Abstract
The active site of the tyrosine family site-specific recombinase Flp contains a conserved catalytic pentad that includes two arginine residues, Arg-191 and Arg-308. Both arginines are essential for the transesterification steps of strand cleavage and strand joining in DNA substrates containing a phosphate group at the scissile position. During strand cleavage, the active site tyrosine supplies the nucleophile to form a covalent 3′-phosphotyrosyl intermediate. The 5′-hydroxyl group produced by cleavage provides the nucleophile to re-form a 3′-5′ phosphodiester bond in a recombinant DNA strand. In previous work we showed that substitution of the scissile phosphate (P) by the charge neutral methylphosphonate (MeP) makes Arg-308 dispensable during the catalytic activation of the MeP diester bond. However, in the Flp(R308A) reaction, water out-competes the tyrosine nucleophile (Tyr-343) to cause direct hydrolysis of the MeP diester bond. We now report that for MeP activation Arg-191 is also not required. In contrast to Flp(R308A), Flp(R191A) primarily mediates normal cleavage by Tyr-343 but also exhibits a weaker direct hydrolytic activity. The cleaved MeP-tyrosyl intermediate formed by Flp(R191A) can be targeted for nucleophilic attack by a 5′-hydroxyl or water and channeled toward strand joining or hydrolysis, respectively. In collaboration with wild type Flp, Flp(R191A) promotes strand exchange between MeP- and P-DNA partners. Loss of a catalytically crucial positively charged side chain can thus be suppressed by a compensatory modification in the DNA substrate that neutralizes the negative charge on the scissile phosphate.
Keywords: DNA Enzymes, DNA-Protein Interaction, DNA Recombination, Mutant, Nucleic Acid Chemistry
Introduction
The hallmark of the active sites of tyrosine site-specific recombinases and type IB topoisomerases is a catalytic pentad (Arg-Lys-(His/Lys)-Arg-(His/Trp)), with a tyrosine residue providing the nucleophile for DNA cleavage (1–6). The primary function of the conserved arginines Arg-191 and Arg-308 in Flp, inferred from crystallographic data, appears to be in balancing the phosphate negative charge in the transition state during the strand cleavage and strand joining steps of DNA recombination and relaxation, respectively. Replacement of alanine, glycine, or the conservative lysine at either of these positions results in the gross impairment of the catalytic activities of the corresponding Flp variants (7–10). Recent studies show that substitution of the scissile phosphate (P)3 by methylphosphonate (MeP), which is charge neutral in the ground state and carries a nominal −1 charge in the transition state, makes Arg-308 dispensable in the MeP activation step by Flp (11). The same holds for the corresponding arginine residues of the Cre recombinase (Arg-292) and vaccinia topoisomerase (Arg-223) (12, 13). The chemical competence of Cre(R292A), Flp(R308A), and Topo(R223A) on their respective MeP substrates is consistent with the suspected electrophilic role of the positively charged arginine side chain in catalysis.
Cre(R292A), wild type topoisomerase, and Topo(R223A) cleave the MeP diester bond using their active site tyrosine nucleophiles (Tyr-324 and Tyr-274, respectively) to yield the 3′-MeP-tyrosyl adduct. This intermediate can then be hydrolyzed to the 3′-MeP product in all three cases, but with one significant difference between Cre and topoisomerase. In the case of topoisomerase, the rate of hydrolysis of the MeP-tyrosyl intermediate is enhanced over 4 orders of magnitude (SP MeP, 7.0 × 10−3 s−1 for topoisomerase; 3.5 × 10−3 s−1 for Topo(R223A) relative to that of the P-tyrosyl intermediate by the wild type enzyme (2.2 × 10−7 s−1) (12, 14). Such a dramatic difference is not observed between the hydrolysis rates of P-tyrosyl and MeP-tyrosyl intermediates by Cre (2.8 × 10−5 s−1) and Cre(R292A) (8.8 × 10−5 s−1; racemic mixture of SP/RP MeP), respectively (13). The endonucleolytic activity of Cre and topoisomerase, directed to the covalent intermediate resulting from strand cleavage, is referred to as type I endonuclease activity (11, 13).
Flp(R308A) is strikingly different from Cre(R292A) or Topo(R223A) in its response to the MeP substituted substrate. In the Flp(R308A) reaction, the activated MeP diester bond is targeted by water rather than the active site nucleophile Tyr-343 and is hydrolyzed predominantly to a DNA product with a 3′-MeP terminus (11). This direct hydrolysis of the DNA phosphodiester bond is referred to as type II endonuclease activity to distinguish it from hydrolysis of the cleaved intermediate (type I). The type II activity is either absent or extremely weak in reactions of wild type Flp on the native P substrate.
The striking enhancement of the type I endonuclease in topoisomerase by MeP substitution and the manifestation of the type II endonuclease in Flp by MeP substitution combined with the R308A mutation suggest that phosphate electrostatics and active site electrostatics may be utilized by these enzymes to repel water and protect the normal reaction from abortive hydrolysis (11, 14). Topoisomerase and Flp differ from each other in how their active sites are organized. The former houses a complete active site within a single monomer (1, 15), whereas the latter must receive the tyrosine nucleophile in trans from a neighboring monomer to complete the active site (16, 17). Because the topoisomerase active site would fully engage the scissile phosphate upon DNA binding by a monomer, the threat of hydrolysis is likely minimal at the strand cleavage step. By contrast, the delay between phosphodiester activation by a bound Flp monomer and the donation of the tyrosine nucleophile by a second Flp monomer may permit water access to the reaction center. The susceptibility of the cleaved intermediate of the topoisomerase reaction to hydrolysis may result from the conformational flexibility associated with strand rotation that precedes strand joining. This risk is perhaps greatly reduced during recombination by the tight packing of the Flp tetramer bound to DNA partners as well as the conformational dynamics of the cleaved strands within it (17). Cre recombinase is analogous to topoisomerase in possessing a cis-active site but resembles Flp in the organization and dynamics of its recombination synapse (5, 18). These features are consistent with the lack of enhanced type I endonuclease activity and the absence of the type II endonuclease activity of Cre(R292A) toward the MeP substrate. Thus, the distinct vulnerabilities of Flp, Cre, and topoisomerase and the pertinent protective strategies they have evolved to deal with them can be rationalized by differences in the assembly of their active sites, the compactness of their reaction complexes, and the conformational dynamics associated with their respective reactions.
In the present work, we have addressed the role of Arg-191 (a second conserved arginine of the Flp pentad) in recombination by assaying its activities on MeP substrates. We find that, like Arg-308, Arg-191 is also not essential for activation of the MeP diester bond. However, unlike Flp(R308A), Flp(R191A) utilizes Tyr-343 to bring about strand cleavage and displays type I endonuclease activity on the cleaved intermediate. A weaker type II endonuclease activity associated with Flp(R191A) can be unveiled by blocking the type I endonuclease with the Y343G mutation. Flp(R191A) is also functional in strand exchange and is thus a recombinase on MeP-DNA, unlike Flp(R308A). We consider the mechanistic implications of these findings.
EXPERIMENTAL PROCEDURES
Oligonucleotides and Assembly of Reaction Substrates
A list of the oligonucleotides used for the assembly of substrates is given in Table 1. Most of these oligonucleotides have been previously described (11). Schematic representations of substrates are included in Figs. 2, 4, 6, and 7. All of the oligonucleotides were purchased from Sigma-Aldrich with the exception of the MeP-containing oligonucleotide (a racemic mixture of the SP and RP forms). The MeP-containing oligonucleotide was supplied by TriLink Technologies (San Diego, CA). Oligonucleotides were 5′-labeled with 32P by the polynucleotide kinase reaction in the presence of γ-32P-labeled ATP as per the supplier's instructions (New England Biolabs). The resultant end-labeled oligonucleotides were purified by either urea-PAGE or by a nucleotide removal kit (Qiagen). Hybridization of oligonucleotides was performed by mixing the oligonucleotides to be annealed in a molar ratio of 10:1 (unlabeled:labeled), placing the tube containing the mixture in 800 ml of 95 °C water, and allowing it to cool overnight to room temperature. The P full-site substrate used in the strand exchange reaction with a MeP half-site was produced by PCR. The sequences of the primers used to amplify this 262-bp DNA fragment are given in Table 1.
TABLE 1.
Oligonucleotides used for assembling the substrates used in this study
The scissile phosphate or methylphosphonate position is indicated by (p) or (mp), respectively. A full-site substrate was obtained by PCR amplification of a plasmid pY9, containing a copy of the Flp recombination target site (FRT) using primers 1 and 2.
| Oligomer | Sequence |
|---|---|
| MeP half-site (top strand) | ACT TGG ATC CGA AGT TCC TAT AC(mp) TTT |
| P half-site (top strand) | ACT TGG ATC CGA AGT TCC TAT AC(p) TTT |
| P or MeP half-site (bottom strand) | TCT AGA AAG TAT AGG AAC TTC GGA TCC AAG T |
| Primer 1 (for full-site assembly by PCR) | GGT ATT TCA CAC CGC ATA TGG TGC ACT CTC |
| Primer 2 (for full-site assembly by PCR) | CTT CTG AGT TCG GCA TGG GGT CAG GTG GG |
FIGURE 2.
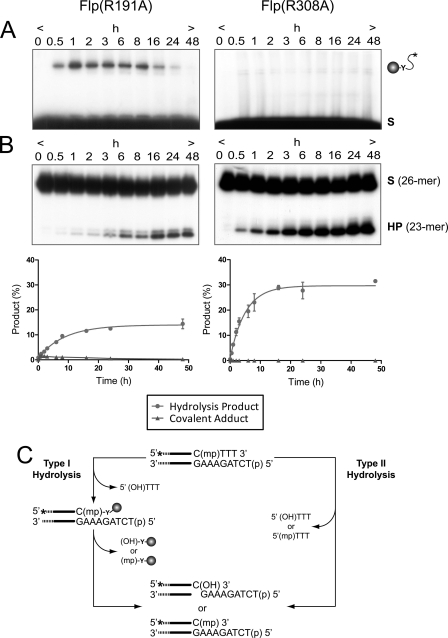
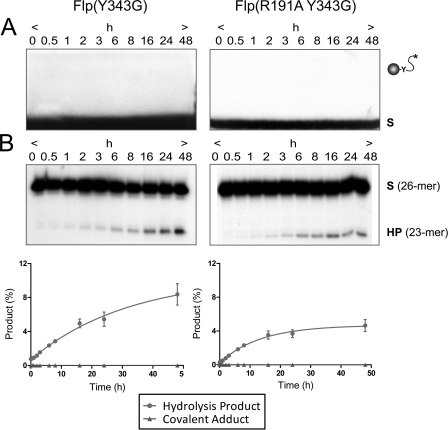
Endonuclease activities of Flp(R191A) and Flp(R308A) on a MeP half-site substrate. A and B, the reactions were analyzed simultaneously by SDS-PAGE (A) for formation of covalent Flp-DNA adduct by Tyr-343-mediated strand cleavage and by denaturing urea-PAGE (B) for formation of the 23-mer hydrolysis product (HP). The end-labeled double-stranded DNA half-site substrate (A) or the labeled single-stranded DNA (26-mer) resulting from its denaturation (B) is denoted by (S). The 5′-OH on the bottom strand of the substrate was phosphorylated to prevent “strand joining.” The respective plots for Flp(R191A) and Flp(R308A) represent the mean values from three independent experiments with the standard errors indicated by bars. C, type I and type II endonuclease activities leading to hydrolysis of the MeP-tyrosyl intermediate and direct hydrolysis of MeP, respectively, are schematically depicted. The asterisk indicates a 32P-label at the 5′-end of the scissile top strand; (mp), (p), and (HO) represent the scissile MeP, 5′-terminal phosphate, and 5′-terminal hydroxyl, respectively. The filled circle represents Flp recombinase with its active site tyrosine (Y).
FIGURE 4.
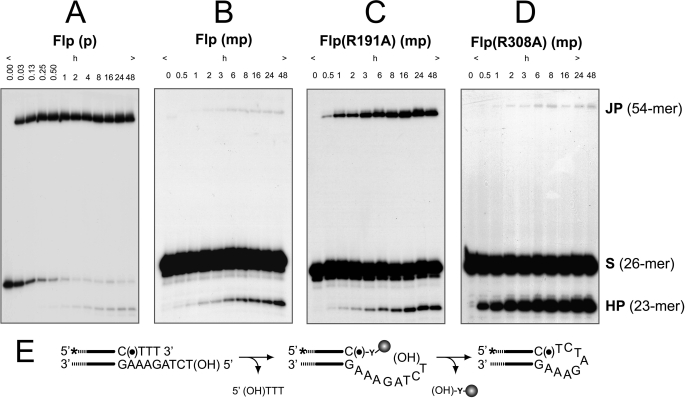
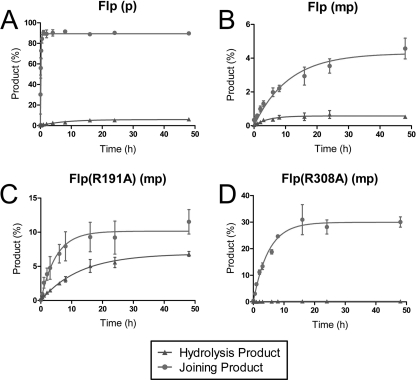
Strand joining by Flp, Flp(R191A), and Flp(R308A) assayed using P half-site and MeP half-site substrates. The half-site substrates in the strand joining assays contained a free 5′-hydroxyl on the bottom (nonscissile) strand. A–D, reactions were analyzed by denaturing urea-PAGE to detect the products of strand joining (JP) and hydrolysis (HP). A and B, the reactions of wild type Flp with the P half-site (A) or the MeP half-site (B) are shown. C and D, only the MeP half-site reactions are shown for Flp(R191A) (C) and Flp(R308A) (D) because they were inactive on the P half-site (data not shown). The strand joining reaction mediated by the attack of the bottom strand 5′-OH on the P- or MeP-tyrosyl intermediate is schematically illustrated in E. The asterisk indicates the 32P label at the 5′-end of the top (scissile) strand, (●) denotes the scissile P or MeP, and (HO) represents the 5′-terminal hydroxyl group.
FIGURE 6.
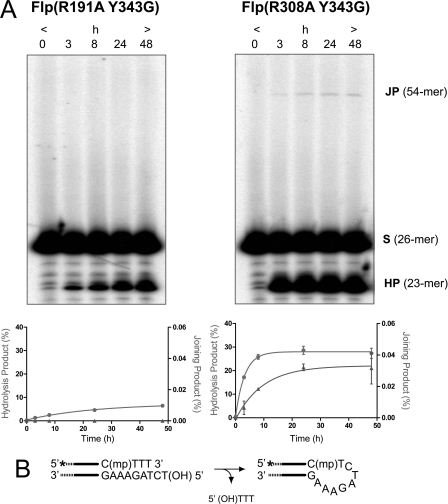
Strand joining activity of Flp(R308A,Y343G) in the MeP half-site substrate by potential direct attack of the DNA backbone by the 5′-OH. A, joining reactions with Flp(R191A,Y343G) and Flp(R308A,Y343G) were performed and analyzed as described for Fig. 4. The plots below represent three independent experiments. B, schematic diagram for a potential strand joining reaction.
FIGURE 7.
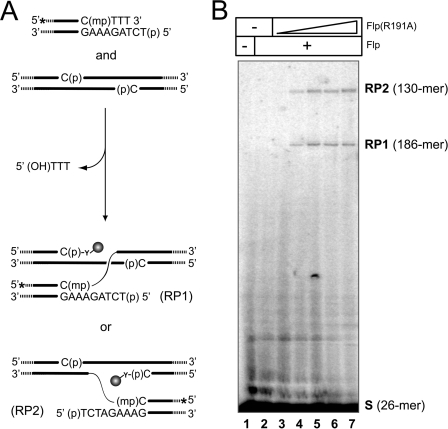
Strand exchange between a P full-site and a MeP half-site promoted collaboratively by Flp and Flp(R191A). A, the exchange reactions between the labeled MeP half-site and the unlabeled P full-site are schematically represented, with the thin wavy line representing the ester bond formed between MeP and the 5′-hydroxyl group. B, the predicted lengths of the labeled recombinant strands resulting from the two possible orientations of the half-site with respect to the full-site are 186 and 130 nucleotides, respectively. The reactions were carried out with a fixed amount of Flp and increasing amounts of Flp(R191A). The reactions were analyzed by 6% urea-PAGE gels. S(26-mer) stands for the labeled strand of the MeP half-site, and RP1(186-mer) and RP2(131-mer) stand for the labeled strands in the recombinant products.
Purification of Flp and Flp Mutants
All of the proteins, wild type Flpe (19) and mutant derivatives, were purified using protocols described previously (20) but with the omission of the DNA-cellulose column chromatography step. The protein concentrations were calculated using the Bradford protein assay (Bio-Rad).
In Vitro Flp Reactions
In vitro Flp reactions were carried out at 30 °C essentially according to the conditions described earlier (11). The reactions were standardized using a range of titrated amounts of Flp or Flp variants/Flp-binding element of the DNA substrates used. All of the subsequent assays employed the optimized amounts of these proteins. The reactions were terminated by the addition of SDS to a final concentration of 0.1%. For the analysis of hydrolysis products, DNA was purified by phenol/chloroform extraction and ethanol precipitation before loading onto 12% urea-PAGE sequencing gels (acrylamide/bis-acrylamide 19:1). The formation of Flp-DNA covalent adducts were detected using 12% SDS-PAGE gels (acrylamide/bis-acrylamide 29:1). All of the gels were dried and exposed to a storage phosphor screen (Bio-Rad) or Blue Autoradiography Film (ISC BioExpress).
Quantification of Reaction Products
Substrate and product bands were detected using a storage phosphor screen (Bio-Rad). Exposure was optimized so as to avoid saturation of the screen but to maximize signal detection. Phosphor screens were scanned using a Typhoon Trio phosphorimager (GE Healthcare). Image analysis was performed using the software Quantity One, version 4.5.1 (Bio-Rad).
Nonlinear Regression Curve Fitting
Data analysis was performed using the software GraphPad Prism (version 5.02) as previously described (13).
RESULTS
Experimental Designs
Most of the experiments were carried out using half-site substrates, as described by Ma et al. (11). A half-site substrate contains only one Flp-binding element and one scissile P or MeP on the top strand, followed by three nucleotides of the strand exchange region (spacer). The bottom strand contains all eight nucleotides of the spacer, thus creating a 5-nucleotide single-stranded DNA overhang. The MeP half-sites employed in all reactions were a racemic mixture of the SP and RP stereoisomers.
The reactions assayed in this study were: strand cleavage by the Tyr-343 nucleophile, hydrolysis of the covalent tyrosyl adduct (type I endonuclease), direct hydrolysis of the DNA backbone at the scissile position (type II endonuclease), strand joining in a half-site, and strand exchange between a MeP half-site and a P full-site. Schematic diagrams depicting the types of reactions studied are included in the figures, as appropriate, for clarity. All of the reactions were carried out using the thermostable wild type Flpe (which differs from native Flp by four amino acid substitutions) (19) and its mutant derivatives.
In a half-site substrate, the trinucleotide product resulting from strand cleavage by the active site Tyr-343 would diffuse away, making the reaction virtually irreversible (see Fig. 2C). If the bottom strand contains a 5′-hydroxyl group, a pseudo-strand joining reaction can proceed by attack of the hydroxyl group on the tyrosyl intermediate (see Fig. 4E). In principle, the scissile P or MeP diester bond activated by a bound Flp or the P- or MeP-tyrosyl intermediate formed by strand cleavage can be hydrolyzed by nucleophilic attack by water. In strand cleavage/hydrolysis reactions, the 5′-end of the noncleaved (bottom) strand was phosphorylated to prevent the joining reaction. In strand joining reactions, the 5′-end harbored a hydroxyl group. Formation of the Flp-DNA (or Flp variant DNA) covalent tyrosyl adduct and the product of hydrolysis were assayed by analyzing reactions by both SDS- and urea-PAGE, respectively. Strand exchange between a half-site and a full-site was also monitored by urea-PAGE.
Flp is a monomer in solution and associates with its binding element as a monomer. Dimerization of Flp occurs between two DNA-bound monomers positioned on either side of the spacer. Because Flp assembles a shared active site between two monomers (16, 17), strand cleavage by Tyr-343 in a half-site will require the association of at least two Flp-bound half-sites. This reaction may also occur in the context of a trimeric or a tetrameric complex of a Flp-bound half-site. However, Tyr-343-independent hydrolysis of the DNA backbone may occur in a single half-site occupied by one Flp monomer.
Strand Cleavage and Hydrolysis by Flp(R191A) in a MeP Half-site
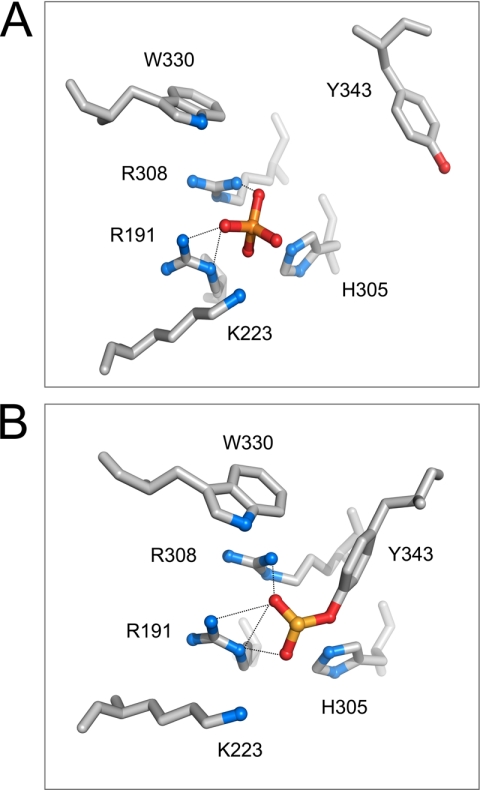
As noted earlier, the MeP diester bond in a half-site substrate is targeted for direct hydrolysis (type II endonuclease activity) by a Flp mutant lacking the conserved Arg-308 of its catalytic pentad. The crystal structures of Flp (Fig. 1 and Ref. 21) reveal that both Arg-191 and Arg-308 are in positions to form hydrogen bonds with the nonbridging oxygen atoms of the scissile phosphate. The specific contacts made by the individual arginines in the pre- and post-cleavage active sites appear to be distinct. Overall, the picture is consistent with the formation of a proton cradle by the twin arginines for stabilizing the negatively charged pentacoordinate transition state. Given the unexpected hydrolytic activity of Flp(R308A) on the MeP diester bond observed earlier (11), we wished to probe the potential catalytic activities of Flp lacking Arg-191 on a MeP half-site. Can Flp(R191A) execute strand cleavage utilizing Tyr-343? Can the cleaved MeP-tyrosyl intermediate be successfully carried through the strand joining step by Flp(R191A)? Or is it vulnerable to hydrolysis (type I endonuclease)? Also, can Flp(R191A), like Flp(R308A), promote direct hydrolysis of the MeP diester bond (type II endonuclease)? The results obtained are assembled in Figs. 2–7.
FIGURE 1.
Organization of active site residues in DNA-bound Flp with the scissile phosphate in its uncleaved and cleaved (covalently linked) states. The catalytic pentad residues (Arg-191, Lys-K223, His-305, Arg-308, and Trp-330) of a Flp monomer and the active site tyrosine (Tyr-343) donated in trans from a second monomer are displayed surrounding the scissile phosphate (gold sphere). In one active site configuration (A), the scissile phosphate is not cleaved; in the other (B), the scissile phosphate is cleaved and linked to the trans-donated Tyr-343. Note that Arg-191 and Arg-308 make hydrogen bonding interactions with the nonbridging oxygens of the scissile phosphate in both A and B (dotted lines). The structural information is from Protein Data Bank entry 1M6X (21) and is displayed using the PyMOL molecular graphics system (DeLano Scientific).
FIGURE 3.
Type II endonuclease activities of Flp(Y343G) and Flp(R191A,Y343G) on the MeP half-site. The reactions were performed and analyzed as described for Fig. 2. The results from three separate experiments are plotted.
FIGURE 5.
Quantitative analysis of the strand joining and endonuclease activities of Flp, Flp(R191A), and Flp(R308A) on half-site substrates containing a 5′-OH on the nonscissile strand. The kinetics of strand joining and hydrolysis are represented by the blue and red circles, respectively.
Previous published and unpublished work shows that Flp mutants lacking a positively charged side chain at position 191 are inactive in performing recombination on native phosphate substrates in vivo and in vitro (7, 8, 10). Additional in vitro assays signified their activities in strand cleavage and strand joining to be almost undetectable on half-site substrates. By contrast, Flp(R191A) readily formed a covalent adduct with the MeP half-site, as was revealed by SDS-PAGE (Fig. 2A, left panel). This reaction product appeared early during the assay and disappeared at later time points. The loss of the DNA-protein adduct was accompanied by the appearance of a 23-nucleotide product (Fig. 2B, left panel). The mobility of the 23-mer, relative to that of the previously characterized hydrolysis product formed by Flp(R308A) (11), suggests the presence of MeP (and not a free hydroxyl) at the 3′-terminus. The mobility of the faint band just above the 23-mer was consistent with a product harboring a 3′-hydroxyl end. The delayed accumulation of the 23-mer proportionate with the disappearance of the DNA adduct was in accordance with a precursor-product relationship. We conclude that Flp(R191A) gives rise to the 23-mer primarily by hydrolyzing the 3′-MeP-tyrosyl DNA-protein linkage, that is, by the Flp type I endonuclease activity.
The pseudo-first order rate constant for 23-mer formation from the MeP half-site by Flp(R191A) (comprising cleavage plus hydrolysis) was estimated as khydro(I) 3.1 × 10−5 s−1 and is within a factor of 3 of that for direct MeP hydrolysis by Flp(R308A) (8.7 × 10−5 s−1). The yield of the 23-mer at 48 h from the Flp(R191A) reaction was 50% more than that from an equivalent Flp reaction: ∼15 and 10% of the input substrate, respectively (this study and Ref. 11). The distinct routes by which the Arg-191 and Arg-308 mutants hydrolyze the MeP half-site are displayed in Fig. 2 with Flp(R191A) proceeding mainly via the cleaved tyrosyl intermediate (type I; Fig. 2A, left panel) and Flp(R308A) via direct attack of the DNAs phosphodiester backbone, bypassing the formation of the cleaved intermediate (type II; Fig. 2A, right panel) (this study and Ref. 11).
The Type II Endonuclease Constitutes a Minor Pathway for MeP Hydrolysis by Flp(R191A): a Scissile MeP Is Sufficient to Unveil Type II Activity in Wild Type Flp without Additional Active Site Mutations
The extent of MeP-tyrosyl intermediate formed by wild type Flp at early time points during the MeP half-site reaction (11) was significantly lower than that formed by Flp(R191A) (Fig. 2A), yet the difference in the yields of the 23-mer by the two proteins was not as prominent, suggesting that MeP at the scissile position may also induce type II activity in wild type Flp. In a previous assay with Flp(Y343G), formation of the 23-mer from the MeP half-site was found to be quite weak (11). A careful re-examination of the Flp(Y343G) hydrolysis reaction revealed that even in the absence of Tyr-343, there is ∼8% conversion of the MeP half-site to the 23-mer product by type II endonuclease activity, that is, without formation of the MeP-tyrosyl intermediate (Fig. 3). These findings indicate that MeP half-site hydrolysis by Flp can occur as a result of both type I and type II endonuclease activities. However, direct MeP hydrolysis by Flp(R308A) and Flp(R308A,Y343G) was much more robust (32% conversion of the input MeP half-site into the 23-mer product) than that by Flp or its variants lacking Tyr-343 (11).
To test whether the type II endonuclease contributed, at least in part, to the hydrolytic activity of Flp(R191A), the MeP half-site was treated with the double mutant Flp(R191A,Y343G). No DNA-protein adduct was detected, as expected for the absence of the cleavage nucleophile (Fig. 3A). Nevertheless, the 23-mer hydrolysis product was formed, indicative of type II endonuclease activity (Fig. 3B). The amount of the 23-mer yielded by Flp(R191A,Y343G) was much lower than that of Flp(R191A) (compare Figs. 3 and 2). Quantitation indicated that direct hydrolysis (type II endonuclease) accounts for only approximately one-third of the total hydrolysis (type I plus type II endonucleases) by Flp(R191A). The type II endonuclease activities of Flp(Y343G) and Flp(R191A,Y343G) were rather similar (8 and 5% conversion, respectively, of the input MeP half-site to the 23-mer) (Fig. 3B) but were significantly lower than that of Flp(R308A) or Flp(R308A,Y343G) observed previously (23-mer yield of 32% of the input MeP half-site) (11). Thus, neutralization of the negative charge on the scissile phosphate is sufficient to cause its susceptibility to direct hydrolysis, when activated by DNA-bound Flp. However, this effect becomes much more dramatic when Arg-308 within the Flp active site is substituted by alanine (11).
Effects of MeP Substitution and Lack of Arg-191 or Arg-308 on the Strand Joining Reaction
The 5′-hydroxyl groups of the bottom strands of the half-sites used in the hydrolysis assays depicted in Figs. 2 and 3 were phosphorylated to prevent them from acting as nucleophiles. As noted earlier, a free 5′-hydroxyl, if present, would be able to attack the P- or MeP-tyrosyl intermediate in a pseudo-strand exchange reaction that forms a hairpin recombinant. This product, along with that formed by a possible inter-half-site joining reaction, can be observed as a 54-mer by urea-PAGE. We had noticed previously that strand joining in a MeP half-site by Flp is slower and less efficient than that in a P half-site (11).4A more detailed analysis of strand joining in the MeP half-site by Flp, Flp(R191A), and Flp(R308A) relative to that in the P half-site by Flp is shown in Fig. 4. Quantitative representations of the data are assembled in Fig. 5.
In the P half-site, joining by Flp proceeded swiftly and efficiently with a rate constant of 2.2 × 10−3 s−1, easily out-competing hydrolysis, whose rate constant was 60-fold lower, 3.6 × 10−5 s−1 (Figs. 4A and 5A). After 1 h of incubation at 30 °C, Flp converted 90% of the substrate to the joined product compared with only a 6% yield from hydrolysis in 24 h. In the Flp/MeP half-site combination (Figs. 4B and 5B), the joined product accounted for 0.5% of the input half-site at 48 h compared with just over 4% of the hydrolysis product in the same duration. This finding is consistent with Tyr-343-mediated strand cleavage (and resultant formation of the tyrosyl intermediate) being a minor reaction compared with direct hydrolysis (type II endonuclease activity) as a result of the MeP substitution. The data are accommodated by the MeP-tyrosyl intermediate being nearly quantitatively converted into the joined product rather than the 23-mer in the presence of the 5′-hydroxyl. Thus, as in the P half-site reaction, the 5′-hydroxyl was a more efficient nucleophile compared with water in the MeP half-site reaction.
Flp(R191A) also promoted efficient strand joining in the MeP half-site (Figs. 4C and 5C). Competition between water and the 5′-hydroxyl was evident by a drop in the amount of hydrolysis product (23-mer) from ∼15% in the absence of the 5′-hydroxyl (Fig. 2) to ∼7% in its presence. Note that the 23-mer formed as a result of direct hydrolysis (∼5%; as inferred from the Flp(R191A,Y343G) reaction; Fig. 3B) would not have contributed to strand joining. Thus, in the presence of a 5′-hydroxyl, the predominant fraction of the MeP-tyrosyl intermediate was converted to the joined product, hydrolysis being reduced to a minor side reaction.
As expected from its inability to form detectable covalent adduct, Flp(R308A) gave very little joining product (0.2% yield) in the MeP half-site (Figs. 4D and 5D). This weak reaction could result either from the formation of a small amount of the tyrosyl intermediate or from a direct attack of the 5′-hydroxyl on the MeP diester bond in DNA. Consistent with the latter possibility, a weak joining reaction (final yield of 0.03% joined product) was also obtained with Flp(R308A,Y343G), an activity not demonstrated by Flp(R191A,Y343G) (Fig. 6). An alternative possibility is that a covalent adduct formed by a surrogate nucleophile either from Flp(R308A) or Flp(R308A,Y343G) or from the reaction buffer (glycerol, for example) was targeted for strand joining. Formation of the hydrolysis product by the type II endonuclease of Flp(R308A) was essentially free from competition by the 5′-hydroxyl, to give a final 32% yield of the 23-mer, the same as was observed for a MeP half-site with the blocked 5′-hydroxyl group (11).
A significant point that emerges from comparing Flp/P half-site and Flp(R191A)/MeP half-site reactions is the efficient suppression of the abortive hydrolysis of the P-tyrosyl or MeP-tyrosyl intermediate in the presence of a joining-competent 5′-hydroxyl group. Clearly, the strand joining reaction in the MeP half-site is independent of Arg-191. The comparable, even though quite low, yields of the MeP-tyrosyl intermediate and the joined product in the MeP half-site by Flp(R308A) (Figs. 4D and 5D and Ref. 11) suggest that Arg-308 may also be dispensable for joining when the scissile phosphate is charge-neutralized. It is probable that the target for strand joining by Flp(R308A) is predominantly the MeP-tyrosyl intermediate rather than the MeP diester bond in DNA, as inferred by the yields of the joined products by Flp(R308A) and Flp(R308A,Y343G): 0.2% and 0.03%, respectively.
Strand Exchange between a MeP Half-site and a P Full-site by Flp-Flp(R191A) Collaboration
Because Flp(R191A) is capable of mediating strand cleavage and joining in a MeP half-site, we wished to know whether it can also catalyze strand exchange between two DNA partners, that is, perform authentic recombination. A positive result would indicate that neither MeP substitution nor the lack of Arg-191 poses an obstacle to the conformational dynamics of recombination. In the assay shown in Fig. 7, we tested whether a full-site, containing phosphate at the scissile positions, can partner with a MeP half-site in DNA exchange in the presence of wild type Flp and Flp(R191A).
The predicted strand exchange reactions between the end-labeled MeP half-site and the unlabeled full-site are schematically diagrammed in Fig. 7A. A normal recombination reaction between two full-site partners is constrained by a strict homology requirement between them in the 8-bp spacer region. However, because the homology rule is relaxed in the case of a half-site by full-site reaction, the labeled strand of the MeP half-site may recombine with the top or the bottom strand of the full-site. The two recombinant strands may be distinguished by their sizes, 186 and 130 bp, respectively.
The MeP half-site was prebound with Flp(R191A) in recombination buffer, and then the P full-site and Flp were added, thus initiating strand exchange between the two DNA substrates. The reactions were analyzed by urea-PAGE. In the control reaction, where the MeP half-site and the P full-site were mixed with Flp, no recombinant product was observed (Fig. 7B, lane 2), as is also the case with Flp(R191A) (data not shown). This lack of reaction is consistent with the very poor formation of the tyrosyl intermediate by Flp and Flp(R191A) on the MeP and P substrates, respectively (Ref. 13 and this study). When the Flp(R191A)-bound half-site was reacted with Flp and the P full-site, the predicted exchange products from the alternative site alignments were formed (Fig. 7B, lanes 3–7). Thus, Flp(R191A) is a functional recombinase for DNA substrates containing MeP at the scissile positions.
DISCUSSION
In this study, we have extended our investigations of the active site mechanism of Flp site-specific recombinase using a modified DNA substrate in which replacement of a nonbridging oxygen atom by a methyl group neutralizes the ground state negative charge on the scissile phosphate. Previous work showed that activation of the MeP diester bond in DNA occurs independently of Arg-308 of the Flp catalytic pentad. However, in the Flp(R308A) reaction, nucleophilic attack on MeP is performed almost exclusively by water (instead of Tyr-343), to yield a dead-end hydrolytic product. This unexpected type II endonuclease activity is also unveiled in wild type Flp by MeP substitution and is considerably enhanced by the R308A mutation. Tyr-343 independence of this activity distinguishes it from type I endonuclease activity, in which the cleaved tyrosyl intermediate is the target of water attack. Type I activity is evident in a Flp/P half-site reaction and is detectable but weak in a Flp/MeP half-site reaction (this study and Ref. 13). Experiments in the present work have examined the effects of replacing the second arginine of the pentad, Arg-191, by alanine and the responses of the variant Flp to MeP-DNA. A summary of the cumulative results from this study given in Table 2 provides the basis for considerations that follow on the electrostatic contributions of the conserved active site arginines to Flp recombination mechanisms.
TABLE 2.
Summary of the catalytic activities of Flp, Flp(R191A), and Flp(R308A) on MeP substrates
The type I and type II endonuclease, strand joining, and strand exchange activities were assayed on MeP half-sites and are presented as percentages of the input substrate converted to product. The hydrolysis assays with Flp, Flp(R191A), and Flp(R308A) measured the sum of the type I plus type II endonuclease activities. The type II activities alone of the aforementioned proteins were deduced using their derivatives harboring the additional mutation Y343G. This mutation blocks the formation of the covalent tyrosyl adduct, which is the target for the type I endonuclease activity. Flp(R308A) was distinct in showing almost exclusively type II activity (Ref. 11 and this study). The extent of type I activity was estimated by subtracting the type II activity from total hydrolysis activity (type I plus type II). In the recombination reactions, strand exchange between a MeP half-site and a P full-site was assayed. ND, not determined.
| Hydrolysis of MeP-tyrosyl adduct (type I endonuclease) | Hydrolysis of MeP diester bond in DNA (type II endonuclease) | Strand joining in MeP half-site | Strand exchange between MeP half-site and P full-site | |
|---|---|---|---|---|
| % | % | % | % | |
| Flp | 2a | 8 | <1 | 0 |
| Flp(R191A) | 9 | 5 | 10 | 1 |
| Flp(R308A) | <1 | 32a | <1 | ND |
a Values taken from Ma et al. (11).
Type I Endonuclease and Strand Joining Activities of Flp(R191A) on a MeP Half-site: Recombination of MeP-DNA by Flp(R191A)
Flp(R191A) cleaves the scissile MeP diester bond in the MeP half-site using the Tyr-343 nucleophile and may subsequently hydrolyze the covalent adduct via a type I endonuclase route (Fig. 2). However, a free 5′-hydroxyl can effectively compete with water for the tyrosyl intermediate to form the strand joined product (Fig. 4). Under the reaction conditions employed in our assays, strand joining is significantly faster and much more efficient than hydrolysis. The strand cleavage and joining activities of Flp(R191A) observed in the half-site reaction are also relevant to the strand exchange reaction between two DNA partners. Flp(R191A), in collaboration with Flp, can promote recombination between a MeP half-site and a P full-site (Fig. 7).
In contrast to Flp(R191A), Flp(R308A) is capable almost exclusively of direct MeP hydrolysis (type II endonuclease activity). Although Flp(R191A) is also competent in directly hydrolyzing MeP, this activity is much weaker than that of Flp(R308A). The catalytic properties of Flp(R191A), in conjunction with those of Flp(R308A) catalogued in a previous work (11), signify that two critical arginine residues of the Flp catalytic pentad can be individually dispensed within the MeP diester bond activation step by chemically compensating for the negative charge in the transition state. The methyl group may be viewed as a suppressor mutation in the substrate that counterbalances the lack of either Arg-191 or Arg-308. The distinct catalytic properties of Flp(R191A) and Flp(R308A) denote that the effects of suppression are nevertheless different in the two cases. Such a difference could not have been anticipated from the crystal structures of Flp in association with DNA substrates containing a scissile phosphodiester bond (17, 21, 22).
Type II Endonuclease of Flp(R191A)
The type II endonuclease activity, direct attack of the scissile phosphodiester bond by water, is almost undetectable in native P substrates in the presence of Flp or Flp(Y343G). However, it becomes prominent in the MeP half-site upon incubation with Flp(R308A) or Flp(R308A,Y343G) (11). Flp and Flp(R191A) also exhibit a type II endonuclease activity, although poorly when compared with Flp(R308A) (13). In Flp(R191A), the type II endonuclease is masked by the higher Tyr-343-dependent activity of the type I endonuclease. The type II reaction can nevertheless be readily unveiled by Flp(R191A,Y343G) (Fig. 3).
The paucity of Tyr-343-mediated cleavage in the MeP half-site by Flp(R308A) despite MeP diester bond activation, as indicated by direct hydrolysis, suggests the possibility that Arg-308 may be involved in proton abstraction from Tyr-343, perhaps through an oriented water molecule (11). The structure of the Leishmania donovani topoisomerase complexed with the vanadate transition state mimic (23) suggests that a well oriented water molecule acts as a specific base with assistance from Arg-410 (equivalent to Arg-308 of Flp), which is hydrogen-bonded to the phenolic oxygen of the tyrosine nucleophile. By analogy, the lack of Arg-308 could result in the impaired activation/orientation of the Tyr-343 nucleophile in the Flp active site. The moderately strong hydrolytic activity of Flp(R308A) on a MeP half-site may reflect the lack of competition by the tyrosine nucleophile.
The yield of the 23-mer hydrolysis product from the Flp(R191A,Y343G) reaction (type II endonuclease activity alone) is only one-third of the corresponding yield from the Flp(R191A) reaction (type I plus type II endonuclease activities) (Figs. 2 and 3). If water and Tyr-343 were truly competitive, one might have expected the yields to be more or less equal for the single and double mutant reactions. An explanation may be found in the shared active site organization of Flp (8, 17). Type II endonuclease activity likely occurs from a half-site bound by a single Flp monomer, with water providing the nucleophile in trans. The type I reaction requires at least a [Flp-half-site] dimer (a [Flp-half-site] trimer or tetramer is also expected to be active), because Tyr-343 has to be delivered in trans from a second Flp monomer. An oriented Tyr-343 in a functional active site would possibly block competition from water.
Direct Attack of the 5′-Hydroxyl on the MeP Diester Bond Activated by Flp(R308A)?
Flp(R308A) may be able to use not only water but also the 5′-hydroxyl group, with much lower efficiency, for nucleophilic attack of the MeP diester bond in DNA. The very weak strand joining reaction promoted by Flp(R308A,Y343G) may be accommodated by this mechanism (Fig. 6). A similar, weak joining activity of Flp(Y343F) in a P half-site has been previously observed (24). An analogous reaction is the type II RNase activity exhibited by Flp and Flp(Y343F) when a ribonucleotide substitution is placed immediately 3′ to the scissile phosphodiester that partakes in DNA recombination (25, 26). In this reaction, a vicinal 2′-hydroxyl attacks the phosphodiester bond to cause strand breakage. The 3′-terminal phosphate left at the strand nick is presumably generated via a 2′,3′-cyclic phosphate intermediate. On the one hand, these activities highlight the catalytic flexibility and versatility of the recombinase active site in being able to target a scissile phosphate using distinct nucleophiles. On the other hand, they illustrate the potential danger from undesirable nucleophiles that threaten an activated phosphodiester bond in a DNA chain.
Potential Steric Perturbations of the Active Site by Arg-to-Ala and P-to-MeP Substitutions
The methyl group has a slightly greater van der Waal's radius (∼1.8 Å) compared with the oxygen atom (∼1.5 Å). Potential steric clashes between the larger nonpolar methyl group and the guanidinium group of Arg-191 or Arg-308 could induce realignment of active site residues and/or the scissile MeP. The consequent adverse effects on transition state stabilization would result in reduced catalytic power. The weaker activity of wild type Flp on MeP substrates relative to Flp(R191A) or Flp(R308A) would be consistent with this scenario (this study and Ref. 11). However, Flp(R191G) and Flp(R191Q) are nearly identical to each other and comparable with Flp(R191A) in their strand cleavage and hydrolytic activities on the MeP half-site.4 Thus, the absence of a side chain or the presence of a rather bulky uncharged one at position 191 makes relatively modest differences in the MeP half-site reaction. Furthermore, differences between Flp(R191A) and Flp(R308A) in their MeP half-site reactions are particularly noteworthy (Table 2 and Ref. 11). Flp(R191A) displays both type I and type II endonuclease activities, type I being more prominent. Flp(R308A), by contrast, shows almost exclusively the type II activity, which is stronger than the combined type I and II activities of Flp(R191A). These qualitative and quantitative differences are more easily accommodated by the distinct mechanistic roles of the individual arginines than a general perturbation of the active site.
Phosphate and Active Site Electrostatics for Protection against Abortive Hydrolysis?
The MeP-tyrosyl intermediate formed by vaccinia topoisomerase or the Topo(R223A) mutant has a half-life of only ∼2 min, being rapidly hydrolyzed at physiological pH to the 3′-MeP product (12, 14). This instability is sharply at odds with the high stability of the P-tyrosyl intermediate formed by wild type topoisomerase, which has a half-life of ∼36 days at physiological pH. Phosphate electrostatics appear to protect the strand joining step of DNA relaxation against hydrolysis and resultant DNA damage. We find that hydrolysis of the tyrosyl intermediate is suppressed in favor of strand joining in Flp/P half-site and Flp(R191A)/MeP half-site reactions when an appropriate 5′-hydroxyl group is available (Fig. 4). Thus, phosphate electrostatics may not have a strong protective effect on the tyrosyl intermediate during recombination by Flp and perhaps other tyrosine site-specific recombinases. Protection is likely accorded by the intimate DNA-protein and protein-protein associations within the recombination synapse and the conformational dynamics of the 5′-hydroxyl containing strand concomitant with cleavage, facilitating rapid progression of recombination.
Protection of the strand cleavage step of Flp recombination against potential hydrolysis appears to be provided principally by Arg-308 (11). In a MeP half-site reaction with Flp(R308A), 32% of the substrate becomes the target for the futile type II endonuclease activity. This activity is also seen in Flp/MeP half-site and Flp(R191A)/MeP half-site reactions, although to a much lesser extent (this study), suggesting that the phosphate negative charge may play a secondary role in preserving the integrity of the strand cleavage reaction. Thus, the complementary electrostatic attributes of the recombinase active site and the DNA substrate may act cooperatively for the dual purpose of promoting the relevant phosphoryl transfer reactions while simultaneously blocking the abundant water nucleophile from aborting them.
This work was supported, in whole or in part, by National Institutes of Health Grant GM035654 (to M. J.). This work was also supported in part by Robert F. Welch Foundation Award F-1274 and by a University of Texas at Austin faculty research award.
P. A. Rowley, A. H. Kachroo, C. H. Ma, A. D. Maciaszek, P. Guga, and M. Jayaram, unpublished data.
- P
- phosphate
- MeP
- methylphosphonate.
REFERENCES
- 1.Perry K., Hwang Y., Bushman F. D., Van Duyne G. D. (2006) Mol. Cell 23, 343–354 [DOI] [PubMed] [Google Scholar]
- 2.Grindley N. D., Whiteson K. L., Rice P. A. (2006) Annu. Rev. Biochem. 75, 567–605 [DOI] [PubMed] [Google Scholar]
- 3.Chen Y., Rice P. A. (2003) Annu. Rev. Biophys. Biomol. Struct. 32, 135–159 [DOI] [PubMed] [Google Scholar]
- 4.Grainge I., Jayaram M. (1999) Mol. Microbiol. 33, 449–456 [DOI] [PubMed] [Google Scholar]
- 5.Guo F., Gopaul D. N., van Duyne G. D. (1997) Nature 389, 40–46 [DOI] [PubMed] [Google Scholar]
- 6.Argos P., Landy A., Abremski K., Egan J. B., Haggard-Ljungquist E., Hoess R. H., Kahn M. L., Kalionis B., Narayana S. V., Pierson L. S., 3rd, Sternberg N., Leon J. M. (1986) EMBO J. 5, 433–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friesen H., Sadowski P. D. (1992) J. Mol. Biol. 225, 313–326 [DOI] [PubMed] [Google Scholar]
- 8.Chen J. W., Evans B. R., Yang S. H., Araki H., Oshima Y., Jayaram M. (1992) Mol. Cell Biol. 12, 3757–3765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parsons R. L., Evans B. R., Zheng L., Jayaram M. (1990) J. Biol. Chem. 265, 4527–4533 [PubMed] [Google Scholar]
- 10.Parsons R. L., Prasad P. V., Harshey R. M., Jayaram M. (1988) Mol. Cell Biol. 8, 3303–3310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma C. H., Rowley P. A., Macieszak A., Guga P., Jayaram M. (2009) EMBO J. 28, 1745–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian L., Claeboe C. D., Hecht S. M., Shuman S. (2005) Structure 13, 513–520 [DOI] [PubMed] [Google Scholar]
- 13.Ma C. H., Kachroo A. H., Macieszak A., Chen T. Y., Guga P., Jayaram M. (2009) PLoS One 4, e7248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian L., Claeboe C. D., Hecht S. M., Shuman S. (2003) Mol. Cell 12, 199–208 [DOI] [PubMed] [Google Scholar]
- 15.Cheng C., Kussie P., Pavletich N., Shuman S. (1998) Cell 92, 841–850 [DOI] [PubMed] [Google Scholar]
- 16.Lee J., Jayaram M., Grainge I. (1999) EMBO J. 18, 784–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y., Narendra U., Iype L. E., Cox M. M., Rice P. A. (2000) Mol. Cell 6, 885–897 [PubMed] [Google Scholar]
- 18.Ghosh K., Lau C. K., Guo F., Segall A. M., Van Duyne G. D. (2005) J. Biol. Chem. 280, 8290–8299 [DOI] [PubMed] [Google Scholar]
- 19.Buchholz F., Angrand P. O., Stewart A. F. (1998) Nat. Biotechnol. 16, 657–662 [DOI] [PubMed] [Google Scholar]
- 20.Prasad P. V., Young L. J., Jayaram M. (1987) Proc. Natl. Acad. Sci. U.S.A. 84, 2189–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conway A. B., Chen Y., Rice P. A. (2003) J. Mol. Biol. 326, 425–434 [DOI] [PubMed] [Google Scholar]
- 22.Chen Y., Rice P. A. (2003) J. Biol. Chem. 278, 24800–24807 [DOI] [PubMed] [Google Scholar]
- 23.Davies D. R., Mushtaq A., Interthal H., Champoux J. J., Hol W. G. (2006) J. Mol. Biol. 357, 1202–1210 [DOI] [PubMed] [Google Scholar]
- 24.Serre M. C., Zheng L., Jayaram M. (1993) J. Biol. Chem. 268, 455–463 [PubMed] [Google Scholar]
- 25.Xu C. J., Grainge I., Lee J., Harshey R. M., Jayaram M. (1998) Mol. Cell 1, 729–739 [DOI] [PubMed] [Google Scholar]
- 26.Xu C. J., Ahn Y. T., Pathania S., Jayaram M. (1998) J. Biol. Chem. 273, 30591–30598 [DOI] [PubMed] [Google Scholar]