Abstract
p50/dynamitin (DM) is a major subunit of the microtubule-associated dynactin complex that is required for stabilization and attachment of its two distinct structural domains, namely the Arp1 rod and the shoulder/sidearm. Here, we define the determinants of p50/DM required for self-oligomerization of the protein and for interactions with other subunits of the dynactin complex. Whereas the N-terminal 1–91-amino acid region of the protein is required and sufficient for binding to the Arp1 rod, additional determinants contained within the first half of the protein are required for optimal recruitment of the p150Glued subunit of the shoulder/sidearm. Overexpression experiments confirmed that the N-terminal 1–91-amino acid region of p50/DM is critical for dynactin functionality, because this fragment acts as a dominant negative to inhibit both dynein-dependent and -independent functions of the complex. Furthermore, the first two predicted coiled-coil motifs of p50/DM contain determinants that mediate self-association of the protein. Interestingly, p50/DM self-association does not contribute to p50/DM-induced disruption of the dynactin complex, but most likely participates in the stabilization of the complex.
Keywords: Dynein, Golgi, Intracellular Trafficking, Microtubules, Protein-Protein Interactions, siRNA, Dynactin, Dynamitin
Introduction
The dynactin complex was initially identified as a multiprotein complex co-purifying with the minus-end-directed microtubular motor dynein and capable of activating membrane vesicle movements (1, 2). Initial genetic studies performed in yeast, filamentous fungi, and Drosophila demonstrated that dynactin was an obligate cofactor of the dynein motor (3–6). To date, many functional studies confirmed that the dynactin complex is required for most, if not all, dynein-based cellular processes (reviewed in Ref. 7). During interphase, dynactin plays a major role in all dynein-based motile events by providing both stable cargo binding and processivity activities, to ensure proper vesicular transport between cellular compartments along the microtubules (8–11). Moreover, the dynactin complex was also shown to interact with and increase the processivity of the kinesin-II plus-ended motor, suggesting an unexpected role in plus-end-directed motile events (12–14). In addition, dynactin plays other roles independently of microtubule motors, by facilitating attachment of microtubules to several subcellular structures. Although initial studies demonstrated that dynactin contributed to microtubule anchoring at the centrosome during interphase (15, 16), recent results provided the first clues concerning its role as an initial receptor for cytoplasmic membrane vesicles at microtubule plus ends (17–20), although this model has been brought into question (21).
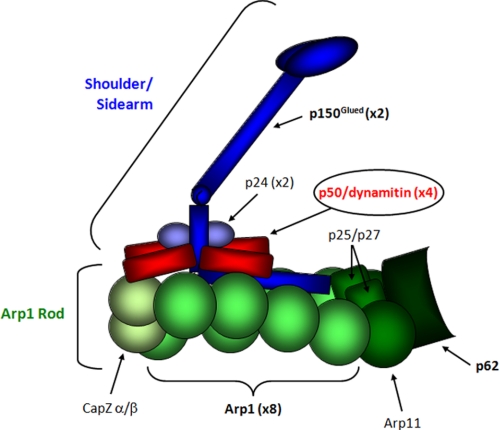
Dynactin is a multiprotein complex of ∼1.2 MDa, composed of 11 different polypeptides that are present as single or multiple copies and organized in two distinct structural domains (see Fig. 1) as follows: (i) the Arp1 rod, consisting of a short polymer of eight copies of Arp1 (actin-related protein-1), capped on its positive barbed end by the CapZ α/β heterodimer and on its negative pointed end by a heterotetrameric complex containing Arp11, p25, p27, and p62; and (ii) the shoulder/sidearm, emanating from the barbed end, composed of the p24, p50/dynamitin (DM),3 and p150Glued subunits, the latter protruding from the shoulder as a flexible extension (7). Although the Arp1 rod is most likely responsible for dynactin recruitment to the surface of several subcellular structures, the extended sidearm links the complex to both dynein and microtubules.
FIGURE 1.
Schematic representation of the overall structure of the dynactin complex. This schema illustrates the location and approximate structural features of individual subunits based on ultrastructural and biochemical characterization; it was adapted from Ref. 7.
The central p50/DM subunit emerged early as a major structural determinant of the dynactin complex by stabilizing attachment of the p150Glued sidearm to the Arp1 minifilament. Indeed, overexpression of either human or chicken p50/DM in mammalian cells leads to a dissociation of the shoulder/sidearm from the Arp1 rod, resulting in a broad range of functional defects of the dynactin complex (8, 9, 15, 22). This dissociation was reproduced in vitro by addition of a large excess of recombinant p50/DM on purified dynactin complexes (23, 24), demonstrating that no additional cytosolic factor was required.
Analysis of the primary sequence of the human p50/DM predicts three coiled-coil (CC) motifs, namely CC1 (amino acids 105–136), CC2 (amino acids 219–251), and CC3 (amino acids 281–308) (22). At least some of these CC motifs are assumed to contribute to oligomerization of the protein as well as interactions with other subunits of the shoulder/sidearm subcomplex, namely p24 and/or p150Glued (25). Moreover, several cellular proteins have been reported to interact directly with p50/DM, including the hZW10 member of the RZZ complex (26), the Golgi membrane-associated BICD2 protein (27), and the centrosomal protein Cep135 (28), most likely allowing dynactin recruitment to specific subcellular structures. Interestingly, these interactions seem to involve the N-terminal moiety of p50/DM, suggesting that it may be exposed at the surface of the complex. The C-terminal moiety of p50/DM has therefore been proposed to participate in the structural organization of the dynactin complex (7).
Although numerous studies have used p50/DM overexpression as a useful tool to identify motile as well as nonmotile cellular processes involving the dynactin complex (reviewed in Ref. 7), little information is available concerning the molecular determinants of p50/DM underlying its structural role within the dynactin complex. The exact mechanism by which p50/DM overexpression disrupts dynactin structure has not yet been fully elucidated, but some studies revealed that it is related to a displacement of p150Glued and p24 from the Arp1 rod (23, 24). Furthermore, p50/DM and p24 were found associated in a single subcomplex, suggesting that these two subunits may provide a structural basis for dynactin assembly, most likely by allowing recruitment of the p150Glued sidearm to the surface of the Arp1 rod.
The aims of this study were as follows: (i) to decipher the molecular determinants of p50/DM responsible for self-association of the protein and its interactions within the dynactin complex; and (ii) to evaluate the contribution of these determinants to dynactin stability and functions. We show that binding of the N-terminal amino acids 1–91 of p50/DM to the Arp1 rod is a major determinant for dynactin functionality. Interestingly, self-association of p50/DM, through cooperation between the first two coiled-coil motifs of the protein, does not contribute to p50/DM-induced disruption of the complex, but rather it participates in the stabilization of the whole dynactin complex in vivo.
EXPERIMENTAL PROCEDURES
Plasmids
Mammalian Expression Vectors
The cDNA encoding the human dynamitin (isoform-2) has been amplified from a human peripheral blood lymphocyte cDNA library using the following primers: EcoF, ACTGGAATTCATGGCGGACCCTAAATACGCCG, and XhoR, ACTGCTCGAGTCACTTTCCCAGCTTCTTCATC. The resulting EcoRI-XhoI insert was cloned into the pAS1b vector (29), allowing expression of the full-length protein fused to the HA tag in mammalian cells (HA-DM). The BamHI-XhoI insert from this construct was then subcloned into the pEGFP-C1 vector (BD Biosciences) for expression of the protein fused to the C terminus of GFP (GFP-DM). Deletion mutants of human p50/DM were generated by PCR using specific primers and subsequently cloned into pAS1B and pEGFP-C1. The cDNA encoding the chicken orthologue of the protein was kindly provided by Ali Saïb (Hôpital Saint-Louis, Paris, France). Specific primers were then used to amplify the chicken p50/DM open reading frame that was subsequently subcloned as described earlier into pAS1B and pEGFP-C1. Specific primers were used to generate deletion mutants of the chicken form of p50/DM.
Bacterial Expression Vectors
The vectors for expression of full-length or deleted forms of the human or chicken p50/DM fused to the glutathione S-transferase (GST) were generated by subcloning the EcoRI-XhoI corresponding fragments, isolated from pAS1b constructs, into pGEX-4T-1 (Amersham Biosciences).
Antibodies
The following antibodies were used: the rat monoclonal antibody (mAb) 3F10 (Roche Applied Science) and mouse mAb 7.1–13.1 (Roche Applied Science) for detection of HA-tagged and GFP-tagged proteins, respectively; anti-p50 mAb 25 (BD Biosciences), anti-p150Glued mouse mAb 1 (BD Biosciences), anti-p62 rabbit polyclonal antibody (pAb) H-300 (Santa Cruz Biotechnology), and anti-Arp1α/Centractin rabbit pAb (Sigma) for detection of dynactin subunits; anti-Giantin rabbit pAb (Covance), anti-α-tubulin mouse mAb DM1A (Sigma) for analysis of Golgi, and microtubule morphology, respectively.
Cell Culture and Transfection
HeLa, 293T, and COS7 cells were maintained in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen), 50 units/ml penicillin/streptomycin, and 125 ng/ml amphotericin B (Invitrogen), at 37 °C under 5% CO2. For dynactin-binding experiments, HeLa cells were grown to 80–90% confluence on the day of transfection. HA- or GFP-tagged p50/DM constructs were transfected using Lipofectamine 2000 (Invitrogen), according to the manufacturer's recommendations. For oligomerization experiments, 3 × 106 293T cells were grown and transfected the following day with HA-tagged constructs using the calcium phosphate method, as described previously (30). For immunofluorescence experiments, HeLa or COS7 cells were grown onto coverslips in 6-well plates and transfected with HA- or GFP-tagged p50/DM constructs using the GeneJuice method (Novagen), according to the manufacturer's recommendations.
siRNAs
The control siRNA targeting the luciferase was purchased from Eurogentec. siRNA targeting the human p50/DM was purchased from Dharmacon, GGAGACAGCUGUACGUUGUUU, corresponding to residues 240–245. Briefly, 2 × 105 HeLa cells were seeded into 6-well plates and transfected twice during the two following days with the appropriate siRNA duplexes using Oligofectamine (Invitrogen), according to the manufacturer's recommendations. Cells were trypsinized 24 h following the second round of siRNA treatment and replated both in 10-cm dishes for Western blot analysis and in 6-well plates containing coverslips for immunofluorescence experiments.
Recombinant Proteins, Pulldown Assay, and Western Blot Analysis
For expression of the full-length or deleted forms of both human or chicken p50/DM fused to GST, E. coli BL21(DE3) cells (Invitrogen) were transformed. Expression of the corresponding recombinant proteins was induced with 0.5 mm isopropyl 1-thio-β-d-galactopyranoside for 3 h at 30 °C. Bacterial pellets were resuspended in phosphate-buffered saline (PBS) containing 2 mm EDTA, 5% Nonidet P-40, 2 mm dithiothreitol, 0.12% lysozyme, and a protease inhibitor mixture (Sigma) and incubated for 1 h at 4 °C. Clarified lysates were then incubated with glutathione (GSH)-Sepharose beads (GE Healthcare) for 1 h at 4 °C. Beads were washed three times in PBS containing 1 m NaCl and three times in PBS. The concentration of each purified recombinant proteins was estimated by SDS-PAGE, followed by a Coomassie staining.
HeLa or 293T cells were recovered 24 h after transfection and lysed for 10 min on ice in a buffer containing 50 mm Tris-HCl (pH 7.6), 150 mm NaCl, 1% Nonidet P-40, 2 mm EDTA, 15 mm MgCl2 and an antiprotease mixture (Sigma). Normalized amounts of each cell lysate (500 μg of proteins) were incubated overnight at 4 °C with equal quantities of the recombinant proteins purified on GSH-Sepharose beads. The beads were then washed three times in lysis buffer and resuspended in 2× sample buffer. Control cell lysates were prepared by removing 6% of the input (30 μg of proteins). Samples were boiled, subjected to SDS-PAGE, and transferred to a polyvinylidene difluoride Hybond-P membrane (GE Healthcare) before immunoblotting with the appropriate antibodies.
Co-immunoprecipitation Assay
293T cells were co-transfected with HA- and GFP-tagged forms of wild type or mutated p50/DM, and HA-tagged forms were immunoprecipitated from cleared lysates containing 400 μg of total proteins with 2 μg of anti-HA on protein A-Sepharose beads. 80% of the precipitated material was then resolved by SDS-PAGE and analyzed by Western blotting using anti-HA and anti-GFP antibodies.
Immunofluorescence
For analysis of Golgi and microtubule morphology, COS7 cells grown on coverslips were fixed 24 h after transfection with 4% paraformaldehyde for 20 min and permeabilized with 0.1% Triton X-100 for 10 min. For analysis of the subcellular localization of dynactin subunits, HeLa cells were fixed 24 h after transfection for 5 min in −20 °C methanol. Cells were subsequently treated with a PBS/bovine serum albumin 0.2% blocking solution containing the appropriate primary antibodies, washed three times in PBS, treated with secondary antibodies diluted in PBS, and washed again in PBS. Cells were mounted in PBS containing 50% glycerol. Images were acquired with a Leica DMRB epifluorescence microscope equipped with a CCD camera (Princeton) controlled by Metamorph version 5.0r6 software.
RESULTS
Chicken Orthologue of p50/DM Provides a More Sensitive Tool than Human Protein for Analysis of Dynactin Functions
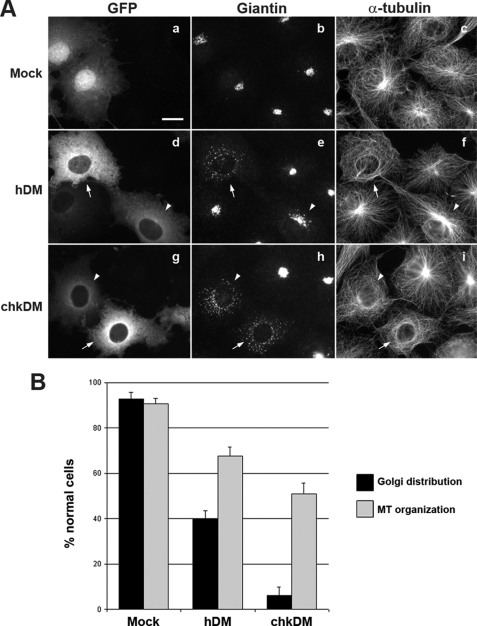
Most of the knowledge obtained regarding dynactin function in mammalian cells came from early studies in which either human (hDM) (8, 22) or chicken (chkDM) (9, 15) p50/DM overexpression was reported to inhibit both dynein-dependent motile processes and dynactin-dependent microtubule anchoring at the centrosome. We therefore performed a comparative analysis of these two p50/DM orthologues regarding their deleterious effect on dynactin functions when overexpressed in COS7 cells. As reported previously (8, 9, 15, 22), COS7 cells show attractive features when addressing subcellular organization issues, because they display a well focused microtubule network radiating from the microtubule-organizing center at the centrosome and a compact perinuclear Golgi distribution. Results shown in Fig. 2 were obtained using p50/DM constructs fused to GFP (GFP-DM), but similar results were obtained when using HA-tagged constructs (data not shown). As expected (8), complete Golgi fragmentation and dispersal was observed in cells expressing high levels of exogenous hDM (Fig. 2A, panels d and e, arrows) but not in cells expressing low levels (arrowheads). In addition, cells expressing high levels of hDM displayed a clear defect in microtubule radial organization, sometimes leading to microtubule bundling (Fig. 2A, panel f, arrow), whereas cells expressing moderate levels typically showed a normal radial microtubule network (arrowhead). Using the chicken orthologue of DM, both high and low levels of exogenous chkDM were capable of altering Golgi distribution (Fig. 2A, panels g and h, arrows and arrowheads), but defects in microtubule network organization were mostly observed in cells expressing high levels of the protein (Fig. 2A, panel i, arrow).
FIGURE 2.
Impact of human and chicken p50/DM overexpression on dynactin functions. A, COS7 cells expressing GFP, GFP-hDM, or GFP-chkDM (panels a, d, and g) were stained with an anti-Giantin pAb (panels b, e, and h) and an anti-α-tubulin mAb (panels c, f, and i). Arrows and arrowheads on panels d–f and g–i show transfected cells expressing high and low levels of the GFP-hDM fusions, respectively. Scale bar, 10 μm. B, Golgi distribution (black bars) and microtubule (MT) organization (gray bars) were quantified over 100 cells expressing GFP (mock), GFP-hDM (hDM), or GFP-chkDM (chkDM). Results are expressed as the percentage of transfected cells showing normal Golgi distribution, either concentrated in the perinuclear region (see nontransfected cells) or showing only slight dispersal (see arrowhead in panel e), and a radial organization of microtubules anchored at the centrosome. Values are the means of three independent experiments; error bars represent 1 standard deviation from the mean.
Quantification of the phenotypes induced by overexpression of either hDM or chkDM confirmed these findings (Fig. 2B) and showed that Golgi distribution and microtubule anchoring remained unaltered in 40 and 70% of cells overexpressing the hDM orthologue, respectively, compared with ∼5 and 50% in cells overexpressing chkDM. This quantification also indicates that the function of dynactin at the centrosome is less susceptible to p50/DM overexpression than the dynein-dependent vesicular trafficking. We conclude from these experiments that overexpression of the chicken orthologue of p50/DM is more efficient than hDM in inhibiting dynactin functions in vivo.
Determinants of the Human p50/DM Mediating Interactions with Other Dynactin Components
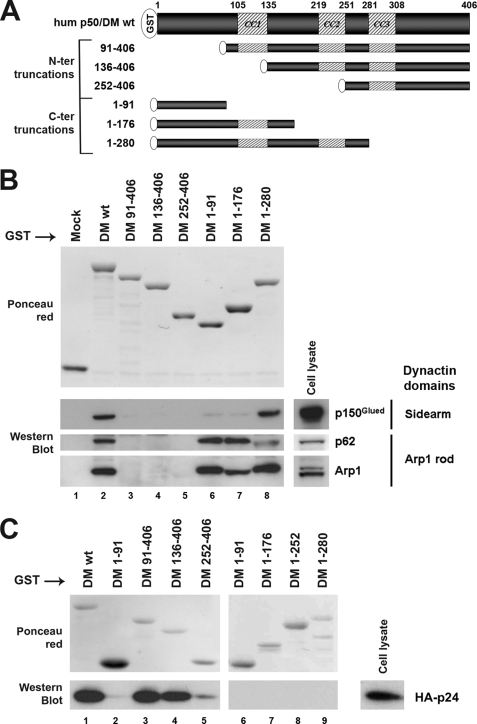
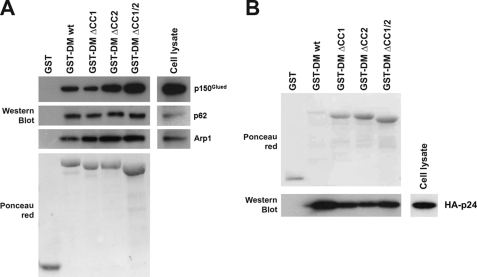
To define the determinants of the human p50/DM implicated in the interactions with other components of the dynactin complex, we designed a simple experimental system in which wild type or deletion mutants of human p50/DM fused to GST were expressed in Escherichia coli (GST-hDM), purified on GSH-Sepharose beads (Fig. 3B, upper panel), and then incubated with a lysate from HeLa cells prepared in a buffer that preserves the integrity of the dynactin complex (8, 9, 15, 22). Bound proteins were eluted, separated by SDS-PAGE, and subsequently analyzed by Western blotting using antibodies against p150Glued and Arp1 for analyzing the ability of immobilized p50/DM to interact with components of the two structural domains of the dynactin complex. A control of Arp1 rod integrity was included by analyzing p62 in precipitates. As shown in Fig. 3B (lane 2), GST-hDM retained all the aforementioned subunits of the dynactin complex, namely p150Glued, p62, and Arp1. These interactions were specific, because no binding was detected with GST alone (Fig. 3B, lane 1).
FIGURE 3.
Characterization of the p50/DM determinants mediating interactions with other dynactin components. A, schematic representation of the human p50/DM deletion mutants fused to GST. Hatched segments represent the three putative coiled-coil motifs (CC1, CC2, and CC3); amino acids are numbered according to Yue et al. (36). wt, wild type; N-ter, N-terminal; C-ter, C-terminal. B, HeLa cell lysates (lower right panels) were incubated with equal amounts of wild type or deleted GST-hDM fusions (upper panel, Ponceau red) immobilized on GSH-Sepharose beads. Bound proteins were analyzed by Western blot with anti-p150, -p62, and -Arp1 antibodies. C, lysates from HeLa cells expressing HA-tagged p24 (lower right panel) were incubated with equal amounts of wild type or deleted GST-hDM fusions (upper panels, Ponceau red) immobilized on GSH-Sepharose beads. Bound proteins were analyzed as in B with anti-HA mAb.
Several deletion mutants of p50/DM were subsequently generated (Fig. 3A) and expressed as GST fusions (Fig. 3B, upper panel). Equal amounts of the purified GST-hDM mutants were then incubated with cell lysate. Compared with the wild type GST-hDM (Fig. 3B, lane 2), the GST-hDM-(91–406) deletion mutant, lacking the highly conserved N-terminal region of hDM, failed to interact with dynactin components (lane 3). In contrast, the recombinant GST-hDM-(1–91) fragment was sufficient to mediate interaction with components of the Arp1 rod (Fig. 3B, lane 6). However, the recruitment of the p150Glued subunit by this fragment was almost totally abrogated compared with that observed with the wild type GST-hDM, indicating that additional molecular determinants are needed for optimal recruitment of the p150Glued sidearm. Analysis of the C-terminal deletion mutants of hDM (GST-DM-(1–176) and -(1–280)) revealed that these additional determinants may be located in the central portion of the protein, between amino acids 176 and 280 (Fig. 3B, see lanes 7 and 8).
Because it was suggested that p50/DM tightly bound to the p24 subunit to form, in association with p150Glued, the shoulder/sidearm moiety of the dynactin complex (23, 24), we hypothesized that the regions involved in p150Glued recruitment may correlate with those mediating binding to p24. As the p24 antibodies currently available do not allow proper detection of endogenous p24 (23, 24), we used GST-DM fusion proteins to pull down an HA-tagged form of p24 from transfected cell lysates. As shown in Fig. 3C, all of the C-terminal truncation mutants, including the 1–280-amino acid mutant that still recruited the whole dynactin complex (see Fig. 3B), failed to pull down HA-p24 (lanes 6–9), suggesting that some determinants located in the C-terminal part of p50/DM may participate in this interaction. This latter observation also suggests that the interaction between p50/DM and p24 may be dispensable for interactions with other components of dynactin. In contrast, the 91–406- and 136–406-amino acid N-terminal truncation mutants efficiently bound to p24 (Fig. 3C, lanes 3 and 4), although the 252–406-amino acid fragment failed (lane 5), indicating that some other crucial determinants required for p24 binding are contained within the 136–252-amino acid central region of p50/DM.
Determinants of the Human p50/DM-mediating Self-association of the Protein
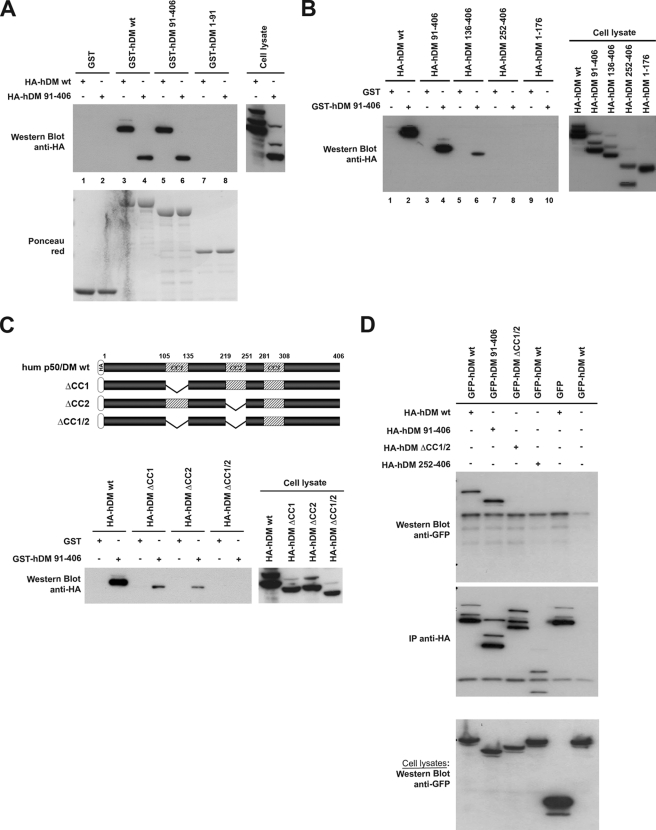
A similar GST pulldown approach was subsequently used to determine the regions of human p50/DM required for self-association of the protein using a lysate from cells expressing HA-tagged p50/DM (HA-hDM). As shown in Fig. 4A (lane 3), HA-hDM indeed associated with immobilized GST-hDM. We first investigated whether an initial interaction between p50/DM and other subunits of the dynactin complex was required for further oligomerization of the protein by evaluating the ability of the C-terminal 91–406-amino acid fragment of p50/DM to mediate self-association (Fig. 4A). Although this fragment failed to exhibit any detectable interaction with components of the dynactin complex in the previous experiment (see Fig. 3B, lane 3), it retained the ability to associate with the full-length HA-tagged hDM expressed in cells (Fig. 4A, lane 5), as well as with the HA-hDM-(91–406) deletion mutant (lane 6). Moreover, no detectable association was revealed with the recombinant GST-hDM-(1–91) fragment (lanes 7 and 8). These results demonstrate that self-association of p50/DM is mediated by the 91–406-amino acid part of the protein.
FIGURE 4.
Characterization of the determinants involved in p50/DM self-association. A, lysates from 293T cells expressing HA-tagged forms of either wild type hDM or the hDM-(91–406) mutant (upper right panel) were incubated with equal amounts of GST, GST-hDM wild type (wt), GST-hDM-(91–406), or GST-hDM-(1–91) (lower panel, Ponceau red) immobilized on GSH-Sepharose beads. Bound proteins were analyzed by Western blot with anti-HA mAb (upper left panel). B, lysates from 293T cells expressing wild type or deleted HA-tagged hDM (right panel) were incubated with equal amounts of GST or GST-hDM-(91–406) immobilized on GSH-Sepharose beads. Bound proteins were analyzed as indicated in A (left panel). C, schematic representation of the HA-tagged p50/DM deletion mutants expressed in 293T cells is shown at the top. Lysates from 293T cells expressing wild type or deleted HA-tagged hDM (right panel) were incubated with equal amounts of GST or GST-hDM-(91–406) immobilized on GSH-Sepharose beads and then analyzed as indicated in A (left panel). D, 293T cells expressing wild type or deleted forms of HA- and GFP-tagged (lower panel, Cell lysates) forms of hDM were lysed, and the HA-tagged forms were precipitated with anti-HA. Precipitates were then analyzed by Western blot with anti-GFP (upper panel) or anti-HA (middle panel). IP, immunoprecipitation.
Fine mapping of the regions of the human p50/DM involved in self-association was then performed using the recombinant GST-DM-(91–406) fragment and several HA-tagged p50/DM deletion mutants expressed in cells (Fig. 4B). Although these mutants were expressed at comparable levels (Fig. 4B, right panel), deletion of the 91–135-amino acid region (HA-DM-(136–406 mutant)) containing the first predicted CC motif resulted in a dramatic decrease of the interaction (Fig. 4B, lane 6). Furthermore, no association was detectable with the HA-hDM-(252–406) and -(1–176) fragments (Fig. 4B, lanes 8 and 10). These results indicate that the region including the first predicted CC1 motif of the human p50/DM largely contributes to self-association of the protein, but other determinants localized between amino acids 176 and 252 also participate in this property. Because the 176–252-amino acid region contains another predictive CC motif, we hypothesized that this motif could contribute, together with CC1, to p50/DM oligomerization. Deletion mutants lacking the CC1 region (ΔCC1), the CC2 region (ΔCC2), or both (ΔCC1/2) were thus constructed and expressed in cells (Fig. 4C). Both HA-hDM ΔCC1 and ΔCC2 mutants were severely affected for association with GST-hDM-(91–406), although residual interactions were clearly detectable (Fig. 4C, left panel). However, no residual association was detected when both domains were simultaneously deleted in the ΔCC1/2 mutant. Similar results were obtained in co-immunoprecipitation experiments performed on 293T cells co-expressing HA- and GFP-tagged forms of wild type or mutated p50/DM (Fig. 4D). Using anti-HA for immunoprecipitation, wild type GFP-DM was co-precipitated with wild type HA-DM, and deletion of the N-terminal 1–90-amino acid region of hDM (DM-(91–406) mutants) had no impact on self-association of the protein. Again, the ΔCC1/2 mutants, lacking both CC1 and CC2 domains, failed to self-associate in this assay.
Because the ΔCC1/2 double mutant, as well as the ΔCC1 and ΔCC2 mutants, was still able to recruit both the Arp1 rod and the p150Glued sidearm of the complex (Fig. 5A), we conclude that deletion of the CC1 and CC2 motifs does not alter the overall structure of the p50/DM protein. Similarly, deletion of both CC1 and CC2 motifs had no impact on p50/DM binding to p24 (Fig. 5B). These results strengthen the idea that self-association of p50/DM does not constitute a prerequisite toward its interaction with other subunits of the dynactin complex.
FIGURE 5.
Contribution of p50/DM self-association to its binding to other dynactin subunits. A, HeLa cell lysates (right panels) were incubated with equal amounts of wild type or deleted GST-hDM fusions (lower left panel, Ponceau red) immobilized on GSH-Sepharose beads. Bound proteins were analyzed by Western blot with anti-p150, -p62, and -Arp1 antibodies. B, lysate from 293T cells expressing HA-tagged p24 (lower right panel) was incubated with equal amounts of the purified wild type or deleted GST-hDM fusions (upper panel, Ponceau red) immobilized on GSH-Sepharose beads. Bound proteins were analyzed by Western blot with anti-HA mAb (lower left panel).
p50/DM Determinants Implicated in Self-association and Binding to Dynactin Components Are Conserved between Human and Chicken Orthologues
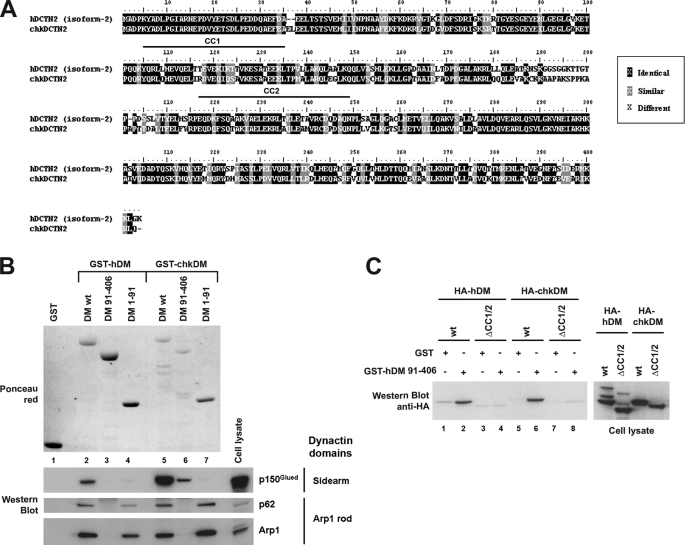
Because the experiments reported in Fig. 2 showed that the inhibition of dynactin functions associated with overexpression of the chicken p50/DM orthologue were exacerbated compared with those observed with the human protein, we decided to use the chicken form to analyze the functional role of the molecular determinants identified in p50/DM. However, we first checked that both p50/DM orthologues shared the same determinants for self-association and binding to other dynactin components, most importantly to the Arp1 rod. Analysis of the primary sequence of the chicken p50/DM confirmed the conservation of the two first CC motifs, namely CC1 and CC2 (Fig. 6A), suggesting that these secondary structures may play a critical role in some aspects of the protein structure and functionality. Three deletion mutants from the p50/DM chicken orthologue (chkDM-(1–91) and -(91–406) and ΔCC1/2) were generated and analyzed for self-association and binding to other dynactin components. Although recombinant wild type GST-chkDM was purified less efficiently than its human counterpart (Fig. 6B, upper panel, compare lanes 2 and 5), similar interactions were repeatedly observed with the Arp1 rod, as revealed by Arp1 and p62 analysis (Fig. 6B, lane 5). However, results from several independent experiments showed a mean 4-fold increase in the ability of GST-chkDM to recruit the p150Glued sidearm (Fig. 6B, lane 5) compared with the human GST-hDM (lane 2), as measured by quantitative densitometry (data not shown). This latter observation suggests that wild type GST-chkDM displays a higher affinity for the p150Glued sidearm than does the human protein. As for the human protein (Fig. 6B, lane 4), the chicken GST-DM-(1–91) fragment was able to recruit components of the Arp1 rod but not the p150Glued sidearm (lane 7). Whereas the human GST-hDM-(91–406) fragment did not interact with dynactin components (Fig. 6B, lane 3), we observed a weak interaction between the chicken GST-chkDM-(91–406) fragment and p150Glued (lane 6). These results indicate that the regions of p50/DM required for interaction with dynactin components are conserved between the human and chicken proteins; most importantly, they confirm that the N-terminal 1–91-amino acid conserved region of the protein is necessary and sufficient to allow interaction with an intact Arp1 rod.
FIGURE 6.
Conservation of the determinants from human and chicken p50/DM implicated in self-association and binding to dynactin components. A, amino acid sequences of human (upper sequence, hDCTN2 isoform 2) and chicken (chkDCTN2) p50/DM. Identical and conservative residues are framed in black and gray, respectively. The human sequence is numbered according to Yue et al. (36); the position of the putative CC1 and CC2 motifs is indicated by an upper black line. B, HeLa cell lysates were incubated with equal amounts of wild type or deleted GST-hDM or GST-chkDM fusions (upper panel, Ponceau red) immobilized on GSH-Sepharose beads. Bound proteins were analyzed by Western blot with anti-p150, -p62, and -Arp1 antibodies (lower panels). C, lysates from 293T cells expressing the human or chicken wild type or ΔCC1/2 HA-tagged p50/DM proteins (right panel) were incubated with equal amounts of GST or GST-hDM-(91–406) immobilized on GSH-Sepharose beads. Bound proteins were analyzed by Western blot with anti-HA mAb (left panel).
Finally, we investigated the contribution of both CC1 and CC2 of the chicken p50/DM for self-association of the protein. The results shown in Fig. 6C confirm that both human and chicken orthologues exhibit similar self-association properties. Consistently with the observations made with the human protein (Fig. 6C, lanes 1–4), deletion of both the CC1 and CC2 predictive motifs of the chicken p50/DM led to a complete loss of association with the 91–406-amino acid fragment of both recombinant human (Fig. 6C, lanes 5–8) and chicken (data not shown) proteins. Together, these results indicate that the main molecular determinants of p50/DM required for oligomerization and binding to dynactin components are contained within the same regions in both human and chicken proteins.
N-terminal 1–91-Amino Acid Region of p50/DM Is Sufficient for Inhibition of Dynactin Functions
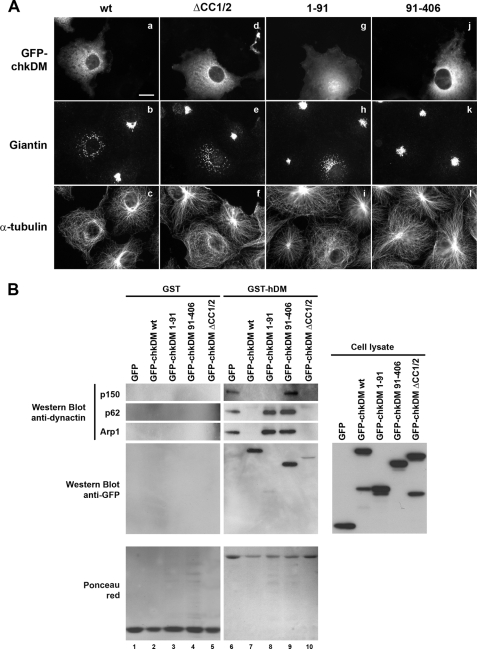
According to our results showing that overexpression of the chicken orthologue of p50/DM is more efficient than the human form in inhibiting dynactin cellular functions (see Fig. 2), and because both forms share the same determinants for self-association and binding to other dynactin components (see Fig. 6), we decided to use the chicken orthologue for further functional analysis of p50/DM molecular determinants. COS7 cells overexpressing the HA-tagged chkDM deletion mutants ΔCC1/2, 91–406, and 1–91 were thus analyzed for Golgi distribution and microtubule organization (Fig. 7A). Surprisingly, the oligomerization-deficient p50/DM ΔCC1/2 mutant (Fig. 7A, panels d–f) was still able to disrupt dynactin-based processes as efficiently as the wild type protein (panels a–c). This finding suggests that self-association of p50/DM does not participate in the p50/DM-induced inhibition of dynactin functions. Although the N-terminal 1–91-amino acid fragment of p50/DM was equally distributed between the cytoplasm and the nucleus (Fig. 7A, panel g), it induced both Golgi dispersal and microtubule release from centrosome (panels h and i). In contrast, deletion of this N-terminal region (HA-chkDM-(91–406)) abolished both phenotypes (Fig. 7A, panels j–l). Quantification revealed that the 1–91-amino acid fragment induced these phenotypes as efficiently as the full-length p50/DM (data not shown), indicating that the 1–91-amino acid fragment of p50/DM is sufficient to inhibit all dynactin-based processes.
FIGURE 7.
Impact of overexpression of chicken p50/DM deletion mutants on dynactin functions. A, immunofluorescence analysis. COS7 cells expressing the wild type (wt) or deleted forms of GFP-chkDM (panels a, d, g, and j) were stained with anti-Giantin (panels b, e, h, and k) and anti-α-tubulin mAbs (panels c, f, i, and l). Scale bar, 10 μm. B, in vitro pulldown assay. Lysates from 293T cells expressing GFP or the wild type or deleted forms of GFP-chkDM (right panel, Cell lysate) were incubated with equal amounts of GST or GST-hDM (lower panels, Ponceau red) immobilized on GSH-Sepharose beads. Bound proteins were analyzed by Western blot with anti-p150, -p62, -Arp1, and -GFP antibodies.
We next assessed whether disruption of the dynactin complex, as induced in cells by p50/DM overexpression, could also affect recruitment of dynactin components observed using the GST pulldown assay (see on Fig. 3B). From immunofluorescence experiments, we estimated that more than 60% of transfected cells efficiently overexpressed p50/DM (data not shown). Interestingly, overexpression of the chicken p50/DM correlated in vitro with a sharp decrease in the pulldown of both structural domains of dynactin by the recombinant GST-hDM (Fig. 7B, lane 7). Similar results were obtained by overexpression of the human p50/DM in this GST pulldown assay (data not shown). Of note, the overexpressed GFP-tagged chicken p50/DM could associate with the recombinant GST-hDM (Fig. 7B, lane 7, Western blot anti-GFP). Because these observations showed that the impairment of dynactin integrity and functionality induced in cells through p50/DM overexpression correlates in our in vitro system with the inhibition of dynactin binding to recombinant p50/DM, we investigated the contribution of the 1–91-amino acid and CC1/2 regions of the protein in this in vitro assay (Fig. 7B). In agreement with the absence of a functional defect of dynactin observed by immunofluorescence in cells overexpressing the GFP-chkDM-(91–406) mutant (see Fig. 7A), this mutant had no impact on dynactin recruitment on recombinant GST-hDM (Fig. 7B, lane 9). Intriguingly, overexpression of the chicken N-terminal 1–91-amino acid fragment did not completely abrogate dynactin binding, but rather it correlated with a selective recruitment of an intact Arp1 rod, with no evidence of p150Glued binding (Fig. 7B, lane 8), suggesting that the 1–91-amino acid mutant uses a distinct mechanism to inhibit dynactin functions in cells. As expected, overexpression of the ΔCC1/2 mutant of chkDM (Fig. 7B, lane 10) totally disrupted dynactin binding as efficiently as the wild type protein (lane 7), confirming that self-association of the protein does not contribute to the phenotype associated with p50/DM overexpression in cells.
Altogether, these results indicate that overexpression of the N-terminal 1–91-amino acid fragment of p50/DM is sufficient to recapitulate most of the phenotypes observed in vivo with the wild type protein, but the detailed mechanism underlying the inhibition of dynactin functions induced by the 1–91-amino acid fragment may differ from that of the wild type p50/DM.
Impact of Endogenous p50/DM Depletion on Dynactin Stability
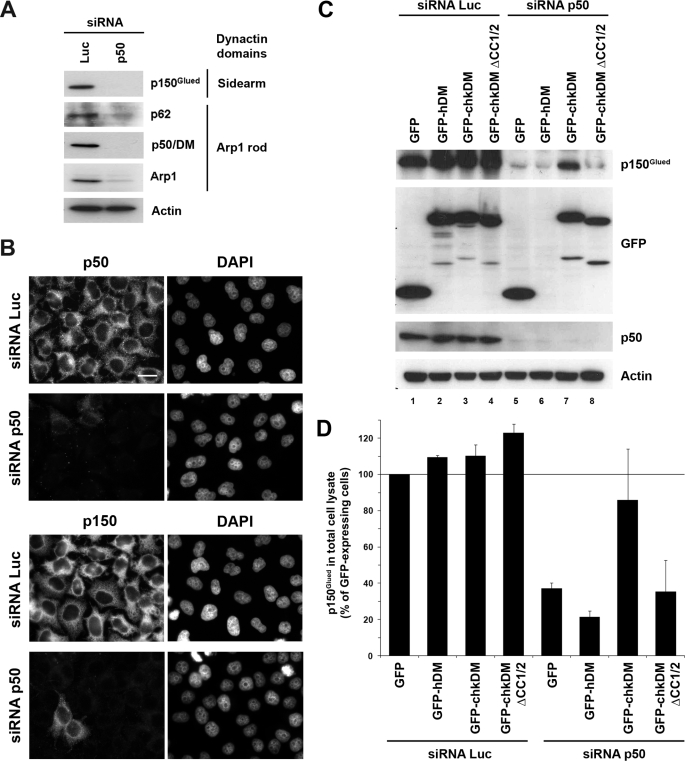
In view of the deleterious effect of p50/DM overexpression on dynactin functions in mammalian cells, we next analyzed the consequence of endogenous p50/DM depletion on the stability of the complex. Human HeLa cells were treated with an siRNA duplex targeting the human form of p50/DM, leading to a nearly complete extinction of p50/DM, monitored by immunoblot in cell lysates (Fig. 8A). Interestingly, immunoblotting with antibodies specific for the p150Glued sidearm or components of the Arp1 rod, namely p62 and Arp1, demonstrated that p50/DM depletion was accompanied by a dramatic reduction of all these subunits. These results show that depletion of the p50/DM subunit of the dynactin complex results in a down-regulation of the whole dynactin complex, indicating that this subunit plays a critical role in dynactin stability.
FIGURE 8.
Impact of p50/DM depletion on dynactin stability. A, HeLa cells treated with control siRNA (Luc) or siRNA targeting p50/DM were analyzed by Western blot (A) or by indirect immunofluorescence (B). A, lysates from siRNA-treated cells were analyzed using anti-p150Glued, -p62, -p50/DM, -Arp1, and -actin antibodies. B, siRNA-treated cells were stained with either anti-p50 (upper panels) or -p150 (lower panels) mAbs; nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (right panels). Scale bar, 10 μm. C and D, HeLa cells were treated with control siRNA (Luc) or siRNA targeting human p50/DM and then transfected with vectors for expression of the human or chicken wild type or ΔCC1/2 p50/DM GFP fusion proteins. Cells were lysed 48 h later for Western blot analysis using anti-p150Glued, -GFP, -p50/DM, and -actin antibodies. D, intensity of the p150Glued bands was quantified by densitometry using Images software (National Institutes of Health) from panels shown in C. p150Glued level was normalized to that of actin. The values represent the percentage of the p150Glued signal intensity relative to control cells treated with siRNA luciferase and complemented 48 h later with the GFP expression vector (lane 1, 100%); they correspond to the mean of three independent experiments, and error bars represent 1 S.D. from the mean.
Down-regulation of dynactin subunits in p50/DM-depleted cells was confirmed by immunofluorescence analysis (Fig. 8B). Specifically, siRNA-mediated depletion of p50/DM (Fig. 8B, top panels) led to a disappearance of the p150Glued (bottom panels) staining in more than 95% of cells treated with the p50 siRNA. Of note, residual amounts of both p50/DM and p150Glued could be detected at the centrosome and microtubules plus ends with longer exposure times (data not shown), suggesting that residual intact dynactin is present at sites where it is expected to function.
Self-association of p50/DM Contributes to Stabilization of the Dynactin Complex
Because the results reported in Figs. 4–6 indicated that self-association of p50/DM was not a prerequisite for interaction with other dynactin components and for disruption of the complex in vivo, we explored the contribution of this property to the stability of the dynactin complex in p50/DM-depleted cells. The strategy used was to express siRNA-insensitive chicken p50/DM in p50/DM-depleted human cells. The stability of the complex was then assayed by immunoblotting detection of the p150Glued subunit (Fig. 8C). As reported in Fig. 8A, depletion of endogenous p50/DM in HeLa cells led to a net reduction of the p150Glued subunit in depleted cells expressing the GFP control (Fig. 8C, lane 5). By contrast, expression of the wild type GFP-chkDM fusion in p50/DM-depleted cells resulted in a significant restoration of p150Glued expression (Fig. 8C, lane 7). Interestingly, the chicken p50/DM ΔCC1/2 mutant, deficient for self-association of the protein, failed to restore p150Glued expression in p50/DM-depleted cells (Fig. 8C, lane 8). Quantification of the p150Glued signal measured in three independent experiments is reported in Fig. 8D. These results suggest that the self-association property of p50/DM is important for the stability of the whole dynactin complex.
DISCUSSION
Although p50/DM emerged early as a central scaffolding subunit of the dynactin complex (8, 9, 15, 16, 22), little information was available concerning the molecular basis related to its contribution to dynactin assembly, stabilization, and functionality. We thus conducted a detailed analysis of the molecular determinants of p50/DM accounting for its interactions with other components of the complex. Our results show that the highly conserved N-terminal 1–91-amino acid region of the protein mediates initial binding to the Arp1 rod. This interaction is independent of the p150Glued and p24 subunits, because p50/DM binding to these subunits rely on other molecular determinants located in the central portion and the C terminus of the protein. We confirmed the functional relevance of these findings, showing that overexpression of the N-terminal 1–91-amino acid fragment of p50/DM in mammalian cells is necessary and sufficient to inhibit both dynein-dependent and dynein-independent functions of the dynactin complex. Interestingly, self-association of p50/DM most likely results from cooperation between molecular determinants contained in both CC1 and CC2 putative motifs, but it is not required for disruption of the complex. Finally, we provide evidence, through RNA interference-based experiments, that the expression of the dynactin complex is tightly regulated as a unique entity that is highly dependent on the expression level of its central p50/DM subunit. These latter experiments suggest that p50/DM oligomerization participates in the assembly and stabilization of newly synthesized complexes.
Previous attempts to analyze the structural role of p50/DM within the dynactin complex were based on sucrose gradient sedimentation analysis of samples in which an excess of recombinant p50/DM was incubated with purified dynactin complexes (23, 24). Although this approach recently allowed us to bring initial clues toward understanding of p50/DM self-association and molecular mechanisms involved in disruption of the dynactin complex (25), a detailed analysis of the molecular determinants involved in p50/DM interactions with other components of the complex was still missing. In this study, we describe a simple in vitro system allowing identification of the p50/DM determinants implicated both in the interaction with other dynactin components, mainly with the intact Arp1 rod, and in self-association of the protein. As expected, the full-length recombinant p50/DM was able to recruit the different subunits of the dynactin complex in this experimental system (Table 1). Our results suggest that the N-terminal 1–91-amino acid region of p50/DM acts as the initial binding site of the shoulder/sidearm at the surface of the Arp1 rod, whereas some other determinants contained in the central part of the protein may be required for optimal recruitment of the p150Glued sidearm. These determinants may allow optimal conformation of a flexible region of p150Glued at the level of the so-called “shoulder” of the dynactin complex (23, 31). In addition, the C-terminal half of p150Glued may also directly contact the Arp1 rod, as proposed in an earlier study (32).
TABLE 1.
Molecular determinants of p50/DM-Dynactin interactions identified in vitro and their impact on dynactin integrity and functionality in cells
ND means not determined.
| p50/DM species |
In vitro |
In vivo |
|||||
|---|---|---|---|---|---|---|---|
| Dynactin binding |
Oligomerization | Dynactin disruption | Inhibition of dynactin functions |
||||
| Arp1 rod | p150/sidearm | p24 | Golgi | MT anchorage | |||
| Full lengtha | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| 91–406a | − | − | +++ | +++ | − | − | − |
| 136–406 | − | − | +++ | + | ND | ND | ND |
| 252–406 | − | − | +/− | − | ND | ND | ND |
| 1–91a | +++ | +/− | − | − | +/− | +++ | +++ |
| 1–176 | +++ | +/− | − | − | ND | ND | ND |
| 1–280 | +++ | +++ | − | ND | ND | ND | ND |
| ΔCC1 | +++ | +++ | ++ | + | ND | ND | ND |
| ΔCC2 | +++ | +++ | ++ | + | ND | ND | ND |
| ΔCC1/2a | +++ | +++ | ++ | − | +++ | +++ | +++ |
a p50/DM mutants were selected for functional analysis in cells.
We found that p50/DM self-association resulted from cooperation between molecular determinants located in both CC1 and CC2 predicted motifs of the protein. These results partially corroborate those of Maier et al. (25), although these authors also identified a dimerization motif located within the first N-terminal 78 amino acids of the protein that would be required for initiation of p50/DM self-association. In our binding assays, no association of the full-length recombinant protein with the N-terminal 1–91-amino acid region could be detected. Of note, our data do not formally prove that the predicted coiled-coil conformation is required for self-association of p50/DM. Accordingly, recent observations indicated that introduction of proline residues in these putative motifs, likely leading to misfolding of the α-helices, did not abrogate p50/DM oligomerization (25). Interestingly, self-association of p50/DM does not account for binding to the Arp1 rod nor recruitment of other components of the shoulder/sidearm, namely p150Glued and p24, because the ΔCC1/2 oligomerization-deficient mutant of p50/DM is still able to bind to both structural domains of the dynactin complex (Table 1).
Relevant functional analyses of the determinants of p50/DM identified here shed light on the molecular mechanisms underlying the structural roles of this protein as a scaffolding subunit for the dynactin complex. As expected, our results show that p50/DM overexpression in COS7 cells inhibits dynein-based dynactin functions, investigated here through Golgi distribution, as well as dynein-independent processes, such as microtubule anchoring at the centrosome. Interestingly, our results indicate that the juxtanuclear localization of the Golgi apparatus is more sensitive to p50/DM overexpression than microtubule anchoring at the centrosome. This observation corroborates previous results showing that Golgi dispersal can occur independently of microtubule release from the centrosome (8), supporting the idea that Golgi dispersal rather results from a specific inhibition of dynein-dependent trafficking events. Furthermore, we show here that the chicken orthologue of p50/DM is a more sensitive tool to analyze dynactin-dependent processes, because both types of functional defects are exacerbated in cells overexpressing the chicken form. Our results demonstrate that the molecular determinants underlying p50/DM interactions with other dynactin components are conserved between both human and chicken orthologues. Taking advantage of this finding, we showed that the N-terminal 1–91-amino acid fragment of chicken p50/DM was necessary and sufficient to inhibit dynein-based dynactin functions but also dynein-independent processes. Thus, in contrast to the model initially proposed (9), our observations demonstrate that inhibition of dynactin functions upon overexpression of the N-terminal part of p50/DM is not related to a specific inhibition of the dynein motor, but rather it results from an alteration of the integrity of the dynactin complex. However, overexpression of the full-length p50/DM correlated with the inhibition of the recruitment of both structural domains of the dynactin complex by recombinant p50/DM, whereas the Arp1 rod was still recruited by p50/DM when the N-terminal 1–91-amino acid fragment was overexpressed. It is therefore clear that the molecular mechanism underlying inhibition of dynactin functions in cells upon overexpression of the 1–91-amino acid fragment is different from that induced by overexpressing the full-length protein. In view of these findings, we propose that dynactin disruption may involve initial binding, or at least transient interaction, of exogenous p50/DM to the Arp1 rod, through its conserved N-terminal domain, and that other molecular determinants located in the central portion of the protein destabilize attachment of the p150Glued, leading to its complete dissociation. This model correlates with previous reports (9, 25) showing that the N-terminal fragment of p50/DM was necessary but not sufficient to induce dynactin disruption, because additional determinants located in the central portion of the protein are required for remodeling of the shoulder/sidearm and its subsequent release from the Arp1 rod.
Finally, using siRNA-based experiments, we provide direct evidence that the endogenous p50/DM subunit of dynactin plays a major role in the overall stability of the complex, most likely by acting as a scaffolding protein. Accordingly, cell depletion of p50/DM leaves other subunits of the complex unstable, leading to the down-regulation of the whole dynactin complex. Only residual amounts of p150Glued, p62, and Arp1 were detected as free subunits in cells missing p50/DM. Although we cannot rule out that the regulation may take place at the transcriptional level, our data suggest that other dynactin subunits are unstable when p50/DM expression is decreased. Interestingly, it was recently shown that mutation or deletion of the Arp1 subunit also led to a concomitant decrease of the expression level of both p150Glued and p50/DM (12). This observation indicated that the Arp1 octamer also represents a crucial structural element of the dynactin complex. These findings clearly strengthen the idea that the stability of the whole dynactin complex is tightly regulated as a unique entity. Similar observations have been made for other cellular protein complexes, such as the clathrin-associated heterotetrameric adaptor protein complexes in which depletion of a single subunit was sufficient for destabilization of the remaining subunits (33–35).
In addition, our results showed that overexpression of the oligomerization-deficient ΔCC1/2 mutant of p50/DM, which still contains all other molecular determinants required for optimal binding to both dynactin domains, was able to recapitulate the phenotype observed both in vitro and in cells with the full-length protein (Table 1). Surprisingly, these observations indicate that dynactin disruption does not result from a binding of exogenous p50/DM to the endogenous protein, as proposed recently (23, 24). In contrast, we documented that oligomerization of p50/DM was required for stabilization of newly synthesized dynactin complexes in cells previously depleted from endogenous dynactin.
In summary, our study provides new insights into how the p50/DM subunit of the dynactin complex contributes to its stability and functionality. Because interactions between the p50/DM N-terminal moiety and some cellular proteins have been identified, it was proposed that this region of p50/DM could be exposed at the surface of the complex, although the C-terminal part would play a structural role inside the dynactin complex (7). Our study rather indicates that the N-terminal moiety of p50/DM contains the major molecular determinants involved in its interaction with other dynactin components of the complex but also determinants required for self-association of the protein. Indeed, we showed that the highly conserved N-terminal 1–91-amino acid portion of the p50/DM subunit mediates initial binding of the protein to the Arp1 rod. This interaction constitutes a preliminary requirement toward optimal recruitment of the extended p150Glued sidearm, which may rely on other molecular determinants located in the central portion of the protein. Unexpectedly, the interaction of p50/DM with p24, which in turn relies on both the central and the C-terminal regions of p50/DM, may not be required for binding to other dynactin subunits but rather for governing appropriate self-association of p50/DM, as proposed recently (25). In addition, oligomerization of p50/DM, which relies on both CC1 and CC2 putative motifs, does not seem to primarily contribute to interaction with other structural components of the dynactin complex but likely contributes to the assembly and stabilization of the endogenous complex.
Acknowledgments
We thank Florence Niedergang, Alexandre Benmerah, and Mark Scott for active support, discussion, and editing of the manuscript. We thank Ali Saïb for the kind gift of reagents.
This work was supported in part by INSERM, CNRS, Université Paris-Descartes, the French National Agency for AIDS Research, and Sidaction Fondation de France (to S. B.).
- DM
- dynamitin
- PBS
- phosphate-buffered saline
- HA
- hemagglutinin
- GST
- glutathione S-transferase
- GFP
- green fluorescent protein
- hDM
- human DM
- chkDM
- chicken DM
- siRNA
- small interfering RNA
- mAb
- monoclonal antibody
- pAb
- polyclonal antibody.
REFERENCES
- 1.Schroer T. A., Sheetz M. P. (1991) J. Cell Biol. 115, 1309–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gill S. R., Schroer T. A., Szilak I., Steuer E. R., Sheetz M. P., Cleveland D. W. (1991) J. Cell Biol. 115, 1639–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark S. W., Meyer D. I. (1994) J. Cell Biol. 127, 129–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harte P. J., Kankel D. R. (1982) Genetics 101, 477–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muhua L., Karpova T. S., Cooper J. A. (1994) Cell 78, 669–679 [DOI] [PubMed] [Google Scholar]
- 6.Plamann M., Minke P. F., Tinsley J. H., Bruno K. S. (1994) J. Cell Biol. 127, 139–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schroer T. A. (2004) Annu. Rev. Cell Dev. Biol. 20, 759–779 [DOI] [PubMed] [Google Scholar]
- 8.Burkhardt J. K., Echeverri C. J., Nilsson T., Vallee R. B. (1997) J. Cell Biol. 139, 469–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valetti C., Wetzel D. M., Schrader M., Hasbani M. J., Gill S. R., Kreis T. E., Schroer T. A. (1999) Mol. Biol. Cell 10, 4107–4120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Presley J. F., Cole N. B., Schroer T. A., Hirschberg K., Zaal K. J., Lippincott-Schwartz J. (1997) Nature 389, 81–85 [DOI] [PubMed] [Google Scholar]
- 11.King S. J., Schroer T. A. (2000) Nat. Cell Biol. 2, 20–24 [DOI] [PubMed] [Google Scholar]
- 12.Haghnia M., Cavalli V., Shah S. B., Schimmelpfeng K., Brusch R., Yang G., Herrera C., Pilling A., Goldstein L. S. (2007) Mol. Biol. Cell 18, 2081–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berezuk M. A., Schroer T. A. (2007) Traffic 8, 124–129 [DOI] [PubMed] [Google Scholar]
- 14.Deacon S. W., Serpinskaya A. S., Vaughan P. S., Lopez Fanarraga M., Vernos I., Vaughan K. T., Gelfand V. I. (2003) J. Cell Biol. 160, 297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quintyne N. J., Gill S. R., Eckley D. M., Crego C. L., Compton D. A., Schroer T. A. (1999) J. Cell Biol. 147, 321–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quintyne N. J., Schroer T. A. (2002) J. Cell Biol. 159, 245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lansbergen G., Komarova Y., Modesti M., Wyman C., Hoogenraad C. C., Goodson H. V., Lemaitre R. P., Drechsel D. N., van Munster E., Gadella T. W., Jr., Grosveld F., Galjart N., Borisy G. G., Akhmanova A. (2004) J. Cell Biol. 166, 1003–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akhmanova A., Hoogenraad C. C. (2005) Curr. Opin. Cell Biol. 17, 47–54 [DOI] [PubMed] [Google Scholar]
- 19.Coquelle F. M., Caspi M., Cordelières F. P., Dompierre J. P., Dujardin D. L., Koifman C., Martin P., Hoogenraad C. C., Akhmanova A., Galjart N., De Mey J. R., Reiner O. (2002) Mol. Cell. Biol. 22, 3089–3102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaughan P. S., Miura P., Henderson M., Byrne B., Vaughan K. T. (2002) J. Cell Biol. 158, 305–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watson P., Stephens D. J. (2006) J. Cell Sci. 119, 2758–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Echeverri C. J., Paschal B. M., Vaughan K. T., Vallee R. B. (1996) J. Cell Biol. 132, 617–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eckley D. M., Gill S. R., Melkonian K. A., Bingham J. B., Goodson H. V., Heuser J. E., Schroer T. A. (1999) J. Cell Biol. 147, 307–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melkonian K. A., Maier K. C., Godfrey J. E., Rodgers M., Schroer T. A. (2007) J. Biol. Chem. 282, 19355–19364 [DOI] [PubMed] [Google Scholar]
- 25.Maier K. C., Godfrey J. E., Echeverri C. J., Cheong F. K., Schroer T. A. (2008) Traffic 9, 11481–11491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Starr D. A., Williams B. C., Hays T. S., Goldberg M. L. (1998) J. Cell Biol. 142, 763–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoogenraad C. C., Akhmanova A., Howell S. A., Dortland B. R., De Zeeuw C. I., Willemsen R., Visser P., Grosveld F., Galjart N. (2001) EMBO J. 20, 4041–4054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uetake Y., Terada Y., Matuliene J., Kuriyama R. (2004) Cell Motil. Cytoskeleton 58, 53–66 [DOI] [PubMed] [Google Scholar]
- 29.Selig L., Pages J. C., Tanchou V., Prévéral S., Berlioz-Torrent C., Liu L. X., Erdtmann L., Darlix J., Benarous R., Benichou S. (1999) J. Virol. 73, 592–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacquot G., Le Rouzic E., David A., Mazzolini J., Bouchet J., Bouaziz S., Niedergang F., Pancino G., Benichou S. (2007) Retrovirology 4, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schafer D. A., Gill S. R., Cooper J. A., Heuser J. E., Schroer T. A. (1994) J. Cell Biol. 126, 403–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waterman-Storer C. M., Karki S., Holzbaur E. L. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 1634–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Motley A., Bright N. A., Seaman M. N., Robinson M. S. (2003) J. Cell Biol. 162, 909–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peden A. A., Rudge R. E., Lui W. W., Robinson M. S. (2002) J. Cell Biol. 156, 327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyer C., Zizioli D., Lausmann S., Eskelinen E. L., Hamann J., Saftig P., von Figura K., Schu P. (2000) EMBO J. 19, 2193–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yue L., Lu S., Garces J., Jin T., Li J. (2000) J. Biol. Chem. 275, 23948–23956 [DOI] [PubMed] [Google Scholar]