Abstract
The thalamus has a key function in processing sensory information, sleep, and cognition. We examined the effects of a common volatile anesthetic, isoflurane, on modulation of neuronal excitability in reticular thalamic nucleus (nRT) in intact brain slices from immature rats. In current-clamp recordings, isoflurane (300–600 µM) consistently depolarized membrane potential, decreased input resistance and inhibited both rebound burst firing and tonic spike firing modes of nRT neurons. The isoflurane-induced depolarization persisted not only in the presence of tetrodotoxin, but after replacement of Ca2+ with Ba2+ ions in external solution; it was abolished by partial replacement of extracellular Na+ ions with N-methyl-D-glucamine. In voltage-clamp recordings, we found that isoflurane slowed recovery from inactivation of T-type Ca2+ current. Thus, at clinically relevant concentrations, isoflurane inhibits neuronal excitability of nRT neurons in developing brain via multiple ion channels. Inhibition of the neuronal excitability of thalamic cells may contribute to impairment of sensory information transfer in the thalamocortical network by general anesthetics. The findings may be important for understanding cellular mechanisms of anesthesia such as loss of consciousness and potentially damaging consequences of general anesthetics on developing mammalian brains.
Keywords: thalamus, low-threshold-activated, isoflurane, calcium, sodium, reticular thalamic nucleus
INTRODUCTION
Recent studies implicate various thalamic nuclei in awareness and cognitive functions.1,2,3,4 The rhythmicity of thalamocortical circuitry depends to a great extent on the ability of thalamic cells to burst in oscillatory patterns at hyperpolarized membrane potentials (burst firing) and to fire sustained-action potentials at depolarized membrane potentials (tonic firing).2,3,4 It has been postulated that during tonic firing, which predominates during awake states, there is a faithful transfer of sensory information to cortical neurons; in contrast, during slow oscillations presented with burst firing pattern of these neurons, there is impairment of sensory transfer and transition to sleep states. A key element in rhythm generation within the thalamus is the GABAergic nucleus reticularis thalami (nRT), which is reciprocally connected to thalamocortical relay neurons and receives collateral excitatory connections from corticothalamic and thalamocortical fibers. In-vivo extracellular recordings have shown that volatile general anesthetics depress the excitability of thalamic neurons, which, in turn, may underlie blockade of thalamocortical information transfer.1 Thus, using whole-cell patch-clamp recordings in brain slices in vitro, we investigated the cellular excitability of nRT neurons in the presence of isoflurane (ISO), the prototypical volatile general anesthetic.
MATERIALS AND METHODS
In-vitro tissue slice preparation
Experiments were done on transverse rat brain slices cut through the middle anterior portion of the nRT. We have detailed elsewhere the procedures for in-vitro recording from intact nRT neurons in brain slices.5,6 Sprague-Dawley rats were housed in a local animal facility in accordance with protocols approved by the University of Virginia Animal Use and Care Committee. Rats (age 7–14 days) of either sex were briefly anesthetized with ISO and decapitated. The brains were rapidly removed and placed in iced cold (4°C) cutting solution consisting, in mM, of 2 CaCl2, 260 sucrose, 26 NaHCO3, 10 glucose, 3 KCl, 1.25 NaH2PO4, and 2 MgCl2 equilibrated with a mixture of 95% O2 and 5% CO2. We glued a block of tissue containing the thalamus to the chuck of a vibrotome (model NVSL, WPI, Sarasota, FL) and cut 250–300 µm slices in a transverse plane. We incubated the slices in 36°C-oxygenated saline for 1 hr before placing them in a recording chamber that had been superfused with external solution at a rate of 1.5 cc/min. The incubating solution consisted, in mM, of 124 NaCl, 4 KCl, 26 NaHCO3, 1.25 NaH2PO4, 2 MgCl2, 10 glucose, and 2 CaCl2 equilibrated with a mixture of 95% O2 and 5% CO2. Slices were maintained at room temperature in the recording chamber, where they remained viable for at least 1 hr.
Recording procedures
The extracellular saline solution used for recording Ca2+ currents in whole-cell experiments consisted, in mM, of 2 CaCl2, 130 NaCl, 2.5 MgCl2, 10 glucose, 26 NaHCO3, 1.25 NaH2PO4, and 0.001 tetrodotoxin (TTX). This solution was equilibrated with a mixture of 95% O2 and 5% CO2 for at least 30 min, until its pH was 7.35–7.45. The extracellular solution for current-clamp experiments was the same as the incubating solution. However, for some current-clamp experiments, we replaced NaCl in the external solution with equimolar N-methyl-D-glucamine (NMDG) and 2 mM Ca2Cl with 2 mM Ba2Cl; we then also included 1 µM TTX in the external solution. To eliminate glutamatergic excitatory currents, most current-clamp recordings were done in the presence of 5 µM NBQX (2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione) and 50 µM d-APV (2R)-amino-5-phosphonovaleric acid; AP5, (2R)-amino-5 phosphonopentanoate) in the bath solution. To eliminate inhibitory synaptic currents, most current-clamp recordings were done in the presence of 20 µM picrotoxin (a noncompetitive GABAA antagonist) in the bath solution.
To record T-currents in brain slices, we used an internal solution (solution 1) of, in mM,, 135–140 tetramethylammonium hydroxide (TMA-OH), 10 EGTA, 40 HEPES, and 2 MgCl2 titrated to pH 7.15–7.25 with hydrofluoric acid (HF).7 Recording electrodes for current-clamp studies contained, in mM, KCl 130, MgCl2 5, EGTA 1, Na-HEPES 40, MgATP 2, and Na3-GTP 0.1 (pH 7.2). For the data presented, membrane potential values were not corrected for the measured liquid junction potential of −10 mV in voltage-clamp experiments and −5 mV in current-clamp experiments. All recordings were obtained from thalamic neurons visualized with an infrared differential interference contrast camera (C2400; Hammamatsu, Hammamatsu City, Japan) on the Zeiss 2 FS Axioscope (Jena, Germany) with a 40X lens and patch-clamp pipette using a Sutter micromanipulator MP-285 (Sutter Instrument Co, Novato, CA).
Aliquots of anesthetic solution were prepared from saturated saline solution incubated with ISO (60 cc of saline with 40 cc of ISO in a closed 100 cc vial) for at least 24 hr.5,6,7 To quantify the actual anesthetic concentrations in solutions, we analyzed samples of saturated stock solutions and aliquots in a gas chromatograph calibrated with the appropriate anesthetic standards.5,6,7 We found a loss of less than 10% if the solution was used within 30 min after preparation. Thus, all anesthetic solutions were used in our experiments within 30 min after preparation. Test solutions were maintained in all-glass syringes tightly sealed with Parafilm punctured with a small escape hole for times when a whole cell was obtained and allowed anesthetic solution to fall by gravity.
Electrophysiological recordings
Recordings were made with standard whole-cell voltage and current-clamp techniques. Electrodes, which were fabricated from thin-walled microcapillary tubes (Drummond Scientific, Broomall, PA), had final resistances of 3–6 MΩ. We recorded membrane currents with an Axoclamp 200B patch-clamp amplifier (Molecular Devices, Foster City, CA). Voltage commands and digitization of membrane currents were done with Clampex 8.2 of the pClamp software package (Molecular Devices, Foster City, CA). Data were analyzed using Clampfit (Molecular Devices, Foster City, CA) and Origin 7.0 (OriginLab Corp, Northampton, MA). We filtered currents at 5–10 kHz.
Analysis of Current
Statistical analysis was done using the Student t test, with statistical significance determined at p < 0.05. Input resistance (Rin) was determined from the slope of the peak voltage versus the current plot that resulted from injecting 80–200 msec-long current ranging from 100–500 pA.
Drugs and Chemicals
TTX was purchased from Alomone Lab (Jerusalem, Israel). All other compounds were purchased from Sigma Chemical (St. Louis, MO). Drugs were delivered with a gravity-driven perfusion system. Manually controlled valves accomplished switching between solutions.
RESULTS
Isoflurane modulates the cellular excitability of nRT neurons
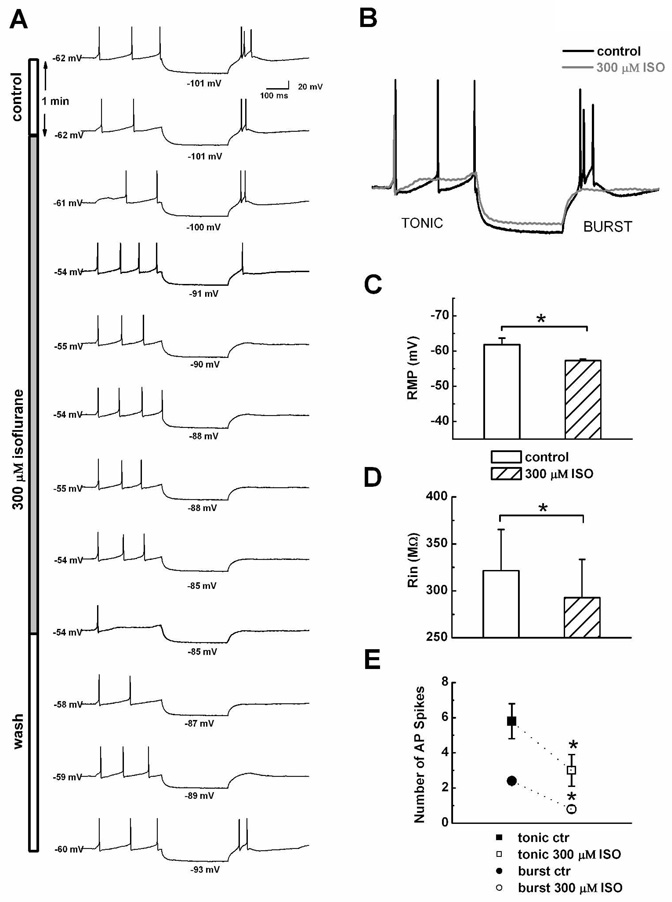
To determine the effects of volatile anesthetics on sensory information transfer in the thalamocortical circuitry, we examined the effects of clinically relevant concentrations of ISO on spike firing of nRT cells in current-clamp experiments. In order to study both functional firing modes of these cells, we first injected a depolarizing current of 300-ms duration to evoke tonic firing of single action potentials (APs); we then injected a hyperpolarizing current of the same duration to asses membrane input resistance (Rin) and evoke rebound burst firing. As demonstrated by the time course of representative nRT shown in Figure 1A and the histogram in Figures 1C and D, bath applications of 300 µM ISO reversibly depolarized resting membrane potential (RMP) in these cells for about 5 mV (average 4.6 ± 0.5 mV, p < 0.001, n = 10) and decreased Rin for about 10% (average 29 ± 6 MΩ, p = 0.001, n = 10). Bath applications of 600 µM ISO further depolarized membranes for about 10 mV (average 9.6 ± 1.0 mV, p < 0.01, n = 3, data not shown). The traces in Figure 1B, which are normalized to baseline membrane potentials (black line), indicate that ISO at 300 µM (gray line) decreased the initial number of APs in tonic mode from 3 to 1 and in burst mode from 3 to 0 with a concomitant decrease of about 10% in the amplitude of the hyperpolarizing pulse. In similar experiments from 10 nRT cells, we counted the number of APs in both tonic and burst firing mode, finding that ISO at 300 µM significantly decreased spike firing in both by about 50% (Fig. 1E).
Figure 1.
A This panel shows time course (from the top to bottom) in control conditions (open bar) during the application of 300 µM ISO (gray solid bar) and after ISO wash (open bar) on (from the left to the right) resting membrane potentials, tonic firing mode, Rin, and rebound burst firing. Note that ISO gradually and reversibly depolarized membranes from −62 mV to −54 mV; decreased Rin, as shown by the decrease in hyperpolarizing potential from −101 mV to −85 mV; and inhibited the number of action potentials during both tonic and burst firing. B: This panel shows normalized traces from the control condition (solid black line) and at the end of ISO application (solid gray line) from the same cell as that show in panel A. C: Histograms indicate the average effects of ISO on RMP in 10 nRT cells. D: Histograms indicate the average effects of ISO on Rin in the same nRT cells as those shown in panel C. E: Points indicate the average effects of ISO on control (ctr) number of action potential (AP) spikes in tonic (squares) and burst (circles) firing modes. In panels C–E, vertical lines indicates SEM and asterisks indicate significance p < 0.05.
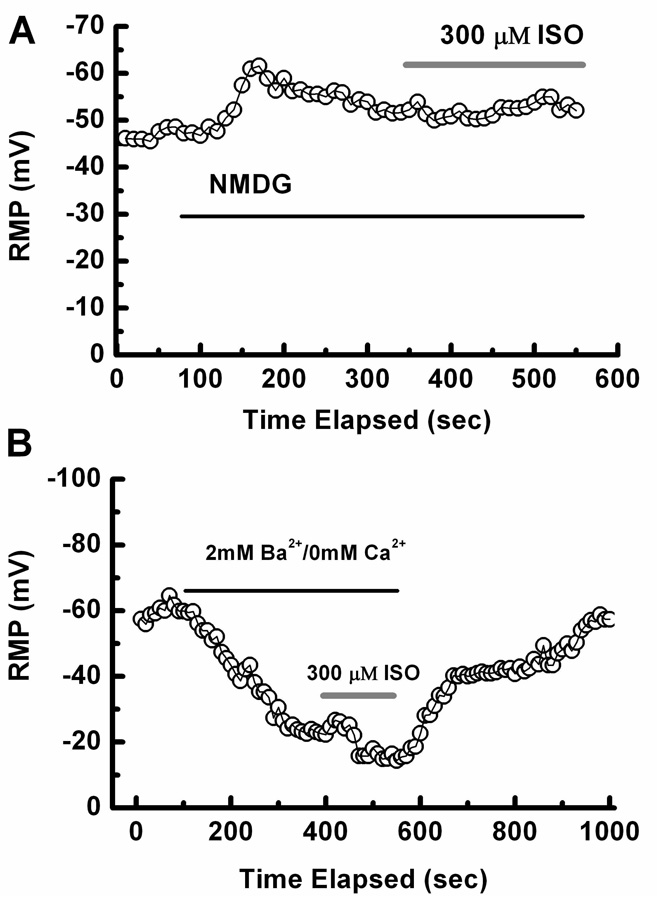
To examine probable ionic conductances involved in ISO-induced depolarization in nRT neurons, we did additional current-clamp experiments. When NaCl in the external solution was replaced with NMDG, ISO-induced depolarization was completely abolished (n = 3). Figure 2 A shows a time course from representative nRT cells; application of NMDG hyperpolarized membranes for about 7 mV, while ISO applied with NMDG had little effect on membrane potential. However, inclusion of 1 µM TTX in the external solution did not have a significant effect on ISO depolarization, suggesting the involvement of TTX-resistant Na+ conductance (n = 4, data not shown). In two experiments, we replaced 2 mM Ca2+ in the external solution with 2 mM Ba2+, which blocks many K+ channels. As shown in Figure 2B, application of Ba2+-containing external solution depolarized membranes for about 40 mV. Under these conditions, ISO further depolarized membranes by an additional 9 mV. These results suggest that resting Ba2+-sensitive K+ conductance is not sensitive to ISO, while resting TTX-resistant Na+ conductance in these cells is enhanced by ISO, which in turn depolarizes the neuronal membrane.
Figure 2.
A: Time course of the effects of NMDG and ISO on RMP. Note that NMDG (black line) hyperpolarized this cell from −45 mV to −52 mV and subsequently co-applied ISO (gray line) had little effect. B: Time course of the effects of 2 mM Ba2+ and ISO on RMP in another nRT cell. Note that the application of Ba2+ (black line) depolarized this cell from about −60 mV to about −20 mV; subsequently co-applied ISO (gray line) further depolarized the cell to RMP of about −11 mV.
Isoflurane modulates recovery from inactivation of isolated T-currents in the nRT neurons
Since, in current-clamp recording, ISO inhibited rebound burst firing, we examined its effects on recovery from inactivation of T-currents, which is important in the generation of burst firing mode in these cells. Inactivation, a major biophysical property of voltage-gated Ca2+ channels, may differ substantially among distinct native T-channels.8 The time- and voltage-dependent process of recovery from inactivation, which controls return of the availability of T-channels and underlying low-threshold calcium spikes (LTS) in the thalamus, is an important factor in determining the rate of repetitive low-threshold burst firing of APs in nRT neurons. By changing recovery from inactivation of T-currents or availability of T-channels at different potentials, anesthetics may influence burst firing of nRT neurons. Therefore, we investigated the influence of ISO on recovery from inactivation in 15 nRT cells.
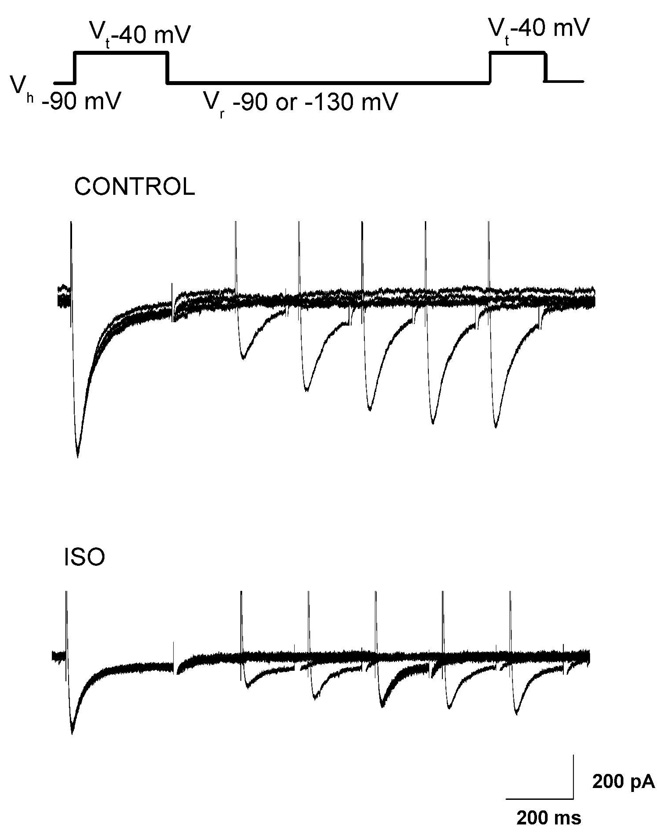
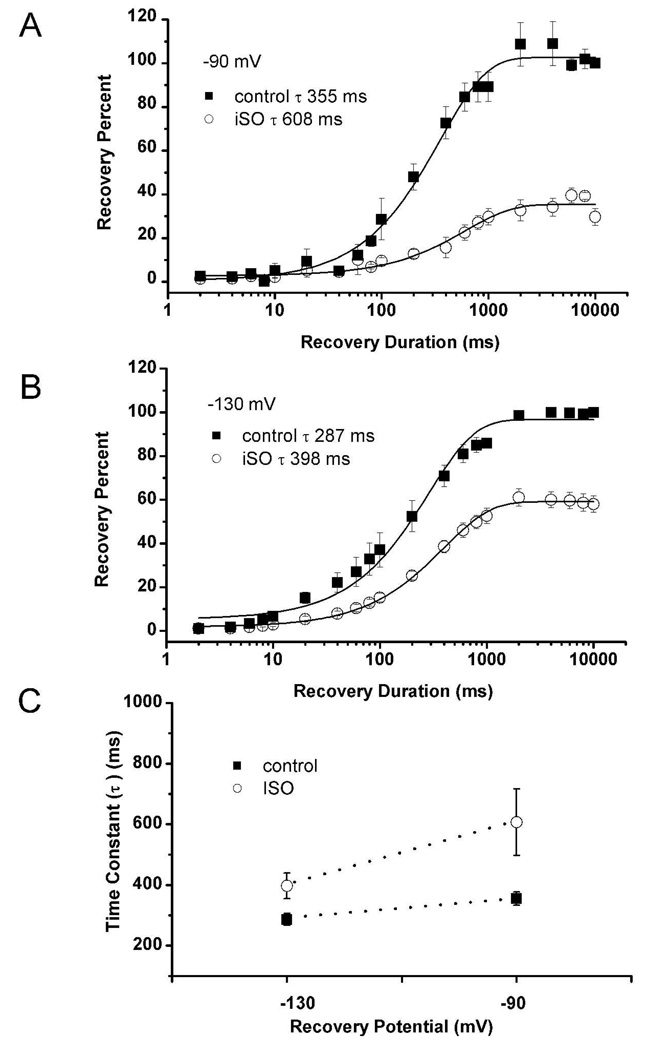
For this purpose, we used a paired-pulse protocol in which a 300-ms step to −40 mV was used to inactivate most of the T-current. After a variable recovery interval (20 to 10,000 msec) at either −90 or −130 mV, a second step to −50 mV was used to determine the amount of T-current that had recovered from inactivation during the recovery period (Fig. 3). The percent recovery in the presence and absence of 600 µM ISO in the same cells (n = 4–5 for each protocol) was then plotted as a function of recovery duration at either 90 mV (Fig. 4A) or −130 mV (Fig. 4B). Recovery time courses with (open symbols) and without (filled symbols) ISO are reasonably well fitted with a single exponential function. Recovery from inactivation at −90 mV was slower in the presence of ISO by about 70%, with a time constant of 608 ± 110 ms with isoflurane as compared to 355 ± 22 ms in controls (Fig. 4 A) and by about 38% at −130 mV (Fig. 4B, 287 ± 20 ms for controls and 398 ± 42 with ISO). Figure 4C summarizes the effects of ISO on recovery from inactivation of T-currents in nRT cells at membrane potentials of −90 and −130 mV. Overall, these results indicate relatively voltage-independent recovery from inactivation of T-currents under control conditions, but the effect of ISO was more prominent at −90 mV (Fig. 4C).
Figure 3.
Paired-pulse protocol depicted on the top of this figure was used to study recovery from inactivation. Original traces show recovery from inactivation in control conditions (middle panel) and after bath application of 600 µM ISO (bottom panel) at −90 mV. In control conditions, T-current recovered to 86% of control after 1 s (last trace on the middle graph) while in the presence of ISO it recovered to 75% of control after same interval (last trace on bottom panel). Calibration bars pertain to both panels.
Figure 4.
Percent recovery of peak inactivating T-current is plotted as a function of recovery duration at −90 mV (A) or −130 mV (B) in the absence (filled squares) and presence (open circles) of ISO. Symbols are average from 4–5 experiments, vertical lines are SEM, and solid lines are fitted by mono-exponential time course. C. This graph shows differences between time constants in control conditions (filled squares) and after application of ISO (open squares) at recovery potentials of −90 and −130 mV from experiments depicted on plots A and B of this figure. Vertical lines indicate ± SEM of fitted values.
A characteristic of inactivation of T-currents in many native and recombinant systems is that recovery is influenced by the duration of the preceding inactivation step.2 Our results confirm this; if a cell is held at −50 mV for 6 sec before the recovery interval at potentials of −90 mV, recovery from inactivation is drastically slowed in comparison to that after a shorter inactivation pulse (from 355 ms to 855 ms, data not shown). However, the recovery time constant became even slower in the presence of ISO (855 ± 40 ms in control and 1150 ± 152 ms for isoflurane, a 34% difference; n = 6, data not shown). These findings indicate that ISO slows recovery from inactivation of T-currents regardless of the duration of the inactivating pulse.
DISCUSSION
A major finding of this study is that ISO at a clinically relevant concentration in vitro at 300–600 µM (corresponding roughly to 1–2 minimum alveolar concentration in vivo) profoundly inhibits neuronal excitability in nRT neurons in intact slices from the brains of young rats. This is manifested as depolarization of membrane potential accompanied by decreased Rin, decreased amplitude of rebound LTS, and diminished numbers of fast APs in both tonic and burst firing modes. ISO-induced depolarization of membrane potential was invariably associated with a decrease in Rin, which in turn indicates that this depolarization is mediated by the opening of some type of ion channels. Since we could completely abrogate this effect of ISO by partial replacement of Na+ ions in external solution, it appears that TTX-resistant Na+ conductance active at resting membrane potentials is increased by ISO, which in turn depolarized membranes.
We previously demonstrated that ISO inhibits isolated T-currents in these cells in voltage-dependent fashion.6 Consistent with this is our present finding that ISO slowed recovery from inactivated states of T-currents, which in turn diminished the amplitude of LTS and underlying burst firing. Somewhat surprisingly, ISO depolarized nRT neurons but inhibited tonic firing of single APs. This could be attributed to several possible effects of ISO. First, a decrease in Rin may shunt APs generated by the injection of depolarizing current. Second, ISO-induced depolarization may enhance inactivation of voltage-gated Na+ currents that control the generation of AP. Third, ISO may directly inhibit voltage-gated Na+ currents.9 This, to the best of our knowledge, is the first report of the effects of ISO on the excitability of rat nRT neurons. However, others have shown that ISO can inhibit the excitability of thalamic relay (TR) cells in rat brain slices.10 Interestingly, given that ISO hyperpolarized TR cells by decreasing Rin, most likely as a consequence of the increase in background K+ conductance, it seems that depolarization is a nucleus-specific effect of ISO.10 However, despite the presumably different ionic mechanisms involved, the effect of ISO in both nRT and TR cells resulted in similar inhibition of tonic and burst firing modes.
Importantly, inhibition of neuronal excitability of nRT cells, together with previously reported inhibition in TR cells, may contribute to the impairment of sensory information transfer in the thalamocortical network by general anesthetics. Since the thalamus has a key function in the processing of sensory information, sleep, and cognition, our results indicate that the effects of ISO on multiple ion conductances involved in the electrogenesis of nRT cells may contribute to the clinical effects of general anesthetics. Extensive molecular and electrophysiological studies will be needed to identify the ion channels in nRT cells that mediate the effects of ISO on neuronal excitability.
Acknowledgments
This work was supported by NIH grant GM070726 to SMT.
REFERENCES
- 1.Angel A. Adventures in anesthesia. Exp. Physiol. 1991;76:1–38. doi: 10.1113/expphysiol.1991.sp003471. [DOI] [PubMed] [Google Scholar]
- 2.Llinas RR, et al. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc. Natl. Acad. Sci. U. S. A. 1999;96(26):15222–15227. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu. Rev. Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- 4.Steriade M. Sleep, epilepsy and thalamic reticular inhibitory neurons. Trends. Neurosci. 2005;28:317–324. doi: 10.1016/j.tins.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Joksovic PM, et al. Contrasting anesthetic sensitivities of slow T-type calcium channels of reticular thalamic neurons and recombinant Ca3.3 channels. Br. J. Pharmacol. 2005;144:59–70. doi: 10.1038/sj.bjp.0706020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joksovic PM, et al. Different kinetic properties of two T-type Ca2+ currents of reticular thalamic neurons and their modulation by enflurane. J. Physiol. 2005;566(1):125–142. doi: 10.1113/jphysiol.2005.086579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Todorovic SM, Lingle CJ. Pharmacological properties of T-type Ca2+ current in adult rat sensory neurons: effects of anticonvulsants and anesthetic agents. J. Neurophysiol. 1998;79:240–252. doi: 10.1152/jn.1998.79.1.240. [DOI] [PubMed] [Google Scholar]
- 8.Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annu. Rev. Physiol. 1996;58:329–358. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- 9.OuYang W, Hemmings HC., Jr Isoform-selective effects of isoflurane on voltage-gated Na+ channels. Anesthesiology. 2007;107(1):91–98. doi: 10.1097/01.anes.0000268390.28362.4a. [DOI] [PubMed] [Google Scholar]
- 10.Ries CR, Puil E. Mechanism of anesthesia revealed by shunting actions of isoflurane on thalamocortical neurons. J. Neurophysiol. 1999;81:1795–1801. doi: 10.1152/jn.1999.81.4.1795. [DOI] [PubMed] [Google Scholar]