Abstract
In skeletal muscle, oxygen (O2) delivery to appropriately meet metabolic need requires mechanisms for detection of the magnitude of O2 demand and the regulation of O2 delivery. Erythrocytes, when exposed to decreases in O2 tension, release both O2 and the vasodilator, adenosine triphosphate (ATP). The aims of this study were to establish that erythrocytes release ATP in response to reduced O2 tension and determine if erythrocytes are necessary for dilation of isolated skeletal muscle arterioles exposed to reduced extra-luminal O2 tension. Rabbit erythrocytes exposed to reduced O2 tension in a tonometer (n = 5, PO2 = 27 ± 3, p<0.01) released ATP in response to reduced O2 tension. ATP release increased proportional to the decrease in O2 tension. The contribution of erythrocytes to the response of skeletal muscle arterioles to reduced extra-luminal O2 tension was determined using isolated hamster cheek pouch retractor muscle arterioles perfused with buffer (n = 11, mean diameter 52 ± 3 μm) in the absence and presence of rabbit erythrocytes. Without erythrocytes, arterioles did not dilate when exposed to reduced extra-luminal O2 tension (PO2 = 32 ± 4 mm Hg). In contrast, when rabbit erythrocytes were present in the perfusate (hematocrit 15%) the same decrease in O2 tension resulted in a 20 ± 4% dilation (p<0.01). These results provide support for the hypothesis that erythrocytes, via their ability to release O2 along with ATP in response to exposure to reduced O2 tension, can participate in the matching of O2 delivery with metabolic need in skeletal muscle.
Keywords: adenosine triphosphate, vasodilation, hypoxia, hamster, microcirculation
Introduction
The oxygen (O2) required to meet the metabolic needs of all tissues is delivered by the erythrocyte, a small flexible cell that contains large amounts of the O2 transporting protein, hemoglobin. Since mammalian erythrocytes lack a nucleus, mitochondria and protein synthetic machinery, these cells were often presumed to be little more than passive O2 transporters. More recently, several groups have suggested that erythrocytes of humans and rabbits could contribute to the control of vascular caliber via their ability to release adenosine triphosphate (ATP) in response to physiological stimuli, including exposure to reduced O2 tension [1,3,7,8,15,17,23].
A signaling pathway that relates physiological stimuli to ATP release from rabbit and human erythrocytes has been described [22–24,29,31]. This pathway includes the heterotrimeric G protein, Gi, adenylyl cyclase, protein kinase A and the cystic fibrosis transmembrane conductance regulator. Importantly, exposure to reduced O2 tension, as would occur when metabolic need is increased in skeletal muscle, activates this signaling pathway resulting in ATP release from erythrocytes of rabbits and humans [1,3,7,8,15,24]. This finding, coupled with reports that ATP stimulates vasodilation in the microcirculation, suggests that the erythrocyte can not only carry O2, but it also participates in matching O2 delivery with metabolic need [3,6,7,17]. Failure of the erythrocyte to release ATP in response to reduced O2 tension could be expected to contribute to peripheral vascular disease.
Here we investigate the hypothesis that rabbit erythrocytes release ATP in response to exposure to reduced O2 tension and that amounts of ATP released correlate with the reduction in O2 tension. In addition, we determined the contribution of rabbit erythrocytes to the response of isolated perfused skeletal muscle arterioles to reduced extraluminal O2 tension. Specifically, we sought to establish that erythrocytes are a necessary component of the perfusate of isolated resistance vessels in order to demonstrate vasodilation in response to exposure to reduced extraluminal O2 tension as would be encountered in vivo.
Materials and Methods
Isolation of Washed Rabbit Erythrocytes
Rabbit blood was obtained from male New Zealand white rabbits (2–3 kg). The animals were anesthetized with ketamine (12.5 mg/kg) and xylazine (1.5 mg/kg) intramuscularly followed by pentobarbital sodium (10 mg/kg) administered intravenously via a catheter placed in an ear vein. After tracheal intubation, the animals were ventilated with room air (tidal volume, 10 ml/kg; rate, 25 breaths/min). A catheter was placed in a carotid artery for administration of heparin (500 units) and for blood removal. The animals were exsanguinated 10 min after heparin administration. Whole anti-coagulated blood was centrifuged at 500 × g for 10 minutes at 4°C. The plasma, buffy coat, and uppermost erythrocyte layer were removed by aspiration and discarded. The remaining erythrocytes were washed three times in buffer containing (in mM) 21.0 Tris-HCl, 4.7 KCl, 2.0 CaCl2, 140.5 NaCl, 1.2 MgSO4, 5.5 glucose and 0.5% bovine albumin fraction V, final pH 7.4. The hematocrit of the washed erythrocytes was determined by micro-centrifugation.
Measurement of ATP
ATP was measured using the luciferin-luciferase assay as described previously [29,32]. Briefly, a 200 μl sample of an erythrocyte suspension was injected into a cuvette containing 100 μl of 10 mg/ml crude firefly tail extract (Sigma) and 100 μl of a 0.5 mg/ml solution of D-luciferin (Sigma). The light emitted from the reaction of ATP with the crude firefly tail extract was measured using a luminometer (TD 20/20, Turner Designs). The peak light emitted was compared to an ATP standard curve generated on the day of the experiment.
Measurement of Free Hemoglobin
Erythrocyte suspensions used to measure ATP were centrifuged at 500 × g for 10 minutes at 4° C. The amount of hemoglobin present in the supernatant was determined by measurement of absorbance at 405 nm (oxyhemoglobin). If any free hemoglobin was detected, the studies were not included.
Exposure of Erythrocytes to Reduced Oxygen Tension
Washed erythrocytes were diluted in buffer containing (in mM) 21.4 NaHCO3, 4.7 KCl, 2.0 CaCl2, 140.5 NaCl, 1.2 MgSO4, 11.0 glucose, and 0.5% bovine albumin fraction V, pH ~ 7.4 at 37 °C when equilibrated with gas containing 6% CO2. Erythrocytes (20% hematocrit) were equilibrated for 30 minutes in a tonometer (model 237, Instrumentation Laboratories) with gas containing 15% O2, 6% CO2, balance N2 (normoxia). Erythrocytes were then exposed to gas containing 5% O2, 6% CO2, balance N2 (reduced O2 tension) for 10 min. ATP released from the erythrocytes was determined as described above. In addition, pH, O2 and CO2 tensions were determined using a blood gas analyzer (model pHOx, Nova Biomedical).
Isolation, Cannulation and Perfusion of Arterioles from Hamster Skeletal Muscle
Male golden hamsters (104 ± 4 gm) were anesthetized with pentobarbital sodium (6.5 mg/100g body wt, ip). The trachea was cannulated to ensure a patent airway for spontaneous breathing. The right cheek pouch retractor muscle was surgically exteriorized as described by Sullivan and Pittman [33]. In brief, the muscle was separated from underlying back muscles and a clip was used to secure two ligatures to the muscle. The muscle was then cut at its spinal end and placed ventral side up, at its in situ dimensions on a Plexiglass platform and covered with plastic film (Saran, Dow Corning) to prevent desiccation of the tissue. Unbranched segments of first and second order arterioles, approximately 1000 μm in length, were surgically removed from the muscle. The vessel was trimmed and cleared of connective tissue while immersed in cold (4°C) modified Ringer’s buffer containing (in mEq/l): 144.0 NaCl, 3.0 KCl, 2.5 CaCl2, 1.5 MgSO4, 5.0 glucose, 2.0 pyruvate, 0.02 ethylenediaminetetraacetic acid (EDTA), 2.0 3-[N-morpholino]-propanesulfonic acid (MOPS), 1.21 NaH2PO4 and 1% bovine serum albumin (dialyzed for 48 hours against distilled water and 48 hours against MOPS-Ringer) with pH adjusted to 7.40. The vessel was then transferred to an organ bath (2.5 ml) mounted on a microscope stage containing the Ringer’s buffer described above but without albumin.
Isolated arterioles were cannulated using concentric glass pipettes (constructed on a Stoelting microforge) and assembled with a larger outer holding pipette and a smaller inner perfusion pipette. The pipette assembly was mounted on micromanipulators attached to the microscope base. Each end of the vessel was, in turn, aspirated into the holding pipette using a controlled vacuum and cannulated with the perfusion pipette filled with the albumin containing Ringer’s buffer described above. The vessel was held in place suspended between the two pipettes. Following stabilization, intraluminal pressure was increased to 60 mmHg while the bath temperature was increased to 37°C. The vessel was allowed to develop spontaneous tone over the next 30–45 minutes. The vessel was viewed using a Zeiss Axiovert 100 inverted microscope with long working distance objectives (10× and 20×). The microscope image was recorded using a high resolution, closed circuit video system consisting of a CCD video camera (model 72, Dage-MTI), video monitor (PVM-137, Sony), S-VHS video recorder (AG1970, Panasonic) and a time-date generator (WJ-810, Panasonic). Vessel diameter was determined off-line using both an automated system (Diamtrax, version 3.5) and direct measurement using a video caliper (model 308, Colorado Video).
Following the development of spontaneous tone, viability of the vessel was determined by the demonstration of constriction in response to alkaline pH (7.65) and dilation to an acidic pH (6.80). Vessels were subsequently perfused with the same albumin containing Ringer’s buffer at 3 μl/min using a 3-syringe microinjection pump (model CMA/100, CMA/Microdialysis). The pump was configured in such a way that the perfusate could be instantly switched by means of a microswitch. Initially, the buffer surrounding the vessel was equilibrated with room air (PO2 ~ 135 mm Hg). The PO2 in the microscope chamber was measured using an oxygen microelectrode (MI 730, Microelectrodes Inc.) polarized to −0.7V and a Chemical microsensor, (Diamond Electro-Tech Inc.). After stability was achieved and vessel diameter recorded, the buffer in the vessel chamber was replaced with buffer equilibrated with 100% nitrogen (PO2 ~ 33 mm Hg). Vessel diameter was again recorded. The chamber buffer was then returned to one equilibrated with room air. Following stabilization, the perfusate was switched to albumin containing buffer to which washed rabbit erythrocytes (hematocrit 15%) were added. Care was taken to ensure that the erythrocytes were well distributed within the syringe. Again, the vessel was allowed to stabilize and the sequence of normal and low PO2 exposures repeated.
Data Analysis
Statistical significance between experiments was determined using either an analysis of variance (ANOVA) or Students t-test, as appropriate. In the case of ANOVA, in the event that the F ratio indicated that a change had occurred, a Fisher’s LSD test was performed to identify individual differences. Results are reported as means ± the standard error of the mean (SEM).
Institutional Approval
The protocols used to obtain blood from rabbits and all surgical and experimental procedures were approved by the Institutional Animal Care and Use Committee of Saint Louis University.
Results
Effect of Reduced O2 Tension on ATP Release from Erythrocytes
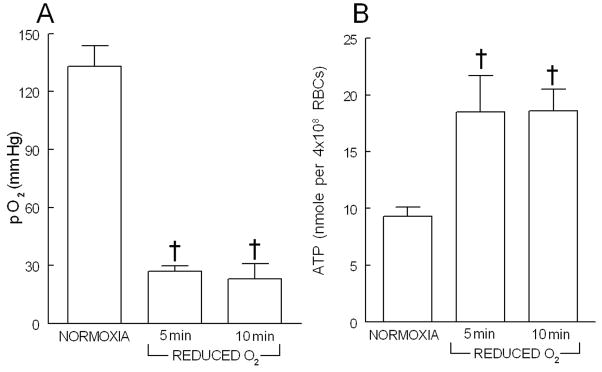
Exposure of erythrocytes (20% hematocrit) to 15% O2, 6% CO2, balance N2 in the tonometer resulted in a PO2 of 133 ± 11 mmHg (figure 1A, n=5). The pH and PCO2 of the erythrocytes were 7.49 ± 0.06 and 34.6 ± 0.8 mm Hg, respectively, and were not altered by exposure to reduced O2 tension. When the equilibrating gas was changed to 0% O2, 6% CO2, balance N2, PO2 decreased by 102 ± 10 and 115 ± 12 mm Hg after 5 and 10 min, respectively (figure 1A). ATP release from erythrocytes increased 5 min after exposure to reduced O2 and remained elevated at 10 min (figure 1B). Figure 2 depicts the relationship between the decrease in PO2 and the increase in ATP. Increases in ATP released correlated with the reduction in O2 tension (r2= 0.73, p<0.02).
Figure 1.
Effect of exposure of washed rabbit erythrocytes (20% hematocrit, n=5) to gas mixtures containing either 15% O2, 6% CO2, balance N2 (NORMOXIA) or 0% O2, 6% CO2, balance N2 (REDUCED O2) on oxygen tension (panel A) and ATP release (panel B). † = p< 0.01 compared to NORMOXIA. Values are mean ± SE.
Figure 2.
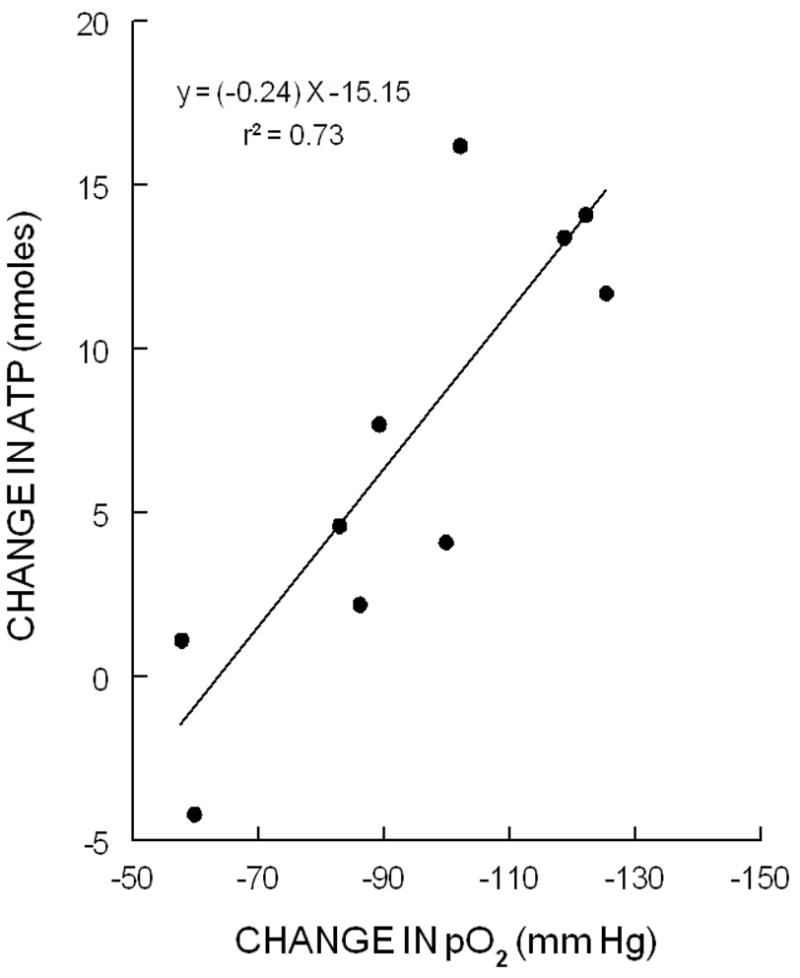
Linear regression relationship between the change (decrease) in O2 tension (pO2) and the change (increase) in ATP release from rabbit erythrocytes in response to exposure to reduced O2 (p<0.02).
Effect of Reduced O2 Tension on Vascular Caliber of Isolated Arterioles in the Absence and Presence of Erythrocytes
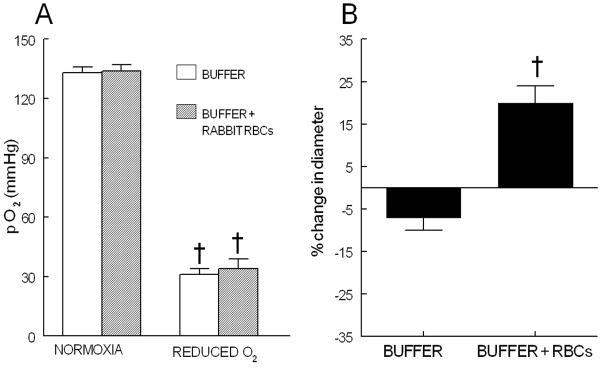
Eleven arterioles having an initial diameter of 28 ± 3 μm were pressurized to 60 mm Hg (initial diameter 76 ± 3 μm) and developed spontaneous tone resulting in a final diameter of 52 ± 3 μm. This level of spontaneous tone (32 ± 2% of the diameter at the time of initial pressurization) was maintained throughout the studies. Vessels included in these studies demonstrated a 14 ± 2 % constriction to pH 7.65 and a 20 ± 4 % dilation to pH 6.80. During perfusion with buffer in the absence of erythrocytes, when the extra-luminal buffer was switched to one equilibrated with 100% N2, there was a decrease in O2 tension from 134 ± 3 to 32 ± 4 mm Hg (figure 3A) that was associated with a small non-significant decrease in vessel diameter of 4 ± 2 μm (figure 3B). The vessel returned to its original diameter when the extra-luminal buffer was returned to one equilibrated with room air.
Figure 3.
Panel A: Buffer PO2 in the isolated vessel chamber when equilibrated with either room air (NORMOXIA) or 100% N2 (REDUCED O2) (n=11). † = p< 0.01 compared to NORMOXIA. Values are mean ± SE.
Panel B: Percent change in vessel diameter in response to exposure to reduced O2 tension in the absence (BUFFER) and presence of rabbit erythrocytes (BUFFER + RBCs, hematocrit 15%) (n=11). † = p< 0.01 compared to diameter during normoxia. Values are mean ± SE.
Similarly, when vessels were perfused with buffer containing washed rabbit erythrocytes (hematocrit of 15%), changing the extra-luminal buffer to one equilibrated with 100% N2 resulted in a decrease in PO2 that was not different from the value measured in vessels perfused with buffer in the absence of erythrocytes (figure 3A). However, in contrast to vessels perfused with buffer in the absence of erythrocytes, when rabbit erythrocytes were present in the perfusate, the vessels dilated when exposed to reduced O2 tension (33 ± 4 mm Hg). Under these conditions, vessel diameter increased from 47 ± 4 to 56 ± 4 μm (figure 3B). Importantly, the vessels again returned to their original diameter when the extra-luminal buffer was returned to one equilibrated with room air.
Discussion
It is well recognized that, within skeletal muscle, blood flow (O2 delivery) is matched with the metabolic requirements of the tissue (O2 utilization). Although several mechanisms that could mediate this fundamental physiological response have been proposed, none has been universally accepted. For example, it was suggested that arterioles feeding capillary beds in areas of increased O2 utilization could respond directly to decreased tissue O2 levels [2,14,19,26] or that a mediator(s) associated with increased O2 utilization could be released into the venous circulation and diffuse to feed arterioles producing dilation [11,12,27,28]. Although there is experimental data in support of both mechanisms, the results do not fully explain the tight coupling of O2 supply with demand observed in skeletal muscle.
The physiological regulation of the supply of O2 to meet exactly the metabolic need in skeletal muscle requires several components including a mechanism to sense and quantify the requirement for O2 and a means to communicate that information to vascular smooth muscle. Since the O2 content of the erythrocyte as it traverses a tissue is directly linked to the level of O2 utilization of that tissue, it has been proposed that the erythrocyte could assume a central role as a sensor of oxygen demand [7]. When exposed to reduced O2 tension, erythrocytes of several species, including rabbits and humans, release ATP. This ATP can then interact with purinergic receptors on the vascular endothelium [4,5,9,13,16,20] resulting in the synthesis and release of vasodilators including nitric oxide and metabolites of arachidonic acid [2,9,17,30,32]. Since ATP is metabolized by ecto-enzymes present in the circulation, its effects on vascular resistance would be expected to be limited to its site of release, in this case, the microcirculation [18,25].
Physiological effects of ATP in the microcirculation of the hamster cheek pouch retractor muscle have been well characterized [3,7,17]. Ellsworth et al. reported that ATP injected into either arterioles or venules produces vasodilation that is conducted to upstream sites [3,17]. The ability of ATP to stimulate conducted vasodilation is a physiologically important property since an increase in upstream arteriolar diameter is required if blood flow to the microcirculation is to be significantly increased. Importantly, in this model, conducted vasodilation was accompanied by a significant increase in erythrocyte supply rate and thus, O2 delivery [3,17].
Here we demonstrate that rabbit erythrocytes release ATP when exposed to reduced O2 tension (figure 1A) and that the increase in ATP released correlates directly with the magnitude of the decrease in O2 tension (figure 1B). That is, the ATP released upon exposure to reduced O2 tension is graded. This relationship between ATP release and the O2 tension would permit the erythrocyte to participate in the precise matching of O2 delivery with demand in skeletal muscle. However, if the release of ATP in response to reduced O2 tension is physiologically important, it must be demonstrated that this ATP produces vasodilation in microvessels.
To address this important issue, we performed a second series of experiments in which hamster skeletal muscle resistance vessels (arterioles) were isolated and perfused with either physiological buffer alone or the same buffer containing rabbit erythrocytes and exposed to reduced extraluminal O2 tension. In the absence of erythrocytes, exposure of the isolated vessels to an O2 tension that stimulates ATP release from erythrocytes (figures 1A and 2A) did not result in a significant change in vascular caliber (figure 2B). In marked contrast, when erythrocytes were present in the vessel perfusate, exposure of the vessels to the same decrease in O2 tension resulted in vasodilation (figure 3B). These studies provide strong support for the hypothesis that erythrocyte-derived ATP, released in response to exposure of these cells to reduced O2 tension, can contribute to the control of vascular caliber in skeletal muscle arterioles.
Numerous studies have evaluated the response of arterial blood vessels to decreased O2 tension. In 1973, Pittman and Duling reported that strips of hog carotid artery relaxed when exposed to reduced PO2 and that the magnitude of the relaxation was dependent upon the thickness of the vessel wall [26]. In these vessels, mechanical tension decreased in these only when the PO2 at the center of the vessel wall reached 2 mmHg. More recently, the O2 sensitivity of resistance arteries has been evaluated using segments of the first branch of the femoral artery of rats [10]. It was reported that, when these small arteries were simultaneously perfused and superfused with buffer equilibrated with low O2 tension in the absence of erythrocytes, the vessels dilated [10]. Although the latter study appears to contradict our finding that erythrocytes are required to demonstrate vasodilation when skeletal muscle arterioles are exposed to reduced O2 tension, several important factors distinguish the two studies. First, the study in rat vessels used muscular arteries which contain a significantly greater proportion of smooth muscle compared with the arterioles used in our study. Second, we continuously perfused the isolated arterioles with buffer equilibrated with room air and only the extraluminal O2 tension was reduced. This is in contrast to the previous study in which PO2 was reduced both intra- and extra-luminally. Differences in design notwithstanding, the results presented here demonstrate that, in isolated perfused hamster skeletal muscle arterioles, erythrocytes that release ATP in response to reduced O2 tension are a required component of the perfusate in order to demonstrate vasodilation when extraluminal PO2 is decreased. Although in the present study ATP release from the erythrocytes present within the isolated arteriole was not measured, previous studies support the hypothesis that the observed dilation was the result of erythrocyte-derived ATP [6]. In these earlier studies using isolated rat cerebral arterioles perfused at the same flow rate as the vessels studied here, similar reductions in extraluminal O2 tension stimulated increases in ATP measured in the vessel effluent. ATP was detected in the effluent only when the perfusate contained erythrocytes that release ATP in response to that stimulus [6]. In addition, these studies in rat cerebral arterioles established that the response was physiologically relevant based on the time required for the dilation to occur.
The finding that erythrocytes release both O2 and ATP when exposed to reduced O2 tension suggests that these cells could be major contributors to the matching of O2 supply with demand in skeletal muscle. At any location within the microcirculation the O2 content of the erythrocyte is directly linked to the level of O2 utilization. Importantly, as the erythrocyte enters an environment in which O2 utilization is increased, that cell releases O2 which addresses the metabolic need of the tissue, but also releases ATP which facilitates increases in blood flow (erythrocyte delivery rate) resulting in increased O2 supply. The finding that the magnitude of ATP release from erythrocytes correlates inversely with decreases in O2 tension suggests that this mechanism can contribute to the tight regulation of O2 supply with demand in skeletal muscle.
Acknowledgments
The authors thank J. L. Sprague for inspiration. This work was supported by grants from the National Institutes of Health (HL64180 and HL089094) and the American Diabetes Association (R-133) as well as an American Heart Association Fellowship Grant (MSH).
Abbreviations
- ATP
adenosine triphosphate
- O2
oxygen
- PO2
partial pressure of oxygen
- PCO2
partial pressure of carbon dioxide
References
- 1.Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnea. Cardiovasc Res. 1992;26:40–47. doi: 10.1093/cvr/26.1.40. [DOI] [PubMed] [Google Scholar]
- 2.Brown IP, Thompson CI, Belloni FL. Mechanisms of coronary vasodilation produced by ATP in guinea-pig isolated perfused heart. Br J Pharmacol. 1992;105:211–215. doi: 10.1111/j.1476-5381.1992.tb14236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins DM, McCullough WT, Ellsworth ML. Conducted vascular responses: Communication across the capillary bed. Microvas Res. 1998;56:43–53. doi: 10.1006/mvre.1998.2076. [DOI] [PubMed] [Google Scholar]
- 4.Communi D, Raspe E, Pirotton S, Boeynaems JM. Coexpression of P2y and P2u receptors on aortic endothelial cells: Comparison of cell localization and signaling pathways. Circ Res. 1991;76:191–198. doi: 10.1161/01.res.76.2.191. [DOI] [PubMed] [Google Scholar]
- 5.Dazel HH, Westfall DP. Receptors for adenine nucleotides and nucleosides: Subclassification, distribution and molecular characterization. Pharmacological Reviews. 1994;46:449–466. [PubMed] [Google Scholar]
- 6.Dietrich HH, Ellsworth ML, Sprague RS, Dacey RG. Red blood cell regulation of microvascular tone through adenosine triphosphate. Am J Physiol. 2000;278:H1294–H1298. doi: 10.1152/ajpheart.2000.278.4.H1294. [DOI] [PubMed] [Google Scholar]
- 7.Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol. 1995;269:H2155–H2161. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- 8.Ellsworth ML. The red blood cell as an oxygen sensor: what is the evidence? Acta Physiol Scand. 2000;168:551–559. doi: 10.1046/j.1365-201x.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- 9.Forsberg EJ, Feuerstein G, Shohami E, Pollard HB. Adenosine triphosphate stimulates inositol phospholipid metabolism and prostacyclin formation in adrenal medullary endothelial cells by means of P2-purinergic receptors. Proc Natl Acad Sci USA. 1987;84:5630–5634. doi: 10.1073/pnas.84.16.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frisbee JC, Maier KG, Falck JR, Roman RJ, Lombard JH. Integration of hypoxic dilation signaling pathways for skeletal muscle resistance arteries. Am J Physiol. 2002;283:R309–R319. doi: 10.1152/ajpregu.00741.2001. [DOI] [PubMed] [Google Scholar]
- 11.Hester RL. Uptake of metabolites by postcapillary venules: mechanism for the control of arteriolar diameter. Microvasc Res. 1993;46:254–261. doi: 10.1006/mvre.1993.1050. [DOI] [PubMed] [Google Scholar]
- 12.Hester RL. Venular-arteriolar diffusion of adenosine in hamster cremaster microcirculation. Am J Physiol. 1990;258:H1918–H1924. doi: 10.1152/ajpheart.1990.258.6.H1918. [DOI] [PubMed] [Google Scholar]
- 13.Houston DA, Burnstock G, Vanhoutte PM. Different P2-purinergic receptor subtypes of endothelium and smooth muscle in canine blood vessels. J Pharmacol Exp Ther. 1987;241:501–506. [PubMed] [Google Scholar]
- 14.Jackson WF. Arteriolar oxygen reactivity: where is the sensor. Am J Physiol. 1987;253:H1120–H1126. doi: 10.1152/ajpheart.1987.253.5.H1120. [DOI] [PubMed] [Google Scholar]
- 15.Jagger JE, Bateman RM, Ellsworth ML, Ellis CG. Role of erythrocyte in regulating local O2 delivery mediated by hemoglobin oxygenation. Am J Physiol. 2001;280:H2833–H2839. doi: 10.1152/ajpheart.2001.280.6.H2833. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy C, Delbro D, Burnstock G. P2-purinoceptors mediate both vasodilation (via the endothelium) and vasoconstriction of the isolated rat femoral artery. Eur J Pharmacol. 1985;107:161–168. doi: 10.1016/0014-2999(85)90055-x. [DOI] [PubMed] [Google Scholar]
- 17.McCullough WT, Collins DM, Ellsworth ML. Arteriolar responses to extracellular ATP in striated muscle. Am J Physiol. 1997;272:H1886–H1891. doi: 10.1152/ajpheart.1997.272.4.H1886. [DOI] [PubMed] [Google Scholar]
- 18.Meghji P, Pearson JD, Slakey LL. Kinetics of extracellular ATP hydrolysis by microvascular endothelial cells from rat heart. Biochem J. 1995;308:725–731. doi: 10.1042/bj3080725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messina EJ, Sun D, Koller A, Wolin MS, Kaley G. Increases in oxygen tension evoke arteriolar constriction by inhibiting endothelial prostaglandin synthesis. Microvasc Res. 1994;48:151–160. doi: 10.1006/mvre.1994.1046. [DOI] [PubMed] [Google Scholar]
- 20.Motte S, Pirotton S, Boeynaems JM. Heterogeneity of ATP receptors in aortic endothelial cells: Involvement of P2y and P2u receptors in inositol phosphate response. Circ Res. 1993;72:504–510. doi: 10.1161/01.res.72.3.504. [DOI] [PubMed] [Google Scholar]
- 21.Olearczyk JJ, Ellsworth ML, Stephenson AH, Lonigro AJ, Sprague RS. Nitric oxide Inhibits ATP release from erythrocytes. J Pharmacol Exp Ther. 2004;309:1079–1084. doi: 10.1124/jpet.103.064709. [DOI] [PubMed] [Google Scholar]
- 22.Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS. Receptor-mediated activation of the heterotrimeric G-protein Gs results in ATP release from erythrocytes. Med Sci Monit. 2001;7:669–674. [PubMed] [Google Scholar]
- 23.Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS. Heterotrimeric G protein Gi is involved in a signal transduction pathway for ATP release from erythrocytes. Am J Physiol. 2004;286:H940–H945. doi: 10.1152/ajpheart.00677.2003. [DOI] [PubMed] [Google Scholar]
- 24.Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS. NO Inhibits signal transduction pathway for ATP release from erythrocytes via its action on heterotrimeric G protein Gi. Am J Physiol. 2004;287:H748–H754. doi: 10.1152/ajpheart.00161.2004. [DOI] [PubMed] [Google Scholar]
- 25.Olsson RA, Pearson JD. Cardiovascular purinoceptors. Physiological Reviews. 1990;70:761–845. doi: 10.1152/physrev.1990.70.3.761. [DOI] [PubMed] [Google Scholar]
- 26.Pittman RN, Duling BR. Oxygen sensitivity of vascular smooth muscle. Microvasc Res. 1973;6:202–211. doi: 10.1016/0026-2862(73)90020-4. [DOI] [PubMed] [Google Scholar]
- 27.Saito Y, Eraslan A, Hester RL. Role of EDRFs in the control of arteriolar diameter during increased metabolism of striated muscle. Am J Physiol. 1994;267:H195–H200. doi: 10.1152/ajpheart.1994.267.1.H195. [DOI] [PubMed] [Google Scholar]
- 28.Sauls BA, Boegehold MA. Adenosine linking reduced O2 to arteriolar NO release in intestine is not formed from extracellular ATP. Am J Physiol. 2001;281:H1193–H1200. doi: 10.1152/ajpheart.2001.281.3.H1193. [DOI] [PubMed] [Google Scholar]
- 29.Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz M, Lonigro A. Deformation-induced ATP release from red blood cells requires cystic fibrosis transmembrane conductance regulator activity. Am J Physiol. 1998;275:H1726–H1732. doi: 10.1152/ajpheart.1998.275.5.H1726. [DOI] [PubMed] [Google Scholar]
- 30.Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. ATP: the red blood cell link to NO and local control of the pulmonary circulation. Am J Physiol. 1996;271:H2717–H2722. doi: 10.1152/ajpheart.1996.271.6.H2717. [DOI] [PubMed] [Google Scholar]
- 31.Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. Participation of cAMP in a signal -transduction pathway relating erythrocyte deformation to ATP Release. Am J Physiol. 2001;281:C1158–C1164. doi: 10.1152/ajpcell.2001.281.4.C1158. [DOI] [PubMed] [Google Scholar]
- 32.Sprague RS, Olearczyk JJ, Spence DM, Stephenson AH, Sprung R, Lonigro AJ. Extracellular ATP signaling in the rabbit lung: Erythrocytes as determinants of vascular resistance. Am J Physiol. 2003;285:H693–H700. doi: 10.1152/ajpheart.01026.2002. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan SM, Pittman RN. Hamster retractor muscle: a new preparation for intravital microscopy. Microvasc Res. 1982;23:329–35. doi: 10.1016/s0026-2862(82)80005-8. [DOI] [PubMed] [Google Scholar]