Abstract
5-Oxo-ETE is a product of the 5-lipoxygenase pathway that is formed by the oxidation of 5-HETE by 5-hydroxyeicosanoid dehydrogenase (5-HEDH). 5-HEDH is a microsomal NADP+-dependent enzyme that is highly selective for 5-HETE. 5-Oxo-ETE synthesis is regulated by intracellular NADP+ levels and is dramatically increased under conditions that favor oxidation of NADPH to NADP+ such as oxidative stress and the respiratory burst in phagocytic cells. 5-Oxo-ETE is a potent chemoattractant for eosinophils and has similar effects on neutrophils, basophils and monocytes. It elicits infiltration of eosinophils and, to a lesser extent, neutrophils into the skin after intradermal injection in humans. It also promotes the survival of tumor cells and has been shown to block the induction of apoptosis by 5-LO inhibitors. 5-Oxo-ETE acts by the Gi/o-coupled OXE receptor, which was also known as TG1019, R527 and hGPCR48. Although the pathophysiological role of 5-oxo-ETE is not well understood, it may play important roles in asthma and allergic diseases, cancer, and cardiovascular disease. The availability of a selective antagonist would help to clarify the role of 5-oxo-ETE and may be of therapeutic benefit.
1. Introduction
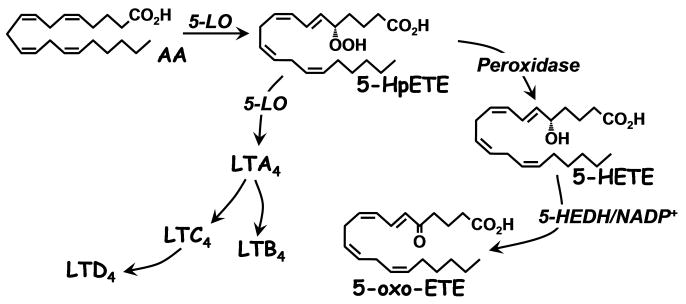
Arachidonic acid (AA) is converted by 5-lipoxygenase (5-LO) to 5-HpETE, which is then either cyclized to LTA4, the precursor of LTB4 and the cysLTs, in a second 5-LO-catalyzed reaction, or reduced to 5-HETE by peroxidase activity [1] (Fig. 1). Although 5-HETE had been shown to activate neutrophils independently of receptors for other lipid mediators [2-4], its rather modest potency was not consistent with what would normally be expected of G-protein coupled receptors (GPCRs) for their preferred ligand. However, we discovered a pathway for the metabolism of 5-HETE to a product (5-oxo-ETE) [5] that is about 100 times more potent than 5-HETE in activating neutrophils [6]. The biological actions of 5-oxo-ETE are mediated by the highly selective OXE receptor, which is expressed on a variety of inflammatory cells as well as tumor cells [7].
Fig. 1.
Formation of 5-oxo-ETE and other 5-lipoxygenase products from arachidonic acid
2. Biosynthesis of 5-oxo-ETE
5-Oxo-ETE is formed by the oxidation of 5-HETE by 5-hydroxyeicosanoid dehydrogenase (5-HEDH). 5-HEDH is a microsomal enzyme that is highly selective for 5S-HETE and requires NADP+ as an obligatory cofactor. Other closely related eicosanoids such as 5R-HETE, 12S-HETE, and 15S-HETE undergo little or no metabolism [5]. 5-HEDH also requires a 6-trans double bond, as 6-trans isomers of LTB4, but not LTB4 itself, are substrates, although they are not metabolized as rapidly as 5-HETE. Further investigation of the selectivity of 5-HEDH revealed that a chain length of at least 16 carbons is required for metabolism [8].
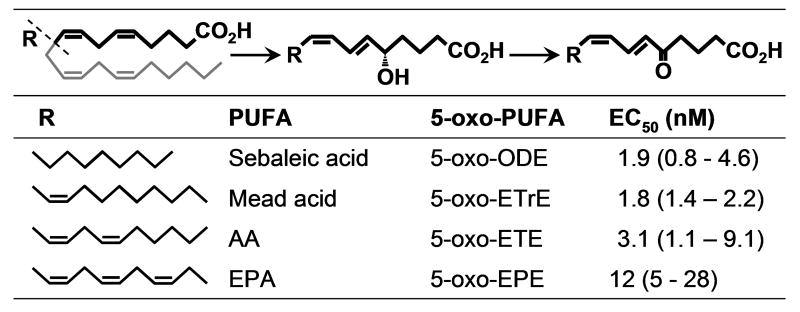
Because of the abundance of AA in cellular lipids 5-oxo-ETE would be the main product of 5-HEDH. However, other endogenously occurring polyunsaturated fatty acids (PUFA) can also be converted to analogous 5-oxo-fatty acids following oxidation by 5-LO (Fig. 2). For example, sebaleic acid, which is the major PUFA in human sebum, is converted to 5-oxo-6,8-octadecadienoic acid (5-oxo-ODE) by human neutrophils [9]. Because it has only two double bonds, sebaleic acid cannot be converted to leukotrienes, so that 5-oxo-ODE is the only potent granulocyte chemoattractant formed by metabolism of this PUFA by the 5-LO pathway. Similarly, the ω9-PUFA Mead acid, which accumulates under conditions of essential fatty acid deficiency, is converted to 5-oxo-6,8,11-eicosatrienoic acid (5-oxo-ETrE) by neutrophils [10]. The latter compound is the major granulocyte chemoattractant formed from Mead acid by the 5-LO pathway because the intermediate LTA3 is a potent inhibitor of LTA hydrolase, thus inhibiting the formation of LTB3 [11]. Finally, EPA is converted to 5-oxo-6,8,11,14,17-eicosapentaenoic acid by a combination of 5-LO and 5-HEDH [12].
Fig. 2. Generation of OXE receptor agonists from endogenous PUFA.
EC50 values (with the 95% confidence limits) are shown for actin polymerization in eosinophils
In addition to neutrophils, 5-HEDH is found in a variety of both inflammatory and structural cells, including monocytes [13], monocyte-derived dendritic cells [14], platelets [15], and endothelial [16], epithelial [17], and airway smooth muscle cells [17]. Thus, as with the leukotriene-forming enzymes LTA4 hydrolase and LTC4 synthase, it is distributed much more widely than 5-LO, which is expressed at high levels only in inflammatory cells. This raises the possibility that, analogous to leukotrienes [18], transcellular biosynthesis could contribute to the synthesis of 5-oxo-ETE, which we have confirmed in experiments in which calcium ionophore-stimulated neutrophils were coincubated with epithelial cells (manuscript in preparation).
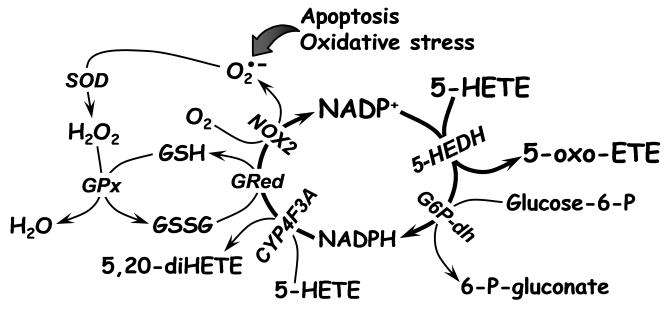
Because NAD+ will not support oxidation of 5-HETE by 5-HEDH except at very high nonphysiological concentrations [30], the synthesis of 5-oxo-ETE is dependent on the intracellular levels of NADP+, which is normally maintained at very low levels in cells in favor of its reduced counterpart NADPH. Furthermore, since NADPH is a potent inhibitor of 5-oxo-ETE formation, this reaction is regulated by the ratio of NADP+ to NADPH rather than the absolute concentration of NADP+, and therefore is normally suppressed in resting cells. Thus resting neutrophils metabolize 5-HETE principally by ω-oxidation to 5,20-diHETE via LTB4 20-hydroxylase (CYP4F3A), which is highly expressed in these cells (Fig. 3) [19,20]. In contrast, elicitation of the respiratory burst with PMA, which activates NADPH oxidase-2 (NOX2), dramatically shifts the metabolism of 5-HETE from 5,20-diHETE to 5-oxo-ETE [21] due to the rapid oxidation of NADPH to NADP+. Similarly, exposure of cells to oxidative stress in the form of H2O2, increases the ratio of NADP+ to NADPH and thereby stimulates 5-oxo-ETE synthesis. A dramatic shift in 5-HETE metabolism favoring 5-oxo-ETE formation is also seen in neutrophils undergoing apoptosis, which is accompanied by oxidative stress and a large increase in the ratio of NADP+ to NADPH [22]. In contrast, oxidation of glucose 6-phosphate by the pentose phosphate pathway results in the reduction of NADP+ to NADPH and thereby inhibits 5-oxo-ETE formation [23].
Fig. 3. Regulation of 5-oxo-ETE synthesis by oxidative stress and the respiratory burst.
5-Oxo-ETE synthesis is regulated by the availability of NADP+, which is increased by oxidative stress through the glutathione redox cycle and the action of NADPH oxidase (NOX2 in phagocytic cells) and reduced by the pentose phosphate pathway, the first step of which is oxidation of glucose-6-phosphate by glucose 6-phosphate dehydrogenase (G6P-dh). Other abbreviations: GRed, glutathione reductase, GPx, glutathione peroxidase, SOD, superoxide dismutase.
3. Metabolism of 5-oxo-ETE
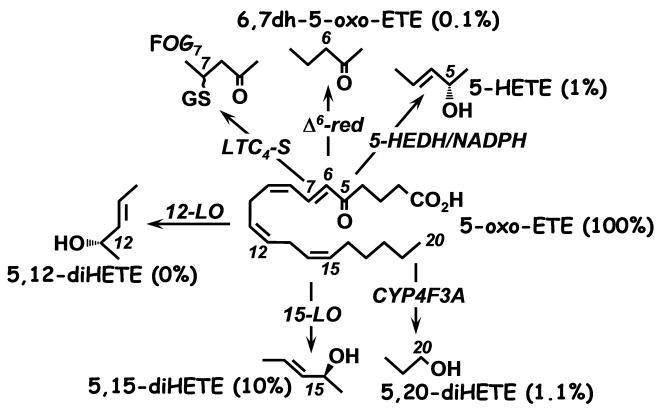
The major pathway for the metabolism of 5-oxo-ETE in neutrophils is via ω-oxidation to 5,20-diHETE as mentioned above [20]. Neutrophils also contain a Ca++/calmodulin-dependent Δ6-reductase that converts 5-oxo-ETE to its 6,7-dihydro metabolite 5-oxo-8,11,14-eicosatrienoic acid [24] (Fig. 4). Human monocytes do not possess neutrophil CYP4F3 and do not convert 5-oxo-ETE to ω-oxidation products. In contrast, mouse macrophages metabolize 5-oxo-ETE by a combination 6,7-reduction and ω-oxidation to 18- and 19- hydroxy derivatives [25]. These cells also convert 5-oxo-ETE to a GSH conjugate, FOG7, by the action of LTC4 synthase [26,27]. 5-Oxo-ETE is a substrate for both 12- and 15- lipoxygenases in platelets [15] and eosinophils [28], respectively, and is consequently metabolized to 5-oxo-12-HETE and 5-oxo-15-HETE in these cells. In addition, 5-oxo-ETE can be incorporated into cellular lipids in neutrophils, although not as well as its precursor 5-HETE [29]. Since 5-HEDH catalyzes a reversible reaction, 5-oxo-ETE can be stereospecifically reduced back to 5S-HETE, although the oxidation reaction is preferred [30].
Fig. 4. Effects of metabolism of 5-oxo-ETE on biological potency.
The major pathways of 5-oxo-ETE metabolism are shown. The potencies of the metabolites are shown in brackets as percentages of the potency of 5-oxo-ETE.
4. The 5-oxo-ETE receptor
The high degree of selectivity of 5-HEDH for 5-HETE suggested that the product of this reaction might serve an important biological function. Because of the previously reported stimulatory effects of 5-HETE on human neutrophils we investigated the actions of 5-oxo-ETE on these cells and found it to be about 100 times more potent than 5-HETE in stimulating calcium mobilization and chemotaxis [6]. These effects were subject to homologous desensitization by pretreatment with 5-oxo-ETE, but not to heterologous desensitization with LTB4, platelet-activating factor (PAF), or other chemoattractants, consistent with mediation by a distinct receptor selective for 5-oxo-ETE [6,28,31,32]. Furthermore, the responses to 5-oxo-ETE could not be blocked by selective LTB4 and PAF antagonists. Binding studies with 5-oxo-ETE in neutrophils were complicated by its esterification into cellular lipids. However, O'Flaherty overcame this problem by conducting binding experiments in the presence of the acyl CoA synthetase inhibitor triacsin C [29].
4.1. Cloning of the 5-oxo-ETE receptor (OXE)
The receptor for 5-oxo-ETE was independently cloned by three groups performing in silico searches for putative orphan GPCRs for which the ligands were unknown. In search of the ligand for the orphan GPCR TG1019, Hosoi et al. screened a library of natural bioactive compounds and related molecules on the basis of the binding of GTPγS to a TG1019-Gαi1-protein fusion product. Of the potential ligands tested 5-oxo-ETE (EC50 6 nM) was the most potent in activating the binding of GTPγS to TG1019-Gαi1 [33]. Other fatty acids (5-HpETE > AA = 5(RS)-HETE) were much less potent, whereas leukotrienes, prostaglandins, 12-HETE and 15-HETE were inactive. Jones et al. independently cloned the orphan GPCR R527 and screened about 2000 potential ligands for Ca++ mobilization in transfected HEK293 [34]. The most potent of these was 5-oxo-ETE, followed by 5-HpETE (100 times less potent) and 5S-HETE. R527 is identical to TG1019 except for the substitution of valine for leucine at position 368 and truncation of the N-terminus by 39 amino acids. These differences did not alter biological activity. Finally, Takeda et al [35], in a search for intronless GPCRs, identified the orphan GPCR hGPCR48, which has a sequence identical to that of TG1019, as a 5-oxo-ETE receptor using a GTPγS binding assay similar to Hosoi et al.
The 5-oxo-ETE receptor was named the OXE receptor by the IUPHAR Nomenclature Committee for Leukotriene and Lipoxin Receptors [36] and the corresponding gene, which maps to 2p21 on chromosome 2 [33,34], is referred to as OXER1. The OXE receptor (OXE-R) is most highly expressed in humans in peripheral leukocytes, lung, kidney, liver and spleen [33,34]. The relative expression of this receptor in eosinophils, neutrophils, and macrophages is 200:6:1 [34].
4.2. Downstream signaling by the OXE receptor
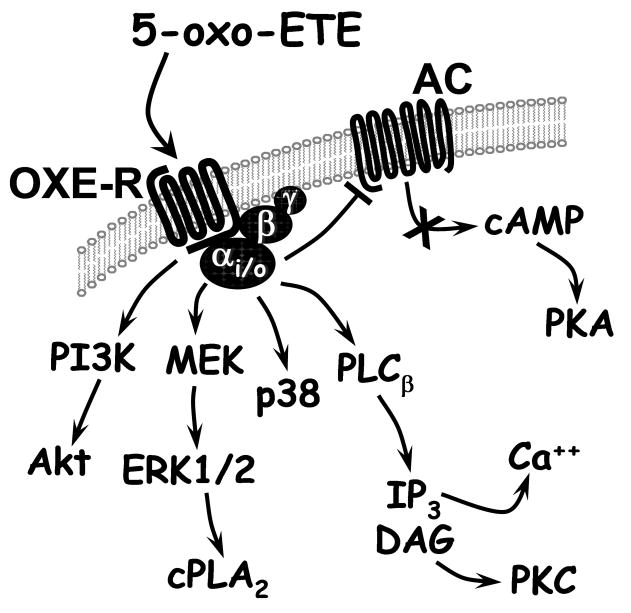
5-Oxo-ETE was initially shown to induce a rapid increase in cytosolic calcium levels in neutrophils [6,31] and later to inhibit forskolin-stimulated cAMP formation in CHO cells transfected with the OXE receptor [33]. These and a variety of other responses to 5-oxo-ETE were inhibited by pertussis toxin indicating that its receptor is coupled to a Gi/o protein [20,37,38]. The effect of 5-oxo-ETE on calcium mobilization, as well as cell migration, in transfected CHO cells was blocked by the phospholipase C inhibitor U73122, suggesting that these responses were mediated by the release of inositol trisphosphate from phosphatidylinositol 4,5-bisphosphate in the cell membrane [39]. 5-Oxo-ETE also activates phosphoinositide-3 kinase (PI3K), since it elevates the levels of its product phosphatidylinositol (3,4,5)-trisphosphate in neutrophils [37]. Activation of PI3K appears to be involved in the chemoattractant effects of 5-oxo-ETE, as this response (but not calcium mobilization) is blocked in transfected CHO cells by LY294002, an inhibitor of this enzyme [39]. Activation of PI3K by 5-oxo-ETE also results in phosphorylation of Akt [33,40], which is blocked by LY294002 [39]. 5-Oxo-ETE also induces the phosphorylation of ERK-1/2 in a variety of cell types including neutrophils [38], eosinophils [41,42], PC3 cells [40] and CHO cells transfected with the OXE-R [39]. ERKs are known to activate cPLA2 [43] and, consistent with this, 5-oxo-ETE stimulates phosphorylation of the latter enzyme along with the release of AA [38], which could lead to further production of proinflammatory AA metabolites. 5-Oxo-ETE-induced cPLA2 phosphorylation is markedly enhanced by GM-CSF [38]. There is also evidence that PKCδ and PKCζ are involved in 5-oxo-ETE-induced cellular responses [41].
4.3. Selectivity of the 5-oxo-ETE receptor
The OXE receptor is highly selective for 5-oxo-PUFA containing at least 2 double bonds in the 6- and 8- positions. The most potent ligands are those derived from the naturally-occurring PUFA AA, Mead acid, and sebaleic acid (i.e. 5-oxo-ETE, 5-oxo-ETrE, and 5-oxo-ODE; Fig. 2), which have EC50 values of 2-3 nM [10]. The product derived from EPA (i.e. 5-oxo-EPE) is also fairly potent, but less so than 5-oxo-ETE.
Metabolism of 5-oxo-ETE by various pathways results in substantial loss of activity (Fig. 4). As noted above, its substrate 5-HETE, which can be formed by reduction of 5-oxo-ETE by 5-HEDH, has only about 1% of the potency of 5-oxo-ETE, whereas the Δ6-reductase product 6,7-dihydro-5-oxo-ETE and the ω-hydroxylation product 5,20-diHETE are only 0.1% and ∼1% as potent, respectively. Metabolism of 5-oxo-ETE by other lipoxygenase pathways also reduces biological activity. The 12-lipoxygenase product 5-oxo-12-HETE is devoid of calcium mobilization activity [15]. Although the 15-lipoxygenase product 5-oxo-15-HETE has been reported to be equipotent with 5-oxo-ETE in stimulating neutrophils [37] and eosinophils [28], we [6] and others [44] have found it to be less potent (Fig. 4). The reason for this discrepancy is not clear. Other modifications of 5-oxo-ETE also result in loss of potency, including esterification of the carboxyl group (20-fold loss in potency) and isomerization of the Δ8-cis double bond to the trans configuration (∼5-fold reduction in potency) [20,44]. In addition, potency drops off dramatically if the carbon chain is reduced in length to below 18 carbons [10].
Another metabolite of 5-oxo-ETE, FOG7, stimulates the migration of neutrophils and eosinophils, but its effects are more limited than those of 5-oxo-ETE [26]. Because of the considerable structural difference due to the presence of the glutathione residue in the 7-position it would seem very unlikely that the response to FOG7 is mediated by the OXE receptor. However, the mechanism of action of this substance has not yet been determined.
4.4. OXE receptor antagonists
No synthetic OXE receptor antagonists have so far been described. However, several endogenously formed substances have been reported to possess antagonist properties. We found that the 12-LO metabolites of 5-oxo-ETE blocked 5-oxo-ETE-induced calcium mobilization in neutrophils with IC50 values of 0.5 μM (5-oxo-12-HETE) and 2.5 μM (8-trans-5-oxo-12-HETE) [15]. In agreement with this, we subsequently found that these compounds could also inhibit 5-oxo-ETE-elicited neutrophil migration. However, when we examined their effects on CD11b expression we found that they acted as weak agonists (100-500 times less potent than 5-oxo-ETE) (unpublished data).
Certain PUFA, including EPA, DHA, dihomo-γ-linolenic acid, and Mead acid have also been reported to have antagonist activity (IC50 ∼2-6 μM) against the OXE receptor on the basis of inhibition of 5-oxo-ETE-induced binding of GTPγS to cells transfected with the receptor [33]. It is not known whether these PUFA can inhibit other 5-oxo-ETE-induced effects in cells that normally express the endogenous receptor.
5. Biological role of 5-oxo-ETE
Although a biological role for 5-oxo-ETE has not yet been clearly demonstrated, it would seem likely that this substance does fulfill important functions in vivo, based on the high degree of selectivity exhibited by both its receptor and its biosynthetic enzyme 5-HEDH. 5-Oxo-ETE is produced by inflammatory cells and acts on a variety of these cells, including eosinophils, neutrophils, basophils, and monocytes. Its effects have been most extensively studied on neutrophils and eosinophils. It has similar effects on these two cell types in inducing chemotaxis, calcium mobilization, actin polymerization, CD11b expression, and L-selectin shedding [7]. However, 5-oxo-ETE has only relatively modest effects on degranulation and superoxide production in untreated cells. In contrast, eosinophils and neutrophils pretreated with cytokines such as GM-CSF and TNFα respond much more strongly to 5-oxo-ETE [38,42]. Expression of the OXE receptor has also been reported in prostate tumor cells [45] in which 5-oxo-ETE induces a proliferative response [46].
5.1. Asthma
Among inflammatory cells, the OXE receptor is most highly expressed in eosinophils, with lower levels of expression being observed in neutrophils, monocytes [34], and basophils [47]. Eosinophils also respond very strongly to 5-oxo-ETE [28,42,48] and it would seem likely that these cells are one of its primary targets. Eosinophils release cytokines and growth factors such as TGFβ and appear to play a role in airway remodeling in asthma [49]. Eosinophils, along with mast cells and basophils, are the major sites of production of cysLTs, which have potent bronchoconstrictor effects, stimulate mucus production and elicit the release of cytokines [50]. These cells also release proteins such as major basic protein, eosinophil cationic protein, and eosinophil peroxidase, which have damaging effects on the lungs [51]. There has been some debate about the precise role of eosinophils in asthma due to the lack of effectiveness of anti-IL-5 in alleviating the symptoms of this disease in humans [52]. However, considerable numbers of eosinophils have been shown to persist in the lungs even after anti-IL-5 treatment, and these may be sufficient to account for the prolongation of the symptoms [53]. Eosinophils thus remain an attractive target in asthma [51,54] and drugs designed to prevent their infiltration into the lungs may have important therapeutic benefits in this disease.
Among lipid mediators, 5-oxo-ETE induces the strongest chemotactic response in human eosinophils [48]. Although it is a bit less potent than eotaxin on a molar basis, it elicits a greater maximal response [55]. Furthermore, it has synergistic effects with eotaxin, RANTES [55], and platelet-activating factor [48] in inducing eosinophil migration. In addition to promoting the migration of these cells through untreated filters, it also elicits migration through filters coated with Matrigel [56] as well as endothelial cell monolayers [57]. Whereas the effects of 5-oxo-ETE on eosinophil movement appear to be mediated primarily by its rapid effects on actin reorganization [58], its ability to stimulate the passage of eosinophils through the basement membrane depends on the release of MMP-9 and activation of the plasmin/plasminogen system [41,56]. 5-Oxo-ETE is also active in vivo in humans, inducing the infiltration of eosinophils into the skin following intradermal injection. This response was more pronounced in asthmatic subjects compared to healthy controls [59].
In addition to eliciting the infiltration of eosinophils, 5-oxo-ETE has other effects that are likely to contribute to the pathophysiology of asthma. Although by itself, it has only a modest effect on eosinophil degranulation, once these cells have been primed with GM-CSF they respond much more strongly to 5-oxo-ETE, resulting in the release of proteins and enzymes that may have damaging effects on the airway epithelium [42]. Furthermore, 5-oxo-ETE strongly enhances eosinophil degranulation in responses to a variety of other mediators, including PAF, C5a, LTB4, and FMLP [42].
Another important effect of 5-oxo-ETE is its ability to stimulate human monocytes to release GM-CSF [60], which is a potent survival factor for eosinophils and appears to play a predominant role among cytokines in prolonging their lifetime in the airways [61]. Thus 5-oxo-ETE does not appear to have a direct effect on eosinophil survival, but when added to cocultures of eosinophils containing small numbers of monocytes, it strongly enhances their survival. A similar response is observed when conditioned medium from 5-oxo-ETE-treated monocytes is added to eosinophils, and this can be blocked with an antibody against GM-CSF [60]. The stimulatory effect of 5-oxo-ETE on GM-CSF release also has the potential to affect a variety of other processes that are affected by this cytokine. In addition to enhancing the responsiveness of eosinophils to 5-oxo-ETE, GM-CSF has been shown to stimulate the formation of 5-LO products at several levels [62-64], which could result in increased formation of both 5-oxo-ETE and cysLTs.
5-Oxo-ETE also has stimulatory effects on basophils, which appear to play an important role in asthma and other allergic diseases, at least in part because they are a source of both IL-4 and IL-13 [65]. Although it has only relatively modest effects on certain responses in these cells, including the surface expression of CD203c and CD11b [66], 5-oxo-ETE is a potent chemoattractant for basophils [47,67] and also stimulates the migration of IL-3-treated cells through Matrigel [68].
Because of the wide-ranging effects of 5-oxo-ETE on eosinophils and its indirect, GM-CSF-mediated effects on their survival, as well as its more limited effects on basophils, blocking the actions of this lipid mediator may be a useful strategy for the treatment of asthma. Drugs targeted specifically at 5-oxo-ETE could either block its synthesis by inhibiting 5-HEDH activity or block its actions by preventing activation of the OXE receptor. We have identified a synthetic 5-HETE analog containing only one double bond, which selectively inhibits 5-oxo-ETE formation in stimulated monocytes without affecting the formation of a variety of other eicosanoids [69]. Although this compound is a substrate for 5-HEDH, its oxidation product has little biological activity. However, a non-competitive inhibitor would be preferable, and further work would be required to develop a useful in vivo inhibitor. Moreover, such a drug would not prevent the formation of 5-oxo-ETE by autoxidation of cellular lipids, which can occur under certain circumstances [70]. As noted above, several naturally-occurring fatty acids have been reported to antagonize some of the effects of 5-oxo-ETE, but these substances are unlikely to be useful therapeutically. The development of a potent and selective OXE receptor antagonist would be an important contribution that could both clarify the pathophysiological role of 5-oxo-ETE and be useful in the treatment of asthma, either as a monotherapy or in combination with currently available drugs.
5.2. Cancer
Epidemiological studies suggest that a diet high in fat may increase the risk for many types of cancers, including prostate cancer [71]. Moreover, arachidonic acid has been reported to increase the rate of proliferation of prostate cancer cells [72]. There is evidence that these effects may be mediated by 5-LO products, as increased 5-LO expression has been reported in prostate tumors [73] and 5-LO inhibitors or FLAP antagonists were found to reduce tumor development in vivo in animal models [74,75] and to induce apoptosis in cancer cells derived from a variety of tissues [46,72,76-78]. In spite of these studies, the involvement of the 5-LO pathway in cancer cell growth is still somewhat controversial, as the concentrations of inhibitors employed are considerably higher than those required to inhibit 5-LO in inflammatory cells. Moreover, the FLAP antagonist MK886 was reported to induce apoptosis in cells that do not express FLAP [79], raising the possibility that its proapoptotic properties may be due to off-target effects. On the other hand, a variety of different agents that inhibit the formation of 5-LO products by different mechanisms have been shown to inhibit tumor cell growth. One possible explanation for the high concentrations of inhibitors required could be high MRP activity associated with tumor cells, which could result in enhanced export from these cells.
Ghosh and Myers have provided evidence that the 5-LO product required to support prostate tumor cell proliferation is 5-oxo-ETE. They found that both 5-oxo-ETE and 5-HETE, the former being more potent, blocked the proapoptotic effects of two agents that block the formation of 5-LO products by two different mechanisms: MK886, which is a FLAP antagonist, and AA861, which is a 5-LO inhibitor [46,72]. In contrast, LTB4 and cysLTs were ineffective. 5-Oxo-ETE also inhibited selenium-induced apoptosis in prostate cancer cells [80] and was found to increase the rates of proliferation of cancer cells derived from a variety of other tissues [81]. The antiapoptotic and proliferative effects of 5-oxo-ETE appear to be mediated by the OXE receptor, which is expressed in a variety of tumor cells [45,81]. Blocking the expression of this receptor with siRNA reduced the viability of PC3 prostate cancer cells [45]. This raises the interesting possibility that an OXE receptor antagonist could be useful in the treatment of cancer.
Although 5-LO and FLAP mRNA have been detected in tumor cells, there is relatively little information about the formation of 5-LO products by these cells. Several studies using immunoassay [46,73] or HPLC [82] have reported the production of 5-HETE by tumor cells, but these await confirmation by more rigorous methods such as mass spectrometry. Production of 5-LO products could also be accomplished by transcellular biosynthesis, as tumors contain large numbers of infiltrating inflammatory cells including macrophages, neutrophils, and eosinophils [83]. We have recently shown that prostate cancer cells contain high levels of 5-HEDH activity, and can synthesize 5-oxo-ETE from neutrophil-derived 5-HETE (manuscript in preparation). It is possible that 5-oxo-ETE could promote the infiltration of eosinophils into the tumor, and could account for the chemoattractant activity that has been reported to be released from dying cells within tumors [83]. Recent studies from our laboratory suggest that 5-oxo-ETE synthesis is enhanced in neutrophils undergoing apoptosis [22] as well as in dying tumor cells (manuscript in preparation).
5.3. Cardiovascular disease
Because of its effects on neutrophils and monocytes 5-oxo-ETE could also be involved in cardiovascular disease as well as various other inflammatory diseases. There has been considerable interest in the role of 5-LO products in cardiovascular disease since the study by Helgadottir et al. linking a SNP in FLAP to increased risk for myocardial infarction and stroke [84]. Since monocyte infiltration of the vessel wall is a key step in the development of atherosclerosis [85], 5-oxo-ETE could contribute to this process because of its chemoattractant effects on these cells. Furthermore, 5-oxo-ETE has synergistic effects with chemokines that stimulate monocyte migration, such as MCP-1 (CCL2), which is believed to play a role in the recruitment of monocytes in this disease [86]. We recently found that endothelial cells contain a high level of 5-HEDH activity and therefore can synthesize 5-oxo-ETE from 5-HETE, especially under conditions of oxidative stress [16], as might be expected to occur in inflammation. Although endothelial cells do not themselves contain appreciable 5-LO activity, it would seem probable that they could synthesize 5-oxo-ETE by transcellular biosynthesis from neutrophil- or monocyte- derived 5-HETE as we have found for other cell types (see above).
5-Oxo-ETE could also contribute to neutrophil infiltration following ischemia-reperfusion due to its chemoattractant effects on neutrophils. Although 5-oxo-ETE is not as potent as LTB4, which is a coproduct of 5-LO activation in various types of inflammatory cells, it activates neutrophils independently of LTB4 and could play a role if neutrophils become desensitized to the latter substance, which has been demonstrated to occur in vivo [87]. Furthermore, 5-oxo-ETE has been shown to sensitize neutrophils to other inflammatory mediators such as PAF [31], which could result in exacerbation of the inflammatory response.
6. Conclusions
5-Oxo-ETE is a product of AA metabolism in a variety of inflammatory cells and can also be formed from 5-HETE by structural cells, possibly by transcellular biosynthesis. Its formation is dependent on the availability of NADP+ and is stimulated by oxidative stress and by the respiratory burst in phagocytic cells, as well as by cell death. These conditions should favor its formation at inflammatory sites, where it may act via its selective OXE receptor to induce the infiltration of eosinophils and other inflammatory cells. 5-Oxo-ETE has been demonstrated by many groups to be a potent chemoattractant for eosinophils and indirectly to promote the survival of these cells by stimulating the release of GM-CSF from monocytes. These effects, along with its ability to elicit basophil migration, suggest that it may be an important mediator in asthma and other allergic diseases. 5-Oxo-ETE has been shown to increase the survival of tumor cells, which could explain the ability of 5-lipoxygenase inhibitors to induce apoptosis in these cells. Because of its chemoattractant effects on neutrophils and monocytes, 5-oxo-ETE could also be involved in atherosclerosis and ischemia-reperfusion injury, as well as a variety of other inflammatory diseases. A selective OXE receptor antagonist could be a useful therapeutic agent in asthma, cancer, and other diseases.
Fig. 5. Major signaling pathways associated with the OXE receptor.
Abbreviations: AC, adenylyl cyclase; PI3K, phosphatidylinositol 3-kinase; MEK, mitogen-activated protein kinase kinase; ERK, extracellular signal-regulated kinase; IP3, inositol triphosphate; DAG, diacyl glycerol; PKA, protein kinase A; PKC, protein kinase C.
Acknowledgments
The authors would like to acknowledge the support of the CIHR (WSP; MOP-6254), the Heart and Stroke Foundation of Quebec (WSP), and the NIH (JR; HL81873). The Meakins-Christie Laboratories - MUHC-RI, are supported in part by a Center grant from Le Fonds de la Recherche en Santé du Québec and by the JT Costello Memorial Research Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294(5548):1871–5. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 2.O'Flaherty JT. Neutrophil degranulation: evidence pertaining to its mediation by the combined effects of leukotriene B4, platelet-activating factor, and 5-HETE. J Cell Physiol. 1985;122:229–39. doi: 10.1002/jcp.1041220211. [DOI] [PubMed] [Google Scholar]
- 3.O'Flaherty JT, Jacobson D, Redman J. Mechanism involved in the mobilization of neutrophil calcium by 5-hydroxyeicosatetraenoate. J Immunol. 1988;140:4323–8. [PubMed] [Google Scholar]
- 4.O'Flaherty JT, Nishihira J. 5-Hydroxyeicosatetraenoate promotes Ca2+ and protein kinase C mobilization in neutrophils. Biochem Biophys Res Commun. 1987;148:575–81. doi: 10.1016/0006-291x(87)90915-6. [DOI] [PubMed] [Google Scholar]
- 5.Powell WS, Gravelle F, Gravel S. Metabolism of 5(S)-hydroxy-6,8,11,14-eicosatetraenoic acid and other 5(S)-hydroxyeicosanoids by a specific dehydrogenase in human polymorphonuclear leukocytes. J Biol Chem. 1992;267:19233–41. [PubMed] [Google Scholar]
- 6.Powell WS, Gravel S, MacLeod RJ, Mills E, Hashefi M. Stimulation of human neutrophils by 5-oxo-6,8,11,14- eicosatetraenoic acid by a mechanism independent of the leukotriene B4 receptor. J Biol Chem. 1993;268:9280–6. [PubMed] [Google Scholar]
- 7.Powell WS, Rokach J. Biochemistry, biology and chemistry of the 5-lipoxygenase product 5-oxo-ETE. Prog Lipid Res. 2005;44(2-3):154–83. doi: 10.1016/j.plipres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Patel P, et al. Substrate Selectivity of 5-Hydroxyeicosanoid Dehydrogenase and Its Inhibition by 5-Hydroxy-Delta(6)-Long-Chain Fatty Acids. Journal of Pharmacology and Experimental Therapeutics. 2009;329(1):335–41. doi: 10.1124/jpet.108.143453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cossette C, et al. Human Neutrophils Convert the Sebum-derived Polyunsaturated Fatty Acid Sebaleic Acid to a Potent Granulocyte Chemoattractant. J Biol Chem. 2008;283(17):11234–43. doi: 10.1074/jbc.M709531200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel P, et al. Structural requirements for activation of the 5-oxo-6E,8Z, 11Z,14Z-eicosatetraenoic acid (5-oxo-ETE) receptor: identification of a mead acid metabolite with potent agonist activity. J Pharmacol Exp Ther. 2008;325(2):698–707. doi: 10.1124/jpet.107.134908. [DOI] [PubMed] [Google Scholar]
- 11.Evans JF, Nathaniel DJ, Zamboni RJ, Ford-Hutchinson AW. Leukotriene A3. A poor substrate but a potent inhibitor of rat and human neutrophil leukotriene A4 hydrolase. J Biol Chem. 1985;260(20):10966–70. [PubMed] [Google Scholar]
- 12.Powell WS, Gravel S, Gravelle F. Formation of a 5-oxo metabolite of 5,8,11,14,17-eicosapentaenoic acid and its effects on human neutrophils and eosinophils. J Lipid Res. 1995;36(12):2590–8. [PubMed] [Google Scholar]
- 13.Zhang Y, Styhler A, Powell WS. Synthesis of 5-oxo-6,8,11,14-eicosatetraenoic acid by human monocytes and lymphocytes. J Leukoc Biol. 1996;59(6):847–54. doi: 10.1002/jlb.59.6.847. [DOI] [PubMed] [Google Scholar]
- 14.Zimpfer U, et al. Human dendritic cells are a physiological source of the chemotactic arachidonic acid metabolite 5-oxo-eicosatetraenoic acid. Inflamm Res. 2000;49(11):633–8. doi: 10.1007/s000110050641. [DOI] [PubMed] [Google Scholar]
- 15.Powell WS, Gravel S, Khanapure SP, Rokach J. Biological inactivation of 5-oxo-6,8,11,14-eicosatetraenoic acid by human platelets. Blood. 1999;93(3):1086–96. [PubMed] [Google Scholar]
- 16.Erlemann KR, et al. Metabolism of 5-hydroxy-6,8,11,14-eicosatetraenoic acid by human endothelial cells. Biochem Biophys Res Commun. 2006;350(1):151–6. doi: 10.1016/j.bbrc.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Erlemann KR, et al. Airway epithelial cells synthesize the lipid mediator 5-oxo-ETE in response to oxidative stress. Free Radic Biol Med. 2007;42(5):654–64. doi: 10.1016/j.freeradbiomed.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folco G, Murphy RC. Eicosanoid transcellular biosynthesis: From cell-cell interactions to in vivo tissue responses. Pharmacol Rev. 2006;58(3):375–88. doi: 10.1124/pr.58.3.8. [DOI] [PubMed] [Google Scholar]
- 19.O'Flaherty JT, Wykle R, Redman J, Samuel M, Thomas M. Metabolism of 5-hydroxyicosatetraenoate by human neutrophils: production of a novel omega-oxidized derivative. J Immunol. 1986;137:3277–83. [PubMed] [Google Scholar]
- 20.Powell WS, MacLeod RJ, Gravel S, Gravelle F, Bhakar A. Metabolism and biologic effects of 5-oxoeicosanoids on human neutrophils. J Immunol. 1996;156(1):336–42. [PubMed] [Google Scholar]
- 21.Powell WS, Gravelle F, Gravel S. Phorbol myristate acetate stimulates the formation of 5-oxo-6,8,11,14-eicosatetraenoic acid by human neutrophils by activating NADPH oxidase. J Biol Chem. 1994;269:25373–80. [PubMed] [Google Scholar]
- 22.Graham FD, Erlemann KR, Gravel S, Rokach J, Powell WS. Oxidative stress-induced changes in pyridine nucleotides and chemoattractant 5-lipoxygenase products in aging neutrophils. Free Radic Biol Med. 2009 doi: 10.1016/j.freeradbiomed.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erlemann KR, Rokach J, Powell WS. Oxidative stress stimulates the synthesis of the eosinophil chemoattractant 5-oxo-6,8,11,14-eicosatetraenoic acid by inflammatory cells. J Biol Chem. 2004;279:40376–84. doi: 10.1074/jbc.M401294200. [DOI] [PubMed] [Google Scholar]
- 24.Berhane K, Ray AA, Khanapure SP, Rokach J, Powell WS. Calcium/calmodulin-dependent conversion of 5-oxoeicosanoids to 6, 7- dihydro metabolites by a cytosolic olefin reductase in human neutrophils. J Biol Chem. 1998;273(33):20951–9. doi: 10.1074/jbc.273.33.20951. [DOI] [PubMed] [Google Scholar]
- 25.Hevko JM, Bowers RC, Murphy RC. Synthesis of 5-oxo-6,8,11,14-eicosatetraenoic acid and identification of novel omega-oxidized metabolites in the mouse macrophage. J Pharmacol Exp Ther. 2001;296(2):293–305. [PubMed] [Google Scholar]
- 26.Bowers RC, Hevko J, Henson PM, Murphy RC. A novel glutathione containing eicosanoid (FOG7) chemotactic for human granulocytes. J Biol Chem. 2000;275(39):29931–4. doi: 10.1074/jbc.C000502200. [DOI] [PubMed] [Google Scholar]
- 27.Hevko JM, Murphy RC. Formation of murine macrophage-derived 5-oxo-7-glutathionyl-8,11,14- eicosatrienoic acid (FOG7) is catalyzed by leukotriene C4 synthase. J Biol Chem. 2002;277(9):7037–43. doi: 10.1074/jbc.M108942200. [DOI] [PubMed] [Google Scholar]
- 28.Schwenk U, Schröder JM. 5-Oxo-eicosanoids are potent eosinophil chemotactic factors -functional characterization and structural requirements. J Biol Chem. 1995;270:15029–36. doi: 10.1074/jbc.270.25.15029. [DOI] [PubMed] [Google Scholar]
- 29.O'Flaherty JT, Taylor JS, Thomas MJ. Receptors for the 5-oxo class of eicosanoids in neutrophils. J Biol Chem. 1998;273(49):32535–41. doi: 10.1074/jbc.273.49.32535. [DOI] [PubMed] [Google Scholar]
- 30.Erlemann KR, et al. Regulation of 5-hydroxyeicosanoid dehydrogenase activity in monocytic cells. Biochem J. 2007;403(1):157–65. doi: 10.1042/BJ20061617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Flaherty JT, Cordes J, Redman J, Thomas MJ. 5-Oxo-eicosatetraenoate, a potent human neutrophil stimulus. Biochem Biophys Res Commun. 1993;192:129–34. doi: 10.1006/bbrc.1993.1391. [DOI] [PubMed] [Google Scholar]
- 32.Powell WS, et al. Effects of 5-oxo-6,8,11,14-eicosatetraenoic acid on expression of CD11b, actin polymerization and adherence in human neutrophils. J Immunol. 1997;159:2952–9. [PubMed] [Google Scholar]
- 33.Hosoi T, et al. Identification of a novel eicosanoid receptor coupled to Gi/o. J Biol Chem. 2002;277:31459–65. doi: 10.1074/jbc.M203194200. [DOI] [PubMed] [Google Scholar]
- 34.Jones CE, et al. Expression and characterization of a 5-oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid receptor highly expressed on human eosinophils and neutrophils. Mol Pharmacol. 2003;63(3):471–7. doi: 10.1124/mol.63.3.471. [DOI] [PubMed] [Google Scholar]
- 35.Takeda S, Yamamoto A, Haga T. Identification of a G protein-coupled receptor for 5-oxo-eicosatetraenoic acid. Biomedical Research-Tokyo. 2002;23(2):101–8. [Google Scholar]
- 36.Brink C, et al. International Union of Pharmacology XLIV. Nomenclature for the oxoeicosanoid receptor. Pharmacol Rev. 2004;56(1):149–57. doi: 10.1124/pr.56.1.4. [DOI] [PubMed] [Google Scholar]
- 37.Norgauer J, et al. Chemotactic 5-oxo-icosatetraenoic acids activate a unique pattern of neutrophil responses - analysis of phospholipid metabolism, intracellular Ca2+ transients, actin reorganization, superoxide-anion production and receptor up-regulation. Eur J Biochem. 1996;236(3):1003–9. doi: 10.1111/j.1432-1033.1996.01003.x. [DOI] [PubMed] [Google Scholar]
- 38.O'Flaherty JT, et al. 5-oxo-eicosanoids and hematopoietic cytokines cooperate in stimulating neutrophil function and the mitogen-activated protein kinase pathway. J Biol Chem. 1996;271(30):17821–8. doi: 10.1074/jbc.271.30.17821. [DOI] [PubMed] [Google Scholar]
- 39.Hosoi T, Sugikawa E, Chikada A, Koguchi Y, Ohnuki T. TG1019/OXE, a Galpha(i/o)-protein-coupled receptor, mediates 5-oxo-eicosatetraenoic acid-induced chemotaxis. Biochem Biophys Res Commun. 2005;334(4):987–95. doi: 10.1016/j.bbrc.2005.06.191. [DOI] [PubMed] [Google Scholar]
- 40.O'Flaherty JT, et al. 5(S)-Hydroxy-6,8,11,14-E,Z,Z,Z-eicosatetraenoate stimulates PC3 cell signaling and growth by a receptor-dependent mechanism. Cancer Res. 2002;62(23):6817–9. [PubMed] [Google Scholar]
- 41.Langlois A, et al. Crucial implication of protein kinase C (PKC)-delta, PKC-zeta, ERK-1/2, and p38 MAPK in migration of human asthmatic eosinophils. J Leukoc Biol. 2009;85(4):656–63. doi: 10.1189/jlb.0808492. [DOI] [PubMed] [Google Scholar]
- 42.O'Flaherty JT, et al. 5-Oxo-eicosatetraenoate is a broadly active, eosinophil- selective stimulus for human granulocytes. J Immunol. 1996;157(1):336–42. [PubMed] [Google Scholar]
- 43.Lin LL, et al. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72(2):269–78. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 44.O'Flaherty JT, Cordes JF, Lee SL, Samuel M, Thomas MJ. Chemical and biological characterization of oxo-eicosatetraenoic acids. Biochim Biophys Acta. 1994;1201(3):505–15. doi: 10.1016/0304-4165(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 45.Sundaram S, Ghosh J. Expression of 5-oxoETE receptor in prostate cancer cells: critical role in survival. Biochem Biophys Res Commun. 2006;339(1):93–8. doi: 10.1016/j.bbrc.2005.10.189. [DOI] [PubMed] [Google Scholar]
- 46.Ghosh J, Myers CE. Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proc Natl Acad Sci U S A. 1998;95(22):13182–7. doi: 10.1073/pnas.95.22.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sturm GJ, et al. 5-Oxo-6,8,11,14-eicosatetraenoic acid is a potent chemoattractant for human basophils. J Allergy Clin Immunol. 2005;116(5):1014–9. doi: 10.1016/j.jaci.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 48.Powell WS, Chung D, Gravel S. 5-Oxo-6,8,11,14-eicosatetraenoic acid is a potent stimulator of human eosinophil migration. J Immunol. 1995;154:4123–32. [PubMed] [Google Scholar]
- 49.Flood-Page P, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112(7):1029–36. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peters-Golden M, Henderson WR., Jr Leukotrienes. N Engl J Med. 2007;357(18):1841–54. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- 51.Rådinger M, Lötvall J. Eosinophil progenitors in allergy and asthma - do they matter? Pharmacol Ther. 2009;121(2):174–84. doi: 10.1016/j.pharmthera.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 52.Leckie MJ, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356(9248):2144–8. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 53.Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil's role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003;167(2):199–204. doi: 10.1164/rccm.200208-789OC. [DOI] [PubMed] [Google Scholar]
- 54.Jacobsen EA, Ochkur SI, Lee NA, Lee JJ. Eosinophils and asthma. Curr Allergy Asthma Rep. 2007;7(1):18–26. doi: 10.1007/s11882-007-0026-y. [DOI] [PubMed] [Google Scholar]
- 55.Powell WS, Ahmed S, Gravel S, Rokach J. Eotaxin and RANTES enhance 5-oxo-6,8,11,14-eicosatetraenoic acid- induced eosinophil chemotaxis. J Allergy Clin Immunol. 2001;107(2):272–8. doi: 10.1067/mai.2001.112847. [DOI] [PubMed] [Google Scholar]
- 56.Guilbert M, et al. 5-Oxo-6,8,11,14-eicosatetraenoic acid induces important eosinophil transmigration through basement membrane components: comparison of normal and asthmatic eosinophils. Am J Respir Cell Mol Biol. 1999;21(1):97–104. doi: 10.1165/ajrcmb.21.1.3517. [DOI] [PubMed] [Google Scholar]
- 57.Dallaire MJ, et al. Endothelial cells modulate eosinophil surface markers and mediator release. Eur Respir J. 2003;21(6):918–24. doi: 10.1183/09031936.03.00102002. [DOI] [PubMed] [Google Scholar]
- 58.Powell WS, Gravel S, Halwani F. 5-oxo-6,8,11,14-Eicosatetraenoic Acid Is a Potent Stimulator of L- Selectin Shedding, Surface Expression of CD11b, Actin Polymerization, and Calcium Mobilization in Human Eosinophils. Am J Respir Cell Mol Biol. 1999;20(1):163–70. doi: 10.1165/ajrcmb.20.1.3141. [DOI] [PubMed] [Google Scholar]
- 59.Muro S, et al. 5-oxo-6,8,11,14-eicosatetraenoic acid induces the infiltration of granulocytes into human skin. J Allergy Clin Immunol. 2003;112(4):768–74. doi: 10.1016/s0091-6749(03)01888-8. [DOI] [PubMed] [Google Scholar]
- 60.Stamatiou PB, et al. 5-Oxo-6,8,11,14-eicosatetraenoic acid stimulates the release of the eosinophil survival factor granulocyte-macrophage colony stimulating factor from monocytes. J Biol Chem. 2004;279:28159–64. doi: 10.1074/jbc.M401537200. [DOI] [PubMed] [Google Scholar]
- 61.Park CS, et al. Granulocyte macrophage colony-stimulating factor is the main cytokine enhancing survival of eosinophils in asthmatic airways. Eur Respir J. 1998;12(4):872–8. doi: 10.1183/09031936.98.12040872. [DOI] [PubMed] [Google Scholar]
- 62.DiPersio JF, Billing P, Williams R, Gasson JC. Human granulocyte-macrophage colony-stimulating factor and other cytokines prime human neutrophils for enhanced arachidonic acid release and leukotriene B4 synthesis. J Immunol. 1988;140(12):4315–22. [PubMed] [Google Scholar]
- 63.Pouliot M, McDonald PP, Khamzina L, Borgeat P, McColl SR. Granulocyte-Macrophage Colony-Stimulating Factor Enhances 5- Lipoxygenase Levels in Human Polymorphonuclear Leukocytes. J Immunol. 1994;152:851–8. [PubMed] [Google Scholar]
- 64.Pouliot M, McDonald PP, Borgeat P, McColl SR. Granulocyte Macrophage Colony-Stimulating Factor Stimulates the Expression of the 5-Lipoxygenase-Activating Protein (FLAP) in Human Neutrophils. J Exp Med. 1994;179:1225–32. doi: 10.1084/jem.179.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gibbs BF. Human basophils as effectors and immunomodulators of allergic inflammation and innate immunity. Clin Exp Med. 2005;5(2):43–9. doi: 10.1007/s10238-005-0064-5. [DOI] [PubMed] [Google Scholar]
- 66.Monneret G, et al. Effects of Prostaglandin D2 and 5-Lipoxygenase Products on the Expression of CD203c and CD11b by Basophils. J Pharmacol Exp Ther. 2005;312(2):627–34. doi: 10.1124/jpet.104.074823. [DOI] [PubMed] [Google Scholar]
- 67.Iikura M, et al. 5-Lipoxygenase products regulate basophil functions: 5-Oxo-ETE elicits migration, and leukotriene B(4) induces degranulation. J Allergy Clin Immunol. 2005;116(3):578–85. doi: 10.1016/j.jaci.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 68.Suzukawa M, et al. Trans-basement membrane migration of human basophils: role of matrix metalloproteinase-9. Int Immunol. 2006;18(11):1575–83. doi: 10.1093/intimm/dxl090. [DOI] [PubMed] [Google Scholar]
- 69.Patel P, et al. Substrate selectivity of 5-hydroxyeicosanoid dehydrogenase and its inhibition by 5-hydroxy-{Delta}6-long chain fatty acids. J Pharmacol Exp Ther. 2009 doi: 10.1124/jpet.108.143453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zarini S, Murphy RC. Biosynthesis of 5-oxo-6,8,11,14-eicosatetraenoic acid from 5-hydroperoxyeicosatetraenoic acid in the murine macrophage. J Biol Chem. 2003;278:11190–6. doi: 10.1074/jbc.M208496200. [DOI] [PubMed] [Google Scholar]
- 71.Rose DP, Connolly JM. Dietary fat, fatty acids and prostate cancer. Lipids. 1992;27(10):798–803. doi: 10.1007/BF02535853. [DOI] [PubMed] [Google Scholar]
- 72.Ghosh J, Myers CE. Arachidonic acid stimulates prostate cancer cell growth: critical role of 5-lipoxygenase. Biochem Biophys Res Commun. 1997;235(2):418–23. doi: 10.1006/bbrc.1997.6799. [DOI] [PubMed] [Google Scholar]
- 73.Gupta S, et al. Lipoxygenase-5 is overexpressed in prostate adenocarcinoma. Cancer. 2001;91(4):737–43. doi: 10.1002/1097-0142(20010215)91:4<737::aid-cncr1059>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 74.Rioux N, Castonguay A. Inhibitors of lipoxygenase: a new class of cancer chemopreventive agents. Carcinogenesis. 1998;19(8):1393–400. doi: 10.1093/carcin/19.8.1393. [DOI] [PubMed] [Google Scholar]
- 75.Moody TW, et al. Lipoxygenase inhibitors prevent lung carcinogenesis and inhibit non-small cell lung cancer growth. Exp Lung Res. 1998;24(4):617–28. doi: 10.3109/01902149809087390. [DOI] [PubMed] [Google Scholar]
- 76.Avis I, et al. Five-lipoxygenase inhibitors can mediate apoptosis in human breast cancer cell lines through complex eicosanoid interactions. FASEB J. 2001;15(11):2007–9. doi: 10.1096/fj.00-0866fje. [DOI] [PubMed] [Google Scholar]
- 77.Hammamieh R, Sumaida D, Zhang X, Das R, Jett M. Control of the growth of human breast cancer cells in culture by manipulation of arachidonate metabolism. BMC Cancer. 2007;7:138. doi: 10.1186/1471-2407-7-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Edderkaoui M, et al. Extracellular matrix stimulates reactive oxygen species production and increases pancreatic cancer cell survival through 5-lipoxygenase and NADPH oxidase. Am J Physiol Gastrointest Liver Physiol. 2005;289(6):G1137–G1147. doi: 10.1152/ajpgi.00197.2005. [DOI] [PubMed] [Google Scholar]
- 79.Datta K, Biswal SS, Kehrer JP. The 5-lipoxygenase-activating protein (FLAP) inhibitor, MK886, induces apoptosis independently of FLAP. Biochem J. 1999;340(Pt 2):371–5. [PMC free article] [PubMed] [Google Scholar]
- 80.Ghosh J. Rapid induction of apoptosis in prostate cancer cells by selenium: reversal by metabolites of arachidonate 5-lipoxygenase. Biochem Biophys Res Commun. 2004;315(3):624–35. doi: 10.1016/j.bbrc.2004.01.100. [DOI] [PubMed] [Google Scholar]
- 81.O'Flaherty JT, et al. 5-Oxo-ETE analogs and the proliferation of cancer cells. Biochim Biophys Acta. 2005;1736(3):228–36. doi: 10.1016/j.bbalip.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 82.Hassan S, Carraway RE. Involvement of arachidonic acid metabolism and EGF receptor in neurotensin-induced prostate cancer PC3 cell growth. Regul Pept. 2006;133(1-3):105–14. doi: 10.1016/j.regpep.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 83.Cormier SA, et al. Pivotal Advance: eosinophil infiltration of solid tumors is an early and persistent inflammatory host response. J Leukoc Biol. 2006;79(6):1131–9. doi: 10.1189/jlb.0106027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Helgadottir A, et al. The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nat Genet. 2004;36(3):233–9. doi: 10.1038/ng1311. [DOI] [PubMed] [Google Scholar]
- 85.Mestas J, Ley K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc Med. 2008;18(6):228–32. doi: 10.1016/j.tcm.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoogeveen RC, et al. Plasma MCP-1 level and risk for peripheral arterial disease and incident coronary heart disease: Atherosclerosis Risk in Communities study. Atherosclerosis. 2005;183(2):301–7. doi: 10.1016/j.atherosclerosis.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 87.Marleau S, Fortin C, Poubelle PE, Borgeat P. In vivo desensitization to leukotriene B4 (LTB4) in the rabbit. Inhibition of LTB4-induced neutropenia during intravenous infusion of LTB4. J Immunol. 1993;150:206–13. [PubMed] [Google Scholar]