Abstract
Enzymatic catalysis of biochemical reactions is essential to all living systems. The “lock and key” and “induced fit” models were early contributions to our understanding of the mechanisms involved in the reaction between an enzyme and its substrate. However, whether a given substrate-induced conformation is rigid or remains flexible has not yet been determined. By measuring the enzyme activity and intrinsic fluorescence of a nonspecific Eisenia fetida protease-I with different chromogenic substrates, we show that in subsequent reactions of protease with substrates, both the “lock and key” and “induced fit” mechanisms are used depending on the degree of conformational change required. Chromozym-Th- or chromosym-Ch-induced protease conformations were unable to bind chromozym-U. The chromosym-U-induced protease conformation remained flexible and could be further induced by chromozym-Th and chromozym-Ch. When low concentrations of guanidine HCl were used to disturb the conformation of the enzyme, only small changes in intrinsic fluorescence of the chromozym-Th-induced protease were detected, in contrast to the native enzyme whose intrinsic fluorescence markedly increased. This indicates that the substrate-induced enzyme was relatively rigid compared with the native protease. Utilizing a lock and key mechanism for secondary substrate reactions may have adaptive value in that it facilitates high efficiency in enzymatic reactions.
Keywords: Enzyme Catalysis, Enzyme Mechanisms, Fluorescence, Protease, Protein Conformation, Eisenia fetida Protease-I, Induced Fit, Lock and Key, Substrate-induced Enzymic Reaction
Introduction
Coupling between ligand binding and protein conformational change is at the heart of all biological interactions. There are several models for explaining how an enzyme binds to a substrate. By studying enzymatic specificity, Fischer and colleagues proposed the “lock and key” model in 1894 to explain the specificity of enzymatic reactions (1). It was suggested that the enzyme and the substrate possess specific complementary geometric shapes that fit exactly into one another. In 1958, Koshland postulated that the reaction between an enzyme and its substrate involves an “induced fit” mechanism (2). Because enzymes are rather flexible structures, the active site is continually reshaped by interactions with the substrate. According to this model, the substrate causes an appreciable conformational change at the active site of the enzyme. This progress in our understanding of the mechanism of enzyme reactions from the “lock and key” to the “induced fit” was considered a milestone in biochemistry at the time. Subsequently, the flexibility of the active site (3), its spatial adaptation (4), and structural plasticity (5) have been demonstrated experimentally, providing evidence in support of the induced fit model. Conformational flexibility is a foundational requirement allowing induced fit (3, 6–11). However, the lock and key model implies that active sites are rigid. There is still ongoing debate about which of these models is correct. Presently, there is practically universal agreement that “conformational selection” is the major mechanism (12–16). Conformational selection postulates that all of the potential conformations of a given protein preexist and that once the ligand selects the most favored conformation, induced fit occurs and conformational change takes place (17).
It is well known that the binding of a ligand molecule to a protein is often accompanied by conformational changes in the protein. However, whether the induced complementary conformation remains flexible after the catalytic reaction or becomes rigid is still to be determined. It is also unclear whether this induced conformation affects subsequent reactions with substrates.
In this laboratory, we have studied the structure and function of earthworm proteases and have isolated an Eisenia fetida protease (EfP-I),2 which has a relatively broad substrate specificity (18–20). It is capable of recognizing and cleaving chromogenic substrates such as chromozym-Th (CTH, a thrombin-like substrate), chromozym-Ch (CCH, a chymotrypsin-like substrate) and chromozym-U (CU, a urokinase-like substrate). Here, we have utilized the broad substrate specificity of this enzyme to investigate the flexibility of substrate-induced conformations. Changes in activity and conformation were observed when an enzyme induced with one substrate was reacted with other substrates in subsequent reactions. Our results indicate that both induced fit and lock and key mechanisms are involved in the subsequent reactions.
EXPERIMENTAL PROCEDURES
Purification of EfP-I
Earthworm EfP-I was purified as described previously (18). Enzyme activity was measured as described previously (20). One enzymatic unit is defined as the conversion of 1 μm substrate per min per mg of enzyme at 25 °C.
Assay of EfP-I with Additional Substrates
The first substrate (1 mm) was mixed with EfP-I (final concentration 100 μm) at 25 °C. Aliquots were taken to measure the absorbance at 405 nm on a Hitachi U-2010 spectrophotometer at different time intervals (1, 2, 4, 8, 16, and 32 min). The second substrate was added when the reaction reached equilibrium, and absorbance was measured again under the same conditions. All assays were carried out as shown in Table 1.
TABLE 1.
Assays of EfP-I with additional substrates
| First substrate | Second substrate | |
|---|---|---|
| 1 | CTH | CU |
| 2 | CU | CTH |
| 3 | CCH | CU |
| 4 | CU | CCH |
| 5 | CTH | CCH |
| 6 | CCH | CTH |
| 7 | CTH | CTH |
| 8 | CU | CU |
| 9 | CCH | CCH |
Assay of Native and Substrate-induced EfP-I
Absorbance of native EfP-I (40 μm) at 405 nm was recorded for 1 min after the addition of CTH (1 mm) at 25 °C. The activities of the enzyme with 1 mm CCH and 1 mm CU were measured under the same conditions. The first substrate CTH (final concentration 1 mm) was incubated with EfP-I (final concentration 40 μm) at 25 °C, and aliquots were taken to monitor the absorbance at 405 nm at different time intervals (1, 2, 4, 8, 16, 32, 64, and 128 min). Incubated samples were then ultrafiltrated four times (4000 rpm, 25 °C, 20 min) to remove the products and residual substrate. The enzyme that had reacted with the first substrate (CTH) is referred to here by the abbreviation EfP-ICTH. The second substrate CU (1 mm) was added to react with the incubated enzyme (40 μm). Absorbance at 405 nm was recorded for 1 min as above. The second substrate and product were removed by ultrafiltration before measuring the activity of the enzyme with CTH. Assays of different combinations of substrates were also carried out: CCH first and CU second, CTH first and CCH second, and vice versa.
Removal of Residual Substrates and Products
The products and residual substrates were removed after catalytic reactions by four rounds of ultrafiltration. Aliquots of ultrafiltrated samples were analyzed on a MALDI-TOF mass spectrometer (AXIMA-CFR PLUS, Kyoto, Japan) to detect whether any substrate or product still contaminated the enzyme.
Kinetic Tests
The activity of 40 μm CTH-induced EfP-I (EfP-ICTH) at different concentrations of CU or CCH (5, 10, 20, and 40 μm) was measured as described above. The activity of EfP-ICU was measured at 405 nm in the presence of CTH and CCH, as was EfP-ICCH in the presence of CU and CTH. Ultrafiltrated native EfP-I was used as a control. Substrate binding constants (Km) and catalytic constants (kcat) were determined using the graphic method of Hanes-Woolf (21). Measurements of the absorbance of nitroaniline from chromogenic substrates (at 405 nm for 1 min) were used to calculate the initial reaction velocity at different concentrations of the substrate. Plots of initial velocity and v/[s] produced a linear relationship, allowing the calculation of Km and maximum velocity (vmax), and thus kcat.
Determination of the Conformation of EfP-I by Fluorescence Spectrophotometry
Each substrate (1 mm) was incubated with EfP-I (40 μm) at 25 °C for 40 min. The spectrum of the intrinsic fluorescence was scanned from 300 to 400 nm by excitation at 292 nm on a Hitachi F-4500 fluorescence spectrophotometer. Native EfP-I was employed as a control.
Determination of the Conformational Flexibility of EfP-I
Different concentrations of guanidine hydrochloride (0, 0.1, 0.25, 0.5, 1, and 2.5 mm) were added to the CTH-induced enzyme solution at 25 °C overnight. Enzyme activity with CU as the substrate was measured at 405 nm, and the intrinsic fluorescence of the CTH-induced enzyme was measured at λem 330 nm; λex 292 nm. Ultrafiltrated uninduced EfP-I was used as a control.
RESULTS
Purification of EfP-I
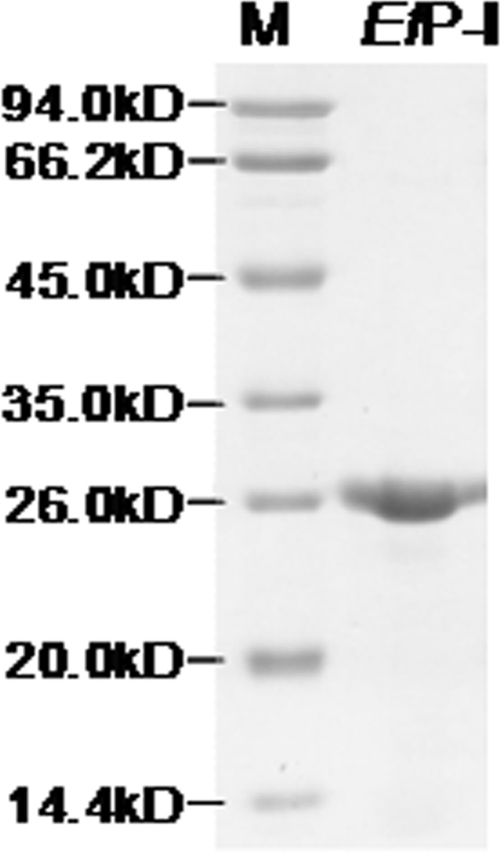
The purified enzyme was present as a single band on SDS-PAGE (Fig. 1). EfP-I was able to recognize and cleave CTH, CCH, and CU, with an activity of 70.2, 28.2, and 32.4 units, respectively.
FIGURE 1.
12% SDS-PAGE of purified EfP-I.
Subsequent Reactions with CU and CTH
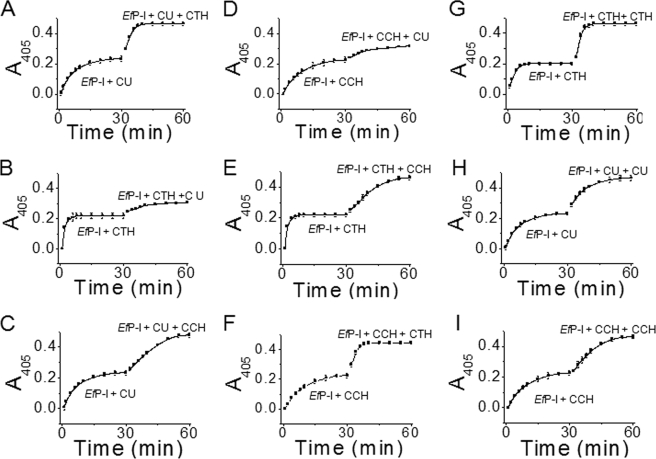
To clarify the effect of a substrate on the enzymic activity with subsequent substrates, CU was added to EfP-I that had been reacted to completion with CTH. The yield of the chromogenic product nitroaniline (Fig. 2B) was markedly less than that from CU hydrolyzed by native EfP-I (Fig. 2A, 0–32 min). When EfP-I was allowed to react to completion with CTH, and additional CTH was then added (Fig. 2G), results indicate that EfP-I was not inactivated by its reaction with CTH and raise the possibility that CTH inhibited the reaction of EfP-I with CU in some way.
FIGURE 2.
Activity of EfP-I on a secondary substrate. EfP-I was first reacted with one substrate and then with another, followed by measurement of absorbance at 405 nm at 25 °C, CU first, CTH second (A); CTH first, CU second (B); CU first, CCH second (C); CCH first, CU second (D); CTH first, CCH second (E); CCH first, CTH second (F); CTH first, CTH second as a control (G); CU first, CU second as a control (H); and CCH first, CCH second as a control (I).
To prove that the decrease in the enzymic activity with CU did not result from competition from residual CTH, the products and residual substrates from the reaction of EfP-I and CTH were removed before addition of CU to the CTH-induced protease (EfP-ICTH). The yield of the nitroaniline product from CU decreased to ∼20% in 120 min compared with that in the reaction with native EfP-I (Fig. 3A).
FIGURE 3.
Changes in the activity of EfP-I induced by the substrates. The first substrate was added to EfP-I and allowed to react to completion. Activity was measured by recording the absorbance of the enzyme reaction at 405 nm. Products and residual substrates were then removed from the incubated samples by four rounds of ultrafiltration (4000 rpm, 25 °C, 20 min). The second substrate was then reacted with the induced enzyme, followed by measurements of absorbance at 405 nm. After the reaction reached completion, products and residual substrates were removed again. The absorbance at 405 nm was measured again in the presence of the first substrate. A, activity of EfP-ICTH and native EfP-I with CU. B, activity of EfP-ICTH+CU and native EfP-I with CTH. C, activity of EfP-ICU and native EfP-I with CTH. D, activity of EfP-ICU+CTH and EfP-I with CU. E, activity of EfP-ICCH and native EfP-I with CU. F, activity of EfP-ICCH+CU and native EfP-I with CCH. G, activity of EfP-ICU and native EfP-I with CCH. H, activity of EfP-ICU+CCH and native EfP-I with CU. I, activity of EfP-ICTH and native EfP-I with CCH. J, activity of EfP-ICTH+CCH and native EfP-I with CTH. K, activity of EfP-ICCH and native EfP-I with CTH. L, activity of EfP-ICCH+CTH and EfP-I with CCH. Error bars represent the S.D.
After the substrate and products were removed once more, EfP-ICTH+CU (CU-induced EfP-ICTH) was allowed to react with CTH again. The results show that EfP-ICTH+CU was still able to react with CTH; that is, the thrombin-like activity of EfP-I was not markedly affected by the experimental process (Fig. 3B).
Subsequent Reactions with CU and CCH
Under the same conditions, EfP-ICCH (CCH-induced enzyme) was also almost totally unable to react with CU (Fig. 2D and Table 2), and the yield of nitroaniline from CU in 120 min was reduced by about 90% compared with that of native EfP-I (Fig. 3E). These results suggest that neither EfP-ICTH nor EfP-ICCH could form a conformation at the active site that was complementary to CU. To confirm that there were no significant conformational changes in EfP-ICCH after induction with CU, CCH was added again to the reaction mixture. As was the case for CTH, the chymotrypsin-like activity of EfP-ICCH+CU did not show any appreciable change (Fig. 3F). Thus, these results suggest that the lock and key model may be applicable when EfP-I induced with CTH or CCH reacts with CU. Under these conditions, EfP-ICCH and EfP-ICTH are misfitting locks for CU.
TABLE 2.
Catalytic parameters for the reaction of EfP-I with different substrates
All measurements were recorded at 25 °C.
| Enzyme-like activity | Treatment | Km | kcat | kcat/Km | Activity |
|---|---|---|---|---|---|
| mol·liter−1 | s−1 | mol−1·s·liter−1 | units | ||
| Thrombin | 1.2 × 10−5 ± 2.8 × 10−7 | 3.0 × 10−2 ± 2.2 × 10−3 | 2.6 × 103 ± 7.6 × 101 | 7.3 × 10−2 | |
| CU induction | 5.1 × 10−5 ± 1.4 × 10−6 | 3.3 × 10−2 ± 7.3 × 10−4 | 6.4 × 102 ± 5.3 | 7.8 × 10−2 | |
| CCH induction | 2.9 × 10−5 ± 2.1 × 10−6 | 2.7 × 10−2 ± 1.3 × 10−3 | 9.2 × 102 ± 2.2 × 101 | 6.4 × 10−2 | |
| Urokinase | 1.4 × 10−5 ± 8.4 × 10−7 | 4.1 × 10−3 ± 2.3 × 10−4 | 3.0 × 102 ± 6.6 | 1.0 × 10−2 | |
| CTH induction | ∞ | ∼0 | ∼0 | 5.2 × 10−4 | |
| CCH induction | ∞ | ∼0 | ∼0 | 1.5 × 10−4 | |
| Chymotrypsin | 1.6 × 10−5 ± 1.1 × 10−6 | 8.9 × 10−3 ± 5.5 × 10−4 | 5.7 × 102 ± 9.6 | 2.1 × 10−2 | |
| CTH induction | 4.7 × 10−5 ± 5.1 × 10−6 | 3.7 × 10−3 ± 5.2 × 10−4 | 8.0 × 101 ± 6.6 | 8.9 × 10−3 | |
| CU induction | 3.4 × 10−5 ± 1.0 × 10−6 | 5.2 × 10−3 ± 1.6 × 10−4 | 1.5 × 102 ± 1.1 × 101 | 1.3 × 10−2 |
To investigate whether the enzyme changes conformation to become a “good fit” for CU but a “bad fit” for CTH or CCH in the subsequent reaction, we prepared CU-induced enzyme (EfP-ICU). Unexpectedly, the activity of EfP-ICU with CTH was as high as that of the native EfP-I (Fig. 3C). Thus, it appears that induction by CU does not affect the reaction between the enzyme and CTH or CCH (Fig. 3G). This suggests that induced fit still occurs in the subsequent reaction of the CU-induced enzyme with CTH or CCH.
However, the initial activity of EfP-ICU+CTH with CU approached zero, and the yield of nitroaniline from CU in 120 min was reduced by about 90% (Fig. 3D and Table 2). When this experiment was carried out with EfP-ICU+CCH under the same conditions, EfP-ICU+CCH did not react initially with CU (Fig. 3H). This demonstrates that after the enzyme had been induced by CCH or CTH, the subsequent reaction followed a lock and key mechanism. The enzyme became a “misfitting lock” for the CU “key” after it was induced by CTH or CCH, even though it had previously been induced by CU.
Subsequent Reactions with CCH and CTH
We examined the interaction of the CTH-induced enzyme with CCH and vice versa. As before, CTH was added to EfP-I, and the products and remnant substrate were removed after the reaction had gone to completion. CCH was then mixed with EfP-ICTH. The chymotrypsin-like activity did not change significantly compared with that of native EfP-I (Fig. 3I). Thus, it appears that another induced fit conformational change can occur when CCH is added to EfP-ICTH. Further studies showed that the activity of EfP-ICTH+CCH was not markedly changed in the presence of CTH (Fig. 3J). In the reverse case, the thrombin-like activity of EfP-ICCH and the chymotrypsin-like activity of EfP-ICCH+CTH were very similar to the native enzyme (Fig. 3, K and L).
Conformational Changes in Subsequent Reactions Involving Substrate-induced EfP-I
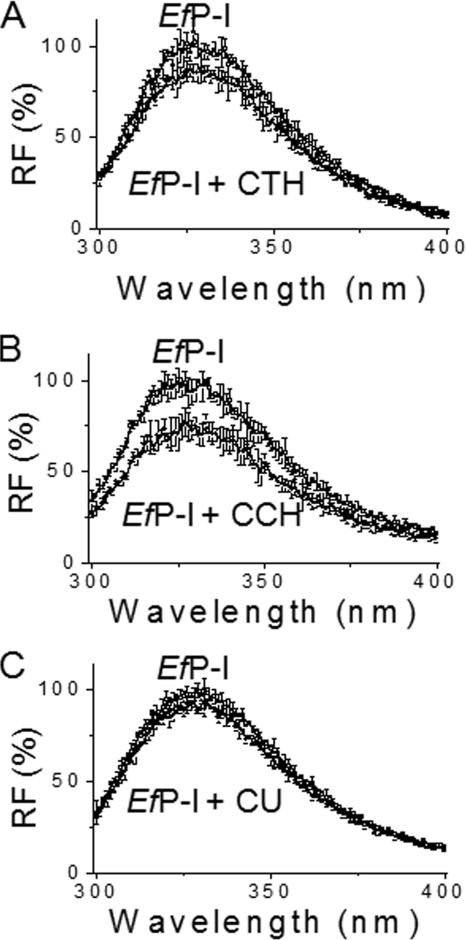
The induced fit model is based on substrate-induced conformational complementation (22, 23). EfP-I contains five tryptophanyl residues, and two of them (Trp169 and Trp205) are close to Ser186, one of the essential residues at the active site. Changes in the intrinsic fluorescence intensity should thus partly reflect conformational change of the active site. Measuring the intrinsic fluorescence around 330 nm (λex 292 nm) thus allows us to observe conformational changes of the enzyme after incubation with substrates. A marked decrease in the intensity of intrinsic fluorescence was observed when CTH or CCH was added to the native enzyme (Fig. 4, A and B). However, only a slight decrease in the fluorescent intensity was detected after the addition of CU (Fig. 4C).
FIGURE 4.
Conformational changes of EfP-I induced by different substrates. EfP-I was incubated with different substrates CTH (A), CCH (B), and CU (C) at 25 °C for 2 h, and the intrinsic fluorescence (λem 330 nm, λex 292 nm) was then measured on a Hitachi F-4500 fluorophotospectrometer. Native EfP-I was used as a control. Error bars represent the S.D.
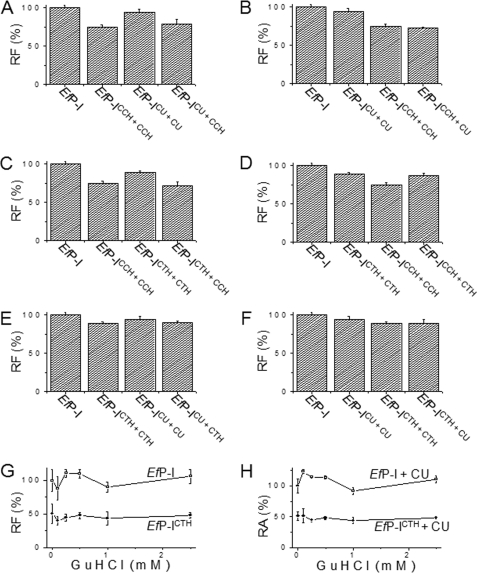
To determine whether the interaction of CCH with EfP-ICTH causes the enzyme to undergo induced fit conformational changes, we measured the protein intrinsic fluorescence during the reaction of EfP-I with the substrates. The emission intensity of EfP-ICTH was stronger than that of EfP-ICCH, suggesting that the CCH-induced conformation is different from the CTH-induced conformation. The fluorescent intensity of EfP-ICTH+CCH decreased to that of EfP-ICCH, and the emission intensity of EfP-ICCH+CTH increased to that of EfP-ICTH (Fig. 5, C and D). This demonstrates that the interaction between CTH and EfP-ICCH and the interaction between CCH and EfP-ICTH uses an induced fit mechanism.
FIGURE 5.
Conformational changes of EfP-I induced repetitively by different substrates. The effects of different substrates on the conformation of the enzyme were determined by comparing the intensity of the intrinsic fluorescence. To exclude disturbance from the residual substrates and products, each sample was ultrafiltrated before fluorescence was measured. EfP-I, native protease; EfP-ICCH, enzyme reacted with CCH and ultrafiltrated to remove residual substrates and products; EfP-ICCH+CCH, enzyme reacted with CCH and ultrafiltrated and then reacted again with CCH and ultrafiltrated; EfP-ICCH+CU, enzyme reacted with CCH and ultrafiltrated and then reacted again with CU and ultrafiltrated. A, effect of CCH on the conformation of EfP-ICU. B, effect of CU on EfP-ICCH. C, effect of CCH on EfP-ICTH. D, effect of CTH on EfP-ICCH. E, effect of CTH on EfP-ICU. F, effect of CU on EfP-ICTH. EfP-I, EfP-ICCH+CCH, EfP-ICU+CU, and EfP-ICTH+CTH were used as controls. Error bars represent the S.D. G, intrinsic fluorescence of EfP-ICTH at different concentrations of guanidine hydrochloride (GuHCl), with native EfP-I used as control. H, urokinase-like activity of EfP-ICTH at different concentrations of guanidine hydrochloride. Error bars represent the S.D.
In addition, the emission intensity of EfP-ICU+CCH or EfP-ICU+CTH decreased to that of EfP-ICCH or EfP-ICTH, and the fluorescence intensity of EfP-ICCH+CU (EfP-ICTH+CU) was similar to EfP-ICCH(EfP-ICTH) (Fig. 5, A, B, E, and F). These results suggest again that the CU-induced protease conformation can be further induced by CTH and CCH and that the CTH- or CCH-induced conformation cannot be further induced by CU.
To demonstrate how the conformation transition occurs, we first incubated EfP-I with CCH for different lengths of time and then assayed enzymic activity with CU. Enzyme activity of EfP-ICCH with CU decreased to about 10% in 8–10 min (supplemental Fig. S1). We then duplicated the experiment in Fig. 2D; EfP-ICCH was used to perform a time course of initial velocities upon addition of CU, and the change in fluorescence between the EfP-ICCH and EfP-ICCH+CU conformations was monitored separately. Changes in the activity of EfP-I with CCH underwent a biphasic procedure containing a fast and slow phase (supplemental Fig. S2). The first order rate constant of the slow phase of native protease was similar to that of EfP-ICCH with CU. Furthermore, the first order increase in initial velocity of EfP-ICCH in reaction with CU and the change in fluorescence that occurred as a result of further induction by CU exhibited similar kinetics (supplemental Table S1). Taken together, these results indicate that a slow conformational transition occurred in the protease on induction by CCH.
Assay of the Flexibility of the Induced Conformation in the Presence of Guanidine HCl
To investigate whether the substrate-induced conformational complementation is flexible, guanidine hydrochloride was employed to disrupt the conformation of the enzyme (6). A small change in the intrinsic fluorescence of the CTH-induced protease was detected in guanidine HCl solutions at concentrations of 0.1–0.5 mm (Fig. 5G). However, the intrinsic fluorescence of the native enzyme increased markedly under the same conditions. This indicates that the structure of the substrate-induced enzyme was less flexible than the native protease. Moreover, low concentrations of guanidine HCl actually improved the activity of the native protease (Fig. 5H), whereas the activity of EfP-ICTH did not increase under the experimental conditions used here. These results suggest again that substrate-induced conformational changes in EfP-ICTH are fixed.
DISCUSSION
According to Berger and colleagues (24), a given native protease has many different conformations, all of which preexist. However, a specific conformation is induced to fit the substrate when the enzyme reacts with a substrate. As shown in our results, whether subsequent reactions of substrate-induced proteases with other substrates occur by the induced fit or lock and key mechanism depends on the degree of conformational change induced by the initial substrate. Relatively fixed substrate-induced conformations such as we have detected here may have adaptive value in that they facilitate high efficiency in enzymatic reactions.
The Km values for the reactions of these three substrates with EfP-I were in the same order of magnitude (10−5 m; Table 2). The Km for CU reached infinity after the enzyme had been induced by either CTH or CCH, and as a result both kcat and kcat/Km approached zero. The affinity of EfP-ICTH for CU approached zero, suggesting that EfP-ICTH and EfP-ICCH had become misfitting locks for CU. However, the CU-induced enzyme was still able to react with CTH and CCH. The Km values for CTH and CCH in reaction with the CU-induced protease were markedly increased, whereas kcat/Km decreased. This suggests that the CU-induced enzyme was induced further by CTH and CCH and that substrate specificity decreased.
The three substrates used here each contain a short peptide and nitroaniline:CTH (tosyl-Gly-Pro-Arg-4-nitroanilide acetate), CCH (N-succinyl-Ala-Ala-Pro-Phe-4-nitroanilide), and CU (Ben-β-Ala-Gly-Arg-4-nitroanilide hydrochloride). The P2 residues of CCH and CTH are proline, in which the nitrogen atom is part of a rigid ring, making rotation about the N-Cα bond impossible (21). However, the P2 residue of CU is glycine, which is the most flexible amino acid residue. Thus, the conformation of CU is flexible because of its P2 residue, whereas that of CTH and CCH are fixed because of the P3–P2 bonds. As mentioned above (Fig. 4C), EfP-I only undergoes a slight conformational change on induction by CU. Thus, EfP-ICU still has the potential to undergo further change in its conformation on induction by CTH or CCH.
The kcat values of EfP-ICU and EfP-ICCH in the presence of CTH were not significantly different from that of the native enzyme. However, their Km values increased. This suggests that the induced conformations of the enzyme have a major affect on the binding of subsequent substrates. The kcat and Km values of EfP-ICTH and EfP-ICCH in reaction with CU were totally different from those of EfP-I (Table 2). This may be due to the failure of the subsequent substrate CU to bind to the enzyme.
The Km values of EfP-ICU in reactions with CTH and CCH changed negligibly, but those of EfP-ICTH and EfP-ICCH reacting with CU approached infinity. Activity of EfP-ICU on CCH and CTH was not significantly different from that of the native enzyme; however, EfP-ICTH and EfP-ICCH did not react with CU. This suggests that the smaller conformational changes of the active site in the presence of CU maintain its flexibility, whereas the larger conformational changes induced by CTH or CCH result in the conformation at the active site becoming rigid (Table 2).
We conclude that the conformational changes in EfP-I induced by CTH and CCH are relatively fixed. This view is based on the following observations: (i) The activity of native EfP-I with CU was 32.4 units/μm whereas that of EfP-ICTH and EfP-ICCH approached zero. (ii) The activity of native EfP-I increased markedly in the presence of guanidine HCl at low concentrations, whereas that of the CTH- or CCH-induced enzyme remained unchanged. (iii) guanidine HCl increased the intrinsic fluorescence of the native protease, but did not markedly alter that of EfP-ICTH or EfP-ICCH. (iv) The first order rate constant for the reaction of EfP-ICCH with CU was similar to that of the slow phase of the native enzyme in the presence of CCH. (v) The first order rate constant for changes in the intrinsic fluorescence of EfP-ICCH on reaction with CU was markedly slower than that for EfP-I in reaction with CCH.
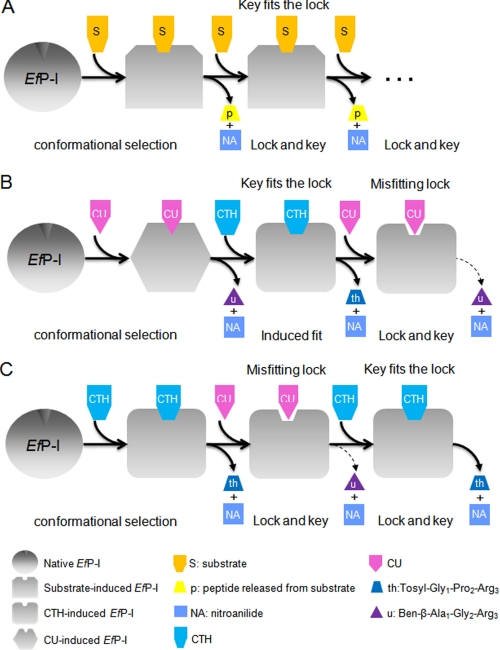
Based on the above results, we propose a model to explain the nature of conformational changes which occur during multi-substrate inductions (Fig. 6). EfP-I undergoes a conformational change to become complementary to any of its substrates via conformational selection. Subsequent reactions with the same substrate will take place by a lock and key mechanism as the conformation of the active site now provides a well fitting lock for this substrate (Fig. 6A). However, our results suggest that different substrates induce different degrees of conformational change. If the change induced by a substrate is small (as is the case for CU), the enzyme will still have enough flexibility to undergo further conformational changes when a subsequent substrate (such as CTH) is introduced. In this case, the rate of the reaction will be similar to that of the native enzyme, as our results from EfP-ICU+CTH reactions indicated (Fig. 6B). However, when the first substrate (for example CTH) causes a relatively marked conformational change, this change becomes fixed, and the conformation of the enzyme can no longer return to its native state. Thus, if a subsequent substrate that requires a smaller conformational change (such as CU) is introduced, the enzyme is no longer flexible enough to adjust its conformation, and the substrate will not fit the lock. In this case, the reaction can only proceed at a greatly reduced rate (Fig. 6C), as was the case in our EfP-ICTH reactions with CU.
FIGURE 6.
Proposed mechanism of EfP-I action involving both induced fit and lock and key models. A, EfP-I reacting with the same chromogenic substrate: first by induced fit and subsequently by lock and key. B, EfP-I reacting sequentially with CU, CTH, and CU, undergoing induced fit and then lock and key. C, EfP-I reacting sequentially with CTH, CU, and CTH, undergoing induced fit and then lock and key.
Supplementary Material
Acknowledgments
We thank Prof. Zhi-Xin Wang for advice on the manuscript, Hai-Jin He and Rong Xiao for critical discussions of the manuscript, Dr. Joy Fleming for editing the language of this manuscript, and Zhen-Sheng Xie for technical help with MALDI-TOF analysis.
This work was supported by National Natural Science Foundation of China Grant 30870544, 973 projects of China Grants 2006CB500703 and 2010CB912303, and the Knowledge Innovation Program of the Chinese Academy of Sciences Grants KSCX2-YW-R-119 and KSCX2-YW-R-256.

The on-line version of this article (available at http://www.jbc.org) contains Figs. S1 and S2, Table S1, and an additional reference.
- EfP-I
- earthworm Eisenia fetida protease-I
- CCH
- chymotrypsin-like substrate (chromozym-Ch)
- CTH
- thrombin-like substrate (chromozym-Th)
- CU
- urokinase-like substrate (chromozym-U)
- EfP-ICTH
- CTH-induced EfP-I
- EfP-ITh+Ch
- induction of EfP-I by chromozym-Th and then by chromozym-Ch
- MALDI-TOF
- matrix-assisted laser desorption ionization time-of-flight.
REFERENCES
- 1.Fischer E. (1894) Ber. Dt. Chem. Ges. 27, 2985–2993 [Google Scholar]
- 2.Koshland D. E. (1958) Proc. Natl. Acad. Sci. U.S.A. 44, 98–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao Q. Z., Tian M., Tsou C. L. (1984) Biochemistry 23, 2740–2744 [DOI] [PubMed] [Google Scholar]
- 4.Davies D. R., Cohen G. H. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 7–128552677 [Google Scholar]
- 5.Sundberg E. J., Mariuzza R. A. (2000) Structure 8, R137–R142 [DOI] [PubMed] [Google Scholar]
- 6.Tsou C. L. (1993) Science 262, 380–381 [DOI] [PubMed] [Google Scholar]
- 7.Somorjai G. A. (2004) Nature 430, 730. [DOI] [PubMed] [Google Scholar]
- 8.Anderson C. M., Zucker F. H., Steitz T. A. (1979) Science 204, 375–380 [DOI] [PubMed] [Google Scholar]
- 9.Hritz J., de Ruiter A., Oostenbrink C. (2008) J. Med. Chem. 51, 7469–7477 [DOI] [PubMed] [Google Scholar]
- 10.Koska J., Spassov V. Z., Maynard A. J., Yan L., Austin N., Flook P. K., Venkatachalam C. M. (2008) J. Chem. Inf. Model 48, 1965–1973 [DOI] [PubMed] [Google Scholar]
- 11.Matsushima N., Yoshida H., Kumaki Y., Kamiya M., Tanaka T., Izumi Y., Kretsinger R. H. (2008) Curr. Protein Pept. Sci. 9, 591–610 [DOI] [PubMed] [Google Scholar]
- 12.Bakan A., Bahar I. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 14349–14354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swift R. V., McCammon J. A. (2009) J. Am. Chem. Soc. 131, 5126–5133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weikl T. R., von Deuster C. (2009) Proteins 75, 104–110 [DOI] [PubMed] [Google Scholar]
- 15.Sullivan S. M., Holyoak T. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 13829–13834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okazaki K., Takada S. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 11182–11187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boehr D. D., Nussinov R., Wright P. E. (2009) Nat. Chem. Biol. 5, 789–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J. X., Zhao X. Y., Pan R., He R. Q. (2007) Int. J. Biol. Macromol. 40, 399–406 [DOI] [PubMed] [Google Scholar]
- 19.Zhao J., Pan R., He J., Liu Y., Li D. F., He R. Q. (2007) J. Biomed. Biotechnol. 40, 97654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao J., Xiao R., He J., Pan R., Fan R., Wu C., Liu X., Liu Y., He R. Q. (2007) Int. J. Biol. Macromol. 40, 67–75 [DOI] [PubMed] [Google Scholar]
- 21.Nelson D. L., Cox M. M. (2000) Lehninger Principles of Biochemistry 3/e Version, Freeman, New York [Google Scholar]
- 22.Li Y. C., Chiang C. W., Yeh H. C., Hsu P. Y., Whitby F. G., Wang L. H., Chan N. L. (2008) J. Biol. Chem. 283, 2917–2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yaremchuk A., Tukalo M., Grøtli M., Cusack S. (2001) J. Mol. Biol. 309, 989–1002 [DOI] [PubMed] [Google Scholar]
- 24.Berger C., Weber-Bornhauser S., Eggenberger J., Hanes J., Plückthun A., Bosshard H. R. (1999) FEBS Lett. 450, 149–153 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.