Abstract
ClC-3 is a Cl−/H+ antiporter required for cytokine-induced intraendosomal reactive oxygen species (ROS) generation by Nox1. ClC-3 current is distinct from the swelling-activated chloride current (IClswell), but overexpression of ClC-3 can activate currents that resemble IClswell. Because H2O2 activates IClswell directly, we hypothesized that ClC-3-dependent, endosomal ROS production activates IClswell. Whole-cell perforated patch clamp methods were used to record Cl− currents in cultured aortic vascular smooth muscle cells from wild type (WT) and ClC-3 null mice. Under isotonic conditions, tumor necrosis factor-α (TNF-α) (10 ng/ml) activated outwardly rectifying Cl− currents with time-dependent inactivation in WT but not ClC-3 null cells. Inhibition by tamoxifen (10 μm) and by hypertonicity (340 mosm) identified them as IClswell. IClswell was also activated by H2O2 (500 μm), and the effect of TNF-α was completely inhibited by polyethylene glycol-catalase. ClC-3 expression induced IClswell in ClC-3 null cells in the absence of swelling or TNF-α, and this effect was also blocked by catalase. IClswell activation by hypotonicity (240 mosm) was only partially inhibited by catalase, and the size of these currents did not differ between WT and ClC-3 null cells. Disruption of endosome trafficking with either mutant Rab5 (S34N) or Rab11 (S25N) inhibited TNF-α-mediated activation of IClswell. Thrombin also activates ROS production by Nox1 but not in endosomes. Thrombin caused H2O2-dependent activation of IClswell, but this effect was not ClC-3- or Rab5-dependent. Thus, activation of IClswell by TNF-α requires ClC-3-dependent endosomal H2O2 production. This demonstrates a functional link between two distinct anion currents, ClC-3 and IClswell.
Keywords: Chloride Channels, Chloride Transport, Reactive Oxygen Species (ROS), Thrombin, Tumor Necrosis Factor (TNF), Chloride-Proton Antiporter, Swelling-activated Chloride Channel
Introduction
ClC-3 is a member of the CLC family of Cl− channels and Cl−/H+ antiporters. When expressed in HEK293 cells, it behaves as an antiporter at neutral pH (1). However, at high extracellular proton concentrations, transport becomes uncoupled, and ClC-3 behaves as an anion-selective pore (2). This mode of ClC-3 activity may account for the native acid-activated current in HEK293 cells (3). The basic biophysical properties of ClC-3 currents are similar under both coupled and uncoupled conditions. They display very sharp outward rectification and time-dependent activation. Mutation of the extracellular fast gate (E224A) alters both of these characteristics, and inhibition of wild type ClC-3 current by alkanethiolation with methanethiosulfonate can be prevented by deletion of cysteine residues in the first extracellular loop of the protein. These properties clearly associate the currents induced by ClC-3 expression with ClC-3 protein (1, 2).
However, heterologous expression of ClC-3 has also been repeatedly associated with a less sharply outwardly rectifying anion current that displays time-dependent inactivation instead of activation. Currents induced in NIH3T3 cells by plasmid-mediated ClC-3 overexpression appeared indistinguishable from the swelling-activated anion current (IClswell),2 and it was suggested that ClC-3 was an IClswell (4). This idea was supported by a demonstration that anti-ClC-3 antibodies placed within the patch pipette block IClswell activation under hypotonic conditions (5, 6) and by the fact that N-terminal deletion mutants of ClC-3 yielded constitutively active, IClswell-like currents (7). Unfortunately, other investigators have either been unable to express ClC-3 currents (8–10) or have observed currents that were insensitive to changes in cell volume (10–14). Importantly, ClC-3 null cells have consistently exhibited normal appearing whole-cell (15–17) and single channel (18) IClswell currents. The regulation of IClswell was, however, clearly altered in ClC-3 null cells (6). Other investigators have identified a calcium-calmodulin kinase II (CamKII)-dependent Cl− current in cells expressing ClC-3 (11). These currents, which look like IClswell, were not responsive to changes in cell volume and were absent in smooth muscle cells (19) and hippocampal neurons from ClC-3 null mice (20). We hypothesized that these apparently disparate data might be reconciled if ClC-3 is required for regulation of IClswell.
Multiple lines of evidence have established a relationship between ClC-3, NADPH oxidase activity, and signaling via reactive oxygen species (ROS). Nox1 and ClC-3 co-localize to early endosomes in vascular smooth muscle (VSM) cells, and both are required for tumor necrosis factor (TNF-α) and interleukin (IL-1β)-dependent ROS generation and for the subsequent activation of the transcription factor nuclear factor κB (NF-κB) (21). Intracellular but not extracellular Nox2 activity in neutrophils also requires ClC-3. The intracellular oxidative burst (22), ROS-dependent endotoxin signaling (23), and shape change during chemotaxis (24) are all impaired in ClC-3-deficient neutrophils. We have proposed that ClC-3 provides charge neutralization of electron flow through NADPH oxidase, into intracellular vesicles (1, 25), but this relationship remains to be directly demonstrated experimentally.
H2O2 activates IClswell under isotonic conditions and provides the signaling mechanism by which epidermal growth factor (EGF) stimulates IClswell in HeLa cells (26–28). Activation of IClswell in ventricular myocytes exposed to hypotonicity (27) or stretch (29) also requires H2O2 production that is triggered via a sequential pathway that involves angiotensin II and EGF receptor activation (28–30). Hypoosmotic stress has also been linked to Nox activation and subsequent ROS production in cultured brain astrocytes (31). Finally, the protein kinase inhibitor staurosporine has been shown to activate IClswell via Nox activation in HeLa cells (32).
We tested the hypothesis that TNF-α-dependent generation of H2O2 activates IClswell in VSM cells. Because TNF-α-induced H2O2 production is ClC-3-dependent, the Cl−/H+ antiporter would be expected to be required for the process. The results of these studies provide evidence that endosomal ClC-3-dependent ROS production is required for cytokine-mediated activation of IClswell.
EXPERIMENTAL PROCEDURES
Cell Culture and Modification of ClC-3 Expression
Murine aortic VSM cells were prepared from ClC-3 wild type and null mice using established methods (19, 21). VSM cells were grown in high glucose Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum, 44 mm NaHCO3, 25 mm HEPES (pH 7.4), 1% minimum Eagle's medium vitamins and 1% non-essential amino acids (Invitrogen). Cells were allowed to reach no more than 70% confluence and were passaged fewer than 10 times. A dual expression adenovirus expressing ClC-3 (Ad-ClC-3) behind the cytomegalovirus promoter and enhanced GFP behind the RSV promoter was prepared and titrated by the University of Iowa Vector Core as reported previously (1). Adenovirus expressing dominant negative Rab5 (S34N) was a generous gift from Dr. Brian Ceresa (University of Oklahoma). Control adenovirus expressed only enhanced GFP. Adenoviruses were infected in serum-free Dulbecco's modified Eagle's medium for 16 h prior to being returned to their standard serum concentration. They were allowed to express for 24–48 h prior to experimentation. Expression of S34N Rab11 was demonstrated by Western blotting performed as previously (23). A 10% gel was loaded with 100 μg/lane of protein, and nitrocellulose blots were probed with rabbit polyclonal anti-Rab5 (Abcam ab18211). Due to the relatively low efficiency of plasmid uptake by cultured VSM cells, plasmid expressing Myc-tagged dominant negative Rab11b (S25N Rab11-Myc) was co-transfected with an independent plasmid expressing enhanced GFP (1 μg of each plasmid/well in 6-well plates containing ∼106 cells/well) using Lipofectamine 2000 (5 μl/ml) in 2% serum. Plasmids were allowed to express for 48–96 h, and only cells expressing GFP were studied.
Electrophysiology
Chloride ion currents were measured at room temperature (22 °C) using perforated patch recording (33) performed with an Axopatch 200B patch clamp amplifier driven by pClamp 9 software (Molecular Devices Corp., Sunnyvale, CA). Pipette resistances were 3–5 megaohms. Pipette and whole-cell capacitance and series resistance compensations were done prior to recording. Currents were elicited from a holding potential of −40 mV to test potentials from −100 mV to +100 mV in 20-mV increments. Test pulses were 1 s in duration, delivered at 3-s intervals. Currents were sampled at 5 kHz and filtered at 1 kHz.
Standard bath solution contained 120 mm NaCl, 2.5 mm MgCl2, 2.5 mm CaCl2, 10 mm HEPES, 5.5 mm glucose, pH 7.35, with NaOH. Osmolality was determined using a μ OSMETTE osmometer and was titrated to 300 mosm using 1 m mannitol. Hypotonic solution (240 mosm) was identical except for the exclusion of mannitol, and hypertonic (340 mosm) solution was prepared using additional mannitol. Low Cl− bath solution contained 32 mm NaCl, 2.5 mm CaCl2, 2.5 mm MgCl2, 10 mm HEPES, and 240 mm glucose. Liquid junction potentials were minimized using 3 m KCl agar bridges and were calculated using pClamp version 9.0 to be 5.0 and 2.4 mV for the 130 and 42 mm Cl− buffer, respectively. Pipette solution contained 125 mm CsCl, 2.5 mm MgCl2, 10 mm HEPES, pH 7.2, with CsOH, and osmolality was adjusted to 290 mosm with 1 m mannitol. Amphotericin was dissolved in DMSO at a concentration of 60 mg/ml, and then 20 μl of this solution was mixed with 5 ml of pipette solution by vortexing. Currents were normalized to cell membrane capacitance and expressed as current density (pA/pF). Identification of GFP-positive cells was done immediately prior to cell selection using a fluorescence-equipped inverted microscope (Zeiss Axiovert 25). Due to significant variability in the size of the currents activated by cytokines or swelling, appropriate controls were repeated in conjunction with each intervention tested. All chemicals were obtained from Sigma.
Data Analysis and Statistics
Peak IClswell currents were measured 20 ms after initiation of the depolarizing pulse, and late currents were quantified 5 ms before the end of the pulse. Peak currents were used to calculate current-voltage relationships for IClswell. Reversal potentials were obtained from each individual I/V relationship by fitting a straight line (y = mx + b) between consecutive data points negative to and positive to zero current density and extrapolating to the x-intercept. Reversal potential estimates were corrected for liquid junction potentials. Results are expressed as means ± S.E. Two-way analysis of variance or unpaired Student's t tests with a Bonferroni correction were used to evaluate differences. A p value of <0.05 was considered to be statistically significant.
RESULTS
Cytokine Activation of IClswell Is ClC-3- and H2O2-dependent
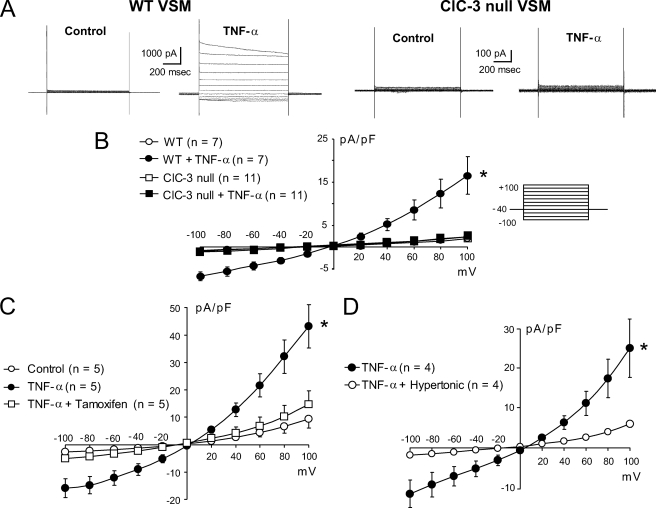
In wild type VSM cells, TNF-α (10 ng/ml) activated an outwardly rectifying current under isotonic conditions. It displayed time-dependent inactivation at positive test potentials and reversed at +0.91 ± 0.35 mV, consistent with the theoretical value under these conditions of 0 mV (Fig. 1, A and B). Reduction of extracellular Cl− to 42 mm caused a +26.0 ± 2.4 mV shift in the reversal potential (n = 5). This is very close to the +28.7 mV shift predicted by the Nernst equation for a Cl−-selective current, thus demonstrating that Cl− is the main permeating ion. The effect of TNF-α required several minutes to begin, reached steady state at 394 ± 22 s (n = 7), and was slowly (>10 min) reversed by washout of the TNF-α. This response could not be consistently elicited using conventional, dialyzed whole-cell recording methods; hence, all experiments were performed using perforated patches. A current similar to that induced by TNF-α was also elicited by IL-1β (10 ng/ml, n = 4; data not shown), which also causes endosomal ROS production (21). No response to TNF-α was observed in ClC-3 null cells. TNF-α-induced currents were inhibited by tamoxifen (10 μm; Fig. 1C) and by hypertonic conditions (340 mosm; Fig. 1D), providing direct evidence that the current activated is indeed IClswell.
FIGURE 1.
TNF-α activates IClswell in WT but not ClC-3 null VSM cells. A, typical currents from WT and ClC-3 null cells ∼10 min after exposure to 10 μm TNF-α. B, I/V plot of the summary data (holding potential −40 mV, test potentials −100 to +100 mV in 20-mV steps; see inset). The response to TNF-α in WT cells is inhibited by either 10 μm tamoxifen (C) or hypertonic (340 mosm) conditions (D). *, significant difference across the full voltage range (p < 0.05). Error bars, S.E.
As observed in other cell types (26, 27), direct exposure to 500 μm H2O2 reversibly activated a current that was consistent with IClswell (Fig. 2A). The magnitude of this current was not different between wild type and ClC-3 null VSM cells. Scavenging of H2O2 by incubation (10 min) in PEG-catalase (1000 units/ml, which converts 2H2O2 to 2H2O + 2O2), prior to exposure to TNF-α completely inhibited current activation (Fig. 2B).
FIGURE 2.
A, H2O2 (500 μm) activates IClswell directly in both WT and ClC-3 null cells. Typical current records (above) are paired with I/V plots of peak currents (below). B, scavenging of H2O2 with catalase (1000 units/ml) inhibits IClswell activation by TNF-α. Error bars, S.E.
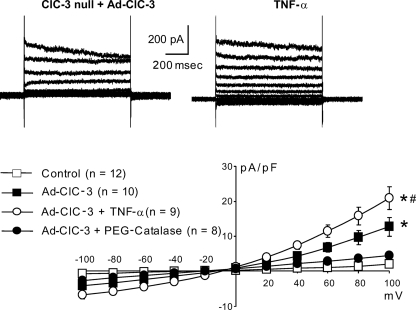
Currents similar to IClswell were also observed in ClC-3 null cells expressing Ad-ClC-3 even in the absence of TNF-α. They were observed immediately after gaining access by amphotericin under isotonic conditions (Fig. 3). The addition of TNF-α caused a small but significant increase in the magnitude of these currents, and they were absent in cells incubated in PEG-catalase. These observations are consistent with the effect of ClC-3 overexpression previously observed in NIH3T3 fibroblasts (4) but in contrast to what is observed in HEK293 cells, where ClC-3 current is observed in the plasma membrane at pH 7.35 (1). This difference may be related to the relative amounts of ClC-3 and IClswell protein that are inserted into the plasma membrane in these two cell types at any one time. Activation of IClswell by ClC-3 overexpression alone is consistent with previous work, demonstrating that in these same murine VSM cells, Ad-ClC-3 expression alone enhances H2O2-dependent NF-κB activation, even in the absence of other stimuli (21). Together, these observations suggest that ClC-3 overexpression enhances endogenous H2O2 production, which activates IClswell in a manner similar to exogenous H2O2.
FIGURE 3.
Ad-ClC-3 expression in ClC-3 null cells activates IClswell even in the absence of cytokine stimulation. This current is further activated by TNF-α, and both responses are inhibited by PEG-catalase. *, difference from non-expressing ClC-3 null cells; #, significant difference from TNF-α. Error bars, S.E.
IClswell Activation by Hypotonicity or Thrombin Is H2O2- but Not ClC-3-dependent
Similar to what others have repeatedly observed in a variety of cell types (15–17), the size of the IClswell elicited by hypotonic conditions was not altered in ClC-3 null VSM cells (Fig. 4). In order to determine the degree to which this response depends upon production of H2O2, both WT and ClC-3 null cells were exposed to hypotonic buffer in the presence of catalase. Scavenging of H2O2 reduced the magnitude of IClswell significantly but did not completely block the response, and cells of both genotypes were equally affected (Fig. 5). Thus, hypotonic swelling appears to activate IClswell partly via H2O2-dependent signaling; however, ROS production in response to cell swelling is not ClC-3-dependent.
FIGURE 4.
The magnitude of IClswell is unaltered in ClC-3 null VSM cells. Typical current records (above) are paired with I/V plots of peak currents (below). Error bars, S.E.
FIGURE 5.
IClswell activation in hypotonic conditions is partially inhibited by PEG-catalase in both WT and ClC-3 null VSM cells. Typical current recordings are shown in A, and I/V summaries for WT (left) and ClC-3 null (right) cells are shown in B. The magnitude of inhibition by catalase is not different between the two groups of cells. *, difference from control or control plus catalase; #, significant difference from hypotonic. Error bars, S.E.
To further investigate the relationship between the currents activated by TNF-α and hypotonicity, we assessed the impact of providing both stimuli sequentially to the same cell. TNF-α increased current density at +100 mV from 3.4 ± 0.6 (control) to 28.0 ± 4.8 pA/pF. Hypotonic conditions in the continued presence of 10 ng/ml TNF-α changed peak current to 38.7 ± 6.6 pA/pF (38 ± 17% increase, n = 5, p = 0.11). A return to isotonic conditions in the presence of TNF-α reduced current back to 29.2 ± 6.1 pA/pF. This hypotonicity-induced increment in current on top of TNF-α persisted and was still reversible even when a higher concentration of TNF-α (100 ng/ml) was used (48 ± 15% increment, n = 5, p < 0.05). The magnitude of the effects of hypotonicity was reminiscent of the proportion of the response to hypotonicity that was catalase-insensitive (40.6 ± 8.6%; Fig. 5A). To better determine if hypotonicity could activate IClswell channels that could not be stimulated by TNF-α, we reversed the order of exposure to the stimuli. Hypotonicity alone activated 28.45 ± 2.2 pA/pF of current, but 100 ng/ml of TNF-α failed to enhance this current at all (29.9 ± 2.27 pA/pF, n = 4, not significant). These results suggest that the population of channels activated by these two stimuli is overlapping, but hypotonicity can activate a subset of channels that are not accessible to TNF-α stimulation.
ROS production in response to TNF-α is endocytosis-, Nox1-, and ClC-3-dependent (21, 34). We assessed the dependence of IClswell activation by TNF-α on endosome internalization by disrupting the formation and/or processing of early endosomes using dominant negative S34N Rab5 (35). Successful expression was documented by Western blotting (Fig. 6A). The presence of the mutant protein blocked activation of IClswell by TNF-α, but hypotonic conditions still activated the current. This supports our previous suggestion that TNF-α-induced H2O2 production occurs in early endosomes (21) and is also consistent with the response to TNF-α, but not to hypotonicity, being ClC-3- and endocytosis-dependent.
FIGURE 6.
A, Western blot demonstrates expression of S34N Rab5 in cells exposed to adenovirus encoding the protein. Disruption of early endosome sorting with S34N Rab5 prevents activation of IClswell by TNF-α but not by hypotonic conditions. B, interfering with endosome recycling using S25N Rab11 also inhibits activation of IClswell by TNF-α. Error bars, S.E.
Dependence of IClswell activation on recycling of proteins back to the plasma membrane was explored using dominant negative S25N Rab11b (36), which disrupts endosome processing in a compartment that is distal to Rab5 (supplemental Fig. 1). This mutant also inhibited the response to TNF-α (Fig. 6B), although it modifies a compartment that is distal to the site of H2O2 production. Dependence of current activation on membrane recycling raises the possibility that IClswell is actually turned on in the early endosome, and functional channels are reinserted into the plasma membrane.
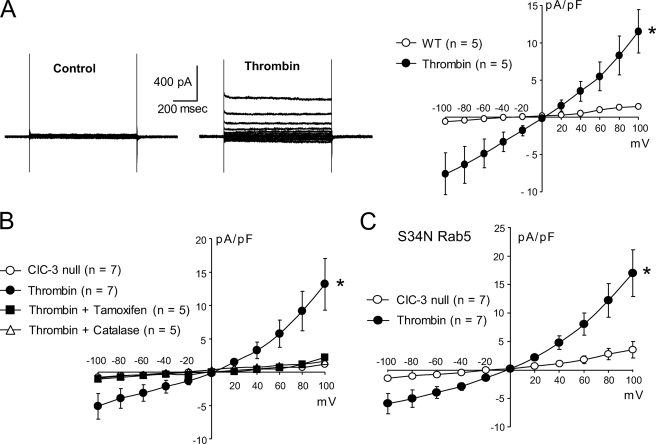
Like TNF-α, thrombin also elicits ROS production by Nox1; however, this effect does not require endocytosis, suggesting that these ROS are made at the cell surface (34). We therefore hypothesized that thrombin could activate IClswell but that this response would be independent of either endocytosis or ClC-3. Thrombin consistently activated IClswell in both WT (Fig. 7A) and ClC-3 null VSM cells (Fig. 7B). Just as with TNF-α, this effect was inhibited by both tamoxifen and PEG-catalase, again suggesting that these are IClswell currents (Fig. 7B). However, consistent with this being an endocytosis-independent process, S34N Rab5 expression had no effect upon the response to thrombin (Fig. 7C).
FIGURE 7.
A, thrombin (1 unit/ml) activates IClswell in wild type VSM cells. B, a similar current is also activated in ClC-3 null cells, and this current is inhibited by either tamoxifen or catalase. C, S34N Rab5 does not interfere with activation of IClswell by thrombin. Error bars, S.E.
ClC-3 and IClswell Currents Are Distinct
We have previously characterized ClC-3 currents in HEK293 cells (1). They are activated, and Cl−/H+ antiport is uncoupled, by low pH. Thus, ClC-3 behaves as an acid-activated anion conductance (2). One of the important historic links between ClC-3 and IClswell has been the ability of antibodies specific for ClC-3 to prevent activation of IClswell (5, 37, 38). We therefore tested the ability of an anti-ClC-3 antibody that was previously shown to inhibit IClswell to inhibit 1) the current induced in HEK293 cells by Ad-ClC-3 expression and 2) native acid-induced currents in HEK293 cells. Unlike the previously described experiments in VSM cells, these experiments were performed in the whole-cell dialyzed configuration so that the antibodies had ready access to the cytoplasm. Both Ad-ClC-3 currents and native IClacid were inhibited by 30 μg/ml anti-ClC-3 antibody in the pipette. This inhibition appeared to be use-dependent. Anti-ClC-4 and anti-ClC-5 antibodies had no effect on the acid-induced current (supplemental Fig. 2).
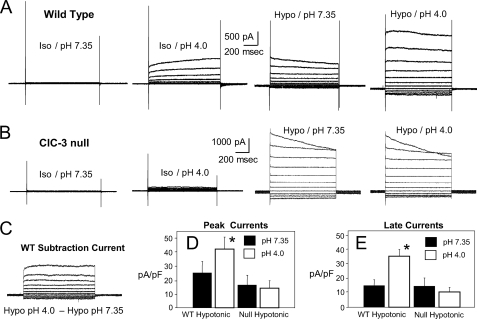
In order to further discriminate the anion current that is carried by ClC-3 from IClswell, we combined two stimuli, low pH (4.0) and hypotonicity, in wild type and ClC-3 null VSM cells. Fig. 8, A and B, show typical current recordings from single cells of each genotype sequentially exposed to pH 4.0 under isotonic conditions, hypotonic buffer at pH 7.35, and hypotonic buffer at pH 4.0. As reported previously, WT cells respond to pH 4.0, but ClC-3 null cells do not (2). These acid-induced currents were easily discernible from the less rectifying and inactivating IClswell elicited by hypotonicity. The combination of low pH and hypotonicity yielded currents of a magnitude and contour that were consistent with a combination of these two currents. ClC-3 null cells had no acid-activated current but again responded to hypotonic buffer with a typical IClswell. The combined stimuli yielded a current that was indistinguishable from the effect of hypotonic buffer alone (Fig. 8B). Subtraction of the current obtained in the wild type cells under hypotonic conditions at pH 7.35 from those observed at the same osmolality at pH 4.0 yielded currents that were very similar to the currents observed at pH 4.0 under isotonic conditions (Fig. 8C). The average peak (Fig. 8D) and late (Fig. 8E) current levels for a group of cells undergoing this protocol revealed the consistency of these observations. ClC-3 null VSM cells responded to low osmolarity but not to low pH, whereas WT cells responded to both stimuli.
FIGURE 8.
Sequential exposure to low pH (4.0), hypotonicity, and finally low pH under hypotonic conditions in WT (A) and ClC-3 null (B) VSM cells reveals that ClC-3 currents (acid-activated (2)) are clearly distinct from those activated under hypotonic conditions. C, when the currents obtained from the WT cell in A under hypotonic conditions at pH 7.35 are subtracted from those observed in the same cell under hypotonic conditions at pH 4.0, the remaining current is very similar to what was initially observed at pH 4.0 under isotonic conditions. Summary data for the magnitude of both peak (D) and late (end of the pulse) currents (E) in WT and ClC-3 null cells confirms that only WT currents are enhanced at low pH. Error bars, S.E.
DISCUSSION
In VSM cells, TNF-α activates Cl− currents that are indistinguishable from those elicited by hypotonic conditions. The response to TNF-α is absent in ClC-3 null cells and is blocked in WT cells by scavenging of H2O2 or impairment of either early endosome processing or endosome recycling to the plasma membrane. Thrombin and cell swelling also cause H2O2-dependent IClswell activation but via pathways that are ClC-3-independent.
The fact that TNF-α activates IClswell is novel but perhaps not so surprising. Cytokines are well known to cause ROS generation (21, 39), and activation of IClswell by hypotonic conditions is H2O2-dependent (26–28). The most significant observation in this study is that TNF-α ROS signaling requires ClC-3, whereas responses to hypotonicity and thrombin do not. This suggests that VSM cells have at least two ROS-generating mechanisms that operate in parallel with respect to activation of IClswell. It is therefore perhaps not surprising that the roles of ClC-3 and IClswell have been difficult to discriminate. ClC-3 independence of the response to hypotonicity is in accordance with the work of multiple other investigators who have also seen no reduction in the magnitude of IClswell in various ClC-3 null cells (6, 16, 17). Thus, in VSM cells, swelling-induced ROS production most likely occurs somewhere other than in early endosomes, where Nox1 activity is ClC-3-dependent (21, 22). Like TNF-α, thrombin also activates Nox1 (34) and causes proinflammatory responses that are very similar to those linked to TNF-α (40). However, the effects of thrombin are endocytosis-independent (34), and accordingly, IClswell activation by thrombin is not impaired in ClC-3 null cells (see Fig. 9 for a schematic overview). Like thrombin, hypotonicity-induced ROS production may utilize Nox1 in the plasma membrane. Alternatively Nox4 has been shown to be activated by hypotonic stress, and the ROS produced in turn can activate IClswell in NIH3T3 cells (41).
FIGURE 9.
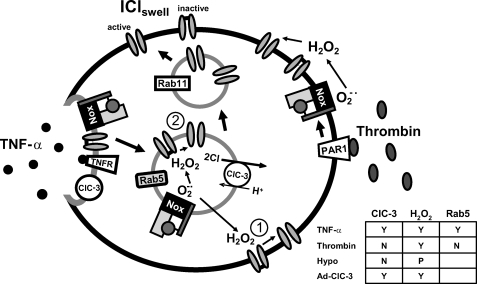
Schematic comparison of proposed pathways for activation of IClswell by TNF-α and thrombin. An activated TNF-α receptor is endocytosed into a Rab5-positive early endosome. This compartment also contains ClC-3 and Nox1 and is the site of TNF-α-mediated ROS production, as previously demonstrated (21). These proteins, including whatever mediates IClswell, may become incorporated into the endosome by endocytosis from the plasma membrane (as shown) but could also come from a different source, such as a post-Golgi vesicle. ClC-3-dependent superoxide and subsequently hydrogen peroxide production within this compartment are required for activation of IClswell. This could occur by several mechanisms: 1) direct activation via diffusion of H2O2 from the endosome to the plasma membrane, 2) direct activation within the early endosome, or 3) indirect activation of IClswell via modification of an associated protein or signaling pathway which has the ability to activate IClswell (not pictured). The ability of S25N Rab11 to interfere with current activation by TNF-α favors the effect of H2O2 on IClswell taking place within endosomes. This mechanism is appealing because it compartmentalizes the H2O2 required for current activation. Thrombin also signals via Nox1 but does not cause endocytosis (34), suggesting that ROS are produced at the plasma membrane. Thrombin-mediated IClswell activation is therefore sensitive to catalase but is not impaired in ClC-3 null cells; nor is it inhibited by S34N Rab5. The dependence of activation of IClswell by TNF-α, thrombin, hypotonicity, and ClC-3 overexpression on ClC-3, H2O2, and Rab5 are summarized in the inset table. Y, yes; N, no; P, partial.
Links between ClC-3 Expression and IClswell
ClC-3 overexpression activates anion currents with biophysical properties very similar to those of IClswell. Observations linking ClC-3 to IClswell include 1) enhanced current in ClC-3-expressing cells (4, 11, 37, 42), 2) altered current regulation in ClC-3 null cells (6), 3) inhibition of current by anti-ClC-3 antibodies (37, 38), and 4) increased magnitude of IClswell induced by a constitutively active mutant of ClC-3 (7). However, as shown in Fig. 4, IClswell has repeatedly been found to be unaltered in cells lacking ClC-3 (6, 16, 17). In previous work, we have characterized ClC-3 currents that are very different from IClswell. They have time-dependent activation rather than inactivation and are much more sharply outwardly rectifying. ClC-3 currents are similar to those produced by ClC-4 and ClC-5 except that the time constants of activation are slower (1, 2). These currents are inhibited by the same anti-ClC-3 antibody that was previously shown to inhibit IClswell (supplemental Fig. 2). Reconciliation of the existence of unique ClC-3 currents with the many observations linking ClC-3 to IClswell is obviously challenging. We believe that much of the association between ClC-3 expression and IClswell can be attributed to effects of altered ClC-3 abundance or function on endosomal ROS signaling, which in turn controls the activity of a distinct anion conductance, IClswell.
TNF-α and IL-1β both failed to activate IClswell in ClC-3 null VSM cells (Fig. 1), yet H2O2 (Fig. 2), swelling (Fig. 4), and thrombin (Fig. 7) all elicited normal responses. Overexpression of ClC-3, in the absence of any other stimulus, activated IClswell in ClC-3 null VSM cells (Fig. 3). Both the current induced by cytokines and by ClC-3 overexpression were blocked by scavenging of H2O2 with catalase. Furthermore, as demonstrated by the relatively small increments in current caused by TNF-α in ClC-3-overexpressing cells and by hypotonic conditions in TNF-α stimulated cells, these three currents were clearly not additive. Taken together, our findings suggest that all three stimuli increase ROS production and thereby activate the independent anion conductance that produces IClswell.
In addition to TNF-α, IL-1β, and thrombin, multiple other receptor-coupled stimuli, including angiotensin II, growth factors (28, 43), and lysophosphatidic acid (41, 42), have been shown to cause ROS production and also activate IClswell. Although not all have been tested, the current activated by lysophosphatidic acid has been shown to be ClC-3 expression-dependent (42). Many of these agonists mobilize intracellular calcium, which can activate CamKII. This enzyme has been linked to regulation of a chloride conductance that shares many basic properties (rectification, time-dependent inactivation, ion selectivity) with IClswell. Activation of this current is dependent upon ClC-3 expression (11) and is absent in ClC-3 null cells (19). Furthermore, a specific serine on the N terminus of ClC-3 has been identified as the site of CamKII regulation (19). It will be important to determine if CamKII phosphorylation of ClC-3 is required for endosomal H2O2 production and thereby IClswell activation. CamKII activation itself is regulated by ROS in a complex manner (44), and both ROS-dependent NF-κB stimulation and CamKII activation are required for induction of vascular cell adhesion molecule expression by IL-1β (45). Unfortunately, the molecular mechanism by which H2O2 actually activates IClswell has not received much attention. In rat hepatoma cells, the mechanism has been proposed to be indirect and involve phospholipase Cγ1 phosphorylation and calcium mobilization (46).
It is important to note that we see a very different response to ClC-3 overexpression in HEK293 cells, where adenoviral expression of ClC-3 results in enough ClC-3 being present in the plasma membrane to allow us to measure ClC-3 current at pH 7.35 (1, 2) but does not activate IClswell. We can only speculate on the reason for this important difference, but it may be related to the relative amount of ClC-3 and/or IClswell that cycles thought the plasma membrane. This is not to say that there is no ClC-3 in the plasma membrane of VSM cells. The ability of low pH to activate endogenous ClC-3 current (Fig. 8 and Ref. 2) demonstrates that ClC-3 is present but is not detectable at physiologic pH and is clearly distinct from IClswell. The fact that ClC-3 expression activates IClswell in VSM cells, but not in HEK293 cells, highlights what may be very significant cell type-dependent variability in the interdependence of ClC-3 and IClswell. This may complicate the reconciliation of all data related to ClC-3 expression and IClswell.
Endosome Dependence of IClswell Activation
Rab guanosine triphosphatases control budding and fission of vesicles and localize to specific intracellular compartments. Rab5 regulates the biogenesis and trafficking of early endosomes (35). Dominant negative S34N Rab5 inhibits early endosomal fusion (47) and impairs proper processing of both transferrin and epidermal growth factor receptors (35). Rab11 controls movement of proteins through a recycling compartment that forms via processing of early endosomes and mediates movement of endocytosed proteins back to the plasma membrane (48, 49). S25N Rab11 disrupts this process and impairs both processing of and signaling via G-protein-coupled receptors, including the lysophosphatidic acid receptor (48, 50). Both S34N Rab5 and S25N Rab11 profoundly impaired the ability of TNF-α (Fig. 6), but not thrombin (Fig. 7) or hypotonic buffer (Fig. 6A), to activate IClswell. There are two potential interpretations of these data. Either proper processing or positioning of H2O2-producing endosomes is required for IClswell activation, or IClswell is activated within endosomes and recycled back to the plasma membrane in an active state. ROS-producing endosomes are Rab5-positive (21), and the Rab5 compartment is “upstream” of Rab11. Therefore, the ability of the Rab11 mutant, which does not impede function until after early endosome formation, to disrupt TNF-α signaling supports the very interesting possibility that IClswell is an endosomal protein and can be activated there.
Localization of intracellular H2O2 to endosomes is an appealing way to minimize exposure of other intracellular proteins to “oxidative stress.” The need to control this exposure is implied by the presence of multiple cytoplasmic antioxidant systems that scavenge H2O2 (catalase, glutathione peroxidase, etc.). These safeguards are likely to impede diffusion-mediated H2O2 signaling from early endosomes to IClswell channels localized in the plasma membrane. It seems more likely that endosomal ROS trigger another signaling cascade that activates IClswell at the membrane. If recycling endosomes indeed return activated IClswell to the plasma membrane, it is also important to consider the possibility that this current plays a physiological role in endosomes. Endosomes are spherical and therefore ill suited to handle osmotic stress created by changes in endosomal ion content by both passive and active transport across the vesicle membrane (reviewed in Ref. 25). Activated endosomal IClswell could also provide a pathway for charge neutralization of proton current generated by the vacuolar ATPase (51). In such a scenario, dependence upon ClC-3 for ROS production and subsequent activation of IClswell could explain why the ClC-3 Cl−/H+ antiporter is required for normal endosome acidification (52). ClC-3 itself seems relatively poorly suited for charge neutralization of the vacuolar type hydrogen-ATPase, based upon its rectification properties and the fact that it is a Cl−/H+ antiporter (25).
An anion conductance has also been shown to provide a pathway for release of superoxide from signaling endosomes. SOD1-mediated dismutation of superoxide at the cytoplasmic surface of endosomes may then produce highly localized H2O2 that is required for redox activation of NF-κB (53). It has been proposed that ClC-3 itself may be able to conduct superoxide (54), but our data suggest that IClswell is another candidate for this role.
Functional Significance
The ability of ClC-3 overexpression alone to activate IClswell suggests that in VSM cells, endosomal ClC-3 is active under “resting” conditions, and ClC-3 activity can be rate-limiting for ROS production and subsequent signaling. This possibility is consistent with prior observations that ClC-3 overexpression activates NF-κB (21) and promotes resistance to apoptosis (55, 56).
Both ClC-3 (55, 57–59) and IClswell (reviewed in Ref. 60) have been linked to proliferation, apoptosis (61), and cell cycle progression in cultured cells. Increased expression of ClC-3 is also seen in association with inflammation-induced cell proliferation in vivo (55). In addition, both ClC-3 expression (62, 63) and IClswell-like currents (64, 65) have been associated with enhanced proliferation and migration of cancer cells. It is well recognized that both the genomic instability induced by “oxidative stress” and ROS-dependent signaling play critical roles in cancer (reviewed in Ref. 66). The interdependence between ClC-3, NADPH oxidase-dependent ROS signaling, and IClswell may link these seemingly disparate modulators of cell growth.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants HL62483 (to F. S. L.), HL081750 (to F. J. M.), AI067533 (to J. G. M.), and DK07690-15 (to J. J. M.). This work was also supported by grants from the American Heart Association (to F. S. L.), and the Department of Veterans Affairs Office of Research and Development (to F. J. M.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
- IClswell
- swelling-induced anion current
- CamKII
- calcium-calmodulin kinase II
- GFP
- green florescent protein
- H2O2
- hydrogen peroxide
- ROS
- reactive oxygen species
- TNF-α
- tumor necrosis factor-α
- VSM
- vascular smooth muscle
- WT
- wild type
- IL
- interleukin
- EGF
- epidermal growth factor
- pF
- picofarad.
REFERENCES
- 1.Matsuda J. J., Filali M. S., Volk K. A., Collins M. M., Moreland J. G., Lamb F. S. (2008) Am. J. Physiol. Cell Physiol. 294, C251–C262 [DOI] [PubMed] [Google Scholar]
- 2.Matsuda J. J., Filali M. S., Collins M. M., Volk K. A., Lamb F. S. (2010) J. Biol. Chem. 285, 2569–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambert S., Oberwinkler J. (2005) J. Physiol. 567, 191–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duan D., Winter C., Cowley S., Hume J. R., Horowitz B. (1997) Nature 390, 417–421 [DOI] [PubMed] [Google Scholar]
- 5.Duan D., Zhong J., Hermoso M., Satterwhite C. M., Rossow C. F., Hatton W. J., Yamboliev I., Horowitz B., Hume J. R. (2001) J. Physiol. 531, 437–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamoto-Mizuma S., Wang G. X., Liu L. L., Schegg K., Hatton W. J., Duan D., Horowitz T. L., Lamb F. S., Hume J. R. (2004) J. Physiol. 557, 439–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossow C. F., Duan D., Hatton W. J., Britton F., Hume J. R., Horowitz B. (2006) Acta. Physiol. 187, 5–19 [DOI] [PubMed] [Google Scholar]
- 8.Friedrich T., Breiderhoff T., Jentsch T. J. (1999) J. Biol. Chem. 274, 896–902 [DOI] [PubMed] [Google Scholar]
- 9.Jentsch T. J., Günther W., Pusch M., Schwappach B. (1995) J. Physiol. 482, 19S–25S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weylandt K. H., Valverde M. A., Nobles M., Raguz S., Amey J. S., Diaz M., Nastrucci C., Higgins C. F., Sardini A. (2001) J. Biol. Chem. 276, 17461–17467 [DOI] [PubMed] [Google Scholar]
- 11.Huang P., Liu J., Di A., Robinson N. C., Musch M. W., Kaezel M. A., Nelson D. J. (2001) J. Biol. Chem. 276, 20093–20100 [DOI] [PubMed] [Google Scholar]
- 12.Ogura T., Furukawa T., Toyozaki T., Yamada K., Zheng Y. J., Katayama Y., Nakaya H., Inagaki N. (2002) FASEB J. 16, 863–865 [DOI] [PubMed] [Google Scholar]
- 13.Shimada K., Li X., Xu G., Nowak D. E., Showalter L. A., Weinman S. A. (2000) Am. J. Physiol. Gastrointest. Liver Physiol. 279, G268–G276 [DOI] [PubMed] [Google Scholar]
- 14.Li X., Shimada K., Showalter L. A., Weinman S. A. (2000) J. Biol. Chem. 275, 35994–35998 [DOI] [PubMed] [Google Scholar]
- 15.Gong W., Xu H., Shimizu T., Morishima S., Tanabe S., Tachibe T., Uchida S., Sasaki S., Okada Y. (2004) Cell Physiol. Biochem. 14, 213–224 [DOI] [PubMed] [Google Scholar]
- 16.Stobrawa S. M., Breiderhoff T., Takamori S., Engel D., Schweizer M., Zdebik A. A., Bösl M. R., Ruether K., Jahn H., Draguhn A., Jahn R., Jentsch T. J. (2001) Neuron 29, 185–196 [DOI] [PubMed] [Google Scholar]
- 17.Arreola J., Begenisich T., Nehrke K., Nguyen H. V., Park K., Richardson L., Yang B., Schutte B. C., Lamb F. S., Melvin J. E. (2002) J. Physiol. 545, 207–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J., Xu H., Morishima S., Tanabe S., Jishage K., Uchida S., Sasaki S., Okada Y., Shimizu T. (2005) Jpn. J. Physiol. 55, 379–383 [DOI] [PubMed] [Google Scholar]
- 19.Robinson N. C., Huang P., Kaetzel M. A., Lamb F. S., Nelson D. J. (2004) J. Physiol. 556, 353–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X. Q., Deriy L. V., Foss S., Huang P., Lamb F. S., Kaetzel M. A., Bindokas V., Marks J. D., Nelson D. J. (2006) Neuron 52, 321–333 [DOI] [PubMed] [Google Scholar]
- 21.Miller F. J., Jr., Filali M., Huss G. J., Stanic B., Chamseddine A., Barna T. J., Lamb F. S. (2007) Circ. Res. 101, 663–671 [DOI] [PubMed] [Google Scholar]
- 22.Moreland J. G., Davis A. P., Bailey G., Nauseef W. M., Lamb F. S. (2006) J. Biol. Chem. 281, 12277–12288 [DOI] [PubMed] [Google Scholar]
- 23.Moreland J. G., Davis A. P., Matsuda J. J., Hook J. S., Bailey G., Nauseef W. M., Lamb F. S. (2007) J. Biol. Chem. 282, 33958–33967 [DOI] [PubMed] [Google Scholar]
- 24.Volk A. P., Heise C. K., Hougen J. L., Artman C. M., Volk K. A., Wessels D., Soll D. R., Nauseef W. M., Lamb F. S., Moreland J. G. (2008) J. Biol. Chem. 283, 34315–34326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamb F. S., Moreland J. G., Miller F. J., Jr. (2009) Antioxid. Redox Signal. 11, 1335–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varela D., Simon F., Riveros A., Jørgensen F., Stutzin A. (2004) J. Biol. Chem. 279, 13301–13304 [DOI] [PubMed] [Google Scholar]
- 27.Ren Z., Raucci F. J., Jr., Browe D. M., Baumgarten C. M. (2008) Cardiovasc. Res. 77, 73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Browe D. M., Baumgarten C. M. (2006) J. Gen. Physiol. 127, 237–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Browe D. M., Baumgarten C. M. (2004) J. Gen. Physiol. 124, 273–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren Z., Baumgarten C. M. (2005) Am. J. Physiol. Heart Circ. Physiol. 288, H2628–H2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reinehr R., Görg B., Becker S., Qvartskhava N., Bidmon H. J., Selbach O., Haas H. L., Schliess F., Häussinger D. (2007) Glia 55, 758–771 [DOI] [PubMed] [Google Scholar]
- 32.Shimizu T., Numata T., Okada Y. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 6770–6773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horn R., Marty A. (1988) J. Gen. Physiol. 92, 145–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller F. J., Jr., Chu X., Stanic B., Tian X., Sharma R. V., Davisson R. L., Lamb F. S. (2010) Antioxid. Redox. Signal. 12, 583–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dinneen J. L., Ceresa B. P. (2004) Exp. Cell Res. 294, 509–522 [DOI] [PubMed] [Google Scholar]
- 36.Schlierf B., Fey G. H., Hauber J., Hocke G. M., Rosorius O. (2000) Exp. Cell Res. 259, 257–265 [DOI] [PubMed] [Google Scholar]
- 37.Zhou J. G., Ren J. L., Qiu Q. Y., He H., Guan Y. Y. (2005) J. Biol. Chem. 280, 7301–7308 [DOI] [PubMed] [Google Scholar]
- 38.Wang G. X., Hatton W. J., Wang G. L., Zhong J., Yamboliev I., Duan D., Hume J. R. (2003) Am. J. Physiol. Heart Circ. Physiol 285, H1453–H1463 [DOI] [PubMed] [Google Scholar]
- 39.Galkina E., Ley K. (2009) Annu. Rev. Immunol. 27, 165–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martorell L., Martínez-González J., Rodríguez C., Gentile M., Calvayrac O., Badimon L. (2008) Thromb. Haemost. 99, 305–315 [DOI] [PubMed] [Google Scholar]
- 41.Friis M. B., Vorum K. G., Lambert I. H. (2008) Am. J. Physiol. Cell Physiol. 294, C1552–C1565 [DOI] [PubMed] [Google Scholar]
- 42.Yin Z., Tong Y., Zhu H., Watsky M. A. (2008) Am. J. Physiol. Cell Physiol. 294, C535–C542 [DOI] [PubMed] [Google Scholar]
- 43.Clempus R. E., Griendling K. K. (2006) Cardiovasc. Res. 71, 216–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trebak M., Ginnan R., Singer H. A., Jourd'heuil D. (2010) Antioxid. Redox Signal. 12, 657–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo S. F., Chang C. C., Lee I. T., Lee C. W., Lin W. N., Lin C. C., Yang C. M. (2009) Toxicol. Appl. Pharmacol. 237, 8–21 [DOI] [PubMed] [Google Scholar]
- 46.Varela D., Simon F., Olivero P., Armisén R., Leiva-Salcedo E., Jørgensen F., Sala F., Stutzin A. (2007) Cell Physiol. Biochem. 20, 773–780 [DOI] [PubMed] [Google Scholar]
- 47.Stenmark H., Parton R. G., Steele-Mortimer O., Lütcke A., Gruenberg J., Zerial M. (1994) EMBO J. 13, 1287–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seachrist J. L., Ferguson S. S. (2003) Life Sci. 74, 225–235 [DOI] [PubMed] [Google Scholar]
- 49.Maxfield F. R., McGraw T. E. (2004) Nat. Rev. Mol. Cell Biol. 5, 121–132 [DOI] [PubMed] [Google Scholar]
- 50.García-Regalado A., Guzmán-Hernández M. L., Ramírez-Rangel I., Robles-Molina E., Balla T., Vázquez-Prado J., Reyes-Cruz G. (2008) Mol. Biol. Cell 19, 4188–4200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faundez V., Hartzell H. C. (2004) Sci. STKE 2004, re8. [DOI] [PubMed] [Google Scholar]
- 52.Hara-Chikuma M., Yang B., Sonawane N. D., Sasaki S., Uchida S., Verkman A. S. (2005) J. Biol. Chem. 280, 1241–1247 [DOI] [PubMed] [Google Scholar]
- 53.Mumbengegwi D. R., Li Q., Li C., Bear C. E., Engelhardt J. F. (2008) Mol. Cell. Biol. 28, 3700–3712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hawkins B. J., Madesh M., Kirkpatrick C. J., Fisher A. B. (2007) Mol. Biol. Cell 18, 2002–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dai Y. P., Bongalon S., Hatton W. J., Hume J. R., Yamboliev I. A. (2005) Br. J. Pharmacol. 145, 5–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang H. N., Zhou J. G., Qiu Q. Y., Ren J. L., Guan Y. Y. (2006) Apoptosis 11, 327–336 [DOI] [PubMed] [Google Scholar]
- 57.Wang G. L., Wang X. R., Lin M. J., He H., Lan X. J., Guan Y. Y. (2002) Circ. Res. 91, E28–E32 [DOI] [PubMed] [Google Scholar]
- 58.Qian J. S., Pang R. P., Zhu K. S., Liu D. Y., Li Z. R., Deng C. Y., Wang S. M. (2009) Cell Physiol. Biochem. 24, 461–470 [DOI] [PubMed] [Google Scholar]
- 59.Tang Y. B., Liu Y. J., Zhou J. G., Wang G. L., Qiu Q. Y., Guan Y. Y. (2008) Cell Prolif. 41, 775–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lang F., Föller M., Lang K., Lang P., Ritter M., Vereninov A., Szabo I., Huber S. M., Gulbins E. (2007) Methods Enzymol. 428, 209–225 [DOI] [PubMed] [Google Scholar]
- 61.Okada Y., Shimizu T., Maeno E., Tanabe S., Wang X., Takahashi N. (2006) J. Membr. Biol. 209, 21–29 [DOI] [PubMed] [Google Scholar]
- 62.Wang L. W., Chen L. X., Jacob T. (2004) Sheng Li Xue Bao 56, 230–236 [PubMed] [Google Scholar]
- 63.Mao J., Chen L., Xu B., Wang L., Li H., Guo J., Li W., Nie S., Jacob T. J., Wang L. (2008) Biochem. Pharmacol. 75, 1706–1716 [DOI] [PubMed] [Google Scholar]
- 64.Sontheimer H. (2008) Exp. Biol. Med. 233, 779–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lemonnier L., Lazarenko R., Shuba Y., Thebault S., Roudbaraki M., Lepage G., Prevarskaya N., Skryma R. (2005) Endocr. Relat. Cancer 12, 335–349 [DOI] [PubMed] [Google Scholar]
- 66.Weinberg F., Chandel N. S. (2009) Cell Mol. Life Sci. 66, 3663–3673 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.