Abstract
Background
Little is known about whether direct angiotensin receptor blockade can reduce atherosclerosis and plaque disruption. This study evaluated the effect of angiotensin receptor blockade on both the development of atherosclerosis and the disruption of plaque in a modified Constantinides animal model.
Methods and Results
Twenty-eight New Zealand White rabbits underwent aortic balloon injury followed by a 1% cholesterol diet for 8 weeks. Thirteen rabbits received candesartan at 0.5 mg · kg−1 · d−1 beginning 2 days before aortic balloon injury and continued for the total 8 weeks of the cholesterol diet. The rabbits were then pharmacologically triggered and humanely killed, and their aortas were analyzed. The degree of atherosclerosis was determined by intima-media ratio of the infrarenal portion of the aorta. The frequency of intra-aortic thrombosis, a measure of plaque disruption, and the percentages of macrophage area and collagen-staining area of the plaque were determined. Candesartan-treated rabbits had less atherosclerosis (intima-media infrarenal aorta ratio of 1.18±0.08 versus 1.57±0.08 [mean±SEM] for the placebo group, P<0.001); fewer thrombi (3 of 13 versus 11 of 15; P<0.05); lower percentage area of macrophages to total plaque (18.8±2.7% versus 27±2.5%, P<0.05); and higher collagen to total plaque area (45±3% versus 35±2%, P<0.01).
Conclusions
These results demonstrate that angiotensin receptor blockade attenuates the degree of atherosclerosis and reduces both plaque disruption and macrophage accumulation while increasing collagen deposition in the aortas of this animal model.
Keywords: atherosclerosis, plaque, thrombosis, angiotensin
Angiotensin II is a potent vasoactive peptide that causes vasoconstriction and can affect blood pressure. More recently, angiotensin II has been shown to contribute to atherogenesis.1 Angiotensin II results in impaired endothelium-dependent vasodilation,2 monocyte recruitment and activation,3 and vascular smooth muscle cell growth and migration,4,5 as well as increased oxidation of LDL, with subsequent uptake of oxidized LDL by macrophages.6 The effect of angiotensin II on the vasculature is mediated by 2 plasma membrane receptors, angiotensin II receptor type 1 and angiotensin II receptor type 2 (AT1 and AT2, respectively).7
The underlying mechanism of most acute coronary syndromes is the disruption of an atherosclerotic coronary plaque with a subsequent overlying thrombus. Although angiotensin II plays an important role in the development of early atherosclerosis, little is known about the role of angiotensin II on plaque disruption. Several animal and clinical studies8 have demonstrated that angiotensin-converting enzyme inhibitors reduce atherosclerosis and may reduce plaque disruption. However, it is not known whether direct angiotensin receptor blockade also reduces atherosclerosis and plaque disruption. To address these questions, we used a modified Constantinides model9,10 of plaque disruption in which atherosclerotic rabbits were pharmacologically triggered, yielding plaques with overlying thrombi similar to those observed in disrupted coronary artery plaques of patients with acute manifestations of cardiovascular disease.
Methods
Rabbit Housing and Diet
Adult New Zealand White (NZW) male rabbits weighing ≈ 3 kg (Millbrook Immunoserv, Amherst, Mass) were continuously housed at the hospital’s animal care facilities. All studies were performed after approval of the hospital Animal Care and Use Committee on Animal Investigations.
Rabbits were fed a 1% high-cholesterol diet incorporated into standard rabbit chow (Purina modified 1% cholesterol diet 5736C-G) beginning immediately after balloon injury and continuing for 8 weeks. Fasting serum cholesterol values were obtained before balloon injury and before pharmacological triggering. Weights were monitored to ensure that the rabbits remained healthy.
Study Protocol
Candesartan-cilexetil was prepared by mixing and emulsifying gum arabic and water. The suspension was administered orally by gavage daily. The study protocol is summarized in Figure 1. A dose-response study was first performed. Four NZW rabbits that received high-dose candesartan (0.5 to 1.5 mg/kg), 6 on low-dose candesartan (0.01 mg/kg, n=3 and 0.10 mg/kg, n=3), and 6 placebo-administered rabbits underwent aortic balloon injury followed by a 1% cholesterol diet for 8 weeks. The rabbits were then pharmacologically triggered and humanely killed (as described below), and their aortas were analyzed.
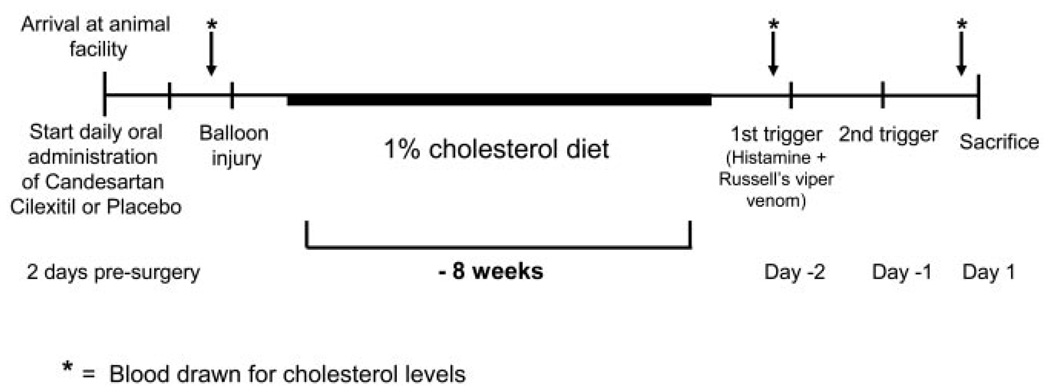
Figure 1.
Schematic of study timeline. Balloon injury was followed by 8 weeks of 1% cholesterol feeding. Pharmacological triggering was performed 48 and 24 hours before euthanization. Serum cholesterol was also monitored at baseline and before triggering.
Two of the 4 rabbits that received high doses (1.0 or 1.5 mg/kg daily) of candesartan died after several days of therapy. Serum from each of the 2 animals demonstrated elevated blood urea nitrogen and creatinine levels (animal 1, 84 and 9.7 mg/dL, respectively; animal 2, 29 and 2.5 mg/dL, respectively). The overall mean blood urea nitrogen and creatinine levels in this high-dose group were normal. All other animals tolerated the candesartan. The highest tolerated dose of candesartan was therefore determined to be 0.5 mg/kg.
Once the highest tolerated dose was established, the rabbits were divided into 2 groups: the experimental arm, in which the rabbits received either 0.5 mg/kg candesartan, and the placebo (control) arm. Candesartan-treated rabbits received candesartan daily for 2 days before balloon injury and the subsequent 8 weeks until triggering. A total of 28 rabbits were used, 15 placebo rabbits and 13 candesartantreated rabbits. Because there were 2 rabbits on 0.5 mg/kg of candesartan and 6 placebo rabbits in the initial dose-response study, an additional 11 candesartan-treated rabbits and 9 placebo rabbits were used.
Balloon Injury and Endothelial Denudation
Rabbits were anesthetized with ketamine (35 mg/kg IM), xylazine (2.5 mg/kg IM), and acepromazine (0.75 mg/kg IM). Anesthesia was maintained during the procedure with isoflurane inhalation via mask. Balloon-induced arterial wall injury of the aorta was performed with a 3F Fogarty catheter introduced through a right femoral artery cutdown. The catheter was first advanced 30 cm to a level just above the aortic valve. The balloon was then inflated with 0.3 mL saline, and the catheter was gently retracted to the iliofemoral artery. This procedure was performed 3 times in each rabbit. The catheter was then removed, and the incision was closed with sutures.
Pharmacological Triggering and Euthanization
After endothelial denudation and 8 weeks of the high-cholesterol diet, plaque disruption was triggered by administration of Russell’s viper venom (0.15 mg/kg IP, Sigma Chemical Co) followed 30 minutes later by histamine (0.02 mg/kg IV) at 48 and at 24 hours before euthanization (see Figure 1). Immediately before euthanization, heparin sulfate (10 U/kg IV) was given to prevent postmortem clotting. Euthanasia was performed with ketamine (35 mg/kg IM) and xylazine (5 mg/kg IM) followed by a bolus injection of sodium pentobarbital (100 mg/kg IV).
Serum Cholesterol Measurement
Blood samples were obtained before beginning candesartan therapy and just before pharmacological triggering. Total serum cholesterol, HDL, and triglycerides were measured (Roche Hitachi 917 and Roche reagents). LDL cholesterol was calculated from the Friedewald equation, whereby LDL=total cholesterol–HDL–(triglycerides/5).
Tissue Preparation
After the animals were killed, 6 rabbits were perfusion-fixed with 10% formalin acetate for a minimum of 2 hours. The aorta from the aortic valve to the femoral bifurcation was removed, cut, and catalogued into 2.5-mm serial sections. These samples then underwent additional fixation overnight in formalin, followed by a second overnight tissue processing and dehydration. The samples were embedded in paraffin the following day. Serial cross sections were processed for general histological staining with hematoxylin-eosin (H&E).
The aortas removed from the remaining 22 rabbits were immediately excised from the animals and cut into 16 serial 2-mm sections, with alternate sections embedded in paraffin and catalogued. The remaining specimens were embedded in OCT compound (Tissue-Tek, Fisher Scientific), snap-frozen, and stored at −70°C for specific immunohistochemical staining as well as general histological staining.
Histological Analysis
Identification of Disrupted Plaques
Sections stained with H&E were analyzed for the presence of disrupted plaques. Disrupted plaques were defined as those with an overlying premortem thrombus. Ruptured plaques were histologically defined as those with either intraplaque hemorrhage or a microscopically visible fissure within the plaque. Plaques that had no overlying thrombus and no intraplaque hemorrhage or plaque fissure were defined as nondisrupted plaques. Comparative analyses of the histological and physical properties of the various types of plaque as outlined were performed.
Determination of Atherosclerotic Burden
The method for determining the intima-media ratio of the aorta, which is used as a measure of atherosclerotic burden, has been previously described.11 Percentage aortic wall thickness [(outer wall area–area of lumen)/(outer wall area)×100%] was determined from the histological sections of the aorta stained with H&E. Because the bulk of the atherosclerotic burden was limited to the infrarenal region, only those regions were analyzed. These slides were scanned microscopically (Olympus DP12) to enable use of computerized image analysis with Adobe Photoshop and NIH Image Analysis software (Scion Image, release 3b). The luminal, intimal, medial, and outer wall areas were manually traced and quantified, which allowed intima-media ratios to be calculated. Six sections from each rabbit aorta were used to determine the intima-media ratio.
Determination of Macrophage and Collagen Areas
Percentage area of macrophages was acquired by using commercially available image analysis computer software (Image Pro Plus). Aortic cross sections (5 µm) were stained immunohistochemically with RAM-11 antibody (DAKO Corp) and developed with a Fast Red TR/naphthol AS-MX alkaline phosphatase substrate tablet kit (Sigma catalog No. F4648). The area of plaque was traced to establish an area of interest, which was set equal to 100%. The areas containing macrophages were then digitally “painted” and detected by the density of red staining. The quantity of digitally painted areas was then calculated electronically as a percentage of the overall selected area of interest. Three slices were chosen at random from each animal.
Similarly, percentage area of collagen was acquired by first staining aortic cross sections with Masson’ s trichome. The blue-stained areas corresponded to collagen. The areas were again digitally painted, and the areas were calculated as a percentage of the overall size of plaque. All pathological measurements were done in a blinded fashion.
Blood Pressure Measurements
Blood pressure measurements were obtained on a biweekly basis by a blood pressure measuring device (Datascope Accutorr 2A) with an NIBP cuff (newborn, Datascope Corp) placed on the rabbit’s hind leg.
Statistical Analysis
All data are reported as mean±SEM. Comparisons and correlations were made with unpaired t test, least-squares linear regression, and Fisher’s exact test, where appropriate. A probability value ≤0.05 was considered significant.
Results
Pretrigger Weights, Cholesterol, and Blood Pressure Levels
All animals underwent initial balloon injury without complications and recovered uneventfully. The weight of the rabbits at baseline was 3.2±0.4 kg, which increased to 3.6±0.3 kg (P=0.012) after the 8-week 1% cholesterol diet period.
The total serum cholesterol at baseline for the control animals was 42.3±11 mg/dL and for the candesartan-treated rabbits, 43.5±13.9 mg/dL. After 8 weeks on the 1% cholesterol diet (just before triggering), the serum total cholesterol level had increased to 961±214 mg/dL, whereas the candesartan-treated rabbits had a mean cholesterol level of 1240±254 mg/dL. These values were not significantly different from each other (P=0.41, t test). Similarly, the pretrigger LDL levels in the control animals were 773.6±190 mg/dL compared with 930.7±206 mg/dL in the candesartantreated group, an insignificant difference (P=0.1125).
The average blood pressure of a subgroup of 9 candesartan-treated rabbits was124±6/52±3 mm Hg and was not significantly lower than that in a subgroup of 9 control rabbits (133±6/53±3 mmHg, P =0.3 for systolic blood pressure).
Degree of Atherosclerosis
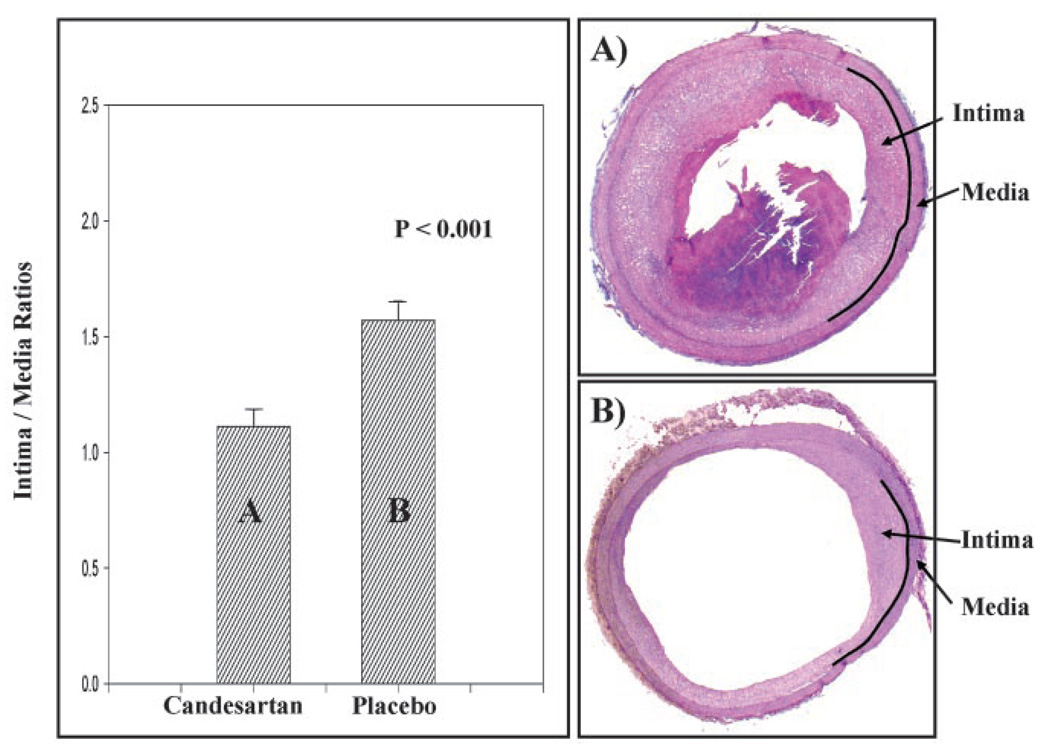
The intima-media ratio of the infrarenal abdominal aorta in the 13 rabbits that received 0.5 mg/kg candesartan daily was examined and compared with those of the control rabbits. Six cross sections of the infrarenal aorta were used from each rabbit and averaged. We limited analysis to the infrarenal aorta because it appeared to be the most atherosclerotic portion, and all thrombi were found within the infrarenal aorta. The intima-media ratio of the infrarenal aortas was 1.57±0.08 for the placebo group compared with 1.18±0.08 in the candesartan-treated group (P<0.001, see Figure 2).
Figure 2.
Bar graph and representative cross sections of infrarenal aortas comparing intima-media ratios of rabbits receiving 0.5 mg/kg candesartan daily with placebo control. Intima-media ratio of infrarenal aortas was 1.57±0.08 (mean±SEM) for placebo group compared with 1.18±0.08 for candesartan-treated animals (P<0.001). Intra-aortic thrombi developed in 11 of 15 rabbits in control group compared with 3 of 13 in candesartan-treated group (P<0.05). Representative histological sections of rabbit aortas after triggering but before euthanization in (A) candesartan group (0.5 mg/kg) showed little intimal thickening with no thrombus seen and in (B) placebo control rabbits, showing significant intimal thickening and thrombus.
Rate of Plaque Disruption and Thrombus Formation
We compared the frequency of plaque disruption with overlying thrombus in rabbits that received 0.5 mg/kg candesartan daily for 8 weeks with that of a placebo group. In the placebo control group of 15 rabbits, 11 animals developed plaque disruption and thrombosis. In contrast, only 3 of 13 rabbits that were treated with 0.5 mg/kg candesartan daily developed plaque disruption and thrombi, a significant difference (P<0.05). This rate of plaque disruption is 70% less than that in control rabbits. All plaque disruption and thrombi occurred in the infrarenal aorta above the bifurcation. There was plaque rupture with hemorrhage into the plaque visible in 6 of 11 control rabbits and in 1 of 3 candesartan-treated rabbits.
Effect of Candesartan on Macrophage Recruitment to the Plaque
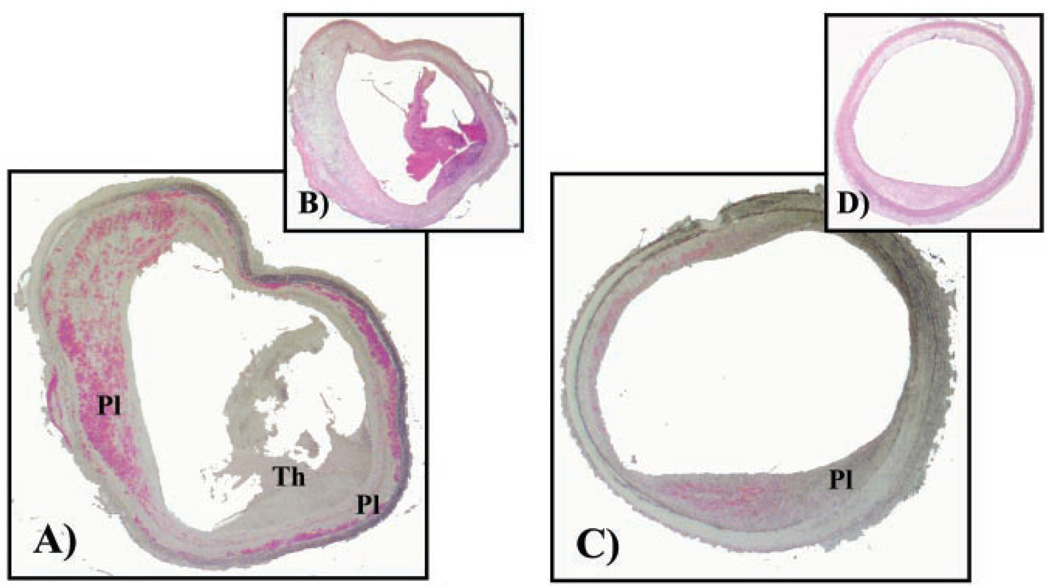
Plaque vulnerability may in part be due to macrophage- and lipid-rich regions in the atheroma. Because fewer candesartan-treated rabbits had plaque disruption than the placebo controls, the ratio of macrophage area to plaque area was examined to determine whether candesartan had an effect on macrophage recruitment into the plaque. We examined 3 random sections stained for macrophages in the infrarenal region of each rabbit aorta and compared the relative proportion of macrophage-stained areas to the plaque area of candesartan-treated rabbits versus the placebo controls. We limited our examination to the infrarenal regions of the aorta because all plaque disruptions occurred in this region. We found a significant difference in the proportion of the macrophage-stained region to the plaque area in the candesartan-treated rabbits (18.8±2.7% versus placebo controls, 27±2.5%, P<0.05, see Figure 3).
Figure 3.
Macrophage immunostaining (RAM1-11, DAKO Corp, fast red stain) and H&E staining of representative rabbit aortic cross section (5 µm) of placebo (A, B) and candesartan-treated (C, D) rabbits, respectively. Plaque area and thrombus are depicted by Pl and Th, respectively. Placebo sections(C, D) depict thrombus. Percentage area of macrophages was determined relative to total plaque area.
Effect of Candesartan on Collagen Deposition in the Plaque
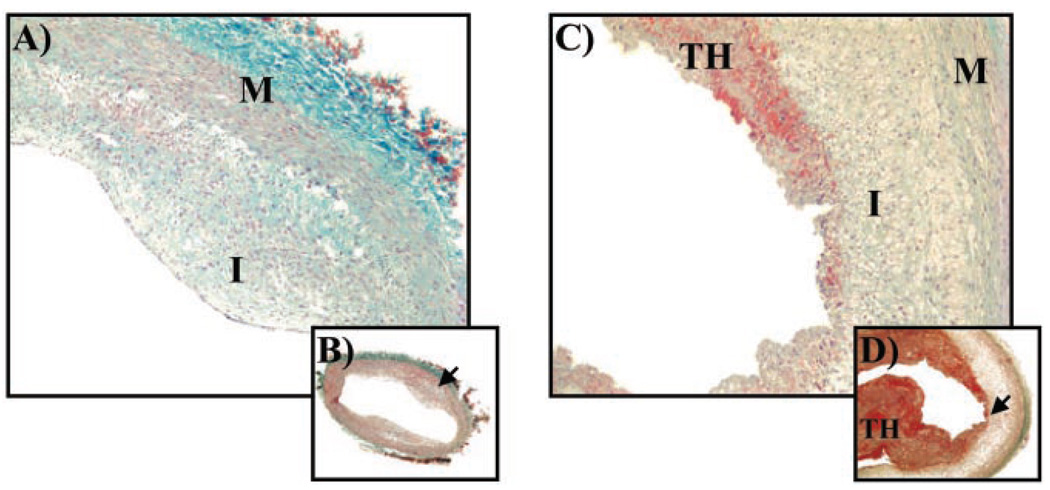
If candesartan improves plaque stability, then it may not only decrease macrophage deposition but also increase collagen deposition within the atheroma. We therefore evaluated the degree of collagen deposition by examining infrarenal aortic tissue sections of both control and candesartan-treated rabbits that were stained with Masson’s trichome, a stain for collagen. We found significantly more collagen in plaques of candesartan-treated rabbits (4±3%) versus placebo control (36±2%, P<0.01, see Figure 4).
Figure 4.
Masson’s trichrome stain of aortic cross sections (5 µm) from candesartan-treated (A, B; high- [×10] and low- [×4] power, respectively) and placebo-control (C, D; high- and low-power, respectively) rabbits. Sections from placebo control rabbit show plaque erosion with overlying thrombus (TH). Blue staining denotes collagen. I indicates intima; M, media.
Discussion
Results from the recent Heart Outcomes Prevention Evaluation (HOPE) trial8 suggest that angiotensin-converting enzyme inhibitors decrease the development of cardiovascular complications of atherosclerosis. Although this would confirm the many studies that demonstrate the role of angiotensin II in atherogenesis, few studies demonstrate that direct angiotensin receptor blockade has a similar effect on the development of atherosclerosis and its effect on acute manifestations of cardiovascular disease. These series of experiments demonstrate that candesartan has an effect on the development of atherosclerosis in the aortas of balloon-injured, high cholesterol–fed rabbits. Furthermore, candesartan appears to decrease the development of thrombosis after pharmacological triggering.
Rabbits that received 0.5 mg/kg candesartan daily had 30% less atherosclerosis than did placebo-control rabbits. The candesartan-treated rabbits’ plaques were less macrophage-rich but more collagen-rich than those in placebo controls. The rate of thrombosis after pharmacological triggering in these rabbits was 25% that of the control rabbits. The systolic blood pressures between the 2 groups were not significantly different. Similarly, there was an insignificant difference with regard to the total cholesterol levels in each group.
This is the first demonstration that angiotensin receptor blockade can decrease the frequency of plaque disruption. This confirms previous reports that angiotensin receptor blockers can attenuate the degree of both atherosclerosis and macrophage recruitment within the plaque. Presumably, this effect is a result of the angiotensin receptor blocker’s preventing angiotensin II from binding to the vascular AT1 receptor. These results also confirm previous studies, which demonstrated the importance of the AT1 receptor in atherogenesis. Yang and colleagues12 found that AT1 receptor expression was increased in hypercholesterolemic atherosclerotic rabbits. Strawn and colleagues13 showed that angiotensin receptor blockers decreased the development of atherosclerosis and vascular cell adhesion molecule expression in high cholesterol–fed monkeys. Similar results were reported in Watanabe rabbits.14 The mechanism underlying the decrease in atherosclerosis after AT1 blockade is unknown. Various groups have reported that angiotensin receptor blockers modulate cellular adhesion molecules in atherosclerosis,15 decrease macrophage accumulation16 and chemokine expression,16 and attenuate LDL oxidation.17 Others have also shown decreases in lipoxygenase-1 expression,18 as well as decreasing NAD(P)H oxidase,19 thereby reducing oxidative stress in the vessel wall. Each of these mechanisms can contribute directly to plaque development and overall plaque stability.
These results support our findings, which demonstrate a decrease in macrophage accumulation in the plaque. This may be explained on the basis that AT1 blockade resulted in a decrease in monocyte chemoattractant protein-13 or a reduction in NADP(H) oxidase, which in turn would reduce macrophage infiltration.20 Alternatively, blocking the AT1 receptor increases stimulation of the AT2 receptor by angiotensin II, thereby reducing the inflammatory response.21 Because macrophage-rich lesions are more prone to disruption, any diminution of macrophage area would lead to plaque stabilization.22 This may have played a role in the reduced plaque disruption in the candesartan-treated rabbits. To our knowledge, this is the first demonstration that angiotensin receptor blockers increase collagen deposition, which is an additional means of plaque stabilization. Recent data23 have suggested that angiotensin receptor blockers can also attenuate the activity of metalloproteinases, which digest collagen.
Vaughan and colleagues24 have demonstrated that angiotensin II promotes the production of plasminogen activator inhibitor-1 in vascular cells, which could contribute to the development of thrombi. Hamdan et al25 showed that angiotensin-converting enzyme inhibitors reduced plasminogen activator inhibitor-1 production, which may partly explain why candesartan reduced the presence of intra-aortic thrombi after triggering. We have recently demonstrated that candesartan can reduce tissue factor expression in rat aortic smooth muscle cells, which may also explain candesartan’ s antithrombotic effect.26 Angiotensin receptor blockade may also suppress platelet activity by antagonizing thromboxane signaling.27
We did not demonstrate a significant effect of candesartan on blood pressure in this rabbit animal model. Although the number of animals studied was small, this could suggest that the angiotensin receptor blocker’s effect on atherogenesis in rabbits is independent of its blood pressure effect.
Modified Constantinides Animal Model
The modified Constantinides animal model10 is one of the few animal models of acute plaque disruption and thrombosis and is relatively easy to use. In this model, the degree of atherosclerosis tends to be greater in the abdominal aorta than the thoracic aorta, which might explain why the thrombi in the aortas of rabbits were consistently located infrarenally but above the aortic bifurcations.
Limitations
The rabbits in the experimental arm were pretreated with candesartan before aortic balloon injury and cholesterol feeding were initiated. This protocol could have served to optimize the effect of angiotensin receptor blockade. Furthermore, candesartan resulted in a small but insignificant reduction in systolic blood pressure. Although the range of blood pressure was normal for that species, a lower blood pressure could reduce the frequency of plaque disruption. The reduction in the frequency of plaque disruption and thrombosis in the candesartan-treated rabbits may, in part, be a consequence of the lesser degree of atherosclerosis. Further studies are needed to demonstrate whether the effect of angiotensin receptor blockers on plaque disruption is a result of decreased atherosclerosis alone. This could be best shown by initiating candesartan treatment in already atherosclerotic animals and then inducing pharmacological triggering. It is, however, important to note that the modified Constantinides animal model has not to date been shown to predict therapeutic effects in humans.
Conclusion
In this study, we demonstrated that candesartan (1) attenuates the development of atherosclerosis; (2) reduces macrophage accumulation; and (3) increases collagen deposition within the plaque, thereby reducing the frequency of plaque disruption. These results suggest that angiotensin receptor blockade may have a similar effect on the reduction of acute cardiovascular events, as angiotensin-converting enzyme inhibitors demonstrated in the HOPE trial.8 Further studies are needed to better elucidate the mechanism underlying its effect on atherogenesis and whether it is similarly effective in clinical application.
Acknowledgments
This research was supported by grants from Astra-Zeneca Pharmaceuticals (to Dr Johnstone) and the National Institutes of Health (RO1 HL-61825, to Drs Hamilton and Johnstone). We thank Ed Feener for his helpful comments.
References
- 1.Murry 1, Dzau VJ, Bernstein K, Celermajer D, Cohen J, Dahlof B, Deanfield J, Diez J, Drexler H, Ferrari R, van Gilst W, Hannson L, Hornig B, Husain A, Johnston C, Lazar H, Lonn E, Luscher T, Mancini J, Pepine C, Rabelink T, Remme W, Ruiolope L, Ruzicka M, Schunkert H, Swedberg K, Unger T, Vaughan D, Weber M. The relevance of tissue angiotensin-converting enzyme: manifestations in mechanistic and endpoint data. Am J Cardiol. 2001;88(suppl):1L–20L. doi: 10.1016/s0002-9149(01)01878-1. [DOI] [PubMed] [Google Scholar]
- 2.de las Heras N, Aragoncillo P, Maeso R, Vasquez-Perez S, Navarro-Cid J, DeGasparo M, Mann J, Ruilope LM, Cachofeiro V, Lahera V. AT1 receptor antagonism reduces endothelial dysfunction and intimal thickening in atherosclerotic rabbits. Hypertension. 1999;34(pt 2):969–975. doi: 10.1161/01.hyp.34.4.969. [DOI] [PubMed] [Google Scholar]
- 3.Chen XL, Tummala PE, Olbrych MT, Alexander RW, Medford RM. Angiotensin II induces monocyte chemoattractant protein-1 gene expression in rat vascular smooth muscle cells. Circ Res. 1998;83:952–959. doi: 10.1161/01.res.83.9.952. [DOI] [PubMed] [Google Scholar]
- 4.Naftilan AJ, Pratt RE, Dzau VJ. Induction of platelet-derived growth factor A-chain and c-myc gene expressions by angiotensin II in cultured rat vascular smooth muscle cells. J Clin Invest. 1989;83:1419–1424. doi: 10.1172/JCI114032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Itoh H, Mukoyama M, Pratt RE, Gibbons GH, Dzau VJ. Multiple autocrine growth factors modulate vascular smooth muscle cell growth response to angiotensin II. J Clin Invest. 1993;91:2268–2274. doi: 10.1172/JCI116454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T, Masaki T. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 7.Vaughan DE. AT1 receptor blockade and atherosclerosis: hopeful insights into vascular protection. Circulation. 2000;101:1496–1497. doi: 10.1161/01.cir.101.13.1496. [DOI] [PubMed] [Google Scholar]
- 8.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Degenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients: the Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 9.Constantinides P, Chakravarti RN. Rabbit arterial thrombosis production by systemic procedures. Arch Pathol. 1961;72:197–208. [PubMed] [Google Scholar]
- 10.Abela GS, Picon PD, Friedl SE, Gebara OC, Miyamoto A, Ferderman M, Tofler GH, Muller JE. Triggering of plaque disruption and arterial thrombosis in an atherosclerotic rabbit model. Circulation. 1995;91:776–784. doi: 10.1161/01.cir.91.3.776. [DOI] [PubMed] [Google Scholar]
- 11.Johnstone MT, Botner RM, Perez AS, Stewart R, Quist WC, Hamilton JA, Manning WJ. In vivo magnetic resonance imaging of experimental thrombosis in a rabbit model. Arterioscler Thromb Vasc Biol. 2001;21:1556–1560. doi: 10.1161/hq0901.094242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang BC, Phillips MI, Mohuczy D, Meng H, Shen L, Mehta P, Mehta JL. Increased angiotensin II type 1 receptor expression in hypercholesterolemic atherosclerosis in rabbits. Arterioscler Thromb Vasc Biol. 1998;18:1433–1449. doi: 10.1161/01.atv.18.9.1433. [DOI] [PubMed] [Google Scholar]
- 13.Strawn WB, Chappell MC, Dean RH, Kivlighn S, Ferrario CM. Inhibition of early atherogenesis by losartan in monkeys with diet-induced hypercholesterolemia. Circulation. 2000;101:1586–1593. doi: 10.1161/01.cir.101.13.1586. [DOI] [PubMed] [Google Scholar]
- 14.Papademetriou V. The potential role of AT1-receptor blockade in the prevention and reversal of atherosclerosis. J Hum Hypertens. 2002;16(suppl 3):S34–S41. doi: 10.1038/sj.jhh.1001437. [DOI] [PubMed] [Google Scholar]
- 15.Prasad A, Koh KK, Schenke WH, Mincemoyer R, Csako G, Fleischer TA, Brown M, Selvaggi TA, Quyyumi AA. Role of angiotensin II type 1 receptor in the regulation of cellular adhesion molecules in atherosclerosis. Am Heart J. 2001;142:248–253. doi: 10.1067/mhj.2001.116699. [DOI] [PubMed] [Google Scholar]
- 16.Dol F, Martin G, Staels B, Mares AM, Cazaubon C, Nisato D, Bidouard JP, Janiak P, Schaeffer P, Herbert JM. Angiotensin AT1 receptor antagonist irbesartan decreases lesion size, chemokine expression, and macrophage accumulation in apolipoprotein E-deficient mice. J Cardiovasc Pharmacol. 2001;38:395–405. doi: 10.1097/00005344-200109000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Rachmani R, Levi Z, Zadok BS, Ravid M. Losartan and lercanidipine attenuate low-density lipoprotein oxidation in patients with hypertension and type 2 diabetes mellitus: a randomized, prospective crossover study. Clin Pharmacol Ther. 2002;72:302–307. doi: 10.1067/mcp.2002.127110. [DOI] [PubMed] [Google Scholar]
- 18.Chen H, Li D, Sawamura T, Inoue K, Mehta JL. Upregulation of LOX-1 expression in aorta of hypercholesterolemic rabbits: modulation by losartan. Biochem Biophys Res Commun. 2000;276:1100–1104. doi: 10.1006/bbrc.2000.3532. [DOI] [PubMed] [Google Scholar]
- 19.Rueckschloss U, Quinn MT, Holtz J, Morawietz H. Dose-dependent regulation of NAD(P)H oxidase expression by angiotensin II in human endothelial cells: protective effect of angiotensin II type 1 receptor blockade in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2002;22:1845–1851. doi: 10.1161/01.atv.0000035392.38687.65. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Yang F, Jankowski M, Pagano PJ. NAD(P)H oxidase mediates angiotensin II-induced vascular macrophage infiltration and medial hypertrophy. Arterioscler Thromb Vasc Biol. 2003;13:776–782. doi: 10.1161/01.ATV.0000066684.37829.16. [DOI] [PubMed] [Google Scholar]
- 21.Wu L, Iwai M, Nakagami H, Li Z, Chen R, Suzuki J, Akishita M, de Gasparo M, Horiuchi M. Roles of angiotensin II type 2 receptor stimulation associated with selective angiotensin II type 1 receptor blockade with valsartan in the improvement of inflammation-induced vascular injury. Circulation. 2001;104:2716–2721. doi: 10.1161/hc4601.099404. [DOI] [PubMed] [Google Scholar]
- 22.Lee RT, Libby P. The unstable atheroma. Arterioscler Thromb Vasc Biol. 1997;17:1859–1867. doi: 10.1161/01.atv.17.10.1859. [DOI] [PubMed] [Google Scholar]
- 23.Cipollone F, Fazia M, Iezzi A, Pini B, Cuccurullo C, Zuchelli M, de Cesare D, Ucchino S, Spigonardo F, De Luca M, Muraro R, Bei R, Bucci M, Cuccurullo F, Mezzetti A. Blockade of the angiotensin II type 1 receptor stabilizes atherosclerotic plaques in humans by inhibiting prostaglandin E2-dependent matrix metalloproteinase activity. Circulation. 2004;109:1482–1488. doi: 10.1161/01.CIR.0000121735.52471.AC. [DOI] [PubMed] [Google Scholar]
- 24.Vaughan DE, Lazos SA, Tong K. Angiotensin II regulates the expression of plasminogen activator inhibitor-1 in cultured endothelial cells: a potential link between the renin-angiotensin system and thrombosis. J Clin Invest. 1995;95:995–1001. doi: 10.1172/JCI117809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamdan AD, Quist WC, Gagne JB, Feener EP. Angiotensin-converting enzyme inhibition suppresses plasminogen activator inhibitor-1 expression in the neointima of balloon-injured rat aorta. Circulation. 1996;93:1073–1078. doi: 10.1161/01.cir.93.6.1073. [DOI] [PubMed] [Google Scholar]
- 26.Johnstone MT, Perez A, Feener EP. Nitric oxide and the angiotensin II receptor blocker candesartan are potent inhibitors of tissue factor expression in vascular smooth muscle cells. J Am Coll Cardiol. 2001;37(suppl A):1078–1184. Abstract. [Google Scholar]
- 27.Schwemmer M, Sommer O, Bassenge E. Angiotensin receptor blocker losartan suppresses platelet activity by interfering with thromboxane signaling. Cardiovasc Drugs Ther. 2001;15:301–307. doi: 10.1023/a:1012750430056. [DOI] [PubMed] [Google Scholar]