Abstract
Hepatitis C virus is a blood-borne virus that typically establishes a chronic infection in the liver, which often results in cirrhosis and hepatocellular carcinoma. Progress in understanding the complete virus life cycle has been greatly enhanced by the recent availability of a tissue culture system that produces infectious virus progeny. Thus, it is now possible to gain insight into the roles played by viral components in assembly and egress and the cellular pathways that contribute to virion formation. This minireview describes the key determining viral and host factors that are needed to produce infectious virus.
Keywords: Hepatitis Virus, Lipid Droplet, Lipoprotein Secretion, Positive-strand RNA Viruses, RNA Viruses, Virus Assembly, Virion Formation, Virus Maturation and Release
Introduction
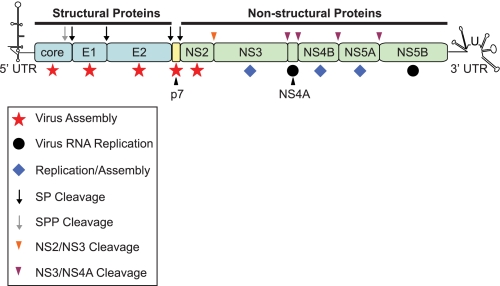
HCV3 possesses a positive-sense single-stranded RNA genome of ∼9.6 kb in length flanked by 5′- and 3′-untranslated regions that are involved in both replication and translation (1–5). The genome contains a single open reading frame encoding a polyprotein of ∼3000 amino acids (6), which is cleaved by cellular and virus-encoded proteases to yield 10 mature viral proteins that are arbitrarily divided into the structural and non-structural proteins (Fig. 1). Prior to 2005, the structural and non-structural proteins could be considered as two distinct functional units; the structural proteins provided physical virion components, whereas the non-structural proteins (with the exception of NS2) were essential for HCV RNA replication (7, 8). Determining the factors needed for viral RNA replication was revolutionized by development of tissue culture systems that supported de novo HCV genome synthesis. By contrast, later stages of the virus life cycle, including virion assembly and release, were not amenable to detailed study because efficient production of infectious virus particles in cell culture was not possible. However, the publication of reports in 2005 demonstrating that genome-length RNA from a genotype 2a HCV strain termed JFH1 could produce infectious virus in cell culture (9, 10) opened a new era for investigating the mechanisms responsible for HCV particle assembly and release. Along with JFH1, studies using chimeric derivatives encoding structural proteins from other HCV genotypes (discussed below) established roles for several non-structural proteins in the production of infectious virus (11–17). Hence, HCV-encoded proteins can no longer be strictly separated by roles in either assembly or RNA replication because some proteins facilitate both processes (Fig. 1). Perhaps more importantly, isolation of JFH1 permitted analysis of the essential contribution of host cell factors to virus production. Here, we summarize the current understanding of assembly and egress of infectious HCV particles.
FIGURE 1.
Schematic representation of the HCV genome. The single open reading frame encodes 10 viral proteins that are divided into the structural (core, E1, and E2; shown in blue) and non-structural (NS2, NS3, NS4A, NS4B, NS5A, and NS5B; shown in green) regions. The small peptide p7 is currently unassigned into either category (shown in yellow). The open reading frame is flanked by untranslated regions (UTR) at the 5′ and 3′ termini that are involved in both replication and translation. Following translation, the viral polyprotein is processed by two cellular protease activities, signal peptidase (SP; black arrow) and signal peptide peptidase (SPP; gray arrow), to yield core, E1, E2, and p7. Two virus-encoded proteases, the NS2-NS3 autoprotease (orange arrowhead) and the NS3 serine protease as a complex with NS4A (purple arrowhead), generate mature proteins from the NS2–NS5B region. Core, E1, and E2 provide physical components of the virion. p7 and NS2 appear to function exclusively in and are essential for virus assembly (red stars). NS4A and NS5B (black circles) are critical for viral RNA replication, but there are no reports suggesting that either protein is required for generation of infectious progeny. NS3, NS4B, and NS5A have dual functions in viral RNA replication and particle production (blue diamonds).
Outline of HCV Assembly and Release
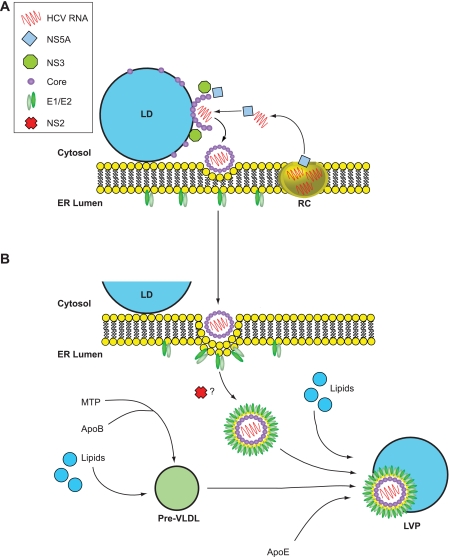
HCV RNA replication occurs at specialized sites on the ER membrane termed the membranous web (18–20). Initiation of virion assembly is thought to require release of replicated genomes from such sites to allow contact with core protein, which forms the virion capsid. Because core is located on the cytosolic side of the ER membrane, assembly probably initiates in the cytosol before further maturation, and release occurs by transfer of nascent particles across the ER membrane to enable access to the secretory pathways in hepatocytes. Thus, production of infectious HCV can be broadly considered as two interconnected processes: an initiation phase, which occurs on the cytosolic side of the ER membrane, followed by maturation and release on the lumenal side of the ER membrane. However, placing the function of viral and/or host factors in either one of these stages of virion formation is not absolute because visualization of assembly intermediates remains problematic. Several studies have identified cytosolic storage organelles, termed LDs, and the VLDL assembly pathway that occurs in the ER lumen as major contributors from the host cell to virion assembly (Fig. 2). For presentation purposes, we have therefore separated HCV virion production into three stages: (i) an initial phase of assembly at LDs, (ii) the contribution made by viral factors that probably takes place after assembly has begun, (iii) and events on the lumenal side of the ER membrane where there is engagement with the VLDL assembly pathway to facilitate virion maturation and egress.
FIGURE 2.
Model for HCV virion assembly. Based on current evidence, assembly initiates on the cytosolic side of the ER membrane (A), and complete maturation occurs in the ER lumen (B) prior to release from the cell. A, in the early steps of assembly, core protein is targeted to LDs, where it coats the organelle surface. Viral RCs are recruited to LD surfaces in a core- and NS5A-dependent manner to enable transfer of replicated RNA from RCs for association with core to permit encapsidation of the genome. During this stage, NS3 is required for the formation of fast-sedimenting core-containing particles, which are presumed to represent non-infectious virions that will undergo further stages of maturation. B, late assembly steps involve the acquisition of a lipid envelope and the incorporation of the E1 and E2 glycoproteins into virions. NS2 appears to confer infectivity to virions, perhaps by mediating an interaction between glycoprotein complexes (E1 and E2) and immature particles. During maturation, nascent virus particles combine with pre-VLDLs (produced by initial lipidation of apoB by MTP), lipids in the form of lumenal LDs, and other lipoprotein components such as apoE to generate LVPs. The stage at which p7 participates is unclear, and therefore, it has not been included in the model.
Initial Phases of HCV Virion Assembly
Association of Core with LDs: A Scaffold for Virus Assembly?
Early stages of HCV assembly are thought to require interaction of the viral core protein with LDs, the intracellular storage sites for triglycerides and cholesterol esters (21). During translation of the HCV polyprotein, the mature form of core is generated by sequential cleavage events at the ER membrane by signal peptidase and signal peptide peptidase, enabling trafficking of the protein to LDs (22–24). Mature core can be divided into two domains (D1 and D2), and it is the D2 domain that mediates the core-LD association (25). D2 contains two amphipathic α-helices that are separated by a hydrophobic loop (26). Hydrophobic residues within each helix are likely to interact with the phospholipid layer surrounding LDs because mutation of amino acids on the hydrophobic face of these helices can abolish the association between core and LDs (26).
The above observations were deduced prior to availability of a tissue culture system that produced infectious progeny. From studies with strain JFH1, it is now clear that association of core with LDs is essential for infectious HCV production because mutations in D2 that disrupt the core-LD interaction also prevent virus release (27, 28). Time course experiments indicate that core progressively coats the surface of LDs, and this process coincides with rises in virus titer (27). Interestingly, single amino acid changes in D2 can also enhance production of infectious virus, an effect that may be linked to the strength of binding of core to LDs (29). Impairing signal peptide peptidase cleavage lowers infectious virus levels, thus providing further evidence that the interaction of fully mature core with LDs is critical for virus production (30, 31). Extensive mutational analysis across the length of core has identified numerous residues that are important for generating infectious virions (32). Although these mutants were not examined for their ability to target LDs, this approach demonstrated that many residues within core contribute to HCV assembly.
Visualization of HCV particles within cells has proven difficult, and the question regarding the extent of virion assembly that occurs at LDs remains unanswered. HCV-LP budding has been observed by expressing core, E1, and E2 with a Semliki Forest virus replicon vector (33–35). HCV-LPs were found at ER membranes located close to LDs, but few particles were observed on LDs (36). Spherical virus-like particles, recognized by core- and E2-specific antibodies and associated with ER membranes, have been found in close proximity to LDs in cells producing JFH1 virions (28), in agreement with the data obtained using HCV-LPs. Therefore, it is likely that HCV virion assembly is dependent on an interaction not only with LDs but also with the ER membranes surrounding these organelles.
Convergence of HCV RNA Replication Sites with Sites of Virus Assembly
If assembly of virus particles initiates at LDs, then it is logical that viral RNA interacts with core protein, which forms the virion capsid, at this stage. HCV RNA is synthesized by RCs, contained within the membranous web that is derived from altered ER membranes (18, 19). RCs contain the components necessary for RNA replication, including non-structural proteins, viral RNA, and a growing list of cellular factors (19, 37–41). Because RCs are embedded within ER membranes, this raises the question as to how replicated RNA molecules are transported from these sites to LDs to enable interaction with core. Available data indicate that non-structural proteins are recruited to the surface of LDs in cells producing infectious virus (28). Thus, RCs may be transported to LDs, aiding the transfer of RNA to core for packaging. Current evidence indicates that NS5A is integral to this process.
NS5A is composed of three domains (DI–DIII), the first two of which are essential for replication of HCV RNA, whereas DIII is dispensable for this process (42, 43). However, both DI and DIII contribute to particle production, although perhaps by distinct mechanisms. Mutations in DI prevent interaction of NS5A with LDs and, moreover, abolish association of other non-structural proteins and viral RNA with these organelles (28). Thus, DI of NS5A may possess a LD-binding capacity that is crucial for the trafficking of viral RCs and RNA to LDs for virus production. By contrast, NS5A containing mutations in DIII retains the ability to traffic to LDs but does not rely on the presence of core protein on LDs for association with the organelles (43). These data suggest that DI is essential for LD association, whereas DIII could be responsible for interaction between NS5A and core on LD surfaces. Supportive evidence for such an interaction comes from studies on mutations in NS5A at a cluster of serine residues in DIII; converting three closely spaced serine residues to alanine or removing a segment of DIII including these amino acids almost abolished interaction with core (44). Phosphorylation of the serine residues, which may be catalyzed by casein kinase II (45), appears to contribute to the interaction of NS5A with core (44). Critically, release of infectious virus was either dramatically reduced or abolished for these NS5A mutants (44, 45). Thus, recruitment of NS5A to LDs to enable interaction with core appears to be essential for virion assembly and may represent one of the earliest events in the pathway.
From the above evidence, association of RCs with LDs occurs in a core- and NS5A-dependent manner. However, it does not explain the processes that allow sites of genome replication to converge with LDs to enable core-NS5A interactions. One proposed hypothesis derives from a clustering of LDs, which is induced by core, at the periphery of the nucleus (46). The change in LD distribution relies on an intact microtubule network and coincides with displacement of adipocyte differentiation-related protein by the HCV core. Adipocyte differentiation-related protein is the major cellular protein found on droplet surfaces (47), and its removal by core appears to preferentially direct LDs toward the nucleus (46). This process may concentrate core-coated LDs in proximity to RCs, thereby enhancing the likelihood of assembly events. Another hypothesis suggests that RCs are transported on the microtubule network, which may be mediated by interaction of NS3 and NS5A with tubulin and actin (48).
Post-initiation Phase: Participation of Other Viral Proteins in Assembly
Events following Recruitment of Core and NS5A to LDs
As described above, core and NS5A apparently act in concert to connect assembly sites with those of RNA synthesis, and core-coated LDs may provide the scaffold for early stages in the assembly pathway. However, it is now apparent that other HCV-encoded proteins, including p7 (11, 14–16), NS2 (12–15, 49, 50), NS3 (14, 51, 52), and NS4B (17), contribute to the production of infectious virions. These studies have revealed that HCV assembly is a complex multistep process with the non-structural proteins participating at different stages in the assembly pathway (summarized in Fig. 2). The involvement of each of the non-structural proteins is outlined below.
p7
p7 is a small hydrophobic peptide that forms oligomers in vitro and exhibits cation channel activity in artificial membranes (53, 54). Although the protein has no obvious function in HCV RNA replication (7), injection of viral RNAs harboring p7 deletions into chimpanzees does not establish productive infection (55), hinting at an involvement in virus assembly or release. Studies in tissue culture cells have now formally demonstrated that p7 is important for virus production because viral RNA genomes containing mutations in the gene or lacking its coding region do not produce infectious particles (15, 16). Conversely, other p7 mutations can enhance virus production (56), and viral genomes harboring p7 sequences from different HCV genotypes differ in their ability to generate virus (16). However, experiments thus far have not identified the stage in assembly that is dependent on p7. Moreover, it is not yet clear whether the cation channel function of the protein is necessary for producing infectious particles (55, 57). Thus, further analyses are required to precisely define the stage at which p7 participates in assembly and its mechanism of action.
More recently, it was suggested that p7 may be a physical component of virions because culturing cells with infectious supernatants in the presence of cation channel inhibitors partially inhibited infection (58). However, the specific infectivity of viruses harboring p7 mutations was unaffected in another study (16). It therefore remains to be conclusively determined whether p7 is a structural component of HCV particles.
NS2
Apart from functioning as an autoprotease, the role of NS2 in the HCV life cycle was undefined because it is dispensable for genome replication (7). Participation of NS2 in virus assembly and release was first assumed through studies with chimeric constructs as higher virus titers were produced by positioning the site for joining chimeric genomes between the first and second transmembrane domains of NS2 compared with the NS2/NS3 boundary (49). Further experiments utilizing chimeric viruses lacking all or portions of NS2 have now formally demonstrated that the protein is essential for virus production (15). Although the NS2 protease domain is important, its catalytic activity is seemingly dispensable for producing infectious virions (15, 50). From studies with mutations introduced at Ser-168, NS2 appears to act at a late stage of infectious particle generation. Conversion of this residue to either alanine or glycine (S168A/G) impairs detection of extracellular infectious virus yet does not prevent generation of intracellular fast-sedimenting core-containing particles reminiscent of those produced by wild-type viruses (13). Furthermore, intracellular core protein expressed from the S168A/G mutants accumulates within cells (13), suggesting that NS2 is essential for a post-assembly step that somehow confers infectivity to the virion or allows the particle to proceed to late infectivity-inducing stages, which can result in egress. A role for NS2 in a post-assembly step is supported further by studies demonstrating that (i) it does not have the same intracellular distribution as core, (ii) it has limited association with NS5A, and (iii) it interacts and colocalizes with the E2 envelope glycoprotein (13). Thus, NS2 may play a role in bringing together glycoproteins and nascent particles to form fully infectious HCV virions.
NS3
NS3 comprises a serine-type protease at the N terminus and an RNA helicase/NTPase domain in the remaining two-thirds of the protein. A connection between NS3 and virion assembly was deduced using an intergenotypic chimera encoding the NS3–NS5B region from JFH1 fused to the core-NS2 segment from a genotype 1a strain, H77. This virus (termed HJ3) gave robust RNA replication but yielded no infectious virus (14, 52). However, infectious progeny was detected following the appearance of a compensatory mutation (Q221L) that lies close to the N terminus of the helicase/NTPase domain (14, 52). HJ3 genomes containing and lacking the Q221L mutation were capable of recruiting core, NS5A, and NS3 to LDs, yet intracellular infectious particles could be detected only in the presence of the compensatory mutation (52). Moreover, the absence of the Q221L mutation prevented assembly of core into high density fast-sedimenting particles (52). Hence, NS3 is important for a stage in virus assembly after the interaction between core and NS5A at LD surfaces but prior to assembly of core-containing particles. It is likely that Q221L corrects an incompatibility between the proteins of different HCV genotypes at sites of critical protein-protein interactions required for virus particle assembly. This incompatibility may exist between NS3 and the C terminus of NS2 because (i) Q221L enhances virus production from HJ3 when the entire NS2 region is derived from H77, but not from a similar chimera in which the C-terminal portion of NS2 originates from JFH1 (52), and (ii) Q221L rescues particle generation from a virus with an NS2-mediated assembly defect (12). Other mutations that enhance production of infectious virus have been mapped within the helicase domain of NS3 (51), providing further evidence that this region provides a key role in particle assembly.
Genetic Interactions between HCV Proteins
As alluded to above, JFH1 and chimeric HCV genomes have proven valuable for providing insight into genetic associations that are necessary for virus production. Multiple passaging of replicating viruses that produce lower or no infectious virus usually leads to selection of compensatory mutations elsewhere within the genome that rescue virus production. These compensatory mutations offer clues regarding protein-protein interactions that are important for particle generation. An example is the possible interaction between the NS3 helicase domain and NS2, as discussed above. Other interactions have been proposed using this method. For example, virus production was abolished or substantially reduced in cells replicating chimeric viral RNA by mutating blocks of amino acids within D1 of core (32). However, infectivity was rescued by compensatory mutations within p7 (F26L/S), NS2 (A67P), and NS3 (T179A) (32). Importantly, the p7 mutations had little effect on virion production when introduced into a virus that did not contain the original core mutations, implying that mutation at residue 26 in p7 restores an interaction between core and p7 that is critical for assembly. By contrast, the NS2 mutation enhanced virus release even in the absence of the core mutations (32). These data suggest that mutation at this position may generally enhance infectivity.
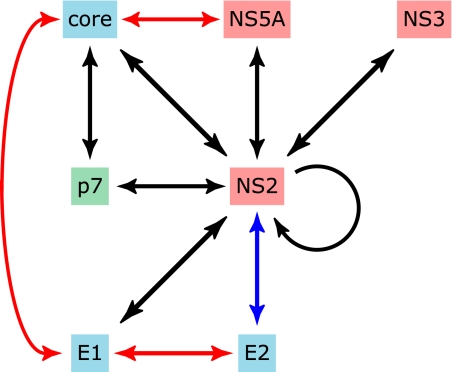
Other studies indicate that NS2 may interact with several other proteins involved in virus production. Compensatory mutations within NS5A can rescue the detection of infectious particles from viral genomes harboring deleterious NS2 mutations (13). In a separate study, several NS2 mutant viruses could be rescued by other compensatory mutations, most notably within NS3 and the E1 glycoprotein (12). Intriguingly, NS2 mutants that could be rescued by changes within NS3 could not be restored by the E1 compensatory mutation, whereas the opposite phenotype was found for viruses containing other NS2 mutations (12). Therefore, opposing epistatic interactions seemingly occur between E1, NS2, and NS3. The significance of these findings is not yet known. Finally, a single amino acid change within NS4B modestly enhances virus production from cells replicating JFH1 RNA (17). Importantly, the same mutation decreases infectious virus titers from the chimeric virus J6-JFH1 (containing the core-NS2 region from genotype 2a HCV strain J6 and JFH1 NS3–NS5B coding sequence). Such results suggest that NS4B, previously implicated only in viral RNA synthesis, may interact with other viral factors to modulate assembly. Thus, it is becoming increasingly clear that a complex set of viral protein-protein interactions is essential for and contributes to generating infectious virus (summarized in Fig. 3).
FIGURE 3.
Interactions between viral proteins that occur during virion assembly. Black arrows indicate genetic interactions between viral components that have been identified by passaging viral genomes, which are defective in one gene (e.g. core), until compensatory mutations arise within other coding regions that restore the generation of infectious progeny (e.g. p7). Red arrows show core-NS5A, core-E1, and E1-E2 interactions that have been determined experimentally. The blue arrow indicates colocalization between NS2 and E2, but there is no available evidence for physical association between the proteins.
Packaging of the Viral Genome
No conclusive data are available for the events that direct packaging of the viral genome within the capsid. Presumably, one of the first stages is transfer of viral RNA from replication sites to core on the surface of LDs. Transfer may involve NS5A because structural analysis of DI of NS5A indicates the presence of a groove formed by dimers of the domain that could accommodate RNA (59). Although the D1 region of core contains a high proportion of positively charged residues (25), the lack of extensive structural data for the domain prevents development of similar models for interaction sites between core and RNA. Moreover, there is no available evidence for a specific signal or motif in the genome that promotes packaging. From studies with trans-encapsidation systems, the core-NS2 region does not apparently contain any cis-acting packaging signals because only RNA encoding the NS3–NS5B replicase unit is encapsidated (60–63). Although the occurrence of specific packaging elements in the viral genome cannot be excluded, it is possible that encapsidation relies on protein-protein rather than protein-RNA interactions (63). Moreover, there is considerable plasticity in the length of RNA sequence that can be packaged because expanding the genome size by incorporating coding sequences for foreign genes does not abolish virion production (64, 65).
HCV Maturation and Release
HCV and Lipoproteins: The Lipoviroparticle
Currently, it is believed that maturation and release of HCV virions coincide with the pathway for producing VLDLs, which export cholesterol and triglyceride from hepatocytes (Fig. 2) (66). In infected individuals, circulating virus particles are complexed with VLDLs and have been termed LVPs (67–69). LVPs display low to very low buoyant densities ranging from <1.03 to ∼1.25 g/ml (9, 10, 67, 68, 70). By contrast, intracellular infectious particles, which are isolated from cells by multiple freeze/thaw cycles, have higher densities (71). This difference in density between intracellular and extracellular viruses suggests that association between virions and lipids may represent a maturation event during egress. LVPs are triglyceride-rich and contain viral RNA, core protein, and the VLDL structural components apoB and apoE (67, 72). In addition to secreted virions, the ER membrane-derived vesicles inside which HCV RNA synthesis occurs also contain apoB and apoE as well as MTP (73, 74), a factor required for VLDL assembly (see below). Hence, an interaction between HCV and VLDL assembly components may occur before or during initial phases in virus assembly. In support of this notion, recent data reveal a specific interaction between NS5A and apoE (75, 76). Indeed, it is possible that NS5A interacts with apoE within RCs, thereby facilitating transport of the lipoprotein to LDs coated with core for inclusion in virion assembly. Such a mechanism may serve to direct nascent particles containing the HCV core and RNA into the VLDL assembly pathway for subsequent maturation before egress.
HCV and the VLDL Assembly Pathway
Given the association between HCV and pathways that release lipid from hepatocytes, recent studies have focused on whether abrogating VLDL assembly would interfere with release of infectious virus. VLDL assembly apparently occurs in two distinct stages. In the first stage, MTP lipidates apoB, creating a lipid-poor pre-VLDL species (77). Subsequent stages in VLDL assembly are obscure but are thought to involve fusion of pre-VLDLs with triglyceride droplets derived from LDs that most likely originate on the cytosolic side of the ER membrane. This process is thought to require apoE (78). Because of the clear importance of apoB, apoE, and MTP in VLDL assembly and secretion, the contribution of the lipoprotein pathway to HCV egress has been tested by targeting these factors. However, experiments have yielded conflicting results. Data from two studies reported reduced infectious virus titers from cells treated with an MTP inhibitor without any effect on viral RNA replication (73, 74). Intracellular infectivity levels were also reduced, suggesting that MTP activity is required for HCV assembly as well as secretion (73). In these studies, small interfering RNA-mediated reduction of apoB levels inhibited secretion of virus particles (73, 74) and was deemed to be a rate-limiting factor for HCV assembly (73). Furthermore, secretion of the E1 and E2 glycoproteins was reduced by treating cells with an MTP inhibitor using an in vitro model (79). Collectively, these results suggest that virus particle release is dependent upon VLDL production and secretion. However, recent data have indicated that apoE is crucial for virus assembly and release, whereas apoB levels and MTP activity have little or no influence (72, 75, 76). Here, small interfering RNAs targeting apoE diminished both intracellular and extracellular infectious titers, whereas those specific for apoB had no appreciable effect. Additionally, MTP inhibitors blocked apoB secretion without preventing apoE/virus release, although higher inhibitor concentrations resulted in lowered virus titers that mirrored the effects of repressed apoE secretion (76). It has been proposed that toxicity and high inhibitor concentrations led to previous results that identified the involvement of apoB and MTP with HCV assembly and release. Thus, there is agreement that VLDL assembly is needed for release of infectious HCV, but the role of specific factors in VLDL production remains a subject of debate.
Other Cellular Pathways Implicated in HCV Release
Recent data have implicated processes governing endosome formation as another cellular pathway involved in particle egress (80). Endosome formation depends upon several multiprotein complexes termed ESCRTs, which function by incorporating target proteins into a multivesicular body for degradation (81). Subsequently, ATP hydrolysis by an oligomeric protein called Vps4 dissociates ESCRTs from the multivesicular body membrane, which are then recycled for further rounds of vesicle formation (82). Disrupting Vps4 function leads to dysfunctional ESCRTs and formation of aberrant endosomes (83). Interestingly, expression of dominant-negative forms of ESCRT components and Vps4 reduces production of HCV particles (80). These results suggest that participation of ESCRT functions in viral egress, although it is unclear how endosomal complexes are involved.
Recently, it has been established that HCV can be transmitted directly from infected donor cells to uninfected recipient cells (84, 85). Such a process is facilitated by seeding monolayers at high density, which enables direct contact between cells. The characteristics of virus particles that promote cell-cell transmission are not known, although it does require the full complement of viral proteins, including the E1 and E2 envelope glycoproteins (84, 85). Moreover, cell-to-cell infection is dependent on claudin-1, a cell-surface receptor that is necessary for entry by cell-released virus (84). By contrast, CD81, which also is essential for infection by secreted virus, is dispensable for transmission of virus between cells (84, 85). Therefore, it is not clear whether HCV virions are generated by several pathways to allow infection by alternative mechanisms.
Future Directions
With the ability to recapitulate the entire HCV life cycle in cell culture, substantial progress has been made toward understanding HCV assembly and release. Nonetheless, the mechanisms by which viral factors interact with each other and with cellular components to construct virions are not fully understood. Moreover, the existing cell-based model undoubtedly has limitations. For example, the cells used to produce infectious virus are derived from a hepatocellular carcinoma and do not possess many characteristics typical of hepatocytes (e.g. they do not release VLDL of authentic size). Alternative cell lines that more readily reflect the properties of hepatocytes in vivo would be invaluable; however, development of such systems has proven challenging (79, 86). Thus, untangling the complexities of the interaction between HCV and the infected cell that contribute to virion assembly and release will certainly remain an active area of study for the foreseeable future.
Supplementary Material
This work was supported by the UK Medical Research Council and funds awarded to the LipidomicNet Consortium (HEALTH-F4–2008-202272) by the European Commission. This is the second article in the Thematic Minireview Series on Hepatitis C Virus. This minireview will be reprinted in the 2010 Minireview Compendium, which will be available in January, 2011.
- HCV
- hepatitis C virus
- NS
- non-structural
- ER
- endoplasmic reticulum
- LD
- lipid droplet
- VLDL
- very low density lipoprotein
- HCV-LP
- HCV-like particle
- RC
- replication complex
- LVP
- lipoviroparticle
- MTP
- microsomal transfer protein
- ESCRT
- endosomal sorting complex required for transport.
REFERENCES
- 1.Friebe P., Bartenschlager R. (2002) J. Virol. 76, 5326–5338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friebe P., Lohmann V., Krieger N., Bartenschlager R. (2001) J. Virol. 75, 12047–12057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim Y. K., Kim C. S., Lee S. H., Jang S. K. (2002) Biochem. Biophys. Res. Commun. 290, 105–112 [DOI] [PubMed] [Google Scholar]
- 4.Murayama A., Date T., Morikawa K., Akazawa D., Miyamoto M., Kaga M., Ishii K., Suzuki T., Kato T., Mizokami M., Wakita T. (2007) J. Virol. 81, 8030–8040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song Y., Friebe P., Tzima E., Jünemann C., Bartenschlager R., Niepmann M. (2006) J. Virol. 80, 11579–11588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Penin F., Dubuisson J., Rey F. A., Moradpour D., Pawlotsky J. M. (2004) Hepatology 39, 5–19 [DOI] [PubMed] [Google Scholar]
- 7.Lohmann V., Körner F., Koch J., Herian U., Theilmann L., Bartenschlager R. (1999) Science 285, 110–113 [DOI] [PubMed] [Google Scholar]
- 8.Blight K. J., Kolykhalov A. A., Rice C. M. (2000) Science 290, 1972–1974 [DOI] [PubMed] [Google Scholar]
- 9.Zhong J., Gastaminza P., Cheng G., Kapadia S., Kato T., Burton D. R., Wieland S. F., Uprichard S. L., Wakita T., Chisari F. V. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 9294–9299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wakita T., Pietschmann T., Kato T., Date T., Miyamoto M., Zhao Z., Murthy K., Habermann A., Kräusslich H. G., Mizokami M., Bartenschlager R., Liang T. J. (2005) Nat. Med 11, 791–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brohm C., Steinmann E., Friesland M., Lorenz I. C., Patel A., Penin F., Bartenschlager R., Pietschmann T. (2009) J. Virol. 83, 11682–11693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phan T., Beran R. K., Peters C., Lorenz I. C., Lindenbach B. D. (2009) J. Virol. 83, 8379–8395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yi M., Ma Y., Yates J., Lemon S. M. (2009) PLoS Pathog. 5, e1000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi M., Ma Y., Yates J., Lemon S. M. (2007) J. Virol. 81, 629–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones C. T., Murray C. L., Eastman D. K., Tassello J., Rice C. M. (2007) J. Virol. 81, 8374–8383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinmann E., Penin F., Kallis S., Patel A. H., Bartenschlager R., Pietschmann T. (2007) PLoS Pathog. 3, e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones D. M., Patel A. H., Targett-Adams P., McLauchlan J. (2009) J. Virol. 83, 2163–2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egger D., Wölk B., Gosert R., Bianchi L., Blum H. E., Moradpour D., Bienz K. (2002) J. Virol. 76, 5974–5984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gosert R., Egger D., Lohmann V., Bartenschlager R., Blum H. E., Bienz K., Moradpour D. (2003) J. Virol. 77, 5487–5492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Appel N., Schaller T., Penin F., Bartenschlager R. (2006) J. Biol. Chem. 281, 9833–9836 [DOI] [PubMed] [Google Scholar]
- 21.Martin S., Parton R. G. (2006) Nat. Rev. Mol. Cell Biol. 7, 373–378 [DOI] [PubMed] [Google Scholar]
- 22.Santolini E., Migliaccio G., La Monica N. (1994) J. Virol. 68, 3631–3641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLauchlan J., Lemberg M. K., Hope G., Martoglio B. (2002) EMBO J. 21, 3980–3988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hüssy P., Langen H., Mous J., Jacobsen H. (1996) Virology 224, 93–104 [DOI] [PubMed] [Google Scholar]
- 25.Hope R. G., Murphy D. J., McLauchlan J. (2002) J. Biol. Chem. 277, 4261–4270 [DOI] [PubMed] [Google Scholar]
- 26.Boulant S., Montserret R., Hope R. G., Ratinier M., Targett-Adams P., Lavergne J. P., Penin F., McLauchlan J. (2006) J. Biol. Chem. 281, 22236–22247 [DOI] [PubMed] [Google Scholar]
- 27.Boulant S., Targett-Adams P., McLauchlan J. (2007) J. Gen. Virol. 88, 2204–2213 [DOI] [PubMed] [Google Scholar]
- 28.Miyanari Y., Atsuzawa K., Usuda N., Watashi K., Hishiki T., Zayas M., Bartenschlager R., Wakita T., Hijikata M., Shimotohno K. (2007) Nat. Cell Biol. 9, 1089–1097 [DOI] [PubMed] [Google Scholar]
- 29.Shavinskaya A., Boulant S., Penin F., McLauchlan J., Bartenschlager R. (2007) J. Biol. Chem. 282, 37158–37169 [DOI] [PubMed] [Google Scholar]
- 30.Okamoto K., Mori Y., Komoda Y., Okamoto T., Okochi M., Takeda M., Suzuki T., Moriishi K., Matsuura Y. (2008) J. Virol. 82, 8349–8361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Targett-Adams P., Hope G., Boulant S., McLauchlan J. (2008) J. Biol. Chem. 283, 16850–16859 [DOI] [PubMed] [Google Scholar]
- 32.Murray C. L., Jones C. T., Tassello J., Rice C. M. (2007) J. Virol. 81, 10220–10231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blanchard E., Hourioux C., Brand D., Ait-Goughoulte M., Moreau A., Trassard S., Sizaret P. Y., Dubois F., Roingeard P. (2003) J. Virol. 77, 10131–10138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roingeard P., Hourioux C., Blanchard E., Brand D., Ait-Goughoulte M. (2004) Biol. Cell 96, 103–108 [DOI] [PubMed] [Google Scholar]
- 35.Hourioux C., Ait-Goughoulte M., Patient R., Fouquenet D., Arcanger-Doudet F., Brand D., Martin A., Roingeard P. (2007) Cell. Microbiol. 9, 1014–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roingeard P., Hourioux C., Blanchard E., Prensier G. (2008) Histochem. Cell Biol. 130, 561–566 [DOI] [PubMed] [Google Scholar]
- 37.Elazar M., Liu P., Rice C. M., Glenn J. S. (2004) J. Virol. 78, 11393–11400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hügle T., Fehrmann F., Bieck E., Kohara M., Kräusslich H. G., Rice C. M., Blum H. E., Moradpour D. (2001) Virology 284, 70–81 [DOI] [PubMed] [Google Scholar]
- 39.Targett-Adams P., Boulant S., McLauchlan J. (2008) J. Virol. 82, 2182–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El-Hage N., Luo G. (2003) J. Gen. Virol. 84, 2761–2769 [DOI] [PubMed] [Google Scholar]
- 41.Moradpour D., Evans M. J., Gosert R., Yuan Z., Blum H. E., Goff S. P., Lindenbach B. D., Rice C. M. (2004) J. Virol. 78, 7400–7409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tellinghuisen T. L., Foss K. L., Treadaway J. C., Rice C. M. (2008) J. Virol. 82, 1073–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Appel N., Zayas M., Miller S., Krijnse-Locker J., Schaller T., Friebe P., Kallis S., Engel U., Bartenschlager R. (2008) PLoS Pathog. 4, e1000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masaki T., Suzuki R., Murakami K., Aizaki H., Ishii K., Murayama A., Date T., Matsuura Y., Miyamura T., Wakita T., Suzuki T. (2008) J. Virol. 82, 7964–7976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tellinghuisen T. L., Foss K. L., Treadaway J. (2008) PLoS Pathog. 4, e1000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boulant S., Douglas M. W., Moody L., Budkowska A., Targett-Adams P., McLauchlan J. (2008) Traffic 9, 1268–1282 [DOI] [PubMed] [Google Scholar]
- 47.Fujimoto Y., Itabe H., Sakai J., Makita M., Noda J., Mori M., Higashi Y., Kojima S., Takano T. (2004) Biochim. Biophys. Acta 1644, 47–59 [DOI] [PubMed] [Google Scholar]
- 48.Lai C. K., Jeng K. S., Machida K., Lai M. M. (2008) J. Virol. 82, 8838–8848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pietschmann T., Kaul A., Koutsoudakis G., Shavinskaya A., Kallis S., Steinmann E., Abid K., Negro F., Dreux M., Cosset F. L., Bartenschlager R. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 7408–7413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jirasko V., Montserret R., Appel N., Janvier A., Eustachi L., Brohm C., Steinmann E., Pietschmann T., Penin F., Bartenschlager R. (2008) J. Biol. Chem. 283, 28546–28562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han Q., Xu C., Wu C., Zhu W., Yang R., Chen X. (2009) Virus Res. 145, 63–73 [DOI] [PubMed] [Google Scholar]
- 52.Ma Y., Yates J., Liang Y., Lemon S. M., Yi M. (2008) J. Virol. 82, 7624–7639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Griffin S. D., Beales L. P., Clarke D. S., Worsfold O., Evans S. D., Jaeger J., Harris M. P., Rowlands D. J. (2003) FEBS Lett. 535, 34–38 [DOI] [PubMed] [Google Scholar]
- 54.StGelais C., Tuthill T. J., Clarke D. S., Rowlands D. J., Harris M., Griffin S. (2007) Antiviral Res. 76, 48–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sakai A., Claire M. S., Faulk K., Govindarajan S., Emerson S. U., Purcell R. H., Bukh J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 11646–11651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Russell R. S., Meunier J. C., Takikawa S., Faulk K., Engle R. E., Bukh J., Purcell R. H., Emerson S. U. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 4370–4375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Griffin S. D., Harvey R., Clarke D. S., Barclay W. S., Harris M., Rowlands D. J. (2004) J. Gen. Virol. 85, 451–461 [DOI] [PubMed] [Google Scholar]
- 58.Griffin S., Stgelais C., Owsianka A. M., Patel A. H., Rowlands D., Harris M. (2008) Hepatology 48, 1779–1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tellinghuisen T. L., Marcotrigiano J., Rice C. M. (2005) Nature 435, 374–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adair R., Patel A. H., Corless L., Griffin S., Rowlands D. J., McCormick C. J. (2009) J. Gen. Virol. 90, 833–842 [DOI] [PubMed] [Google Scholar]
- 61.Friebe P., Bartenschlager R. (2009) J. Virol. 83, 11989–11995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ishii K., Murakami K., Hmwe S. S., Zhang B., Li J., Shirakura M., Morikawa K., Suzuki R., Miyamura T., Wakita T., Suzuki T. (2008) Biochem. Biophys. Res. Commun. 371, 446–450 [DOI] [PubMed] [Google Scholar]
- 63.Steinmann E., Brohm C., Kallis S., Bartenschlager R., Pietschmann T. (2008) J. Virol. 82, 7034–7046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koutsoudakis G., Kaul A., Steinmann E., Kallis S., Lohmann V., Pietschmann T., Bartenschlager R. (2006) J. Virol. 80, 5308–5320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schaller T., Appel N., Koutsoudakis G., Kallis S., Lohmann V., Pietschmann T., Bartenschlager R. (2007) J. Virol. 81, 4591–4603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gibbons G. F., Wiggins D., Brown A. M., Hebbachi A. M. (2004) Biochem. Soc. Trans. 32, 59–64 [DOI] [PubMed] [Google Scholar]
- 67.André P., Komurian-Pradel F., Deforges S., Perret M., Berland J. L., Sodoyer M., Pol S., Bréchot C., Paranhos-Baccalà G., Lotteau V. (2002) J. Virol. 76, 6919–6928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nielsen S. U., Bassendine M. F., Burt A. D., Martin C., Pumeechockchai W., Toms G. L. (2006) J. Virol. 80, 2418–2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nielsen S. U., Bassendine M. F., Martin C., Lowther D., Purcell P. J., King B. J., Neely D., Toms G. L. (2008) J. Gen. Virol. 89, 2507–2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lindenbach B. D., Evans M. J., Syder A. J., Wölk B., Tellinghuisen T. L., Liu C. C., Maruyama T., Hynes R. O., Burton D. R., McKeating J. A., Rice C. M. (2005) Science 309, 623–626 [DOI] [PubMed] [Google Scholar]
- 71.Gastaminza P., Kapadia S. B., Chisari F. V. (2006) J. Virol. 80, 11074–11081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chang K. S., Jiang J., Cai Z., Luo G. (2007) J. Virol. 81, 13783–13793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gastaminza P., Cheng G., Wieland S., Zhong J., Liao W., Chisari F. V. (2008) J. Virol. 82, 2120–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang H., Sun F., Owen D. M., Li W., Chen Y., Gale M., Jr., Ye J. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 5848–5853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Benga W. J., Krieger S. E., Dimitrova M., Zeisel M. B., Parnot M., Lupberger J., Hildt E., Luo G., McLauchlan J., Baumert T. F., Schuster C. (2010) Hepatology 51, 43–53 [DOI] [PubMed] [Google Scholar]
- 76.Jiang J., Luo G. (2009) J. Virol. 83, 12680–12691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Olofsson S. O., Borèn J. (2005) J. Intern. Med. 258, 395–410 [DOI] [PubMed] [Google Scholar]
- 78.Mensenkamp A. R., Havekes L. M., Romijn J. A., Kuipers F. (2001) J. Hepatol. 35, 816–822 [DOI] [PubMed] [Google Scholar]
- 79.Icard V., Diaz O., Scholtes C., Perrin-Cocon L., Ramière C., Bartenschlager R., Penin F., Lotteau V., André P. (2009) PLoS One 4, e4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Corless L., Crump C. M., Griffin S. D., Harris M. (2010) J. Gen. Virol. 91, 362–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Teis D., Saksena S., Emr S. D. (2009) Cell 137, 182–182.e1 [DOI] [PubMed] [Google Scholar]
- 82.Babst M., Katzmann D. J., Estepa-Sabal E. J., Meerloo T., Emr S. D. (2002) Dev. Cell 3, 271–282 [DOI] [PubMed] [Google Scholar]
- 83.Babst M., Katzmann D. J., Snyder W. B., Wendland B., Emr S. D. (2002) Dev. Cell 3, 283–289 [DOI] [PubMed] [Google Scholar]
- 84.Timpe J. M., Stamataki Z., Jennings A., Hu K., Farquhar M. J., Harris H. J., Schwarz A., Desombere I., Roels G. L., Balfe P., McKeating J. A. (2008) Hepatology 47, 17–24 [DOI] [PubMed] [Google Scholar]
- 85.Witteveldt J., Evans M. J., Bitzegeio J., Koutsoudakis G., Owsianka A. M., Angus A. G., Keck Z. Y., Foung S. K., Pietschmann T., Rice C. M., Patel A. H. (2009) J. Gen. Virol. 90, 48–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ploss A., Khetani S. R., Jones C. T., Syder A. J., Trehan K., Gaysinskaya V. A., Mu K., Ritola K., Rice C. M., Bhatia S. N.Proc. Natl. Acad. Sci. U.S.A. (2010) 107, 3141–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.