254-003-0021-1.Csermely P, Schnaider T, Söti C, Proháska Z, Nardai G. Pharmacol Ther. 1998;79:129. doi: 10.1016/s0163-7258(98)00013-8.
- 2.For a short overview of these client proteins see: Goetz MP, Toft DO, Ames MM, Erlichman C. Ann Oncol. 2003;14:1169. doi: 10.1093/annonc/mdg316. and references therein.
- 3.(a) Sasaki K, Yasuda H, Onodera K. J Antibiot. 1979;32:849. doi: 10.7164/antibiotics.32.849. [DOI] [PubMed] [Google Scholar]; (b) Uehara Y. Curr Cancer Drug Targets. 2003;3:325. doi: 10.2174/1568009033481796. [DOI] [PubMed] [Google Scholar]
- 4.(a) De Boer C, Meulman PA, Wnuk RJ, Peterson DH. J Antibiot. 1970;23:442. doi: 10.7164/antibiotics.23.442. [DOI] [PubMed] [Google Scholar]; (b) Rinehart KL, Sasaki K, Slomp G, Grostic MF, Olson EC. J Am Chem Soc. 1970;92:7591. doi: 10.1021/ja00729a018. [DOI] [PubMed] [Google Scholar]
- 5.Solit DB, Rosen N. Curr Top Med Chem. 2006;6:1205. doi: 10.2174/156802606777812068. [DOI] [PubMed] [Google Scholar]
- 6.(a) Biamonte MA, Van de Water R, Arndt JW, Scannevin RH, Perret D, Lee WC. J Med Chem. 2010;53:3. doi: 10.1021/jm9004708. [DOI] [PubMed] [Google Scholar]; (b) Thepchatri P, Eliseo T, Cicero DO, Myles D, Snyder JP. J Am Chem Soc. 2007;129:3127. doi: 10.1021/ja064863p. [DOI] [PubMed] [Google Scholar]
- 7.(a) Dikalov S, Landmesser U, Harrison DG. J Biol Chem. 2002;277:25480. doi: 10.1074/jbc.M203271200. [DOI] [PubMed] [Google Scholar]; (b) Tudor G, Gutierrez P, Aguilera-Gutierrez A, Sausville EA. Biochem Pharm. 2003;65:1061. doi: 10.1016/s0006-2952(03)00013-3. [DOI] [PubMed] [Google Scholar]
- 8.For most recent reviews see: Workman P. Trends Mol Med. 2004;10:47. doi: 10.1016/j.molmed.2003.12.005.Bagatell R, Whitesell L. Mol Cancer Ther. 2004;3:1021.Isaacs JS, Xu W, Neckers L. Cancer Cell. 2003;3:213. doi: 10.1016/s1535-6108(03)00029-1.Neckers L. Trends Mol Med. 2002;8:S55. doi: 10.1016/s1471-4914(02)02316-x.
- 9.Ge J, Normant E, Porter JR, Ali JA, Dembski MS, Gao Y, Georges AT, Grenier L, Pak RH, Patterson J, Sydor JR, Tibbitts TT, Tong JK, Adams J, Palombella VJ. J Med Chem. 2006;49:4606. doi: 10.1021/jm0603116.8,9-Amido analogs of Geldanamycin; Andrus MB, Wong Y, Liu J, Beebe K, Neckers LM. Tet Lett. 2009;50:6705.
- 10.(a) Schulte TW, Neckers LM. Cancer Chemother Pharmacol. 1998;42:273. doi: 10.1007/s002800050817. [DOI] [PubMed] [Google Scholar]; (b) Banerji U. Proc Am Assoc Cancer Ther. 2003;44:677. [Google Scholar]; (c) Sausville EA, Tomaszewski JE, Ivy P. Curr Cancer Drug Targets. 2003;3:377. doi: 10.2174/1568009033481831. [DOI] [PubMed] [Google Scholar]; (d) Jez JM, Chen JCH, Rastelli G, Stroud RM, Santi DV. Chem Biol. 2003;10:361. doi: 10.1016/s1074-5521(03)00075-9. [DOI] [PubMed] [Google Scholar]; (e) Goetz MP, Toft D, Reid J, Ames M, Stensgard B, Safgren S, Adjei AA, Sloan J, Atherton P, Vasile V, Salazaar S, Adjei A, Croghan G, Erlichman C. J Clin Oncol. 2005;23:1078. doi: 10.1200/JCO.2005.09.119. [DOI] [PubMed] [Google Scholar]
- 11.Kaur G, Belotti D, Burger AM, Fisher-Nielson K, Borsotti P, Riccardi E, Thillainathan J, Hollingshead M, Sausville EA, Giavazzi R. Clin Cancer Res. 2004;10:4813. doi: 10.1158/1078-0432.CCR-03-0795. [DOI] [PubMed] [Google Scholar]
- 12.(a) Guo W, Reigan P, Siegel D, Zirrolli J, Gustafson D, Ross D. Cancer Res. 2005;65:10006. doi: 10.1158/0008-5472.CAN-05-2029. [DOI] [PubMed] [Google Scholar]; (b) Maroney AC, Marugan JJ, Mezzasalma TM, Barnakow AN, Garrabrant TA, Weaner LE, Jones WJ, Barnakova LA, Koblish HK, Todd MJ, Masucci JA, Deckman IC, Galemmo RA, Jr, Johnson DL. Biochemistry. 2006;45:5678. doi: 10.1021/bi0524969. [DOI] [PubMed] [Google Scholar]; (c) Onuoha SC, Mukund SR, Coulstock ET, Sengerovà B, Shaw J, McLaughlin SH, Jackson SE. J Mol Biol. 2007;372:287. doi: 10.1016/j.jmb.2007.06.065. [DOI] [PubMed] [Google Scholar]
- 13.(a) Zhang M-Q, Gaisser S, Nur-E-Alam M, Sheehan LS, Vousden WA, Gaitatzis N, Peck G, Coates NJ, Moss SJ, Radzom M, Foster TA, Sheridan RM, Gregory MA, Roe AM, Prodormou C, Pearl L, Boyd SM, Wilkinson B, Martin CJ. J Med Chem. 2008;51:5494. doi: 10.1021/jm8006068. [DOI] [PubMed] [Google Scholar]; (b) Eichner S, Floss HG, Sasse F, Kirsching A. ChemBioChem. 2009;10:1801. doi: 10.1002/cbic.200900246. [DOI] [PubMed] [Google Scholar]; (c) Roe SM, Prodromou C, O’Brien R, Ladbury JE, Piper PW, Pearl LH. J Med Chem. 1999;42:260. doi: 10.1021/jm980403y. [DOI] [PubMed] [Google Scholar]
- 14.Takatsu T, Ohtsuki M, Muramatsu A, Enokita R, Kurakata SI. J Antibiot. 2000;53:1310. doi: 10.7164/antibiotics.53.1310. [DOI] [PubMed] [Google Scholar]
- 15.Li MG, Wu SH, Zhao LX, Zhang Q, Li WJ, Cui XL, Xu LH, Wu DG, Jiang CL. Chin Chem Lett. 2001;12:903. [Google Scholar]
- 16.Stead P, Latif S, Blackaby AP, Sidebottom PJ, Deakin A, Taylor NL, Life P, Spaull J, Burrell F, Jones R, Lewis J, Davidson I, Mander T. J Antibiot. 2000;53:657. doi: 10.7164/antibiotics.53.657. [DOI] [PubMed] [Google Scholar]
- 17.Onodera H, Kaneko M, Takahashi Y, Uochi Y, Funahashi J, Nakashima T, Soga S, Suzuki M, Ikeda S, Yamashita Y, Rahayu ES, Kanda Y, Ichimura M. Bioorg Med Chem Lett. 2008;18:1588. doi: 10.1016/j.bmcl.2008.01.072. [DOI] [PubMed] [Google Scholar]
- 18.Wrona IE, Gabarda AE, Evano G, Panek JS. J Am Chem Soc. 2005;127:15026. doi: 10.1021/ja055384d.We rationalize that the reversible addition of a second equivalent of Cp2ZrHCl, followed by elimination across the C10–C11 positions may lead to partial epimerization. For mechanistic precedence for this pathway, see: Schwartz J, Labinger J. Angew Chem Int Ed Engl. 1976;15:333.Wailes P, Weigold H, Bell AP. J Organomet Chem. 1971;27:373.
- 19.Review on chiral metal enolates: Evans DA, Shaw JT. L′actualité Chimique. 2003:35–38.Lin G-Q, Li Y-M, Chan ASC. Principles and Applications of Asymmetric Synthesis. Wiley & Sons; New York: 2001. pp. 135–193.Chiral allyl boranes: Ramachamdran PV. Aldrichchimica Acta. 2002;35:23.
- 20.For a recent review on copper-mediated coupling reactions and their applications in natural products synthesis see: Evano G, Blanchard N, Toumi M. Chem Rev. 2008;108:3054. doi: 10.1021/cr8002505.
- 21.Klapars A, Huang X, Buchwald SL. J Am Chem Soc. 2002;124:7421. doi: 10.1021/ja0260465. [DOI] [PubMed] [Google Scholar]
- 22.(a) Wipf P, Xu W. Tetrahedron Lett. 1994;35:5197. [Google Scholar]; (b) Wipf P, Jahn H. Tetrahedron. 1996;52:12853. [Google Scholar]; (c) Wipf P, Xu W, Takahashi H, Jahn H, Coish PDG. Pure Appl Chem. 1997;69:639. [Google Scholar]; (d) Wipf P, Kendall C. Chem Eur J. 2002;8:1778. doi: 10.1002/1521-3765(20020415)8:8<1778::aid-chem1778>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 23.(a) Hu T, Panek JS. J Am Chem Soc. 2002;124:11368. doi: 10.1021/ja0206700. [DOI] [PubMed] [Google Scholar]; (b) Panek JS, Hu T. J Org Chem. 1997;62:4912. [Google Scholar]
- 24.(a) Qin HL, Lowe JT, Panek JS. J Am Chem Soc. 2007;129:38. doi: 10.1021/ja067234o. [DOI] [PubMed] [Google Scholar]; (b) Qin HL, Panek JS. Org Lett. 2008;10:2477. doi: 10.1021/ol800749w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.(a) Prepared in three-steps from commercially available 2,3-dihydroxybenzaldehyde; See Supporting Information for experimental details. (b) For selective benzylation of 2,3-dihydroxy benzaldehyde see: Parker KA, Georges AT. Org Lett. 2000;2:497. doi: 10.1021/ol991346l.
- 26.3-benzyloxy-5-bromobenzaldehyde was prepared in two steps from commercially available 3,5-dibromophenol. See Supporting Information for experimental details.
- 27.(a) Lowe JT, Panek JS. Org Lett. 2005;7:1529. doi: 10.1021/ol0501875. [DOI] [PubMed] [Google Scholar]; (b) Huang H, Panek JS. Org Lett. 2003;5:1991. doi: 10.1021/ol034582b. [DOI] [PubMed] [Google Scholar]; (c) Huang H, Panek JS. J Am Chem Soc. 2000;122:9836. [Google Scholar]
- 28.For related electrophilic additions to dihydropyrans see: Su Q, Panek JS. J Am Chem Soc. 2004;126:2425–2430. doi: 10.1021/ja037957x.Su Q, Panek JS. Angew Chem Int Ed Engl. 2005;44:1223. doi: 10.1002/anie.200462408.
- 29.For a synthetic review of ansamycin antibiotics see: Wrona IE, Agouridas V, Panek JS. Comptes-Rendus Académie des Sciences, Paris. 2008;11:1483–1522.
- 30.Roush WR, Ando K, Powers DB, Palkowitz AD, Halterman RL. J Am Chem Soc. 1990;112:6339. [Google Scholar]
- 31.(a) Roush WR, Palkowitz AD, Ando K. J Am Chem Soc. 1990;112:6348. [Google Scholar]; (b) Roush WR, Hoong LK, Palmer MAJ, Park JC. J Org Chem. 1990;55:4109. [Google Scholar]
- 32.Gilbert JC, Weerasooriya U. J Org Chem. 1982;47:1837. [Google Scholar]; (b) Brown DG, Velthuisen EJ, Commerford JR, Brisbois RG, Hoye TR. J Org Chem. 1996;61:2540. [Google Scholar]
- 33.For examples of chiral pool reagents see: Blaser HU. Chem Rev. 1992;92:935.
- 34.Herdeis C. Synthesis. 1986:232. [Google Scholar]
- 35.González IC, Forsyth CJ. J Am Chem Soc. 2000;122:9099. [Google Scholar]
- 36.Omura K, Swern D. Tetrahedron. 1978;34:1651. [Google Scholar]
- 37.For preparation of 2,3-O-isopropylidene-D-glyceraldehyde from D-mannitol see: Schmid C, Bryant JD. Org Synth. 1995;72:6.
- 38.(a) Mann J, Weymouth-Wilson AC. Org Syn. 1998;75:139. [Google Scholar]; (b) Ghosh AK, Leshchenko S, Noetzel M. J Org Chem. 2004;69:7822. doi: 10.1021/jo049156y. [DOI] [PubMed] [Google Scholar]
- 39.De Mico A, Margarita R, Parlanti L, Vescovi A, Piancatelli G. J Org Chem. 1997;62:6974. [Google Scholar]
- 40.(a) Reetz MT. Acc Chem Res. 1993;26:462. [Google Scholar]; (b) Mengel A, Reiser O. Chem Rev. 1999;99:1191. doi: 10.1021/cr980379w. [DOI] [PubMed] [Google Scholar]
- 41.(a) Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH. Cell. 1997;90:65. doi: 10.1016/s0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]; (b) Stebbins CE, Russo AA, Schneider C, Rosen N, Hartl FU, Pavletich NP. Cell. 1997;89:239. doi: 10.1016/s0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]; (c) Jez JM, Chen JCH, Rastelli G, Stroud RM, Santi DV. Chem Biol. 2003;10:361. doi: 10.1016/s1074-5521(03)00075-9. [DOI] [PubMed] [Google Scholar]
- 42.(a) Hu T, Li C. Org Lett. 2005;7:2035. doi: 10.1021/ol0505555. [DOI] [PubMed] [Google Scholar]; (b) Cai Q, Zou B, Ma D. Angew Chem Int Ed. 2006;45:1276. doi: 10.1002/anie.200503538. [DOI] [PubMed] [Google Scholar]; (c) Toumi M, Couty F, Evano G. J Org Chem. 2007;72:9003. doi: 10.1021/jo070517u. [DOI] [PubMed] [Google Scholar]; (d) Toumi M, Couty F, Evano G. Angew Chem Int Ed. 2007;46:572. doi: 10.1002/anie.200602865. [DOI] [PubMed] [Google Scholar]
- 43.Kocovsky P. Tetrahedron Lett. 1986;27:5521. [Google Scholar]
- 44.Attempt to deprotect the C11 MOM-ether using the following procedures failed: Catecholborane: Boeckman RK, Jr, Potenza JC. Tetrahedron Lett. 1985;26:1411.MgBr2: Kim S, Kee IS, Park YH, Park JH. Synlett. 1991:183.CBr4/i-PrOH: Lee ASY, Hu YJ, Chu SF. Tetrahedron. 2001;57:2121.TMSBr: Hanessian S, Delomre D, Dufresne Y. Tetrahedron Lett. 1984;25:2515.BCl3•SMe2: Congreve MS, Davison EC, Fuhry MAM, Holmes AB, Payne AN, Robinson RA, Ward SE. Synlett. 1993:663.Me2BBr: Guindon Y, Morton HE, Yoakim C. Tetrahedron Lett. 1983;24:3969.AcCl/MeOH/Et2O: Tius MA, Gomex-Galeno J, Gu XQ, Zaidi JH. J Am Chem Soc. 1991;113:5775.
- 45.Akiyama T, Hirofuji H, Ozaki S. Tetrahedron Lett. 1991;32:1321. [Google Scholar]
- 46.See Supporting Information for comparison of spectral data (1H-NMR and 13C-NMR).
- 47.Recent examples: Beeler AB, Acquilano DE, Su Q, Yan F, Roth BL, Panek JS, Porco JA., Jr J Combi Chem. 2005;7:673–681. doi: 10.1021/cc050064b.Dandapani S, Beeler AB, Lan P, Beischel S, Abbas A, Roth RB, Porco JA, Jr, Panek JS. J Org Chem. 2006;71:8934. doi: 10.1021/jo061758p.
- 48.Tietze LF, Bell HP, Chandrasekhar S. Angew Chem Int Ed. 2003;42:3996. doi: 10.1002/anie.200200553. [DOI] [PubMed] [Google Scholar]
- 49.Neckers L. Trends Mol Med. 2002;8:S55. doi: 10.1016/s1471-4914(02)02316-x. [DOI] [PubMed] [Google Scholar]
- 50.See Supporting Information for details.
- 51.For competition assays see: Moulick K, Clement CC, Aguirre J, Kim J, Kang Y, Felts S, Chiosis G. Bioorg Med Chem Lett. 2006;16:4515. doi: 10.1016/j.bmcl.2006.06.025.For growth assays see: O’Brien J, Wilson I, Orton T, Pognan F. Eur J Biochem. 2000;267:5421. doi: 10.1046/j.1432-1327.2000.01606.x.
- 52.Tian ZQ, Liu Y, Zhang D, Wang Z, Dong SD, Carreras CW, Zhou Y, Rastelli G, Santi DV, Myles DC. Bioorg Med Chem. 2004;12:5317. doi: 10.1016/j.bmc.2004.07.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.