Abstract
Background
Ventilatory efficiency (VE/VCO2 ratio) and the partial pressure of end-tidal carbon dioxide (PETCO2), obtained during moderate to high levels of physical exertion demonstrate prognostic value in heart failure (HF). The present investigation assesses the clinical utility of these variables during low-intensity exercise.
Methods and Results
One hundred and thirty subjects diagnosed with HF underwent a 2-minute, constant-rate treadmill session at 2 miles per hour. Both the VE/VCO2 ratio and PETCO2 were recorded during exercise (30-second average) and their change (Δ) from rest. B-type and atrial natriuretic peptide (BNP and ANP) were also determined. Only PETCO2 and ΔPETCO2 emerged from the multivariate Cox regression. Receiver operating characteristic curve analysis revealed the prognostic classification schemes were significant with thresholds of </≥34 mm Hg (hazard ratio: 4.2, 95% CI: 2.2–8.0, P < .001) and </≥1 mm Hg (hazard ratio: 3.5, 95% CI: 1.9–6.6, P < .001) being optimal for PETCO2 and Δ PETCO2, respectively. Moreover, subjects with a PETCO2≥34 mm Hg had a significantly lower BNP (214.1 ± 431.9 vs. 1110.5 ± 1854.0 pg/mL, P =.005) and ANP (108.2 ± 103.6 vs. 246.2 ± 200.4 pg/mL, P < .001).
Conclusions
The results of this pilot study indicate ventilatory expired gas analysis during a short bout of low-intensity exercise may provide insight into prognosis and cardiac stability.
Keywords: Cardiopulmonary, prognosis, ventilatory efficiency, carbon dioxide
The use of cardiopulmonary exercise testing (CPX) for prognostic purposes is a well-accepted practice in patients with heart failure (HF).1,2 Variables obtained from CPX are also associated with other makers of pathophysiology such as diminished cardiac output and elevated pulmonary pressure and neurohormonal markers.3 The implementation of this assessment technique traditionally consists of symptom-limited exercise testing where patients are progressed to their maximal exercise tolerance through either incremental or ramped workload adjustments. From such testing, peak aerobic capacity,4 and more recently ventilatory efficiency,5,6 are the most frequently assessed exercise parameters. It does appear that ventilatory efficiency, typically expressed as the minute ventilation/carbon dioxide production (VE/VCO2) slope or ratio, is prognostically superior to the more established peak oxygen consumption (VO2).3
Although most previous investigations focused on measurements requiring maximal exertion, some groups have demonstrated the potential clinical value of CPX variables obtained during submaximal exercise.7–10 Even so, the majority of submaximal analyses to this point have required exertion to the point of ventilatory threshold, which requires progression toward maximal exertion to ensure the patient has passed the point of increasing anaerobic metabolism. Moreover, the achievement of ventilatory threshold relative to maximal aerobic capacity is not consistent from one individual to the next, negating the ability to devise a standard submaximal exercise protocol at a given workload. Last, poor subject effort and ventilatory abnormalities, such as oscillatory ventilation,11 make attainment or detection of ventilatory threshold a challenge in a number of patients with HF.
To date, the limited analysis of the value of ventilatory expired gas analysis during low-intensity workloads has focused on the oxygen uptake kinetic response. Koike et al12 found the time constant for oxygen uptake during a 4-minute bout of lower extremity ergometry exercise at a constant-rate 20 watt workload was prognostically significant. Brunner-La Rocca et al13 reported similar results using a 6-minute submaximal treadmill protocol at a constant-rate speed and grade of 1 mile per hour and 6%, respectively.
Both the VE/VCO2 ratio and the partial pressure of end-tidal carbon dioxide (PETCO2) have demonstrated prognostic value in previous investigations, although calculations of these variables were made at ventilatory threshold.8–10 To our knowledge, no previous investigations have examined the potential clinical value of the VE/VCO2 ratio or the PETCO2 obtained during constant-rate low-intensity exercise. We hypothesized that CPX abnormalities, as measured by the VE/VCO2 ratio and PETCO2, will already become apparent at constant-rate low-intensity exercise, be related to natriuretic peptide levels and provide prognostic value.
Methods
This study, conducted at the Mayo Clinic in Rochester, Minnesota, included a total of 130 patients diagnosed with compensated HF and 106 apparently healthy controls. Sample size calculation for the HF group was based on the ability to detect a 20% difference in event-free survival according to a dichotomous CPX variable threshold with >80% power. The primary goal of apparently healthy subject recruitment was to approximate the number, age, and gender distribution of the HF group. Subjects in the HF group were recruited from the Mayo Cardiovascular Health Clinic, whereas subjects in the control group were recruited from the community in and surrounding Rochester, Minnesota. Inclusion criteria for the HF group consisted of a diagnosis of HF15 and evidence of left ventricular systolic or diastolic dysfunction by 2-dimensional echocardiography obtained within 4 days of exercise testing. Subjects were classified as having systolic HF if they presented with a left ventricular ejection fraction (LVEF) <50%. Subjects with an LVEF ≥50% and indications of an abnormal filling pattern were classified as having diastolic dysfunction. Inclusion criteria for the control group included no previous history of cardiac and/or pulmonary disease and normal findings on echocardiography. Echocardiography was performed in the control group to confirm normal left ventricular function. Current tobacco use or a previous history of use > 15 years, severe obesity (body mass index > 40 kg/m2) or orthopedic limitations to exercise served as exclusion criteria for both the HF and control groups. All subjects completed a written informed consent and institutional review board approval was obtained.
Cardiopulmonary Exercise Testing Procedures
All subjects completed an initial 2-minute, constant-rate treadmill bout at 2 miles per hour with a 0% grade. After this initial workload, speed and grade were incrementally increased at 2-minute intervals to maximal exertion. Blood pressure and electrocardiogram were monitored throughout the exercise test. Ventilatory expired gas analysis was collected at rest and throughout exercise using a metabolic cart (Medgraphics CPX-D, Rochester, MN). Before each test, the equipment was calibrated in standard fashion using reference gases as per manufacturer specifications. Minute ventilation, VO2, and VCO2 were acquired breath by breath, and averaged over 30-second intervals. Immediately after exercise, subjects remained in the seated position for 1 minute and resting ventilatory expired values were calculated as averaged data over that entire period. The averaged value during the last 30 seconds of exercise during the initial constant-rate low-intensity workload and at peak exercise was used to calculate the VE/VCO2 ratio and PETCO2. The change (Δ) in the VE/VCO2 ratio and PETCO2 from rest to the terminal portion of the constant-rate low-intensity exercise bout was also determined. To assess level of exertion and metabolic cost, the averaged value during the last 30 seconds of constant-rate low-intensity exercise was also used to determine the respiratory exchange ratio (RER) and VO2 in mLO2●kg−1●min−1. Peak VO2, in mLO2●kg−1●min−1, was defined as the 30-second averaged value at maximal exertion. The 6-20 Borg scale14 was used to assess rating of perceived exertion (RPE) at the terminal portion of constant-rate low-intensity as well as peak exercise. Exercise testing was supervised by a doctorally prepared exercise physiologist and conducted by an RN and an American College of Sports Medicine Exercise Specialist.
Measurement of Natriuretic Peptides
All blood draws were performed at rest in a supine position. Brain natriuretic peptide (BNP), and atrial natriuretic peptide (ANP) were assessed according to previously published methods.15,16 As with echocardiography, blood draws were preformed within 4 days of exercise testing.
End Points
Subjects were tracked for major cardiac events (mortality and urgent heart transplantation) via hospital and outpatient medical chart review. Subjects continued to receive medical care through Mayo Cardiovascular Health Clinic. This provided detailed information on their clinical status. Clinicians conducting the exercise test were not involved in decisions regarding urgent heart transplant.
Statistical Analysis
All continuous data are reported as mean ± standard deviation. Unpaired t-testing compared key baseline characteristics as well as CPX and natriuretic peptide values that were on a continuous scale between the HF and control group. The chi-square test compared differences in gender between groups. The Mann-Whitney U test assessed differences in RPE between the HF and control groups.
Receiver operating characteristic (ROC) curve analysis assessed the prognostic classification schemes of CPX variables obtained during constant-rate low-intensity exercise and determined optimal dichotomous threshold values in the HF group. Optimal thresholds were defined as the value that provided the highest combination of sensitivity and specificity. ROC curve analysis was also performed on CPX variables obtained at peak exercise for comparative purposes.
Univariate and multivariate (forward stepwise method, entry and removal values 0.10 and 0.05, respectively) Cox regression analysis assessed the prognostic characteristics of resting and constant-rate low-intensity CPX variables in patients with HF. Multivariate Cox regression was also used to assess the combined prognostic value of constant-rate low-intensity CPX and key resting variables. Lastly, univariate Cox regression analysis assessed the prognostic strength of CPX variables at peak exercise.
Pearson product moment correlation assessed the relationship amongst CPX variables in the HF group. In the presence of Collin-earity between resting and constant-rate low-intensity CPX variables (r≥0.70), the variable with higher prognostic strength was included in the multivariate Cox regression.
Kaplan-Meier analysis assessed survival characteristics of dichotomous expressions of CPX variables retained in the multivariate Cox regression analysis. The log-rank test determined statistical significance of the Kaplan-Meier analysis.
Unpaired t-testing compared BNP and ANP levels according to dichotomous CPX thresholds as defined by ROC curve analysis. Pearson product moment correlation assessed the relationship between continuous expressions of neurohormones and constant-rate low-intensity CPX variables retained in the multivariate Cox regression as well as those obtained at peak exercise. All statistical tests with a P value < .05 were considered significant.
Results
Baseline characteristics for the HF and control groups are listed in Table 1. Control subjects were slightly younger and presented with a lower body mass index. As expected, BNP and ANP were significantly lower, whereas LVEF was significantly higher in control subjects compared with patients with HF. As for the HF cohort, the majority were diagnosed with a nonischemic etiology and prescribed an angiotensin-converting enzyme inhibitor, β-blocker, and diuretic. Although none of the subjects in the control group had a previous diagnosis of cardiac or pulmonary disease and demonstrated normal left ventricular function by echo-cardiogram, 1 was prescribed an antihypertensive medication (diuretic) and 13 others were prescribed a statin.
Table 1.
Baseline Characteristics for the Heart Failure and Control Groups
| Heart Failure (n = 130) |
Control (n = 106) |
P Value |
|
|---|---|---|---|
| Age (y) | 55 ± 11 | 51 ± 15 | .02* |
| Body mass index (kg/m2) | 28 ± 4 | 25 ± 3 | <.001* |
| Gender (male/female in %) | 64/36 | 60/40 | .41 |
| Left ventricular ejection fraction (%) |
28 ± 11 | 63 ± 7 | <.001* |
| Heart failure etiology (ischemic/ nonischemic in %) |
32/68 | N/A | N/A |
| New York Heart Association Class (I/II/III/IV in %) |
34/32/27/7 | N/A | N/A |
| B-type natriuretic peptide (pg/mL) | 501.3 ± 1179.5 | 41.8 ± 145.4 | <.001* |
| Atrial natriuretic peptide (pg/mL) | 153.0 ± 155.8 | 30.2 ± 22.4 | <.001* |
| β-blocker (%) | 76 | N/A | N/A |
| Angiotensin-converting enzyme inhibitor (%) |
73 | N/A | N/A |
| AII receptor blocker (%) | 12 | N/A | N/A |
| Diuretic (%) | 68 | <1 | <.001* |
AII, angiotensin II.
Cardiopulmonary exercise test variables at rest, constant-rate low-intensity, and peak exercise are listed in Table 2. Resting PETCO2 and the VE/VCO2 ratio were not significantly different between groups. There were no complications warranting premature exercise test termination in either group. Although constant-rate low-intensity VO2 was comparable between groups, PETCO2 and Δ PETCO2 were significantly lower, whereas the VE/VCO2 ratio, RER, and RPE were significantly higher in patients with HF compared with healthy controls. Moreover, while successfully completing the constant-rate low-intensity component of exercise, 6 subjects in the HF group, 5 with New York Heart Association Class designation of II and one with a designation of IV, presented with a RER that surpassed 1.0. The ΔVE/VCO2 ratio approached but did not reach statistical significance. All CPX variables at peak exercise were significantly different between groups. With the exception of peak RPE, trends in these differences were similar to those observed at constant-rate low-intensity exercise.
Table 2.
Low-Intensity Constant-Rate and Peak Cardiopulmonary Exercise Testing Variables for the Heart Failure and Control Groups
| Heart Failure (n = 130) |
Control (n = 106) |
P Value |
|
|---|---|---|---|
| Resting PETCO2 (mm Hg) | 33.4 ± 4.4 | 33.8 ± 4.4 | .44 |
| Resting VE/VCO2 ratio | 41.2 ± 5.9 | 40.0 ± 4.9 | .11 |
| Constant-rate PETCO2 (mm Hg) |
35.4 ± 4.7 | 37.7 ± 3.2 | <.001* |
| Δ PETCO2 (mm Hg) | 2.0 ± 3.4 | 4.0 ± 2.7 | <.001* |
| Constant-rate VE/VCO2 ratio |
34.7 ± 6.4 | 32.3 ± 3.4 | <.001* |
| Δ VE/VCO2 ratio | 6.5 ± 4.8 | 7.5 ± 3.6 | .08 |
| Constant-rate VO2 (mLO2●kg−1●min−1) |
10.6 ± 2.1 | 11.1 ± 1.8 | .07 |
| Constant-rate RER | 0.83 ± 0.10 | 0.78 ± 0.08 | <.001* |
| Constant-rate RPE (6–20 scale) |
9 ± 2 | 8 ± 1 | <.001* |
| Peak PETCO2 (mm Hg) | 34.8 ± 5.9 | 38.9 ± 4.5 | <.001* |
| Peak VE/VCO2 ratio | 34.4 ± 7.0 | 29.3 ± 3.6 | <.001* |
| Peak VO2 (mLO2●kg−1 ●min−1) |
18.9 ± 6.0 | 34.7 ± 8.7 | <.001* |
| Peak RER | 1.13 ± 0.12 | 1.15 ± 0.08 | .04 |
| Peak RPE (6–20 scale) | 17 ± 1 | 18 ± 1 | <.001* |
PETCO2, partial pressure of end-tidal carbon dioxide; VE, ventilatory efficiency; VCO2, carbon dioxide production; VO2, peak oxygen consumption; RER, respiratory exchange ratio; RPE, rating of perceived exertion.
There were 29 deaths and 10 urgent heart transplants during a mean and median follow-up of 5.4 ± 2.7 and 5.0 years, respectively (crude annual event rate: 5.4%). All subjects who did not suffer an adverse event were tracked for a minimum of 3 years. The ROC and univariate Cox regression analyses listed in Table 3 demonstrate constant-rate low-intensity PETCO2, Δ PETCO2, the VE/VCO2 ratio and ΔVE/VCO2 were all significant predictors of adverse events. Area under the ROC curve and univariate chi-square values were likewise significant for the peak PETCO2 (area: 0.74, 95% CI: 0.63–0.84, univariate chi-square: 24.5, P < .001), the VE/VCO2 ratio (area: 0.73, 95% CI: 0.63–0.84, univariate chi-square: 27.5, P < .001), and VO2 (area: 0.72, 95% CI: 0.62–0.81, univariate chi-square: 16.7, P < .001) prognostic classification schemes. Pearson product moment correlation results listed in Table 4 revealed, for the constant-rate low-intensity variables, the relationships amongst PETCO2, Δ PETCO2, the VE/VCO2 ratio and ΔVE/VCO2 ratio was significant. The correlation between resting values of PETCO2 and the VE/VCO2 ratio and their constant-rate low-intensity counterparts surpassed an r value of 0.70 in both instances. During exercise, only the correlation between constant-rate low-intensity PETCO2 and the VE/VCO2 ratio surpassed an r value of 0.70. Uni-variate Cox regression revealed chi-square values were significant for both resting PETCO2 (4.2, P = .04) and the VE/VCO2 ratio (4.2, P = .04) and constant-rate low-intensity PETCO2 (22.0, P < .001) and the VE/VCO2 ratio (7.3, P = .007). Given collinearity issues between resting and constant-rate low-intensity CPX values and differences in prognostic strength, only measures obtained during exercise were employed in the multivariate Cox regression. Moreover, given constant-rate low-intensity PETCO2 was a superior univariate prognostic marker; the VE/VCO2 ratio was also excluded from the multivariate Cox regression. Table 4 additionally lists the correlations among resting, constant-rate low-intensity and peak CPX variables of which there are numerous statistically significant relationships.
Table 3.
Univariate Cox Regression and Receiver Operating Characteristic Curve Analysis Results for Low-Intensity Constant-Rate Cardiopulmonary Exercise Testing Variables
| Area Under ROC Curve (95% CI) |
Optimal Threshold |
Sensitivity/ Specificity |
Chi- Square |
Hazard Ratio (95% CI) |
P Value |
|
|---|---|---|---|---|---|---|
| Constant-rate PETCO2 (mm Hg) |
0.70 (0.60–0.81) | </≥34 | 79/61 | 22.0 | 4.1 (2.2–8.0) | <.001 |
| Δ PETCO2 (mm Hg) | 0.67 (0.57–0.78) | ≤/>1 | 68/67 | 15.0 | 3.5 (1.8–6.8) | <.001 |
| Constant-rate VE/VCO2 ratio |
0.63 (0.57–0.74) | </≥35 | 65/61 | 7.3 | 2.4 (1.2–2.5) | .009 |
| Δ VE/VCO2 ratio | 0.60 (0.49–0.71) | </≥5 | 67/54 | 5.6 | 2.1 (1.2–4.0) | .02 |
PETCO2, partial pressure of end-tidal carbon dioxide; VE, ventilatory efficiency; VCO2, carbon dioxide production; ROC, receiver operating characteristic.
Table 4.
Pearson Product Moment Correlation for Cardiopulmonary Exercise Testing Variables
| Resting PETCO2 |
Resting VE/VCO2 ratio |
Submaximal PETCO2 |
Δ PETCO2 | Submaximal VE/VCO2 ratio |
Δ VE/VCO2 ratio |
Peak PETCO2 | Peak VE/VCO2 ratio |
Peak VO2 | |
|---|---|---|---|---|---|---|---|---|---|
| Resting PETCO2 | — | −0.67 P < .001 | 0.71 P < .001 | 0.28 P =.001 | −0.50 P < .001 | 0.22 P =.01 | 0.58 P < .001 | −0.54 P < .001 | 0.03 P =.72 |
| Resting VE/VCO2 ratio |
— | — | −0.66 P < .001 | −0.07 P =.42 | 0.72 P < .001 | −0.36 P < .001 | −0.58 P < .001 | 0.65 P < .001 | −0.15 P =.09 |
| Constant-rate PETCO2 | — | — | — | 0.51 P < .001 | −0.79 P < .001 | −0.19 P =.02 | 0.82 P < .001 | −0.80 P < .001 | 0.41 P =.09 |
| Δ PETCO2 | — | — | — | — | −0.46 P < .001 | −0.52 P < .001 | 0.40 P < .001 | −0.42 P < .001 | 0.51 P < .001 |
| Constant-Rate VE/VCO2 ratio |
— | — | — | — | — | 0.39 P < .001 | −0.65 P < .001 | 0.78 P < .001 | −0.35 P < .001 |
| Δ VE/VCO2 ratio | — | — | — | — | — | — | −0.10 P =.27 | 0.18 P =.04 | −0.27 P =.002 |
| Peak PETCO2 | — | — | — | — | — | — | — | −0.91 P < .001 | 0.45 P < .001 |
| Peak VE/VCO2 ratio | — | — | — | — | — | — | — | — | −0.46 P < .001 |
PETCO2, partial pressure of end-tidal carbon dioxide; VE, ventilatory efficiency; VCO2, carbon dioxide production; VO2, peak oxygen consumption; RER, respiratory exchange ratio; RPE, rating of perceived exertion.
Multivariate Cox regression analysis revealed constant-rate low-intensity PETCO2 was the strongest prognostic marker (chi-square: 22.0, P < .001), whereas Δ PETCO2 added predictive value (residual chi-square: 5.2, P = .02) and was thus retained. The change in the VE/VCO2 ratio from rest to exercise, however, did not add predictive value (residual chi-square: 0.53, P = .47) and was removed from the multivariate regression. A subsequent multivariate Cox regression analysis was performed including BNP, age, LVEF, New York Heart Association Class, and constant-rate low-intensity PET-CO2 and Δ PETCO2. B-type natriuretic peptide was the strongest prognostic marker (chi-square: 28.8, P < .001), whereas constant-rate PETCO2 added prognostic value and was retained in the regression (residual chi-square: 11.1, P = .001). All other variables included in the analysis did not add prognostic value and were thus removed (residual chi-square: ≥3.5, P ≥ .06).
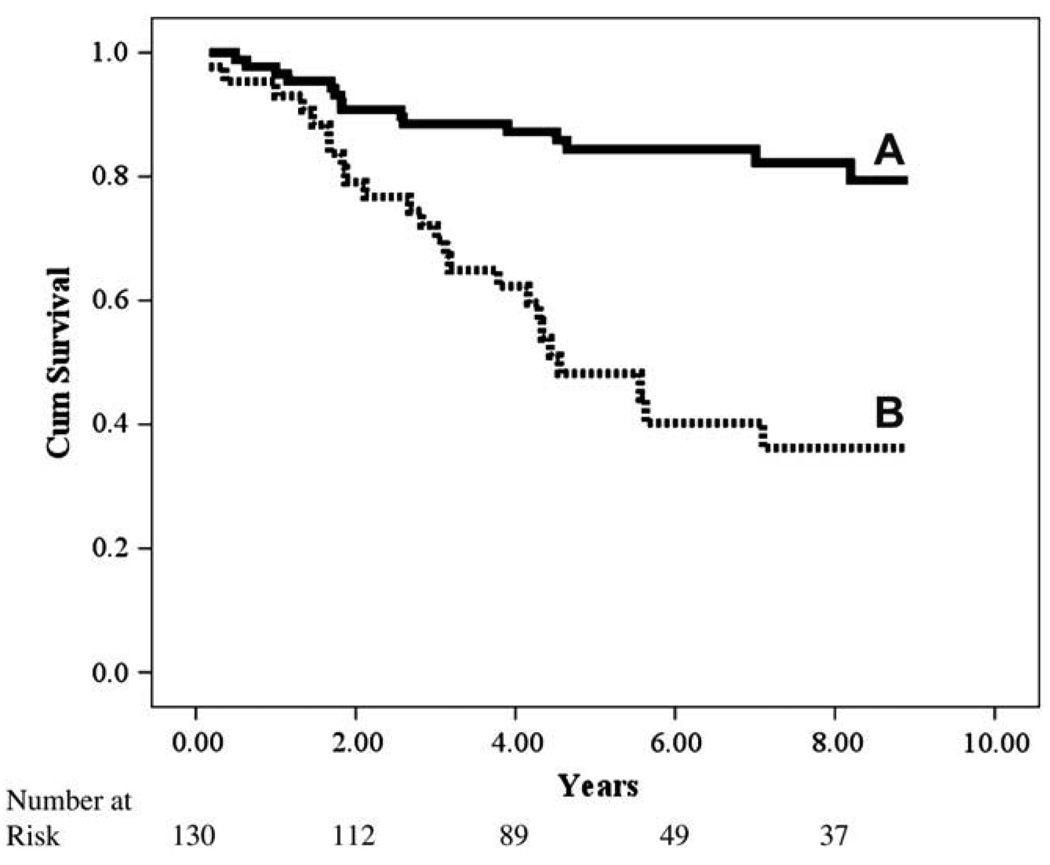
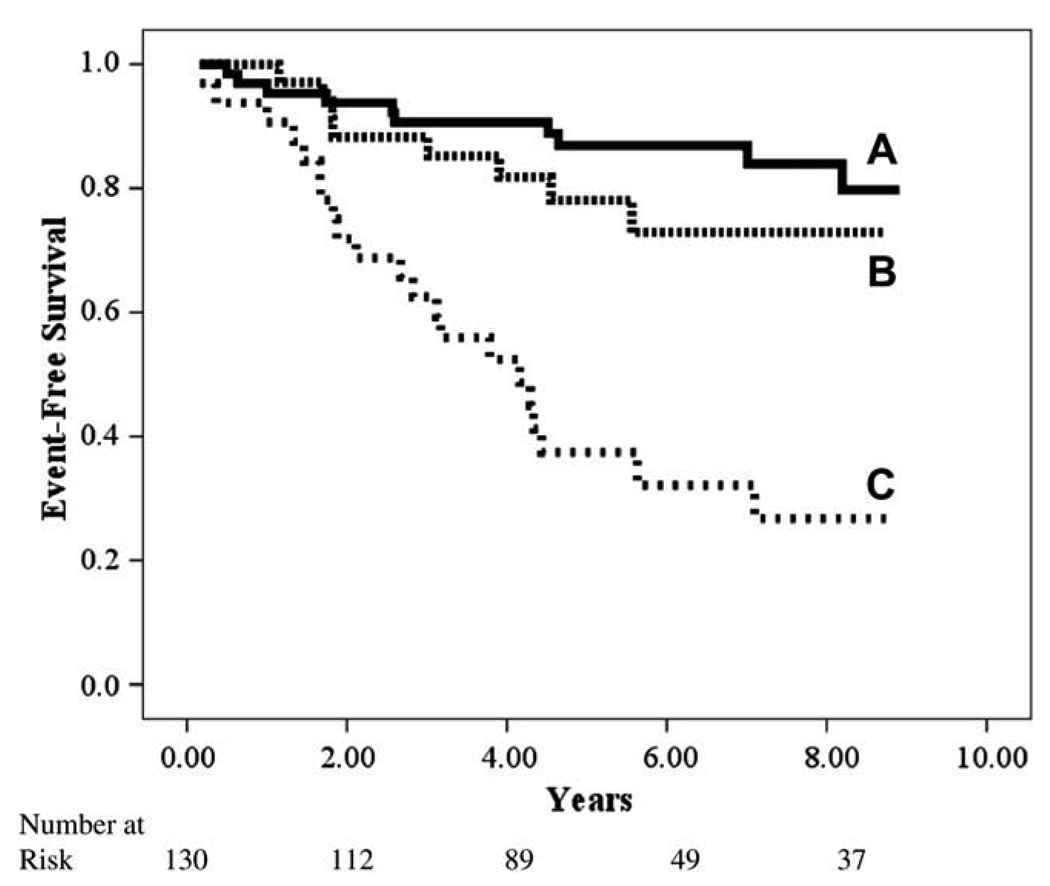
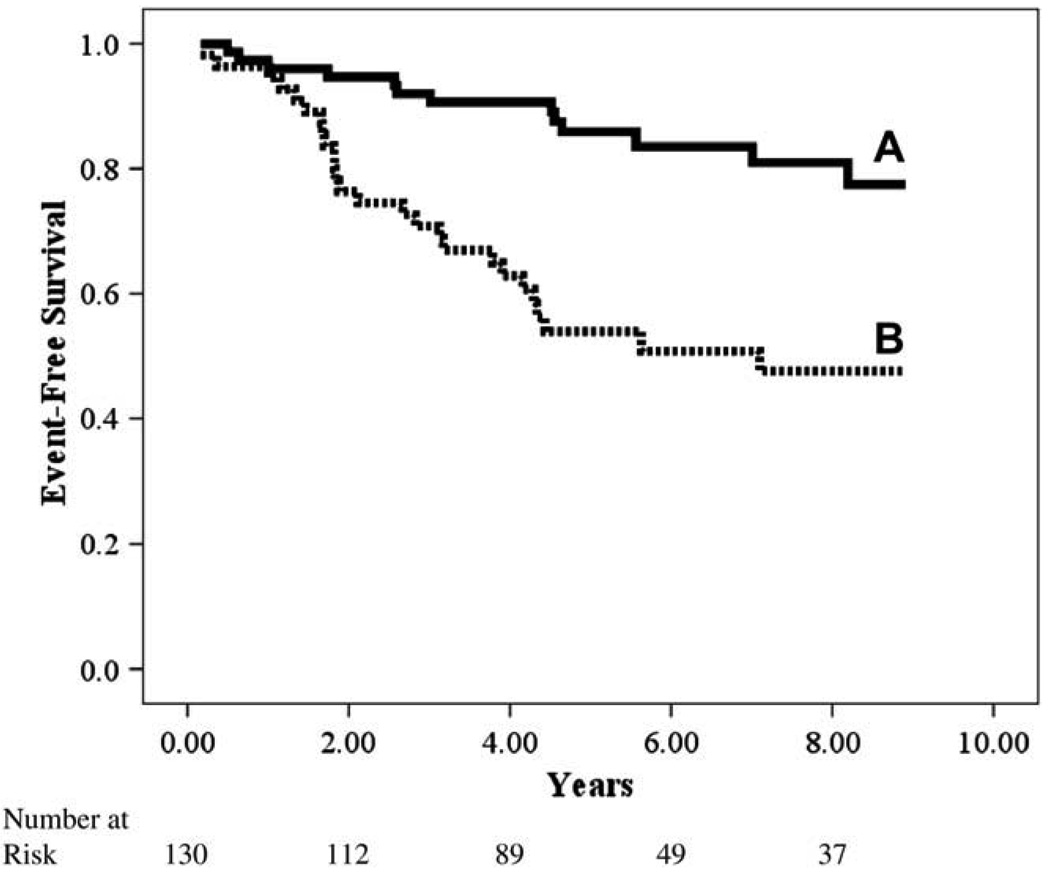
Kaplan-Meier analyses for both CPX variables retained in the initial multivariate regression are illustrated independently and in combination in Figures 1–3. Although both constant-rate low-intensity PETCO2 and Δ PETCO2 independently identified those subjects at greater risk for adverse events, analysis of these variables in combination, which were both retained in the multivariate Cox regression, improved the classification of individuals more likely to have a negative outcome.
Fig. 1.
Kaplan-Meier analysis for constant-rate partial pressure of end-tidal carbon dioxide (PETCO2).
Fig. 3.
Kaplan-Meier analysis for combination of constant-rate partial pressure of end-tidal carbon dioxide (PETCO2) and change in PETCO2 from rest to exercise.
Differences in BNP and ANP were also assessed according to the optimal prognostic threshold values for constant-rate low-intensity PETCO2 and Δ PETCO2. Subjects with a PETCO2≥34 mm Hg had a significantly lower BNP (214.1 ± 431.9 vs. 1110.5 ± 1854.0 pg/mL, P =.005) and ANP (108.2 ± 103.6 vs. 246.2 ± 200.4 pg/mL, P < .001). This trend was consistent in subjects with a ΔPETCO2≥1 mm Hg for both BNP (192.8 ± 282.1 vs. 1149.8 ± 1893.8 pg/mL, P = .003) and ANP (101.5 ± 93.4 vs. 264.3 ± 200.3 pg/mL, P < .001). When expressed as continuous variables, constant-rate low-intensity PETCO2 and ΔPETCO2 were both significantly correlated with BNP (r =−0.43 and −0.34, P < .001) and ANP (r =−0.46 and −0.45, P < .001). Moreover, peak PETCO2, VO2 and the VE/VCO2 ratio were likewise correlated with BNP (r =−0.36, −0.31, and 0.43, P < .001) and ANP (r =−0.40, −0.43, and 0.43, P < .001).
Discussion
The present pilot study demonstrates the VE/VCO2 ratio and PETCO2 obtained during constant-rate low-intensity exercise will: already demonstrate abnormalities compared to healthy control subjects, be related to natriuretic peptide levels, and provide prognostic value, thus allowing for acceptance of all 3 alternate hypotheses tested in this study.
The clinical utility of symptom-limited maximal exercise testing in patients with HF is firmly established and well supported by a robust body of original research and subsequent scientific statements.1,2,17–19 Exercising a patient to maximal exertion allows for the assessment of a number of variables with substantial prognostic value, such as peak VO220 and heart rate recovery.21 Moreover, other variables that could be obtained via submaximal testing, such as the VE/VCO2 ratio or slope, appear to provide superior prognostic information when calculated with exercise data collected to the point of maximal exertion.22–24 Given the value of peak exercise variables obtained during symptom-limited CPX, which is further supported by the findings of the present study, the use of this assessment will certainly continue in the HF population. The logistics of performing a symptom-limited maximal exercise test, including laboratory staffing, consideration of direct physician supervision and test duration, in addition to the increased level of patient discomfort, does not lend to conducting this procedure in a serial fashion over short time intervals (ie, several weeks or months). The results of the present investigation indicate that a constant-rate low-intensity exercise session, which can conceivably be performed with lower staffing requirements in a shorter period with minimal patient discomfort, provides prognostic information and reflects varying degrees of cardiac stability. Although we are not proposing such a protocol be used to take the place of symptom-limited maximal exercise testing, particularly given the stronger prognostic value of CPX variables attained at peak exercise, a low-intensity approach does lend itself to tracking a patient serially, during, for example, routine clinic visits.
Although both the VE/VCO2 ratio and PETCO2 were significant univariate prognostic markers, the latter CPX variable appears to provide superior prognostic information during low-intensity exercise. Moreover, constant-rate low-intensity PETCO2 added prognostic value to BNP and outperformed other established prognostic markers such as age, New York Heart Association Class and LVEF. There has been one previous investigation demonstrating the prognostic significance of PETCO2 at ventilatory threshold and Δ PETCO2 from rest to ventilatory threshold.9 In this previous investigation, optimal threshold values for PETCO2 and Δ PETCO2 were 36 and 1.8 mm Hg, respectively. The lower prognostic threshold values for PETCO2 in the present study compared with the previous analysis can be expected given this CPX variable progressively increases to the point of ventilatory threshold. Analysis of the PETCO2 response for prognostic and or diagnostic purposes therefore depends on the exercise intensity used. Specifically, optimal PETCO2 threshold values during a constant-rate low-intensity exercise protocol are likely to be lower than progressive exercise tests to the point of ventilatory threshold or higher. Future investigations are required to verify this hypothesis and more firmly establish optimal clinical threshold values for PETCO2.
Several previous investigations have demonstrated a significant correlation between BNP and both peak VO2 and the VE/VCO2 ratio and slope, which is also demonstrated in the present study.25,26 To our knowledge, the present investigation is the first to demonstrate a relationship between BNP, as well as ANP and PETCO2. Furthermore, BNP and ANP were significantly different according to the optimal prognostic threshold values for constant-rate low-intensity PETCO2 and Δ PETCO2. Our results add to previous investigations27,28 that found constant-rate PETCO2 is able to noninvasively reflect varying degrees of cardiac function/stability. That the results of the present investigation were obtained during a constant-rate low-intensity exercise bout adds to the novelty of these findings.
Similar to symptom-limited maximal exercise testing, there is no universally accepted protocol for constant-rate low-intensity exercise assessments. Our preliminary findings indicate that a workload of 2 miles per hour at no grade, which was a function of the exercise testing protocol employed in this cohort, was sufficient to elicit ventilatory expired gas abnormalities compared with healthy controls, although VO2 was comparable between groups. The RER and RPE were significantly higher in patients with HF compared with control subjects, indicating the physiologic and perceived level of exertion was greater in the former group. In the HF group, mean RER was, however, substantially lower than 1.0, and only 6 subjects surpassed this threshold, supporting the assertion that the predefined workload (2 miles per hour/no grade) produced a low-intensity response in the majority of subjects. However, that a small number of subjects were more challenged from an effort standpoint at this low exercise intensity does indicate flexibility may be needed in defining the workload for this type of assessment. Moreover, the possibility that constant-rate low-intensity protocols that are longer in duration provide more valuable information cannot be discounted. Future research should be directed toward comparing the ventilatory expired gas response and diagnostic/prognostic characteristics of different constant-rate low-intensity protocols on both a treadmill and lower extremity ergometer. Such work is necessary to develop universally accepted protocols, allowing for translation of findings across different clinical and research centers.
The small sample size is a significant limitation of the present investigation, particularly for a prognostic analysis. It would therefore be appropriate to view the findings presented here as novel, but preliminary. Additional investigations utilizing similar constant-rate low-intensity exercise protocols are required to support the results of reported in this pilot study. Moreover, constant-rate low-intensity exercise assessments, which potentially provide relevant physiologic and prognostic information, will not be able to provide an accurate reflection of maximal functional capacity, a highly valuable clinical metric. Therefore, as previously mentioned, this inherent limitation of the submaximal exercise assessment obviates its ability to replace the symptom-limited maximal exercise test. More appropriately, submaximal and maximal exercise assessments should be viewed as complimentary evaluation techniques.
In conclusion, symptom-limited maximal exercise testing, using protocols that progress a patient to maximal exertion, are commonly employed during the clinical assessment of patients with HF. The results of the present study indicate ventilatory expired gas analysis data obtained during constant-rate low-intensity exercise may also provide valuable information in this patient population. Future investigations should continue to explore the potential clinical utility of constant-rate low-intensity CPX in patients with HF.
Fig. 2.
Kaplan-Meier analysis for change in partial pressure of end-tidal carbon dioxide (PETCO2) from rest to constant-rate exercise.
References
- 1.Gibbons RJ, Balady GJ, Timothy BJ, Chaitman BR, Fletcher GF, Froelicher VF, et al. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) J Am Coll Cardiol. 2002;40:1531–1540. doi: 10.1016/s0735-1097(02)02164-2. [DOI] [PubMed] [Google Scholar]
- 2.Arena R, Myers J, Williams MA, Gulati M, Kligfield P, Balady GJ, et al. Assessment of functional capacity in clinical and research settings: a scientific statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology and the Council on Cardiovascular Nursing. Circulation. 2007;116:329–343. doi: 10.1161/CIRCULATIONAHA.106.184461. [DOI] [PubMed] [Google Scholar]
- 3.Arena R, Myers J, Guazzi M. The clinical and research applications of aerobic capacity and ventilatory efficiency in heart failure: an evidence-based review. Heart Fail Rev. 2008;13:245–269. doi: 10.1007/s10741-007-9067-5. [DOI] [PubMed] [Google Scholar]
- 4.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–786. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed] [Google Scholar]
- 5.Arena R, Myers J, Abella J, Peberdy MA, Bensimhon D, Chase P, et al. Development of a Ventilatory Classification System in Patients With Heart Failure. Circulation. 2007;115:2410–2417. doi: 10.1161/CIRCULATIONAHA.107.686576. [DOI] [PubMed] [Google Scholar]
- 6.Francis DP, Shamim W, Davies LC, Piepoli MF, Ponikowski P, Anker SD, et al. Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO(2)slope and peak VO(2) Eur Heart J. 2000;21:154–161. doi: 10.1053/euhj.1999.1863. [DOI] [PubMed] [Google Scholar]
- 7.Gitt AK, Wasserman K, Kilkowski C, Kleemann T, Kilkowski A, Bangert M, et al. Exercise anaerobic threshold and ventilatory efficiency identify heart failure patients for high risk of early death. Circulation. 2002;106:3079–3084. doi: 10.1161/01.cir.0000041428.99427.06. [DOI] [PubMed] [Google Scholar]
- 8.MacGowan GA, Janosko K, Cecchetti A, Murali S. Exercise-related ventilatory abnormalities and survival in congestive heart failure. Am J Cardiol. 1997;79:1264–1266. doi: 10.1016/s0002-9149(97)00097-0. [DOI] [PubMed] [Google Scholar]
- 9.Arena R, Guazzi M, Myers J. Prognostic value of end-tidal carbon dioxide during exercise testing in heart failure. Int J Cardiol. 2007;117:103–108. doi: 10.1016/j.ijcard.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 10.Koike A, Itoh H, Kato M, Sawada H, Aizawa T, Fu LT, et al. Prognostic power of ventilatory responses during submaximal exercise in patients with chronic heart disease. Chest. 2002;121:1581–1588. doi: 10.1378/chest.121.5.1581. [DOI] [PubMed] [Google Scholar]
- 11.Corra U, Giordano A, Bosimini E, Mezzani A, Piepoli M, Coats AJ, et al. Oscillatory ventilation during exercise in patients with chronic heart failure: clinical correlates and prognostic implications. Chest. 2002;121:1572–1580. doi: 10.1378/chest.121.5.1572. [DOI] [PubMed] [Google Scholar]
- 12.Koike A, Koyama Y, Itoh H, Adachi H, Marumo F, Hiroe M. Prognostic significance of cardiopulmonary exercise testing for 10-year survival in patients with mild to moderate heart failure. Jpn Circ J. 2000;64:915–920. doi: 10.1253/jcj.64.915. [DOI] [PubMed] [Google Scholar]
- 13.Brunner-La Rocca HP, Weilenmann D, Schalcher C, Schlumpf M, Follath F, Candinas R, et al. Prognostic significance of oxygen uptake kinetics during low level exercise in patients with heart failure. Am J Cardiol. 1999;84:741–744. doi: 10.1016/s0002-9149(99)00426-9. A9. [DOI] [PubMed] [Google Scholar]
- 14.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–881. [PubMed] [Google Scholar]
- 15.Yasue H, Yoshimura M, Sumida H, Kikuta K, Kugiyama K, Jougasaki M, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90:195–203. doi: 10.1161/01.cir.90.1.195. [DOI] [PubMed] [Google Scholar]
- 16.Burnett JC, Jr, Kao PC, Hu DC, Heser DW, Heublein D, Granger JP, et al. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science. 1986;231:1145–1147. doi: 10.1126/science.2935937. [DOI] [PubMed] [Google Scholar]
- 17.Piepoli MF, Corra U, Agostoni PG, Belardinelli R, Cohen-Solal A, Hambrecht R, et al. Statement on cardiopulmonary exercise testing in chronic heart failure due to left ventricular dysfunction: recommendations for performance and interpretation Part II: how to perform cardiopulmonary exercise testing in chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2006;13:300–311. doi: 10.1097/00149831-200606000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Piepoli MF, Corra U, Agostoni PG, Belardinelli R, Cohen-Solal A, Hambrecht R, et al. Statement on cardiopulmonary exercise testing in chronic heart failure due to left ventricular dysfunction: recommendations for performance and interpretation. Part I: definition of cardiopulmonary exercise testing parameters for appropriate use in chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2006;13:150–164. doi: 10.1097/01.hjr.0000209812.05573.04. [DOI] [PubMed] [Google Scholar]
- 19.Piepoli MF, Corra U, Agostoni PG, Belardinelli R, Cohen-Solal A, Hambrecht R, et al. Statement on cardiopulmonary exercise testing in chronic heart failure due to left ventricular dysfunction: recommendations for performance and interpretation Part III: interpretation of cardiopulmonary exercise testing in chronic heart failure and future applications. Eur J Cardiovasc Prev Rehabil. 2006;13:485–494. doi: 10.1097/00149831-200602000-00003. [DOI] [PubMed] [Google Scholar]
- 20.O’Neill JO, Young JB, Pothier CE, Lauer MS. Peak oxygen consumption as a predictor of death in patients with heart failure receiving β-blockers. Circulation. 2005;111:2313–2318. doi: 10.1161/01.CIR.0000164270.72123.18. [DOI] [PubMed] [Google Scholar]
- 21.Lipinski MJ, Vetrovec GW, Gorelik D, Froelicher VF. The importance of heart rate recovery in patients with heart failure or left ventricular systolic dysfunction. J Card Failure. 2005;11:624–630. doi: 10.1016/j.cardfail.2005.06.429. [DOI] [PubMed] [Google Scholar]
- 22.Arena R, Myers J, Aslam S, Varughese EB, Peberdy MA. Technical considerations related to the minute ventialtion/carbon dioxide output slope in patients with heart failure. Chest. 2003;124:720–727. doi: 10.1378/chest.124.2.720. [DOI] [PubMed] [Google Scholar]
- 23.Bard RL, Gillespie BW, Clarke NS, Egan TG, Nicklas JM. Determining the best ventilatory efficiency measure to predict mortality in patients with heart failure. J Heart Lung Transplant. 2006;25:589–595. doi: 10.1016/j.healun.2005.11.448. [DOI] [PubMed] [Google Scholar]
- 24.Tabet JY, Beauvais F, Thabut G, Tartiere JM, Logeart D, Cohen-Solal A. A critical appraisal of the prognostic value of the VE/VCO2 slope in chronic heart failure. J Cardiovasc Risk. 2003;10:267–272. doi: 10.1097/00149831-200308000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Kruger S, Graf Ju, Kunz D, Stickel T, Hanrath P, Janssens U. brain natriuretic peptide levels predict functional capacity in patients with chronic heart failure. J Am Coll Cardiol. 2002;40:718–722. doi: 10.1016/s0735-1097(02)02032-6. [DOI] [PubMed] [Google Scholar]
- 26.Scardovi AB, De MR, Coletta C, Aspromonte N, Perna S, Infusino T, et al. Brain natriuretic peptide is a reliable indicator of ventilatory abnormalities during cardiopulmonary exercise test in heart failure patients. Med Sci Monit. 2006;12:CR191–CR195. [PubMed] [Google Scholar]
- 27.Tanabe Y, Hosaka Y, Ito M, Ito E, Suzuki K. Significance of end-tidal P(CO(2)) response to exercise and its relation to functional capacity in patients with chronic heart failure. Chest. 2001;119:811–817. doi: 10.1378/chest.119.3.811. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto A, Itoh H, Eto Y, Kobayashi T, Kato M, Omata M, et al. End-tidal CO2 pressure decreases during exercise in cardiac patients: association with severity of heart failure and cardiac output reserve. J Am Coll Cardiol. 2000;36:242–249. doi: 10.1016/s0735-1097(00)00702-6. [DOI] [PubMed] [Google Scholar]