Abstract
The unique properties of cancer- and metastasis-initiating cells endowed with a high self-renewal and aberrant differentiation potential (including their elevated expression levels of anti-apoptotic factors, multidrug transporters, and DNA repair and detoxifying enzymes) might be associated with their resistance to current clinical cancer therapies and disease recurrence. The eradication of cancer- and metastasis-initiating cells by molecular targeting of distinct deregulated signaling elements that might contribute to their sustained growth, survival, and treatment resistance, therefore, is of immense therapeutic interest. These novel targeted approaches should improve the efficacy of current therapeutic treatments against highly aggressive, metastatic, recurrent, and lethal cancers.
Introduction
Major progress toward the identification of new therapeutic targets in cancer cells in recent years has led to the discovery and development of new classes of anti-cancer drugs [1-7]. These anti-cancer drugs hold great promise for improving the efficacy of current clinical treatments by surgical resection, anti-hormonal therapy, radiation therapy, and/or chemotherapy. In particular, the progression of organ-confined cancers to locally invasive or metastatic disease stages is generally associated with resistance to current conventional treatments, disease relapse, and the death of cancer patients within a short period [2-9]. Thus, the molecular targeting of distinct oncogenic products in the cancer cells involved in primary cancer progression and metastases at distant tissues and organs might constitute more promising therapeutic approaches than monotherapies for developing new combination therapies against aggressive and recurrent cancers [2-8].
Importantly, a growing body of experimental evidence has revealed that the accumulation of genetic and/or epigenetic alterations in tissue-resident adult stem/progenitor cells or their early progenies can result in their malignant transformation into cancer stem/progenitor cells, also known as ‘cancer-initiating cells’ or ‘tumor-initiating cells’ [10-20] (Figure 1). Based on the cancer stem/progenitor cell concept of carcinogenesis, it is suggested that highly leukemic or tumorigenic cancer-initiating cells can provide crucial functions for primary cancer initiation and progression by giving rise to the bulk mass of differentiated tumor cells. In support of this hypothesis, it has been shown that the induction of genetic and/or epigenetic alterations in adult stem cells and/or their more committed progenitors can culminate in their malignant transformation and leukemia or tumor development in animal models [14,15,17,19-30]. These genetic aberrations include specific mutations in distinct tumor suppressor genes (p53, p16INK4A, retinoblastoma and/or phosphatase, and tensin homolog deleted on chromosome 10, or PTEN), activating mutations in oncogenic products such as RAS, chromosomal rearrangements generating oncogenic fusion proteins, and/or the persistent activation of diverse developmental signaling cascades such as hedgehog, epidermal growth factor receptor (EGFR), HER2, Wnt/β-catenin, and/ or Notch [14,15,17,19-30]. In particular, cumulative telomere shortening and mutations, sustained oxidative stress, chronic inflammation, and/or fibrosis occurring over time during chronological aging might culminate in a genomic instability and the malignant transformation of these immature cells [12,15,19,20,25-28].
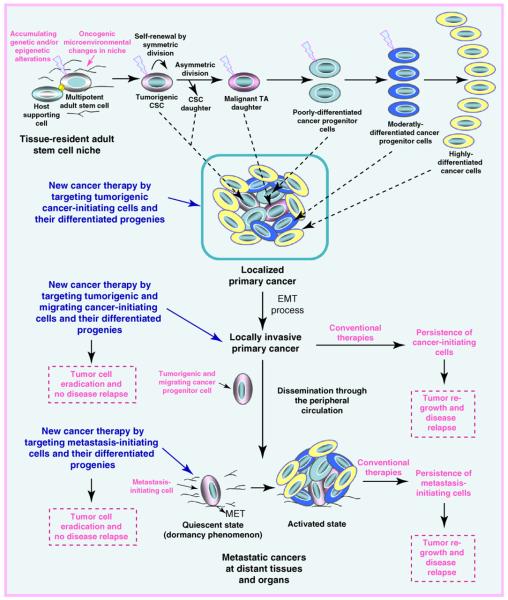
FIGURE 1.
Proposed model of cancer initiation, progression, and metastasis and treatment resistance mediated through cancer- and metastasis-initiating cells. This scheme shows the malignant transformation of adult stem/progenitor cells into tumorigenic cancer stem/progenitor cells, also known as ‘cancer-initiating cells’, which may be induced through genetic and/or epigenetic alterations in these immature cells and changes in their local microenvironments, including the activated stromal cells. The symmetric or asymmetric division of cancer stem cells (CSCs) into transit-amplifying (TA) also designated as intermediate cells that, in turn, may generate the bulk mass of poorly, moderately, and highly differentiated cancer cells is also illustrated. Moreover, the acquisition of a migratory phenotype by tumorigenic cancer-initiating cells, which may be induced by the sustained activation of distinct oncogenic signaling pathways during the epithelial–mesenchymal transition (EMT) program, is shown. The possible invasion of certain tumorigenic and migrating cancer-initiating cells in the activated stroma, which might lead to their dissemination through the peripheral circulation at distant tissues and organs, is also illustrated. Moreover, the possible loss of the migratory phenotype of metastasis-initiating cells via the occurrence of mesenchymal–epithelial transition (MET) at secondary tumor sites is indicated. The dormancy phenomenon of metastasis-initiating cells and their possible reactivation associated with the formation of secondary tumor formation under specific microenvironmental conditions at distant sites is also illustrated. New cancer therapies, which use molecular targeting of cancer- and metastasis-initiating cells to counteract cancer progression and metastases at distant tissues and organs, are also indicated.
Importantly, subpopulations of immature cancer cells with stem cell-like properties have been isolated from primary malignant tissues of cancer patients and established cancer cell lines [14-17,31-47]. Among the cancer types harboring a subpopulation of cancer-initiating cells, there are leukemias, lymphomas, sarcomas, melanoma, brain tumors, and a variety of epithelial cancers including skin, head and neck, thyroid, lung, cervical, renal, hepatic, esophageal, gastrointestinal, colon, bladder, pancreatic, prostate, mammary, and ovarian cancers [14-17,31-45,48-55]. The cancer-initiating cells typically expressed several stem cell-like markers including telomerase, aldehyde dehydrogenase (ALDH), CD133, CD44, CXC chemokine receptor 4 (CXCR4), KIT, ATP-binding cassette (ABC) multidrug transporters, and/or transcription factors such as OCT-3/4, Nanog, and SOX2 [14-17,31,33-36,38-43,50,52-55]. The highly leukemic or tumorigenic cancer stem/progenitor cells were able to give rise in vitro and in vivo to the total mass of differentiated cancer cells that recapitulated the complex morphological characteristics and heterogeneous phenotype of the original patient's tumors [14,16,31,33-37,39-43,52-55] (Figure 1).
In addition, the acquisition of a migratory phenotype by tumorigenic cancer stem/progenitor cells during the epithelial–mesenchymal transition (EMT) process concomitant with the changes in the activated stroma might also lead to their invasion from primary neoplasms, dissemination through the peripheral circulation, and the formation of aggressive and metastatic cancers at distant tissues and organs [10,11,15,19,20,35,36,43,47,53,56-65] (Figure 1). Consistently, tumorigenic and migrating cancer stem/progenitor cells with stem cell-like properties have been detected at invasion sites in primary tumors, as well as isolated from peripheral blood [15,35,36,39,43,66,67]. In addition, immature cancer cells with stem cell-like properties, also known as ‘metastasis-initiating cells’, have been detected and isolated from secondary tumor samples from cancer patients and metastatic cancer cell lines [10,11,15,35,36,39,47,49,65]. Although the cancer- and metastasis-initiating cells generally represent minorities within the tumor cell population (Figure 1), it is important to note that their numbers and phenotypic and functional features can vary with cancer subtype, during cancer progression, after treatment initiation, and after disease relapse [10,11,36,38,46,53,68-80]. Furthermore, the number of cancer stem/progenitor cells endowed with stem cell-like properties may depend on the methods used for their isolation and in vitro and in vivo characterization [46,60,68,73,74,81,82]. In particular, a greater proportion of cancer-initiating cells might be detected in total cancer cell mass after the induction of an EMT program, as well as after cancer therapy initiation, which may lead to an elimination of the bulk cancer cell mass concomitant with an enrichment of cancer cells with stem cell-like properties that are resistant to conventional treatments [10,11,36,38,46,60,68-72,81]. In addition, the metastasis-initiating cells detected in metastatic cancers may undergo a mesenchymal–epithelial transition and acquire specific phenotypic and functional features at their novel microenvironment prevalent at certain metastatic sites [10,11,61]. Hence, the metastasis-initiating cells might show different phenotypic and functional features and respond differently to current therapeutic treatments as compared with their malignant counterpart detected at primary cancers [10,11,61].
Of therapeutic interest, it has also been shown that the intrinsic and/or acquired resistance of cancer- and metastasis-initiating cells to current clinical therapies could result in their persistence at primary and/or secondary neoplasms after treatment initiation and, thereby, be responsible for tumor regrowth and disease relapse [4,15-17,31,36,38,39,42-46,50,52,54,55,69,75,76,83-91] (Figure 1). It seems, therefore, that the molecular targeting of these immature cancer- and metastasis-initiating cells must also be considered for overcoming treatment resistance. In this regard, we summarize the recent investigations undertaken to identify new molecular targets in cancer- and metastasis-initiating cells. The information provided should help in the design of new therapeutic strategies for eradicating the total tumor cell mass, including cancer- and metastasis-initiating cells and their differentiated progenies, and thereby improve current clinical therapies against aggressive, metastatic, recurrent, and lethal cancers.
Intrinsic and acquired phenotypes of cancer- and metastasis-initiating cells associated with their resistance to current cancer treatments
Numerous lines of experimental evidence have indicated that tumorigenic cancer- and metastasis-initiating cells might be intrinsically resistant to certain chemotherapeutic drugs or radiation at the start of treatment and/or acquire an enhanced multidrug resistance (MDR) phenotype with cancer development or after treatment initiation [4,15-17,31,36,38-40,42-46,50,52,54,75,76,84-90,92,93]. Several intrinsic properties of cancer- and metastasis-initiating cells common with their normal counterpart, tissue-resident adult stem/progenitor cells, might contribute to their intrinsic resistance to current clinical therapeutic treatments and disease recurrence. In particular, immature cancer- and metastasis-initiating cells, such as adult stem/progenitor cells, can exist in a quiescent and less metabolically active state (Figure 1). Thus, they can display a slow division and be more resistant than their differentiated progenies to radiation or cytotoxic drugs targeting the proliferative cancer cells [4,10,11,15,59,88]. The adoption of a quiescent state by cancer- and metastasis-initiating cells might explain, at least in part, the long-term dormancy phenomenon associated with their persistence at primary neoplasms and micrometastases for a long period without clinical or histopathologic signs of apparent metastases [4,10,11,15,59] (Figure 1). The changes in the microenvironment of cancer- and metastasis-initiating cells leading to the reactivation of mitogenic signaling pathways, however, could trigger their proliferation and tumor regrowth and culminate in disease recurrence [4,10,11,15,59] (Figure 1).
In addition, the high expression levels of multidrug transporters, DNA mismatch repair, and detoxifying enzymes in cancer-and metastasis-initiating cells might be responsible, in part, for their resistance to ionizing radiation and certain chemotherapeutic drugs. More specifically, the ABC transporters localized at the plasmic or endolysosomal membrane might protect these immature cancer cells from the cytotoxic effects induced by diverse structurally and functionally non-related chemotherapeutic drugs and, in this manner, contribute to their MDR phenotype [4,38,39,44,45,55,86,88,92,94,95] (Figure 2). In fact, the ABC efflux pumps can actively remove intracellular cytotoxic agents out from cells at the expense of ATP hydrolysis, and thereby reduce intracellular drug accumulation (Figure 2). In regard to this, the high expression of ABC transporters in cancer stem/progenitor cells is notably the basis of the Hoechst dye exclusion method, which is useful to isolate a small cell fraction designated as ‘side population’ (SP), displaying a high capacity to efflux Hoechst 33342 dye from the bulk cancer cell mass [15,44,45,52,55,72,86,88,92,96-105]. It has been observed that a small subset of SP cells isolated from patients' tumors or different cancer cell lines, which displayed stem cell-like properties and expressed high levels of distinct ABC drug efflux pumps and anti-apoptotic factors, were more tumorigenic in animal models in vivo and resistant to chemotherapeutic drugs or irradiation therapy than non-SP cells [15,44,45,52,55,72,86,88,92,97-100,102-106]. For example, hepatoma HuH7 SP cells expressing high levels of multidrug resistance 1 (MDR1/ABCB1) encoding P-glycoprotein, breast cancer resistant protein (BCRP-1/ABCG2) and CEACAM6 displayed a higher resistance to doxorubicin, 5-fluorouracil and gemcitabine than the non-SP cell fraction [44]. A SP cell subpopulation detected in neuroblastoma cells from 15 out of 23 patients (65%), which expressed high levels of BCRP/ABCG2 and ABCA3 transporters, also showed a greater capacity to efflux cytotoxic drugs, such as mitoxantrone, than the non-SP cell fraction [45]. It is important to note that like other enrichment and isolation methods of small subpopulations of cancer stem/progenitor cells – including fluorescence-activated cell sorting using specific antibodies directed against one or several stem cell-like surface markers, and flow cytometric isolation of cells based on ALDH enzymatic activity, as assessed by Aldehfluor assay – the Hoechst dye efflux method can have certain limitations. Indeed, it has been noted that the number of cancer cells detected in the SP cell subpopulation versus the non-SP fraction can be influenced considerably by experimental conditions, including the source of patients' malignant tissues (untreated, treated, and/or relapsed patients), and the culture conditions used [45,107-109]. Moreover, it has been reported that the Hoechst dye might be cytotoxic to certain cell types and the SP cell subpopulation may be undetectable in certain non-malignant or malignant cells [106,110].
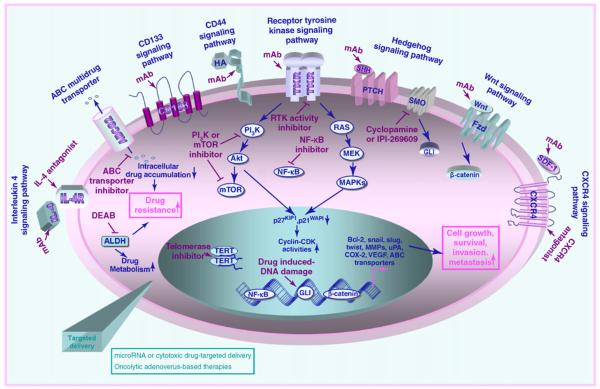
FIGURE 2.
Potential signaling transduction pathways involved in the aggressive behavior and multidrug resistance phenotype of cancer- and metastasis-initiating cells and new cancer therapies by molecular targeting distinct signaling elements. The mitotic effects induced through the activation of distinct intracellular signaling pathways initiated by different growth factors, cytokines and chemokines in cancer stem/progenitor cells are illustrated. In particular, the possible upregulation of the expression levels of numerous gene products involved in the sustained growth, survival, invasion, and metastasis of cancer stem/progenitor cells are indicated. Moreover, a decrease in intracellular drug accumulation mediated via the ATP-binding cassette (ABC) multidrug efflux pump and enhanced drug metabolism via aldehyde dehydrogenase (ALDH) is also shown. The potential inhibitory effect induced by diverse pharmacological agents such as a selective inhibitor of receptor tyrosine kinase (RTK) activity (gefitinib, erlotinib, or lapitinib), smoothened (SMO) co-receptor of hedgehog (cyclopamine or IPI-269609), NF-κB, PI3K or mTOR signaling element, telomerase, ABC transporters, and ALDH (diethylaminobenzaldehyde, DEAD) in cancer cells is indicated. In addition, the inhibitory effect induced by a monoclonal antibody (mAb) directed against interleukin-4 (IL-4) ligand, CD44 receptor, RTK, sonic hedgehog ligand (SHH), Wnt ligand, stromal cell-derived factor-1 (SDF-1), or an antagonist of IL-4R and CXCR4 chemokine receptor are indicated. Abbreviations: COX-2, cylooxygenase-2, MMPs, matrix metalloproteinases, VEGF, vascular endothelial growth factor, uPA, urokinase plaminogen activator.
In addition, a high expression level of O6-methylguanine DNA methyltransferase in cancer- and metastasis-initiating cells might protect them against the cytotoxic effects induced by methylating and chloroethylating agents [carmustine, 1,3-bis (2-chloroethyl)-1-nitrosourea, methyl methanesulfonate and temozolomide] by repairing alkyl adducts at the O6-position of guanine in DNA [38,92]. Furthermore, the cancer stem/progenitor cells found in leukemia, retinoblastoma, lung, head and neck squamous, liver, pancreatic, colorectal, breast, and prostate cancers also show high ALDH expression levels and/or enzymatic activity [10,11,16,50,78,85,111-114]. ALDHs are a group of enzymes that have a crucial role in the metabolic process and cellular detoxification by catalyzing the oxidation (dehydrogenation) of aldehydes into carboxylic acids. Hence, ALDHs may contribute to the detoxification of a variety of compounds, such as the active metabolite of an alkylating chemotherapeutic agent, cyclophosphamide and treatment resistance [50,78,111,112]. In this regard, it has been reported that certain ALDH isoenzymes (including cytosolic ALDH1 acidic protein), which are expressed in certain stem/progenitor cells and progenies, can play a crucial role in retinoic acid (RA) biosynthesis and the regulation of stem cell fate during development and adult life [115-118]. RA biosynthesis from vitamin A, also known as retinol, involves two sequential oxidation steps consisting of the oxidation of retinol to retinaldehyde followed by an irreversible conversion of retinaldehyde to RA, which might be catalyzed by NADP+-dependent ALDHs, including ALDH1 [115]. Despite the important role of ALDHs in RA biosynthesis in certain adult stem/progenitor cells, including hematopoietic stem cells and neuronal stem cells, few studies have investigated the relationship between ALDH expression levels and RA biosynthesis in cancer cells with an aberrant differentiation potential, including cancer stem/ progenitor cells during cancer development [116,117,119-126].The results from a recent study have indicated that the inhibition of ALDH enzymatic activity by using a specific inhibitory agent, diethylaminobenzaldehyde, in breast cancer cell lines promoted tumor sphere formation [120]. By contrast, the treatment of breast cancer cells with exogenous all-trans RA led to cell differentiation and inhibition of tumor sphere formation ability [120]. These observations suggest that the activation of RA signaling, which could be induced through certain ALDH isoenzymes expressed in cancer-initiating cells (including breast cancer initiating cells), might result in a reduction of this immature cell population. Conversely, the inhibition of ALDH1 in a conditioned medium from MiaPaCa-2 pancreatic cell clonal subpopulation by small interference RNA (siRNA) has been observed to inhibit their invasive ability, suggesting other potential mechanism(s) of action of ALDHs, including ALDH1, in promoting the invasion of pancreatic cancer cells [121]. Importantly, it has also been reported that defects in the RA signaling pathway, such as a methylation of the RA receptor-β2, and a feedback inhibition of ALDH expression induced at high intracellular RA concentration can occur in certain cancer cell lines (including breast cancer cells) and impede the differentiating potential of RA [117,122,124-126]. Moreover, only certain ALDH isoenzymes can catalyze the conversion of vitamin A into RA and, therefore, their expression levels in normal and cancer stem/progenitor cells and their progenies might be a determinant factor controlling their specific functions in normal and pathological conditions and the carcinogenesis process [118,123]. Future studies are essential to establish the connections between the expression levels of different ALDH isoenzymes and RA biosynthesis, and their specific function(s) in cancer-initiating cells and the differentiated progenies versus normal cells in the development of different cancer subtypes, as well as their implications in the resistance of cancer cells to current cancer therapies and disease relapse.
Other phenotypic features that might contribute to the survival and treatment resistance of these immature cells include the sustained activation of diverse growth factor signaling pathways and their high expression levels of anti-apoptotic factors such as Bcl-2, survivin, NF-κB, and EMT process-associated molecules, including snail, slug, and/or twist [4,15,17,38,45,53,58,75,76,78,90,92,94,99,127]. These signaling elements may confer survival advantages to these immature cells in stress and hypoxia conditions prevalent in primary tumors. With respect to this, we describe here new targeting approaches that have been developed to overcome treatment resistance and eradicate the total tumor cell mass, including cancer- and metastasis-initiating cells and their differentiated progenies.
Molecular targeting of cancer- and metastasis-initiating cells and their differentiated progenies
The molecular targeting of specific biomarkers and distinct deregulated signaling elements that mediate the transforming events occurring in cancer- and metastasis-initiating cells during disease progression – and, more specifically, during the EMT process and metastases at distant sites – represents new promising therapeutic strategies to improve current cancer therapies [4,15,17,38,45,50,53,58,71,75,76,78,90,92,99,127] (Figure 2). Interestingly, recent data have revealed that the stem cell-like markers (including CD133 and CD44 proteins) that are expressed by numerous cancer stem/progenitor cell types can contribute to their malignant behavior and, thereby, could constitute potential molecular therapeutic targets [35,71,128,129]. For instance, it has been observed that an anti-CD133 monoclonal antibody (mAb) directed against epitope 1 (AC133/W6B3C1) or epitope 2 (AC141) induced cytotoxic effects in FEMX-I melanoma cells in vitro and decreased their metastatic capacity in a mouse model in vivo [128]. In the same way, the use of small hyaluronan oligosaccharides that can effectively compete with natural ligand hyaluronan for binding to the CD44 receptor also inhibited the in vivo growth of spinal cord gliomas formed after engraftment of the SP cell fraction from the C6 glioma cell line in rats [129]. Moreover, it has been observed that treatment of CD90+ cells endowed with stem cell-like properties and CD90− cell fractions from the MHCC-97L liver cell line with an anti-CD44 mAb induced apoptosis in vitro, whereas only the CD90+ cell fraction from the PLC cell line was sensitive to this treatment type [35]. Importantly, anti-CD44 mAb also suppressed the local and systemic tumor formation induced by the CD90+ cell fraction from the MHCC-97L cell line subcutaneously or ortho-topically implanted in the liver of nude mice in vivo [35]. In spite of the great interest in targeting the cascades initiated through the stem cell-like markers expressed on cancer-initiating cells, the potential cytotoxic effects of these agent types on normal tissue-resident adult stem/progenitor cells must also be considered before their potential translation in clinical trials. In this regard, the targeted delivery of these pharmacological agents, as described below, could constitute an alternative therapeutic approach to preventing systemic toxicity. In addition, among other potential molecular targets often altered in cancer- and metastasis-initiating cells, there are diverse growth factor, cytokine and chemokine signaling elements that provide crucial functions in the stringent regulation of self-renewal, differentiation, and/or migration of normal tissue-resident adult stem/progenitor cells.
Targeting growth factor, cytokine and chemokine signaling pathways
Several studies have revealed that the malignant transformation of cancer- and metastasis-initiating cells is frequently associated with the sustained activation of telomerase and diverse developmental cascades initiated by distinct growth factors, cytokines or chemokines through their cognate receptors along disease progression [7,15,19,20,46,47,54,58,59,108,111,130-132]. These deregulated pathways include hedgehog, EGFR, HER2, Wnt/β-catenin, Notch, transforming growth factor-β (TGF-β)/TGFRβ, interleukin (IL)-4/IL-4Rα, IL-6/IL-6R, IL-8/IL-8 receptor CXCR1, BMI-1, stem cell factor/KIT, extracellular matrix components/integrin, and/or stromal cell-derived factor-1 (SDF-1)/CXCR4 [7,15,19,20,24,46,47,54,58,59,108,111,130,131,133,134] (Figure 2). In particular, it has been reported that the targeting telomerase and blockade of these tumorigenic pathways by using mAb, antisense oligonucleotides, siRNA, or specific inhibitory agent led to growth inhibition, apoptotic cell death, and/or a reduction of invasiveness or metastatic spread of cancer-initiating cells and their progenies in vitro or in animal models in vivo [7,15,19,20,54,58,59,70,108,111,112,130-133,135-137] (Figure 2). For instance, the use of a specific inhibitor of smoothened (SMO) co-receptor, cyclopamine or IPI-269609, as well as siRNA GLI-1 transcriptional effector of hedgehog cascade, has been observed to reduce the number of cancer-initiating cells and their progenies and inhibit tumor growth in vitro and/or in vivo [70,80,97, 111,112,130,131,135-139]. Moreover, cyclopamine treatment improved the cytotoxic and/or anti-metastatic effects induced by current chemotherapeutic drugs on gliomasphere cells and prostate and pancreatic cancer cells [70,97,111,135,138,139]. In the same way, it has been reported that a specific inhibitor of EGFR tyrosine kinase activity, gefitinib or erlotinib, induced anti-proliferative and cytotoxic effects on EGFR+/CD133+ tumor-initiating cells from five patients with gliomablastomas (GBM TICs). It has been noted that two cases of GBM TICs with high Akt activation were insensitive to both drugs or only sensitive to high concentrations of erlotinib [80]. This observation suggests that the combined use of EGFR and Akt inhibitors could be more effective in certain GBM patients than the single drugs. Of clinical interest, the treatment of 40 women with erbB-2-positive breast cancer with oral dual EGFR and the erbB-2 tyrosine kinase inhibitor lapitinib alone for six weeks followed by six weeks with the standard chemotherapy and the anti-erbB2 mAb trastuzumab also caused complete tumor regression in 63% of patients [79]. These data imply that this treatment type might be effective in reducing the number of CD44+/CD24low breast cancer-initiating cell-like cells. The molecular targeting of IL-4, whose cytokine may protect the tumorigenic CD133+ cancer stem/progenitor cells from human colon carcinoma of apoptotic death, by using IL-4Rα antagonist or anti-IL-4 neutralizing antibody, also sensitized these tumor-initiating cells to the antitumoral effects induced by standard chemotherapeutic drugs [54].
Of particular interest, the use of pharmacological agents that are able to interfere with the invasion, recruitment, homing, and/or adhesion of tumorigenic and migrating cancer stem/progenitor cells to the endothelial surface and stromal components at distant metastatic sites, including bone marrow (BM), also constitute potential cancer therapies [10,11,36,59] (Figure 1). More specifically, the blockade of chemoattractant gradient systems such as SDF-1/CXCR4 could lead to the eradication of tumorigenic and migrating cancer-initiating cells and metastasis-initiating cells and, thereby, prevent metastatic spread, secondary tumor initiation and disease relapse. Consistent with this, it has been shown that treatment of CD133+/CXCR4+ prostate cancer stem cells from a primary patient tumor with an anti-CXCR4 mAb abrogated their migration induced by the exogenous SDF-1 protein in vitro [140]. Similarly, the treatment of highly metastatic human pancreatic cancer cell line L3.6 pl harboring a CD133+/CXCR4+ cancer cell subpopulation with an anti-CXCR4 mAb also resulted in a reduction of their metastatic capacity in vivo [36]. In this regard, other attractive molecular therapeutic targets to counteract the invasion of cancer-initiating cells also comprise the gene products that are frequently induced during the EMT process and involved in their survival and treatment resistance.
Targeting of EMT process- and treatment resistance-associated molecules
The acquisition of more malignant phenotypes by cancer-initiating cells and their progenies occurring along primary cancer progression, and more particularly during the EMT process, might provide them with migratory properties essential for their invasion and metastatic spread at distant sites [10,11,15,20,36,53,56-65,76,81]. In general, the EMT program is associated with a disruption of cell–cell interactions, extensive remodeling of the extracellular matrix components, and selection of cancer cells endowed with survival advantages [11,58]. More specifically, the occurrence of the EMT process may lead to a downregulation of adhesion molecules such as E-cadherin, concomitant with the enhanced expression of diverse mesenchymal genes such as vimentin, β-catenin, snail, slug, and twist that may contribute in an important manner to their resistance to current conventional cancer therapies and disease relapse [11,58]. Consistently, some recent investigations have indicated the therapeutic benefit of targeting different signaling elements contributing to the survival of cancer-initiating cells to overcome radioresistance and intrinsic and/or acquired MDR phenotype and counteract tumor growth and metastases [4,15,39,58,76,78,86,87,90,92,127,141-143].
Among the molecular therapeutic targets altered during cancer progression are the cellular signaling effectors of the EMT program (Cripto-1, tenacin C, snail, slug, and/or twist), PI3K/Akt/mTOR, NF-κB, ABC multidrug efflux pumps, ALDHs, Bcl-2 and survivin, as well as deregulated apoptotic signaling elements such as ceramide and caspases [4,15,39,58,71,76,78,86-88,90,92,102,127,141-143] (Figure 2). For instance, a high-throughput screening strategy aiming to identify the cytotoxic agents specifically targeting epithelial cancer cells that express high levels of EMT-associated molecules has led to the discovery of a compound, salinomycin, exhibiting a selective toxicity for breast cancer-initiating cells [81]. It has been shown that salinomycin inhibited mammary tumor growth and metastatic nodule formation in vivo and reduced the number of CD44+/CD24low breast cancer cells while it induced the expansion of differentiated breast cancer epithelial cells in vivo and in vivo [81]. Conversely, the clinical chemotherapeutic drug paclitaxel, commonly used to treat breast cancer patients, displayed the cytotoxic effect only on the differentiated breast cancer epithelial cells, while the proportion of CD44+/CD24low cell subpopulation was enriched after this treatment type [81]. These data underline the importance of using the drugs or combination of cytotoxic agents targeting the highly tumorigenic cancer stem/progenitor cells and their more committed progenies to develop more effective therapeutic regimens for treating patients diagnosed with aggressive and recurrent cancers. Moreover, it has been reported that dofequidar fumarate, an orally active quinoline compound that can inhibit diverse ABC transporters and is under clinical investigation, reduced the efflux of chemotherapeutic drugs and increased the sensitivity of SP cells with stem cell-like properties isolated from various cancer cell lines to cytotoxic effects induced by anticancer drugs [102]. Hence, dofequidar fumarate might represent a potential therapeutic agent for reversing the MDR phenotype of immature cancer cells.
In addition, the sustained activation of PI3K/Akt/mTOR signaling elements induced by diverse growth factors and/or genetic inactivation of the PTEN tumor suppressor gene through either gene deletion or mutation is a frequent transforming event in a wide variety of human cancers [88,90,142,144]. Therefore, the use of a specific inhibitor of Akt (perifosine) and/or mTOR (rapamycin or HSP90) downstream signaling effectors, which provide crucial functions for sustained cell growth, survival, and migration, is of great interest in reducing tumor growth and the metastatic spread of cancer-initiating cells (Figure 2). As a matter of fact, it has been shown that the inhibition of Akt kinase activity by using perifosine reduced the number of ALDH1-expressing breast cancer-initiating cells and primary and secondary tumor growth in vivo [144]. Moreover, inhibition of the PI3K/Akt pathway sensitized highly invasive brain tumors established by orthotopic transplantation of human GBM cells from a patient to immunodeficient rats in vivo [142]. It has been reported, however, that the natural broccoli compound sulforaphane induced the apoptotic effect on pancreatic cancer-initiating cells through the inhibition of the NF-κB-induced anti-apoptotic pathway and sensitized these immature cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced cytotoxicity in vitro [143]. Sulforaphane was also effective, alone or in combination with TRAIL, in inhibiting tumor growth and angiogenesis in a pancreatic cancer xenograft model in vivo without an apparent cytotoxic effect on normal cells [143].
In view of the fact that the ABC multidrug transporters can have a major role in the MDR phenotype of cancer stem/progenitor cells, several pharmacological agents have been investigated for inhibiting their multidrug efflux pump activity [39,71,86,87,94,95, 98,108,141]. It has been observed that the treatment of the SP cell fraction from human oral squamous cell carcinoma cell line H357 with a broad-spectrum ABC transporter inhibitor, verapamil, blocked the mitoxantrone efflux-mediated via ABCG2 and multi-drug resistance associated protein-1 (MPR-1/ABCC1) pumps and restored the growth inhibitory effect induced by mitoxantrone on this cancer stem cell-like population [86]. Importantly, the molecular targeting of melanoma-initiating cells by using mAb directed against ABCB5 transporter significantly reversed the resistance of G3361 melanoma cells to doxorubicin and induced an inhibitory effect on the growth of melanoma stem cell xenograft-derived tumor in vivo [39]. In the same pathway, the inhibition of ABCG2 with 4-fumitremorgin C or PI3K/Akt/mTOR signaling pathway by using LY294002 or rapamycin in the SP cell fraction sorted from MHCC-97L hepatocellular carcinoma cells also induced an intra-cellular translocation of ABCG2 transporter, attenuated the doxorubicin efflux and increased doxorubicin-induced cytotoxicity [141]. In addition, inhibition of the NF-κB signaling pathway using parthenolide and pyrrolidinedithiocarbamate and its analog diethyldithiocarbamate preferentially inhibits the proliferation and colony formation of MCF7 mammosphere cell cultures and vera-pamil-sensitive SP cell population enriched in breast cancer stem-like cells [87]. Although this is important advance, further investigations are required for improving the efficacy of these drug types in vivo. In this regard, other potential approaches to eradicating cancer stem/progenitor cells and their progenies include the targeted delivery of anticancer drugs into these immature cancer cells, immunotherapies, and targeting of their microenvironment to counteract the angiogenic process [1,145,146].
Targeted delivery strategies
The targeted delivery of therapeutic agents into cancer- and metastasis-initiating cells by using a conjugation of drugs to tumor-specific antibodies, encapsulation of cytotoxic drugs in liposomes or other carriers such as nanoparticles, as well as genetically engineered stem cells as drug vehicles, are also promising strategies [1,145,147]. These delivery techniques might enhance drug bioavailability, improve selectivity, and continue delivering drugs into particular tumoral tissues and, thereby, decrease systemic toxicity [1,145,147]. Furthermore, the oncolytic adenovirus-based therapies represent another attractive approach to eliminating cancer- and metastasis-initiating cells [93,148-150].
Of particular interest, recent studies have revealed that the small single-stranded and non-coding RNA molecules of approximately 20–23 nucleotides known as ‘microRNAs’ (miRNAs), which contribute to the stringent control of the gene expression, are often deregulated during etiology and progression of diverse diseases and cancers [127,151-155]. The miRNA can interact with the 3′ untranslated regions of specific target RNAs and, thereby, induce their translational repression or rather stimulate the rapid degradation of the target transcripts [127,151-153]. Thus, miRNAs that can control the expression of a wide variety of proteins might be potential therapeutic targets to modulate diverse gene products contributing to the malignant behavior of cancer-initiating cells [127,152,153,155]. Consistently, it has been observed that the transfection of miRNA-124 or miRNA-137 induced the differentiation of adult mouse oligodendroglioma-derived stem cells and human GBM-derived stem cells and a growth arrest of GBM cells [152]. Importantly, the treatment of CD44+/CD24−/low/Lin− breast tumor-initiating cells isolated from a patient with breast cancer treated with chemotherapy plus let-7-lentivirus noticeably abrogated their proliferation and ability to form mammospheres in vitro, as well as the tumor formation and metastasis in non-obese diabetic severe combined immunodeficiency mice in vivo [155]. The enhanced let-7 expression was accompanied by a reduced expression of H-RAS and a high mobility group AT-hook 2, the molecular targets of which might contribute to reduced self-renewal and enhanced differentiation properties of breast tumor-initiating cells, respectively [155]. In this way, the induction of the differentiation of cancer stem/progenitor cells using agents such as RA and its synthetic analogs, interferons (IFNs), or histone deacetylase inhibitor might also represent a promising adjuvant therapeutic strategy [156-158]. For instance, it has been reported that IFN-α treatment caused a dramatic reduction in the verapamil-sensitive SP cell fraction from diverse ovarian cancer cell lines [157]. It is noteworthy that differentiation cancer therapy should also be combined with the cytotoxic agents targeting the cancer stem/progenitor cells to prevent disease relapse associated with the persistence of residual cancer-initiating cells.
Molecular targeting of the microenvironment of cancer- and metastasis-initiating cells and their differentiated progenies
Because the local microenvironment of cancer stem/progenitor cells also plays an active part in their malignant transformation at primary neoplasms and metastases at distant sites and the angiogenic process, the molecular targeting of host stromal cells is also a potential approach to counteracting the tumor development and angiogenic process [159-161]. In fact, it has been observed that treatment of the mice-bearing orthotopic U87 glioma cell xeno-grafts with an anti-VEGF mAb, bevacizumab, markedly reduced microvasculature density and tumor growth [160]. This anti-carcinogenic effect was accompanied by a decrease in the number of vessel-associated self-renewing CD133+/nestin+ tumor-initiating cells [160]. A combination of anti-angiogenic inhibitor, VEGFR2 antibody DC101 and a cytotoxic agent (cyclophosphamide) was more effective in reducing the number of tumor-sphere-forming cells in C6 glioma xenografts in vivo than individual agents [159].
Some studies, however, have indicated that the recruitment and incorporation of circulating BM-derived immature cells and tissue-resident mesenchymal stem cells and endothelial progenitor cells in tumor stroma might contribute to the promotion of the neo-plastic development and neovascularization process [162-164]. Thus, the molecular targeting of these tumor-associated stem cells or their use as a delivery vehicle might represent a potential adjuvant strategy for counteracting cancer progression and metastasis formation. In support of this, it has been shown that the use of BM-derived endothelial progenitor cells engineered to express a soluble truncated form of VEGFR2 impaired tumor growth in vivo [163]. A significant reduction in metastatic tumor burden and metastasis formation was also induced by using inducible TRAIL-expressing mesenchymal stem cells [164].
In addition to the oncogenic effects induced through the activation of the hedgehog pathway in cancer cells, it has been reported that hedgehog signaling may contribute to pathogenesis of diverse human epithelial cancers, including pancreatic, colon, prostate, breast, and ovarian cancers, by acting on the surrounding stoma and promoting the tumor neovascularization process [139,165-168]. More specifically, it has been observed that blockade of hedgehog signaling by using the SMO inhibitor IPI-926 induced an anti-angiogenic effect and improved the tumor delivery and anti-tumoral efficacy of gemcitabine, at least in part, by disrupting the desmoplastic stroma on the pancreatic cancer cell model in vivo [139]. Moreover, the results from another recent study have revealed that the inhibition of hedgehog cascade by using cyclopamine inhibited the tumor neoangiogenic process and growth of pancreatic cancer cell-derived xenografts [168]. This anti-angiogenic effect of cyclopamine was mediated, in part, by an impairment of the homing of BM-derived pro-angiogenic cells at primary pancreatic tumor sites [168].
Concluding remarks
Significant advances have been made in the development and the in vitro and in vivo validation of novel drugs targeting cancer- and metastasis-initiating cells and their tumor microenvironment. Hence, the use of these novel anticancer drugs represents a potential strategy for eradicating these immature cancer cells. In particular, the combination of therapeutic agents targeting the highly leukemic or tumorigenic cancer stem/progenitor cells, as well as the bulk mass of differentiated cancer cells, seems to be important for improving the efficacy of current cancer treatments against aggressive, metastatic, and recurrent cancers and, thereby, preventing disease relapse.
Future investigations are still essential to more precisely determine the specific biomarkers and altered gene products in cancer- and metastasis-initiating cells and the changes in their local microenvironment occurring during cancer initiation and progression to locally invasive and metastatic disease stages. It will be important to further establish the phenotypic and functional features of cells at the origin of various human cancers, including the implications of tissue-resident adult stem cells versus their more committed progenies, as well as the deregulated regulatory mechanism(s) involved in their malignant transformation, in considering the heterogeneity of cancers.Inaddition,thecomparativeanalysesofgeneexpressionprofiles observed for cancer-initiating cells during primary tumor development and the EMT process versus their respective normal tissue-resident stem/progenitor cells should shed light on the molecular transforming events occurring in these malignant cells and their pathological consequences. Furthermore, the establishment of molecular mechanisms associated with the specific migration of metastasis-initiating cells to pre-determined metastatic sites, dormancy phenomenon, and reactivation of metastasis-initiating cells at distant sites after a long latency is also of great interest. These studies should lead to the identification of novel drug targets and therapeutic strategies to overcome treatment resistance and prevent tumor regrowth, metastases, and disease relapse without major systemic cytotoxic effects on normal cells, including tissue-resident adult stem/progenitor cells. These multitargeted approaches could beexploitedto developnewandeffective combination therapiesfora potential translation for treating and even curing patients diagnosed with locally advanced, metastatic, recurrent, and lethal cancers.
Acknowledgements
We apologize to the researchers that have contributed to the advancements in cancer stem/progenitor cell research and therapies whose works have not been cited due to space limitations. The authors on this work are supported by grants from the National Institutes of Health (CA78590, CA111294, CA133774 and CA131944). We thank Kristi L. Berger for editing the manuscript.
References
- 1.Schrama D, et al. Antibody targeted drugs as cancer therapeutics. Nat. Rev. Drug Discov. 2006;5:147–159. doi: 10.1038/nrd1957. [DOI] [PubMed] [Google Scholar]
- 2.Gray-Schopfer V, et al. Melanoma biology and new targeted therapy. Nature. 2007;445:851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 3.Sorscher SM. Biological therapy update in colorectal cancer. Expert Opin. Biol. Ther. 2007;7:509–519. doi: 10.1517/14712598.7.4.509. [DOI] [PubMed] [Google Scholar]
- 4.Mimeault M, et al. Recent advances on the molecular mechanisms involved in the drug resistance of cancer cells and novel targeting therapies. Clin. Pharmacol. Ther. 2008;83:673–691. doi: 10.1038/sj.clpt.6100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sathornsumetee S, et al. Molecularly targeted therapy for malignant glioma. Cancer. 2007;110:13–24. doi: 10.1002/cncr.22741. [DOI] [PubMed] [Google Scholar]
- 6.Le Tourneau C, et al. New developments in multitargeted therapy for patients with solid tumours. Cancer Treat. Rev. 2008;34:37–48. doi: 10.1016/j.ctrv.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Rosa DD, et al. Molecular-targeted therapies: lessons from years of clinical development. Cancer Treat. Rev. 2008;34:61–80. doi: 10.1016/j.ctrv.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Spangenberg HC, et al. Targeted therapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2009;6:423–432. doi: 10.1038/nrgastro.2009.86. [DOI] [PubMed] [Google Scholar]
- 9.Jemal A, et al. Cancer statistics, 2008. CA Cancer J. Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 10.Mimeault M, Batra SK. New advances on<– critical implications of tumor- and metastasis-initiating cells in cancer progression, treatment resistance and disease recurrence. Histol. Histopathol. 2010:25. doi: 10.14670/hh-25.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mimeault M, Batra SK. Novel therapies against aggressive and recurrent epithelial cancers by molecular targeting tumor- and metastasis-initiating cells and their progenies. Anticancer Agents Med. Chem. 2010;10:137–151. doi: 10.2174/187152010790909353. Special issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaish M. Mismatch repair deficiencies transforming stem cells into cancer stem cells and therapeutic implications. Mol. Cancer. 2007;6:26. doi: 10.1186/1476-4598-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rizo A, et al. Signaling pathways in self-renewing hematopoietic and leukemic stem cells: do all stem cells need a niche? Hum. Mol. Genet. 2006;15:R210–R219. doi: 10.1093/hmg/ddl175. [DOI] [PubMed] [Google Scholar]
- 14.Kim CF, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 15.Mimeault M, et al. Recent advances on cancer stem/progenitor cell research: therapeutic implications for overcoming resistance to the most aggressive cancers. J. Mol. Cell. Med. 2007;11:981–1011. doi: 10.1111/j.1582-4934.2007.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ginestier C, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsui W, et al. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer Res. 2008;68:190–197. doi: 10.1158/0008-5472.CAN-07-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mimeault M, Batra SK. Recent progress on tissue-resident adult stem cell biology and their therapeutic implications. Stem Cell Rev. 2008;4:27–49. doi: 10.1007/s12015-008-9008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mimeault M, Batra SK. Recent progress on normal and malignant pancreatic stem/progenitor cell research: therapeutic implications for the treatment of type 1 or 2 diabetes mellitus and aggressive pancreatic cancer. Gut. 2008;57:1456–1468. doi: 10.1136/gut.2008.150052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mimeault M, et al. Functions of normal and malignant prostatic stem/progenitor cells in tissue regeneration and cancer progression and novel targeting therapies. Endocr. Rev. 2008;29:234–252. doi: 10.1210/er.2007-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiba T. Enhanced self-renewal capability in hepatic stem/progenitor cells drives cancer initiation. Gastroenterology. 2007;133:937–950. doi: 10.1053/j.gastro.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 22.Chen BY, et al. Hedgehog is involved in prostate basal cell hyperplasia formation and its progressing towards tumorigenesis. Biochem. Biophys. Res. Commun. 2007;357:1084–1089. doi: 10.1016/j.bbrc.2007.04.091. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korkaya H, et al. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008;27:6120–6130. doi: 10.1038/onc.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mimeault M, Batra SK. Aging of tissue-resident adult stem/progenitor cells and their pathological consequences. Panminerva Med. 2009;51:57–79. [PubMed] [Google Scholar]
- 26.Mimeault M, Batra SK. Recent insights into the molecular mechanisms involved in aging and the malignant transformation of adult stem/progenitor cells and their therapeutic implications. Ageing Res. Rev. 2009;8:94–112. doi: 10.1016/j.arr.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mimeault M, Batra SK. Targeting of cancer stem/progenitor cells plus stem cell-based therapies: the ultimate hope for treating and curing aggressive and recurrent cancers. Panminerva Med. 2008;50:3–18. [PMC free article] [PubMed] [Google Scholar]
- 28.Mimeault M, Batra SK. Recent advances on skin-resident stem/progenitor cell functions in skin regeneration, aging and cancers and novel anti-aging and cancer therapies. J. Cell. Mol. Med. 2010;14:116–134. doi: 10.1111/j.1582-4934.2009.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alcantara LS, et al. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell. 2009;15:45–56. doi: 10.1016/j.ccr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu Y, et al. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell. 2005;8:119–130. doi: 10.1016/j.ccr.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galli R, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 32.Singh SK. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 33.Al-Hajj M, et al. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. U.S.A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eramo A, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 35.Yang ZF, et al. Significance of CD90(+) cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 36.Hermann PC, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Zhang S, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu G, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol. Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schatton T, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maitland NJ, et al. Prostate cancer stem cells: a target for new therapies. Ernst. Schering. Found. Symp. Proc. 2006;5:155–179. doi: 10.1007/2789_2007_050. [DOI] [PubMed] [Google Scholar]
- 41.Ricci-Vitiani L, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 42.Alvero AB, et al. Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance. Cell Cycle. 2009;8:158–166. doi: 10.4161/cc.8.1.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baba T, et al. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene. 2009;28:209–218. doi: 10.1038/onc.2008.374. [DOI] [PubMed] [Google Scholar]
- 44.Haraguchi N, et al. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells. 2006;24:506–513. doi: 10.1634/stemcells.2005-0282. [DOI] [PubMed] [Google Scholar]
- 45.Hirschmann-Jax C, et al. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc. Natl. Acad. Sci. U.S.A. 2004;101:14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levina V, et al. Drug-selected human lung cancer stem cells: cytokine network, tumorigenic and metastatic properties. PLoS One. 2008;3:e3077. doi: 10.1371/journal.pone.0003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klarmann GJ, et al. Invasive prostate cancer cells are tumor initiating cells that have a stem cell-like genomic signature. Clin. Exp. Metastasis. 2009;26:433–446. doi: 10.1007/s10585-009-9242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hope KJ, et al. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat. Immunol. 2004;5:738–743. doi: 10.1038/ni1080. [DOI] [PubMed] [Google Scholar]
- 49.Fang D, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 50.Wright MH, et al. Brca1 breast tumors contain distinct CD44+/CD24− and CD133+ cells with cancer stem cell characteristics. Breast Cancer Res. 2008;10:R10. doi: 10.1186/bcr1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi GM, et al. Identification of side population cells in human hepatocellular carcinoma cell lines with stepwise metastatic potentials. J. Cancer Res. Clin. Oncol. 2008;134:1155–1163. doi: 10.1007/s00432-008-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang D, et al. Isolation and identification of cancer stem-like cells in esophageal carcinoma cell lines. Stem Cells Dev. 2009;18:465–473. doi: 10.1089/scd.2008.0033. [DOI] [PubMed] [Google Scholar]
- 53.Huang Q, et al. Glioma stem cells are more aggressive in recurrent tumors with malignant progression than in the primary tumor, and both can be maintained long-term in vitro. BMC Cancer. 2008;8:304. doi: 10.1186/1471-2407-8-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Todaro M, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, et al. Identification of cancer stem cell-like side population cells in human nasopharyngeal carcinoma cell line. Cancer Res. 2007;67:3716–3724. doi: 10.1158/0008-5472.CAN-06-4343. [DOI] [PubMed] [Google Scholar]
- 56.Brabletz T, et al. Opinion: migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat. Rev. Cancer. 2005;5:744–749. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 57.Tso CL, et al. Primary glioblastomas express mesenchymal stem-like properties. Mol. Cancer Res. 2006;4:607–619. doi: 10.1158/1541-7786.MCR-06-0005. [DOI] [PubMed] [Google Scholar]
- 58.Mimeault M, Batra SK. Interplay of distinct growth factors during epithelial-mesenchymal transition of cancer progenitor cells and molecular targeting as novel cancer therapies. Ann. Oncol. 2007;18:1605–1619. doi: 10.1093/annonc/mdm070. [DOI] [PubMed] [Google Scholar]
- 59.Mimeault M, Batra SK. Functions of tumorigenic and migrating cancer progenitor cells in cancer progression and metastasis and their therapeutic implications. Cancer Metastasis Rev. 2007;26:203–214. doi: 10.1007/s10555-007-9052-4. [DOI] [PubMed] [Google Scholar]
- 60.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spaderna S. Epithelial-mesenchymal and mesenchymal-epithelial transitions during cancer progression. Verh. Dtsch. Ges. Pathol. 2007;91:21–28. [PubMed] [Google Scholar]
- 62.Storci G, et al. The basal-like breast carcinoma phenotype is regulated by SLUG gene expression. J. Pathol. 2008;214:25–37. doi: 10.1002/path.2254. [DOI] [PubMed] [Google Scholar]
- 63.Shah AN, et al. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann. Surg. Oncol. 2007;14:3629–3637. doi: 10.1245/s10434-007-9583-5. [DOI] [PubMed] [Google Scholar]
- 64.Morel AP, et al. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Balic M, et al. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin. Cancer Res. 2006;12:5615–5621. doi: 10.1158/1078-0432.CCR-06-0169. [DOI] [PubMed] [Google Scholar]
- 66.Aktas B, et al. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 2009;11:R46. doi: 10.1186/bcr2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Theodoropoulos PA, et al. Circulating tumor cells with a putative stem cell phenotype in peripheral blood of patients with breast cancer. Cancer Lett. 2010;288:99–106. doi: 10.1016/j.canlet.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 68.Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008;10:R25. doi: 10.1186/bcr1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li X, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J. Natl. Cancer Inst. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 70.Jimeno A, et al. A direct pancreatic cancer xenograft model as a platform for cancer stem cell therapeutic development. Mol. Cancer Ther. 2009;8:310–314. doi: 10.1158/1535-7163.MCT-08-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hong SP, et al. CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int. J. Cancer. 2009;125:2323–2331. doi: 10.1002/ijc.24573. [DOI] [PubMed] [Google Scholar]
- 72.Wang YH, et al. A side population of cells from a human pancreatic carcinoma cell line harbors cancer stem cell characteristics. Neoplasma. 2009;56:371–378. doi: 10.4149/neo_2009_05_371. [DOI] [PubMed] [Google Scholar]
- 73.Kennedy JA, et al. Comment on “Tumor growth need not be driven by rare cancer stem cells”. Science. 2007;318:1722. doi: 10.1126/science.1149590. [DOI] [PubMed] [Google Scholar]
- 74.Quintana E, et al. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bao S, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 76.Das B, et al. Hypoxia enhances tumor stemness by increasing the invasive and tumorigenic side population fraction. Stem Cells. 2008;26:1818–1830. doi: 10.1634/stemcells.2007-0724. [DOI] [PubMed] [Google Scholar]
- 77.Shmelkov SV, et al. CD133 expression is not restricted to stem cells, and both CD133+ and CD133− metastatic colon cancer cells initiate tumors. J. Clin. Invest. 2008;118:2111–2120. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dylla SJ, et al. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS One. 2008;3:e2428. doi: 10.1371/journal.pone.0002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schmidt C, et al. Lapatinib study supports cancer stem cell hypothesis, encourages industry research. J. Natl. Cancer Inst. 2008;100:694–695. doi: 10.1093/jnci/djn168. [DOI] [PubMed] [Google Scholar]
- 80.Griffero F, et al. Different response of human glioma tumor-initiating cells to EGFR kinase inhibitors. J. Biol. Chem. 2009;284:7138–7148. doi: 10.1074/jbc.M807111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gupta PB, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kelly PN, et al. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- 83.Ponti D, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 84.Yang ZF, et al. Identification of local and circulating cancer stem cells in human liver cancer. Hepatology. 2008;47:919–928. doi: 10.1002/hep.22082. [DOI] [PubMed] [Google Scholar]
- 85.Morimoto K, et al. Stem cell marker aldehyde dehydrogenase 1-positive breast cancers are characterized by negative estrogen receptor, positive human epidermal growth factor receptor type 2, and high Ki67 expression. Cancer Sci. 2009;100:1062–1068. doi: 10.1111/j.1349-7006.2009.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Loebinger MR, et al. Squamous cell cancers contain a side population of stem-like cells that are made chemosensitive by ABC transporter blockade. Br. J. Cancer. 2008;98:380–387. doi: 10.1038/sj.bjc.6604185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou J, et al. NF-kappaB pathway inhibitors preferentially inhibit breast cancer stem-like cells. Breast Cancer Res. Treat. 2008;111:419–427. doi: 10.1007/s10549-007-9798-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou J, et al. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc. Natl. Acad. Sci. U.S.A. 2007;104:16158–16163. doi: 10.1073/pnas.0702596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Phillips TM, et al. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J. Natl. Cancer Inst. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 90.Ma S, et al. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27:1749–1758. doi: 10.1038/sj.onc.1210811. [DOI] [PubMed] [Google Scholar]
- 91.Schatton T, et al. Modulation of T-cell activation by malignant melanoma initiating cells. Cancer Res. 2010;70:697–708. doi: 10.1158/0008-5472.CAN-09-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dean M, et al. Tumour stem cells and drug resistance. Nat. Rev. Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 93.Zhang X, et al. Treatment of radioresistant stem-like esophageal cancer cells by an apoptotic gene-armed, telomerase-specific oncolytic adenovirus. Clin. Cancer Res. 2008;14:2813–2823. doi: 10.1158/1078-0432.CCR-07-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shervington A, Lu C. Expression of multidrug resistance genes in normal and cancer stem cells. Cancer Invest. 2008;26:535–542. doi: 10.1080/07357900801904140. [DOI] [PubMed] [Google Scholar]
- 95.Frank NY, et al. ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res. 2005;65:4320–4333. doi: 10.1158/0008-5472.CAN-04-3327. [DOI] [PubMed] [Google Scholar]
- 96.Hadnagy A, et al. SP analysis may be used to identify cancer stem cell populations. Exp. Cell Res. 2006;312:3701–3710. doi: 10.1016/j.yexcr.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 97.Mimeault M, et al. Cytotoxic effects induced by docetaxel, gefitinib and cyclopamine on side population and non-side population cell fractions from human tumorigenic and invasive prostate cancer cells. Mol. Cancer Ther. 2010;9:617–630. doi: 10.1158/1535-7163.MCT-09-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sung JM, et al. Characterization of a stem cell population in lung cancer A549 cells. Biochem. Biophys. Res. Commun. 2008;371:163–167. doi: 10.1016/j.bbrc.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 99.Ho MM, et al. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67:4827–4833. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- 100.Tanaka H, et al. The Hedgehog signaling pathway plays an essential role in maintaining the CD44+ CD24−/low subpopulation and the side population of breast cancer cells. Anticancer Res. 2009;29:2147–2157. [PubMed] [Google Scholar]
- 101.Mimeault M, Batra SK. Characterization of non-malignant and malignant prostatic stem/progenitor cells by Hoecsht side population method. Methods Mol. Biol. 2009;568:139–149. doi: 10.1007/978-1-59745-280-9_8. [DOI] [PubMed] [Google Scholar]
- 102.Katayama R, et al. Dofequidar fumarate sensitizes cancer stem-like side population cells to chemotherapeutic drugs by inhibiting ABCG2/BCRP-mediated drug export. Cancer Sci. 2009;100:2060–2068. doi: 10.1111/j.1349-7006.2009.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang M, et al. Whole genome expression profiling reveals a significant role for the cell junction and apoptosis pathways in breast cancer stem cells. Mol. Biotechnol. 2010 doi: 10.1007/s12033-010-9241-1. [DOI] [PubMed] [Google Scholar]
- 104.Zhou J, et al. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc. Natl. Acad. Sci. U. S. A. 2007;104:16158–16163. doi: 10.1073/pnas.0702596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Christgen M, et al. Identification of a distinct side population of cancer cells in the Cal-51 human breast carcinoma cell line. Mol. Cell. Biochem. 2007;306:201–212. doi: 10.1007/s11010-007-9570-y. [DOI] [PubMed] [Google Scholar]
- 106.Xu JX, et al. High tolerance to apoptotic stimuli induced by serum depletion and ceramide in side-population cells: high expression of CD55 as a novel character for side-population. Exp. Cell Res. 2007;313:1877–1885. doi: 10.1016/j.yexcr.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 107.Kondo T, et al. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc. Natl. Acad. Sci. U.S.A. 2004;101:781–786. doi: 10.1073/pnas.0307618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen JS, et al. EGFR regulates the side population in head and neck squamous cell carcinoma. Laryngoscope. 2006;116:401–406. doi: 10.1097/01.mlg.0000195075.14093.fb. [DOI] [PubMed] [Google Scholar]
- 109.Mitsutake N, et al. Characterization of side population in thyroid cancer cell lines: cancer stem-like cells are enriched partly but not exclusively. Endocrinology. 2007;148:1797–1803. doi: 10.1210/en.2006-1553. [DOI] [PubMed] [Google Scholar]
- 110.Dey D, et al. Phenotypic and functional characterization of human mammary stem/progenitor cells in long term culture. PLoS One. 2009;4:e5329. doi: 10.1371/journal.pone.0005329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Feldmann G, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67:2187–2196. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bar EE, et al. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25:2524–2533. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tanei T, et al. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential Paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin. Cancer Res. 2009;15:4234–4241. doi: 10.1158/1078-0432.CCR-08-1479. [DOI] [PubMed] [Google Scholar]
- 114.Charafe-Jauffret E, et al. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin. Cancer Res. 2010;16:45–55. doi: 10.1158/1078-0432.CCR-09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Duester G, et al. Cytosolic retinoid dehydrogenases govern ubiquitous metabolism of retinol to retinaldehyde followed by tissue-specific metabolism to retinoic acid. Chem. Biol. Interact. 2003;143–144:201–210. doi: 10.1016/s0009-2797(02)00204-1. [DOI] [PubMed] [Google Scholar]
- 116.Chute JP, et al. Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells. Proc. Natl. Acad. Sci. U.S.A. 2006;103:11707–11712. doi: 10.1073/pnas.0603806103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wagner E, et al. Retinoic acid delineates the topography of neuronal plasticity in postnatal cerebral cortex. Eur. J. Neurosci. 2006;24:329–340. doi: 10.1111/j.1460-9568.2006.04934.x. [DOI] [PubMed] [Google Scholar]
- 118.Marchitti SA, et al. Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily. Expert Opin. Drug Metab. Toxicol. 2008;4:697–720. doi: 10.1517/17425250802102627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rice KL, et al. Overexpression of stem cell associated ALDH1A1, a target of the leukemogenic transcription factor TLX1/HOX11, inhibits lymphopoiesis and promotes myelopoiesis in murine hematopoietic progenitors. Leuk. Res. 2008;32:873–883. doi: 10.1016/j.leukres.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 120.Ginestier C, et al. Retinoid signaling regulates breast cancer stem cell differentiation. Cell Cycle. 2009;8:3297–3302. doi: 10.4161/cc.8.20.9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Walsh N, et al. Aldehyde dehydrogenase 1A1 and gelsolin identified as novel invasion-modulating factors in conditioned medium of pancreatic cancer cells. J. Proteomics. 2008;71:561–571. doi: 10.1016/j.jprot.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 122.Widschwendter M, et al. Methylation and silencing of the retinoic acid receptor-beta2 gene in breast cancer. J. Natl. Cancer Inst. 2000;92:826–832. doi: 10.1093/jnci/92.10.826. [DOI] [PubMed] [Google Scholar]
- 123.Rexer BN, et al. Retinoic acid biosynthesis by normal human breast epithelium is via aldehyde dehydrogenase 6, absent in MCF-7 cells. Cancer Res. 2001;61:7065–7070. [PubMed] [Google Scholar]
- 124.Moreb JS, et al. Retinoic acid down-regulates aldehyde dehydrogenase and increases cytotoxicity of 4-hydroperoxycyclophosphamide and acetaldehyde. J. Pharmacol. Exp. Ther. 2005;312:339–345. doi: 10.1124/jpet.104.072496. [DOI] [PubMed] [Google Scholar]
- 125.Elizondo G, et al. Retinoic acid modulates retinaldehyde dehydrogenase 1 gene expression through the induction of GADD153-C/EBPbeta interaction. Biochem. Pharmacol. 2009;77:248–257. doi: 10.1016/j.bcp.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Epping MT, et al. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835–847. doi: 10.1016/j.cell.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 127.Kurrey NK, et al. Snail and slug mediate radio- and chemo-resistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells. 2009;27:2059–2068. doi: 10.1002/stem.154. [DOI] [PubMed] [Google Scholar]
- 128.Rappa G, et al. The stem cell-associated antigen CD133 (Prominin-1) is a molecular therapeutic target for metastatic melanoma. Stem Cells. 2008;26:3008–3017. doi: 10.1634/stemcells.2008-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gilg AG, et al. Targeting hyaluronan interactions in malignant gliomas and their drug-resistant multipotent progenitors. Clin. Cancer Res. 2008;14:1804–1813. doi: 10.1158/1078-0432.CCR-07-1228. [DOI] [PubMed] [Google Scholar]
- 130.Karhadkar SS, et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–712. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 131.Tanaka H, et al. The Hedgehog signaling pathway plays an essential role in maintaining the CD44+ CD24−/low subpopulation and the side population of breast cancer cells. Anticancer Res. 2009;29:2147–2157. [PubMed] [Google Scholar]
- 132.Marian CO, et al. The telomerase antagonist, imetelstat, efficiently targets glioblastoma tumor-initiating cells leading to decreased proliferation and tumor growth. Clin. Cancer Res. 2010;16:154–163. doi: 10.1158/1078-0432.CCR-09-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ginestier C, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J. Clin. Invest. 2010;120:485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Magnifico A, et al. Tumor-initiating cells of HER2-positive carcinoma cell lines express the highest oncoprotein levels and are sensitive to trastuzumab. Clin. Cancer Res. 2009;15:2010–2021. doi: 10.1158/1078-0432.CCR-08-1327. [DOI] [PubMed] [Google Scholar]
- 135.Clement V, et al. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr. Biol. 2007;17:165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Feldmann G, et al. An orally bioavailable small-molecule inhibitor of Hedgehog signaling inhibits tumor initiation and metastasis in pancreatic cancer. Mol. Cancer Ther. 2008;7:2725–2735. doi: 10.1158/1535-7163.MCT-08-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xu Q, et al. Hedgehog signaling regulates brain tumor-initiating cell proliferation and portends shorter survival for patients with PTEN-coexpressing glioblastomas. Stem Cells. 2008;26:3018–3026. doi: 10.1634/stemcells.2008-0459. [DOI] [PubMed] [Google Scholar]
- 138.Mimeault M, et al. Improvement of cytotoxic effects of mitoxantrone on hormone-refractory metastatic prostate cancer cells by co-targeting epidermal growth factor receptor and hedgehog signaling cascades. Growth Factors. 2007;25:400–416. doi: 10.1080/08977190801930935. [DOI] [PubMed] [Google Scholar]
- 139.Olive KP, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Miki J, et al. Identification of putative stem cell markers, CD133 and CXCR4, in hTERT-immortalized primary nonmalignant and malignant tumor-derived human prostate epithelial cell lines and in prostate cancer specimens. Cancer Res. 2007;67:3153–3161. doi: 10.1158/0008-5472.CAN-06-4429. [DOI] [PubMed] [Google Scholar]
- 141.Hu C, et al. Analysis of ABCG2 expression and side population identifies intrinsic drug efflux in the HCC cell line MHCC-97L and its modulation by Akt signaling. Carcinogenesis. 2008;29:2289–2297. doi: 10.1093/carcin/bgn223. [DOI] [PubMed] [Google Scholar]
- 142.Johannessen TC, et al. Highly infiltrative brain tumours show reduced chemosensitivity associated with a stem cell-like phenotype. Neuropathol. Appl. Neurobiol. 2009;35:380–393. doi: 10.1111/j.1365-2990.2008.01008.x. [DOI] [PubMed] [Google Scholar]
- 143.Kallifatidis G, et al. Sulforaphane targets pancreatic tumour-initiating cells by NF-kappaB-induced antiapoptotic signalling. Gut. 2009;58:949–963. doi: 10.1136/gut.2008.149039. [DOI] [PubMed] [Google Scholar]
- 144.Korkaya H, et al. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009;7:e1000121. doi: 10.1371/journal.pbio.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schatzlein AG, et al. Delivering cancer stem cell therapies—a role for nanomedicines? Eur. J. Cancer. 2006;42:1309–1315. doi: 10.1016/j.ejca.2006.01.044. [DOI] [PubMed] [Google Scholar]
- 146.Mine T, et al. Breast cancer cells expressing stem cell markers CD44+ CD24 lo are eliminated by Numb-1 peptide-activated T cells. Cancer Immunol. Immunother. 2009;58:1185–1194. doi: 10.1007/s00262-008-0623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bansal T, et al. Novel formulation approaches for optimizing delivery of anticancer drugs based on P-glycoprotein modulation. Drug Discov. Today. 2009 doi: 10.1016/j.drudis.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 148.Nandi S, et al. Low-dose radiation enhances survivin-mediated virotherapy against malignant glioma stem cells. Cancer Res. 2008;68:5778–5784. doi: 10.1158/0008-5472.CAN-07-6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Eriksson M. Oncolytic adenoviruses kill breast cancer initiating CD44+ CD24−/low cells. Mol. Ther. 2007;15:2088–2093. doi: 10.1038/sj.mt.6300300. [DOI] [PubMed] [Google Scholar]
- 150.Cripe TP, et al. Targeting cancer-initiating cells with oncolytic viruses. Mol. Ther. 2009;17:1677–1682. doi: 10.1038/mt.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Esau CC, Monia BP. Therapeutic potential for microRNAs. Adv. Drug Deliv. Rev. 2007;59:101–114. doi: 10.1016/j.addr.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 152.Silber J, et al. miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med. 2008;6:14. doi: 10.1186/1741-7015-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ji Q, et al. Restoration of tumor suppressor miR-34 inhibits human p53-mutant gastric cancer tumorspheres. BMC Cancer. 2008;8:266. doi: 10.1186/1471-2407-8-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ji Q, et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 2009;4:e6816. doi: 10.1371/journal.pone.0006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yu F, et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 156.Sell S, et al. Stem cell origin of cancer and differentiation therapy. Crit. Rev. Oncol. Hematol. 2004;51:1–28. doi: 10.1016/j.critrevonc.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 157.Moserle L, et al. The side population of ovarian cancer cells is a primary target of IFN-alpha antitumor effects. Cancer Res. 2008;68:5658–5668. doi: 10.1158/0008-5472.CAN-07-6341. [DOI] [PubMed] [Google Scholar]
- 158.Petrie K, et al. Differentiation therapy of acute myeloid leukemia: past, present and future. Curr. Opin. Hematol. 2009;16:84–91. doi: 10.1097/MOH.0b013e3283257aee. [DOI] [PubMed] [Google Scholar]
- 159.Folkins C, et al. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 2007;67:3560–3564. doi: 10.1158/0008-5472.CAN-06-4238. [DOI] [PubMed] [Google Scholar]
- 160.Calabrese C, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 161.Borovski T, et al. Tumor microvasculature supports proliferation and expansion of glioma-propagating cells. Int. J. Cancer. 2009;125:1222–1230. doi: 10.1002/ijc.24408. [DOI] [PubMed] [Google Scholar]
- 162.Rafii S, et al. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat. Rev. Cancer. 2002;2:826–835. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- 163.Davidoff AM, et al. Bone marrow-derived cells contribute to tumor neovasculature and, when modified to express an angiogenesis inhibitor, can restrict tumor growth in mice. Clin. Cancer Res. 2001;7:2870–2879. [PubMed] [Google Scholar]
- 164.Loebinger MR, et al. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res. 2009;69:4134–4142. doi: 10.1158/0008-5472.CAN-08-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Theunissen JW, de Sauvage FJ. Paracrine Hedgehog signaling in cancer. Cancer Res. 2009;69:6007–6010. doi: 10.1158/0008-5472.CAN-09-0756. [DOI] [PubMed] [Google Scholar]
- 166.Shaw A, et al. The Sonic Hedgehog pathway stimulates prostate tumor growth by paracrine signaling and recapitulates embryonic gene expression in tumor myofibroblasts. Oncogene. 2009;28:4480–4490. doi: 10.1038/onc.2009.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kasper M, et al. Hedgehog signalling in breast cancer. Carcinogenesis. 2009;30:903–911. doi: 10.1093/carcin/bgp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nakamura K, et al. Hedgehog promotes neovascularization in pancreatic cancers by regulating Ang-1 and IGF-1 expression in bone-marrow derived proangiogenic cells. PLoS One. 2010;5:e8824. doi: 10.1371/journal.pone.0008824. [DOI] [PMC free article] [PubMed] [Google Scholar]