Abstract
The Rho GTPase members and their effector proteins, such as the Wiskott-Aldrich syndrome protein (WASP), play critical roles in regulating actin dynamics that affect cell motility, endocytosis, cell division, and transport. It is well established that Caenorhabditis elegans wsp-1 plays an essential role in embryonic development. We were interested in the role of the C. elegans protein WSP-1 in the adult nematode. In this report, we show that a deletion mutant of wsp-1 exhibits a strong sensitivity to the neuromuscular inhibitor aldicarb. Transgenic rescue experiments demonstrated that neuronal expression of WSP-1 rescued this phenotype and that it required a functional WSP-1 Cdc42/Rac interactive binding domain. WSP-1-GFP fusion protein was found localized presynaptically, immediately adjacent to the synaptic protein RAB-3. Strong genetic interactions with wsp-1 and other genes involved in different stages of synaptic transmission were observed as the wsp-1(gm324) mutation suppresses the aldicarb resistance seen in unc-13(e51), unc-11(e47), and snt-1 (md290) mutants. These results provide genetic and pharmacological evidence that WSP-1 plays an essential role to stabilize the actin cytoskeleton at the neuronal active zone of the neuromuscular junction to restrain synaptic vesicle release.
Keywords: C. elegans, C. elegans Genetics, Cytoskeleton, Neurobiology, Synapses
Introduction
The regulation of actin cytoskeleton plays important roles in the proper execution of a number of dynamic processes, such as cell motility, endocytosis, cell division, and transport (1). Assembly and disassembly of specific actin structures are under the control of phosphoinositides and the Rho GTPase family of small GTP-binding proteins (2). For example, the activation of Rac and Cdc-42 play roles in promoting the formation of lamellipodia and filopodia, whereas Rho activation causes stress fiber formation (3). In the case of Cdc-42, filopodia formation is stimulated upon activation of this GTPase through the effector protein Wiskott-Aldrich syndrome protein (WASP).2
The human WASP family consists of WASP, N-WASP, and three Scar/WAVE molecules where WASP is expressed solely in hematopoietic cells, whereas N-WASP and Scar/WAVE are expressed more generally (4). Members of the WASP family are multidomain actin nucleating-promoting factors and activate the actin-related protein 2 and 3 (Arp2/3) complex at the correct time and place (5). The ability for proteins such as WASP and WAVE to activate Arp2/3 is tightly controlled by a combination of many signaling complexes such as binding of activated Cdc42 to the Cdc42/Rac interactive binding (CRIB) domain of WASP, phospholipid interactions, and Src homology 3 domain binding to proline-rich regions. Once activated, the Arp2/3 proteins enhance the cellular assembly of actin and modulate actin dynamics (5).
We were interested in determining the role of Caenorhabditis elegans WSP-1 at the neuromuscular junction. C. elegans has proven to be a useful model organism to study the genetics of synaptic function (6). To evaluate synaptic transmission in C. elegans, we used the acetylcholinesterase inhibitor aldicarb complemented with transgenic and mutational analysis, as done previously (7). In this report, we demonstrated that wsp-1 mutants rapidly became paralyzed in the presence of the aldicarb. Transgenic experiments showed that the activity of WSP-1 was required in neurons to rescue this phenotype, and mutational analysis showed that a functional CRIB domain in WSP-1 was required. We also show that wsp-1 demonstrates genetic interactions with other genes involved in synaptic transmission at the neuromuscular junction as the wsp-1(gm324) mutation suppressed the aldicarb resistance seen in unc-13(e51), unc-11(e47), and snt-1(md290) mutants and enhanced the aldicarb sensitivity of tom-1(ok285). Together, these results show that WSP-1 plays a role in neuromuscular synaptic regulation by maintaining the actin cytoskeletal network at the neuromuscular junction of the adult nematode to prevent aberrant release of synaptic vesicles.
EXPERIMENTAL PROCEDURES
C. elegans Strains
Routine growth and maintenance of C. elegans were done as described previously (8). Mutants and alleles used in this study were: tom-1(ok285) I, unc-13(e51) I, unc-11(e47) I, snt-1(md290) II, and wsp-1(gm324) IV. Double mutants of wsp-1 animals were generated using standard techniques. Strains generated for this study: YF78 [unc-13(e51); wsp-1(gm324)], YF79 [unc-11(e47); wsp-1(gm324)], YF80 [snt-1(md290); wsp-1(gm324)], YF81 [tom-1(ok285); wsp-1(gm324)], and YF82 [wsp-1(gm324); jsIs219[pSY3[sng-1::GFP], pRF4[rol-6(su1006)]]. The transgenic worms created were: YF74 [wsp-1(gm324); tqEx36], YF75 [wsp-1(gm324); tqEx39], YF78 [wsp-1(gm324); tqEx37], YF83 [wsp-1(gm324); tqEx38], YF84 [wsp-1(gm324); tqEx41], and YF85 [tqEx42]. All mutants were identified by either their visible phenotypes (e.g. unc) or by single worm PCR for the deletion mutants.
Construction of Transgenic Constructs
For somatic expression of WSP-1, full-length wsp-1 cDNA was amplified from expressed sequence tag yk576d2, and the PCR product was introduced into vector pPD103.05 at the XmaI site to generate the plasmid pJZ2. This plasmid was mixed at a concentration of 25 μg/ml with 25 μg/ml pTG96_1 plasmid (9) and microinjected into N2 worms to make tqEx36. For muscle-specific expression of WSP-1, the full-length wsp-1 cDNA was amplified, and the PCR product was introduced into vector pPD95.86 at the SalI site to generate the plasmid pJZ3. This plasmid was mixed at a concentration of 25 μg/ml with 25 μg/ml GFP containing co-injection marker pAC12 (10) to select for lines with the array expressed in body muscles (tqEx37). For neuronal expression of WSP-1, the full-length wsp-1 cDNA was amplified and introduced into the vector pJH1045 at the BamHI and KpnI sites to generate the plasmid pJZ4. This plasmid was mixed at a concentration of 25 μg/ml with 25 μg/ml Psur-5::mCherry plasmid to select for lines with the array expressed in neurons (tqEx39). For hypodermal expression of WSP-1, the full-length wsp-1 cDNA was introduced into vector containing a synthetic intron and the promoter of dpy-7 (11) in pSL301 at the BamHI site to generate the plasmid pJZ5. This plasmid was mixed at a concentration of 25 μg/ml with 25 μg/ml GFP containing co-injection of a Pdpy-7::Venus construct to select for lines with the array expressed in hypodermal cells (tqEx40). For the transgene containing the CRIB H266D mutation (tqEx41), site-directed mutagenesis was carried out using the QuikChange method, and the mutated wsp-1 cDNA was amplified and introduced into the vector pPD103.05 at the XmaI site to generate the plasmid pJZ6. This plasmid was mixed at a concentration of 25 μg/ml with 25 μg/ml PF25B3.3::GFP plasmid to select for lines with the array expressed in neurons. The In-Fusion Dry down PCR cloning kit from Clontech was used for subcloning. To visualize co-localization of WSP-1 with the synaptic protein RAB-3, the tqEx42 transgene was made by mixing 25 μg/ml plasmid pJZ4 and 25 μg/ml Pmig-13::mCherry::rab-3 (12).
Drug Sensitivity Assays
Aldicarb was purchased from CHEM Service. Fifteen to 30 synchronized adult worms were put to the aldicarb plates and left at room temperature for 2–3 h depending on the sensitivity of the test strain. The worms were assayed for paralysis, which was defined as absence of movement when prodded three times with a platinum wire on the head (13). For transgenic rescue experiments, one plate was used for all the strains being assayed at one time to minimize the variation that may occur between aldicarb-containing plates.
Protein Mixing Protocol
pMal-C2 and pMal-CeWSP-1 CRIB domain wild-type and H266D mutation were transformed into Escherichia coli BL21(DE3) and induced with isopropyl 1-thio-β-d-galactopyranoside. After a 3-h induction, pelleted cells were resuspended in lysis buffer consisting of 20 mm Tris, 150 mm NaCl, 10% glycerol, 1 mm dithiothreitol, 0.1% Nonidet P-40, and protease inhibitors mixture. The cells were lysed by sonication, and 20 μl of amylose resin (New England Biolabs) was added to the lysate followed by a 1-h incubation at 4 °C, and the beads were then washed three times with the lysis buffer. For CDC-42 production, 2 μg of vector for mammalian expression of Myc-CDC-42 was transfected into HEK293T cells using polyethylenimine (Sigma). After 72 h, the cells were harvested and lysed in 0.75 ml of HNTG lysis buffer (20 mm Hepes (pH 7.5), 150 mm NaCl, 1% Nonidet P-40, 5% glycerol, 2 mm MgCl2, proteinase inhibitor mixture, and GTPγS). The supernatant was then incubated for 1 h with the beads containing maltose-binding protein (MBP), MBP-WASP CRIB domain wild-type, and H266D mutation at 4 °C, washed for five times, and then boiled in 2 × SDS-polyacrylamide gel loading buffer. SDS-PAGE and membrane transfer was performed with the Bio-Rad mini gel system and semidry apparatus. After transfer, the membrane was blocked for 1 h in 5% milk in TBST (10 mm Tris-HCl, pH 7.5, 150 mm NaCl, 0.05% Tween-20), probed with antibody 9E10 or anti-MBP antibody (New England Biolabs) followed by goat anti-mouse horseradish peroxidase, and was developed with Super Signal West Pico Chemiluminescent Substrate (Thermo Scientific).
Statistical Analysis
To determine significant differences between strains exposed to aldicarb, a minimum of three repeated experiments (each individual experiment containing at least n = 20 for each strain) were subjected to a two-way repeated-measure analysis of variance comparing groups (different strains) over time. The level of p < 0.05 was considered to be the cutoff value for significance (14).
RESULTS
wsp-1 Mutant Displays Aldicarb Sensitivity
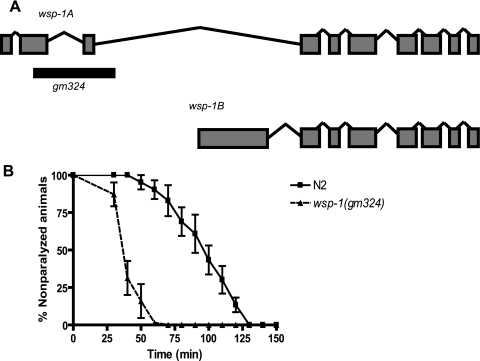
We set out to investigate the postdevelopmental signaling function of WSP-1 in C. elegans. The N-WASP homolog in C. elegans (wsp-1) was previously identified and corresponds to transcripts C07G1.4a and C07G1.4b. It generates two splicing variants that produce transcripts of 2.1 kb (isoform A) and 3.5 kb (isoform B). Isoform A is predicted to produce a protein of 608 amino acids and contains the WASP homology 1 (WH1) domain, IQ motif, CRIB binding motif, and two WASP homology 2 (WH2) domains. Isoform B is a protein consisting of 781 amino acids that has the same C-terminal region of isoform A but differs in its N-terminal region such that the WASP homology 1 domain and IQ motifs are replaced with amino acids that have no homology to any known protein. We obtained from the CGC a strain that contains a homozygous deletion that removes 1892 bp from the genomic sequence of wsp-1. This deletion targets the second and third exons of the gene, removing the WH1 and IQ motifs, and would be predicted to affect only the major isoform A (Fig. 1A).
FIGURE 1.
Schematic structure C. elegans wsp-1 in wild-type and deletion mutant. A, the wsp-1 gene is predicted to produce two isoforms, WSP-1A and WSP-1B. The gm324 allele deletes a portion of the second and third exons, which would affect the WSP-1A isoform only. The dark bar represents the genomic region deleted. B, wsp-1(gm324) deletion increases the aldicarb sensitivity of C. elegans. Synchronized 1-day-old adult wsp-1(gm324) (total n = 96) and wild-type N2 nematodes (n = 102) were placed on plates containing 1 mm aldicarb and assayed for paralysis over time (minutes). The wsp-1(gm324) nematodes are more sensitive to aldicarb because 50% of these nematodes were paralyzed at 37.1 min, respectively, compared with 78.0 min for wild type (p = 0.0023).
We used aldicarb-induced paralysis to evaluate the synaptic transmission in C. elegans in wsp-1 mutant worms. Aldicarb is a chemical analog of the neurotransmitter acetylcholine and prevents efficient breakdown of acetylcholine, leading to acetylcholine accumulation at synaptic clefts, overactivation of muscular acetylcholine receptors, muscle hypercontraction, and paralysis (15). A mutant with a defect in acetylcholine synaptic transmission demonstrates either resistance or sensitivity to aldicarb compared with wild type. We placed 1-day-old adult homozygous wsp-1(gm324) mutants and N2 wild-type worms on plates containing 1 mm aldicarb and assayed for paralysis over time. As shown in Fig. 1B, wsp-1 mutant worms demonstrated significant sensitivity (p = 0.0023) to aldicarb because 50% were paralyzed at 37.1 min compared with 78.0 min for the wild-type worms. Therefore, wsp-1 mutation results in higher sensitivity of this strain to aldicarb, suggesting that WSP-1 plays a role in acetylcholine synaptic transmission.
CRIB Domain of wsp-1 Is Required to Rescue the Aldicarb Hypersensitivity Phenotype of wsp-1(gm324) Mutant
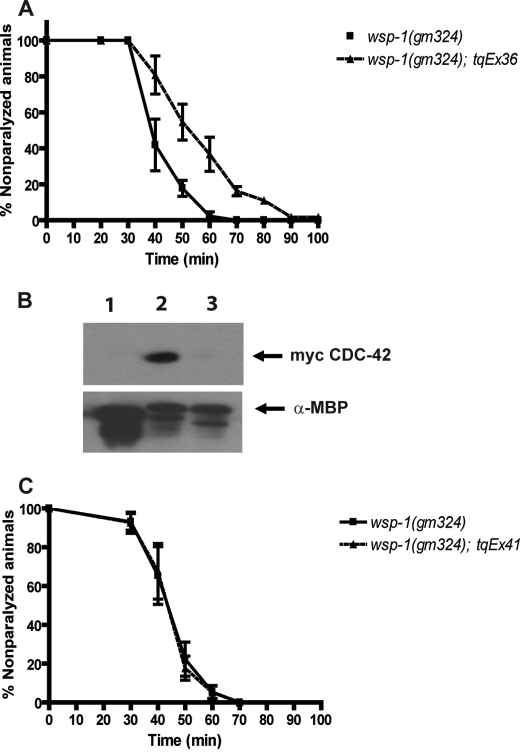
To demonstrate that the aldicarb sensitivity phenotype of wsp-1 mutant worms was due to the deletion in the wsp-1 gene and not due to a secondary, unidentified mutation in the genomic background, we carried out rescue experiments using transgenic constructs of wsp-1. A transgene capable of expressing in all somatic cells was made, injected, and crossed into wsp-1 mutant animals. Transgenic wsp-1 mutant worms that expressed WSP-1 in somatic cells were significantly (p = 0.02) more resistant to aldicarb than the mutant worms, as 50% of the transgenic strain was paralyzed at 52.5 min compared with 50% of the wsp-1(gm324) worms that were paralyzed at 39.1 min (Fig. 2A). Therefore, we conclude that there was rescue and that the deletion in the wsp-1 gene is responsible for the aldicarb sensitivity phenotype.
FIGURE 2.
Somatic cell expression of WSP-1 rescues the aldicarb sensitivity phenotype of wsp-1(gm324) and requires an intact CRIB domain. A, graph of synchronized 1-day-old adult wsp-1(gm324) and wsp-1(gm324); tqEx36 nematodes were placed on plates containing 1 mm aldicarb and assayed for paralysis over time (minutes). The wsp-1(gm324) nematodes were more sensitive to aldicarb because 50% of these nematodes were paralyzed at 39.1 min compared with 52.5 min for wsp-1(gm324); tqEx36 nematodes (p = 0.0184). B, WSP-1 CRIB interacts with CDC-42, but the CRIB H266D mutation does not. Western blot of protein-mixing experiment of recombinant WSP-1 protein bound to amylose beads and mixed with myc-CDC-42 containing HEK293 cell lysates is shown. Lane 1, MBP control protein; lane 2, MBP-WSP-1 CRIB domain; lane 3, MBP-WSP-1 CRIB (H266D). Upper panel, Western blot probed with antibody 9E10 (α-myc). Lower panel, Western blot probed with anti-MBP. C, expression of WSP-1 (H266D) in somatic cells does not rescue the aldicarb sensitivity phenotype of wsp-1(gm324). Synchronized 1-day-old adult wsp-1(gm324) (n = 73) and wsp-1(gm324); tqEx41 (somatic cell expression of wsp-1 (H266D) transgene) nematodes (n = 78) were placed on plates containing 1 mm aldicarb and assayed for paralysis over time (minutes) as described. The wsp-1(gm324) nematodes have the same level of sensitivity to aldicarb (no significant difference) because 50% of these nematodes were paralyzed at 43.4 min compared with 43.2 min for wsp-1(gm324); tqEx41 nematodes.
Activated mammalian Cdc42 and Rac1 interact with N-WASP through the CRIB motif (16). We wanted to determine whether this interaction is conserved between the C. elegans orthologs CDC-42 and WSP-1. We fused the WSP-1 CRIB domain with MBP, expressed it, purified it with amylose resin, and then mixed it with HEK293T lysate that contained transfected myc-tagged wild-type CDC-42. We saw a strong and specific interaction between the WSP-1 CRIB domain and CDC-42 (Fig. 2B), whereas no interaction was seen with MBP alone, indicating that the interaction between CDC-42 and WSP-1 is conserved between mammals and C. elegans.
Next, we asked whether the rescue of wsp-1 aldicarb sensitivity phenotype required an intact CRIB domain. We made a mutation in the WSP-1 CRIB domain (H266D) and expressed the mutated CRIB domain in bacteria and carried out a protein-mixing experiment with lysate of HEK293T cells transfected with wild-type myc-tagged CDC-42 as described above. We found that the H266D mutation in the CRIB domain abolished its interaction with CDC-42 in vitro (Fig. 2B).
Subsequently, we made a WSP-1 transgene that contained the H266D mutation. The transgene was designed for expression in somatic cells of nematodes, for which the wild-type WSP-1 expression in the wsp-1 mutant background was capable of rescuing. We generated transgenic animals and performed the aldicarb assay with this strain. Expression of the WSP-1 protein carrying the H266D mutation was unable to rescue the aldicarb sensitivity of the wsp-1 mutant worms because 50% of the transgenic worms were paralyzed at 43.2 min, similar to 43.4 min of the wsp-1 mutant worms (Fig. 2C). Therefore, the CRIB domain on WSP-1 capable of interacting with CDC-42 was required for proper function of WSP-1 at the neuromuscular junction.
Tissue-specific Rescue of the wsp-1 Mutant
To determine the site of function within the nematode that is responsible for the aldicarb sensitivity phenotype, we created transgenes that expressed WSP-1 in specific tissues. We considered the possibility that there could be different tissues responsible for the aldicarb sensitivity phenotype. The first could be that wsp-1 is required in nerve cells and has a presynaptic role in neuromuscular function. The second alternative could be that WSP-1 is required in muscle cells, and perhaps the aldicarb hypersensitivity phenotype was due to the absence of wsp-1 in postsynaptic tissues. Finally, we also considered the possibility that wsp-1 plays an important role in hypodermal cells, which are responsible for forming the cuticle surrounding the worm. It is possible that if the wsp-1 worms have a weaker cuticle than normal, it would result in higher concentrations of aldicarb at the neuromuscular junction, resulting in the aldicarb sensitivity phenotype.
For specific expression of wsp-1 in these three tissues, the wsp-1 cDNA was subcloned into vectors downstream of established tissue specific promoters. These constructs were co-injected with plasmids coding for specific GFP expression patterns to ensure that the transgene was expressed in the desired tissues. The transgenes were crossed into wsp-1 mutant worms, and they were tested for rescue of the aldicarb sensitivity phenotype.
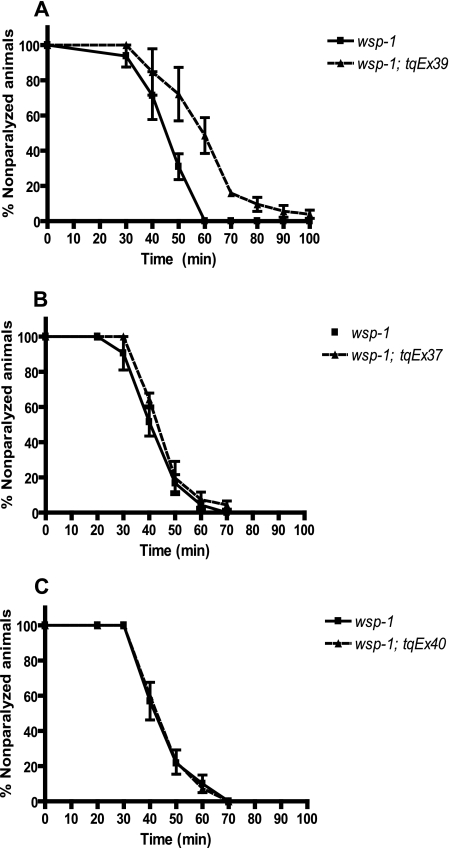
We found that only the neuronal specific expression of WSP-1 was capable of rescuing the aldicarb-sensitive phenotype, as 50% of these worms were paralyzed at 57.7 min compared with 44.6 min of the wsp-1 mutant worms (p = 0.004), whereas the transgenic expression of WSP-1 in muscle or in hypodermal cells individually was not able to rescue the aldicarb phenotype (Fig. 3). Therefore, neuronal expression of WSP-1 is required to rescue (at least partially) the defect at the neuromuscular junction present in wsp-1 mutant worms, although these experiments do not rule out that WSP-1 may play a role postsynaptically in muscle or in hypodermal cells that could contribute to the aldicarb sensitivity phenotype.
FIGURE 3.
Expression of WSP-1 in neurons, but not muscle or hypodermal cells, rescues aldicarb sensitivity phenotype of wsp-1(gm324) mutant worms. Transgenic worms expressing the wsp-1 gene in neurons (tqEx39), body wall muscles (tqEx37), and hypodermal cells (tqEx40) in the wsp-1(gm324) background assayed for their sensitivity to paraquat. Synchronized 1-day-old adult worms were placed on plates containing 1 mm aldicarb and assayed for paralysis over time (minutes). The neuronal transgene expressing WSP-1 (tqEx39) significantly (p = 0.0037, n = 81) rescued the wsp-1(gm324) nematodes (n = 80) because 50% of these nematode were paralyzed at 57.7 min compared with 44.6 min for wsp-1(gm324). The muscle and hypodermal transgenes did not rescue the aldicarb sensitivity of the wsp-1 mutant worms because 50% of the wsp-1(gm324); tqEx37 nematodes (n = 63) were paralyzed at 43.6 min, and the wsp-1(gm324); tqEx40 nematodes (n = 60) were paralyzed at 41.8 min.
WSP-1 Localizes to the Neuromuscular Junction
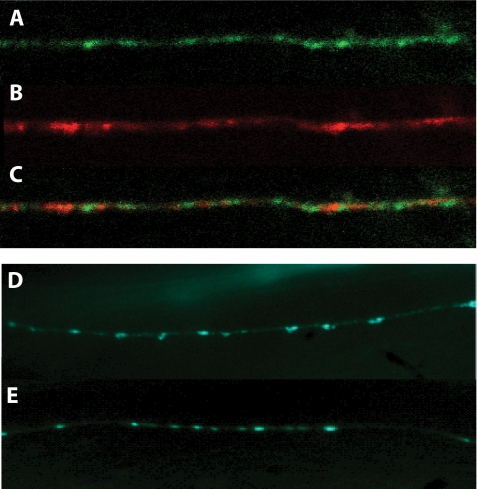
Because rescue of the wsp-1 aldicarb sensitivity occurs with neuronal expression of WSP-1, we were interested in determining the subcellular localization of WSP-1. For this, we used the neuronal wsp-1-expressing transgene that has GFP fused to the coding region of WSP-1. When this strain was examined, GFP expression was observed diffused in the nerve cords but was also enriched in discrete punctate structures along the ventral nerve cord (Fig. 4A). We compared the localization of WSP-1-GFP with the synaptic vesicle protein RAB-3 tagged with the mCherry fluorescent protein (17). We found that WSP-1 localized to regions immediately adjacent to RAB-3 puncta (Fig. 4, A–C). This region of WSP-1 localization corresponds to periactive zone localization, which is the F-actin-rich region that regulates synaptic growth and plasticity (18). Therefore, WSP-1-GFP fusion protein localizes to the presynaptic region of the neuromuscular junction.
FIGURE 4.
WSP-1 is localized at the presynaptic terminal in neuronal cells, and wsp-1 mutants exhibit normal synaptogenesis. A, representative image of the ventral cord of wild-type animals containing the tqEx42 transgene mounted on agarose pads in 2 mm levamisole and viewed with an Olympus BX61 confocal microscope. WSP-1-GFP localization is in the ventral nerve cord. B, RAB-3::mCherry localization in the same worm. C, localization of both GFP and mCherry fusion proteins shows that WSP-1 resides immediately adjacent to RAB-3 containing puncta. D and E, wsp-1(gm324) display normal synaptogenesis. For visualizing the synaptic specializations of wsp-1 mutant, we used the expression of SNG-1-GFP in wild-type (E) and wsp-1(gm324) (D) nematodes. The wsp-1 mutant carrying the SNG-1-GFP marker has the similar synapses pattern compared with the wild-type worm carrying the SNG-GFP marker.
One possibility for the aldicarb sensitivity of the wsp-1 mutant nematodes is that phenotype may be due to improper synaptogenesis. One commonly used synaptic marker is the C. elegans ortholog of synaptogyrin, SNG-1. We examined the synapse of wsp-1 mutants by introducing the SNG-1-GFP transgene into wsp-1(gm324) and checked the number and strength of puncta. We observed that there were no gross abnormalities detected between wild-type worms carrying the SNG-1-GFP fusion marker and the wsp-1 mutants in terms of number of synapses, shape, or intensity of puncta (Fig. 4, D and E). Therefore, the wsp-1 mutants worms are not defective in synaptogenesis.
wsp-1 Mutant Genetically Interacts with Known C. elegans Genes Involved in Synaptic Vesicle Release and Recycling
There are many C. elegans proteins involved with synaptic function, such as unc-13, unc-11, snt-1, and tom-1 (6, 7). The proteins produced from these genes are involved in different steps of the synaptic vesicle cycle. UNC-13 regulates neurotransmitter release by altering the conformation of syntaxin and is required for the priming of synaptic vesicles (19). UNC-11 encodes for multiple isoforms of the clathrin adaptor protein AP180 and functions in clathrin-mediated endocytosis (20), whereas SNT-1 encodes for the ortholog of the synaptic vesicle protein synaptotagmin, which regulate both synaptic vesicle endocytosis and exocytosis (21). TOM-1 is a syntaxin-binding partner involved in regulating exocytosis and can inhibit the release of neurotransmitter and neuropeptide (7).
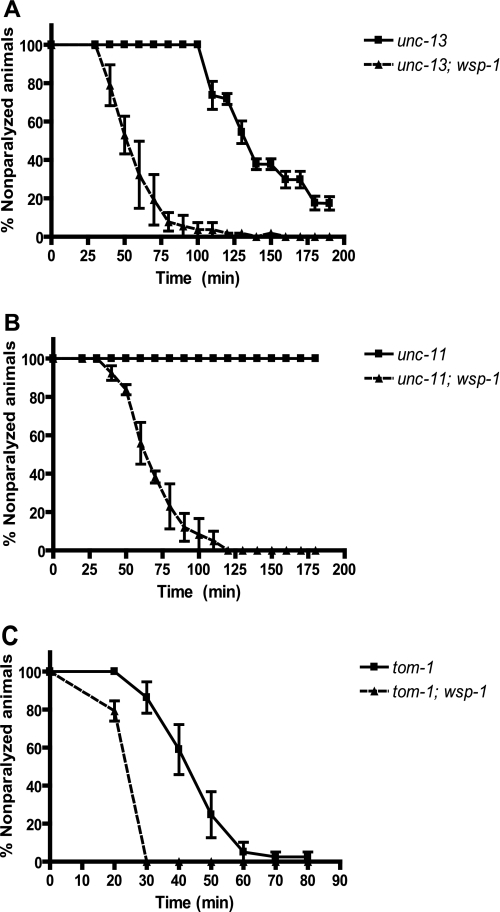
We created double mutant worms containing these genes with the wsp-1 deletion allele and tested the aldicarb sensitivity of the double mutants. We found that the double mutants all showed increased aldicarb sensitivity compared with the single mutants that do not contain the wsp-1 mutation. As demonstrated in Fig. 5A, 50% of the unc-13(e51); wsp-1(gm324) double mutant worms were paralyzed at 51.4 min whereas 50% unc-13(e51) worms were resistant to aldicarb and paralyzed at 125.9 min, showing significant rescue of the unc-13-resistant phenotype by wsp-1 mutation (p = 0.0005). In Fig. 5B, 50% of the unc-11(e47); wsp-1(gm324) double mutants were paralyzed at 64.1 min whereas the unc-11(e47) single mutant worms were completely resistant to aldicarb (p < 0.0001). Also, 50% of the snt-1(md290); wsp-1(gm324) double mutant worms were paralyzed at 60.6 min whereas 50% of the snt-1(md290) single mutant worms were paralyzed at 95.2 min (p = 0.001; data not shown). Therefore, wsp-1 is epistatic to unc-11, unc-13, and snt-1. This is reminiscent of tom-1, which is also epistatic to unc-13 and is a gene involved in inhibiting dense core vesicle fusion (17). Furthermore, tom-1(ok285) mutant worms were shown previously to exhibit sensitivity to aldicarb (7). When we looked at the tom-1(ok285); wsp-1(gm324) double mutant, 50% of the worms were paralyzed at 21.1 min whereas 50% of the tom-1 single mutant worms were paralyzed at 42.0 min (p = 0.0062; Fig. 5C), showing that the wsp-1 mutation enhances the aldicarb sensitivity of tom-1. Therefore, in the double mutants, the wsp-1 hypersensitivity phenotype is expressed with respect to the phenotypes of other mutations, regardless of which step of the synaptic cycling pathway was otherwise affected.
FIGURE 5.
wsp-1 deletion in unc-13, unc-11, and tom-1 mutants increases their the aldicarb sensitivity. Synchronized 1-day-old adult unc-13(e51) mutant and unc-13(e51); wsp-1(gm324) double mutant (A), unc-11(e47) mutant and unc-11(e47); wsp-1(gm324) double mutant (B), and tom-1(ok285) mutant and tom-1(ok285); wsp-1(gm324) double mutant (C) were placed on plates containing 1 mm aldicarb and assayed for paralysis over time (minutes). The unc-13(e51); wsp-1(gm324) double mutant nematodes (n = 62) had an increased aldicarb sensitivity because 50% of these nematodes were paralyzed at 51.4 min compared with 125.9 min for the unc13 (e51) single mutant nematodes (p = 0.0005, n = 63). The unc-11(e47); wsp-1(gm324) double mutant nematodes (n = 60) have increased aldicarb sensitivity because 50% of these nematodes were paralyzed at 64.1 min whereas unc-11(e47) single mutant nematodes were resistant to aldicarb (p < 0.0001, n = 67). The tom-1(ok285); wsp-1(gm324) double mutant nematodes (n = 58) have an increased sensitivity because 50% of the worms were paralyzed at 21.1 min compared with 42.0 for the tom-1(ok285) single mutant nematodes (p = 0.0062, n = 62).
DISCUSSION
In C. elegans, previous studies of the N-WASP homolog (WSP-1) homozygous mutant worm NG324 show slight neuronal migration and axon outgrowth defects (22, 23). In this study, we demonstrated that the C. elegans wsp-1 mutant showed strong aldicarb sensitivity that was probably due to excess cholinergic neurotransmission. This mutation was rescued with transgenic expression of WSP-1 in neuronal cells, indicating that the altered synaptic transmission in wsp-1 mutant worms originates from a defect in the nervous system. Furthermore, WSP-1 containing a mutation in its CRIB domain did not bind to activated CDC-42, whereas the wild-type CRIB domain could. This experiment was fashioned after a series of experiments that has been used extensively in the studies of CDC-42 regulation of WASP in mammalian cells and Drosophila (16, 24, 25) and demonstrated that the nature of the physical association between activated CDC-42 and WASP is conserved in C. elegans. Furthermore, we observed that the transgene to express this mutant form of WSP-1 did not rescue the aldicarb sensitivity of the wsp-1(gm324) mutant, indicating that an intact CRIB domain of WSP-1 is required to maintain the normal synaptic function.
We also examined the genetic interactions between wsp-1 and known regulators of synaptic function, such as unc-13, unc-11, snt-1, and tom-1, and showed that wsp-1 rescued the aldicarb resistance phenotype of unc-13, unc-11, and snt-1 and further enhanced the aldicarb sensitivity of tom-1, indicating that wsp-1 is epistatic to C. elegans genes involved in multiple steps of synaptic transmission, such as vesicle priming, exocytosis, and endocytosis (6, 7). These results suggest that WSP-1 plays a general role at the neuromuscular junction and not a specific role in any of these individual cellular processes.
The excess in cholinergic transmission seen with wsp-1 mutants is similar to a number of previous studies that demonstrated that treatment of actin-depolymerizing agents on neuronal cell culture promotes exocytosis of synaptic vesicles (26, 27, 28). Actin is present as a dense network that requires remodeling during exocytosis, most notably a local disassembly that permits synaptic vesicles to gain access to the plasma membrane for docking and fusion, leading to the hypothesis that the cortical actin network is a physical barrier that prevents neurotransmitter release (29). However, actin is also believed to play a positive role in propulsion of exocytotic vesicles, with a number of studies demonstrating decreased secretion in presence of high concentrations of actin-depolymerizing agents, indicating that at least a minimal amount of actin structure is required (30).
Mammalian N-WASP is a well established regulator of actin structure, and upon activation by Cdc42, it activates the Arp2/3 complex to nucleate actin polymerization (18). Previous reports showed that overexpression of N-WASP in PC12 cells produced a stimulatory effect on secretion and that the stimulatory effect was dependent on its ability to induce actin polymerization (31). Therefore, based on previous results that WASP is an actin cytoskeleton modulator and our genetic and pharmacological evidence presented here, we propose that C. elegans WSP-1 is required for the proper formation of the actin cytoskeleton at the neuromuscular junction that allows it to restrain fusion of synaptic vesicles at the active zone. The model we suggest is that the absence of WSP-1 causes the depolymerization of actin, probably through the disruption of the actin-nucleating Arp2/3 complex, to the extent that it relieves the actin “barrier” resulting in an increase in synaptic transmission, thus causing the wsp-1 mutant to be epistatic to a number of aldicarb resistant genes. Although WSP-1 appears to have a role in maintaining the actin cytoskeleton to prevent synaptic vesicle release, a positive role for WSP-1 for the propulsion of synaptic vesicles cannot be ruled out and remains to be tested.
In conclusion, we have shown that mutants of wsp-1 had a high sensitivity to aldicarb because of the absence of WSP-1 in neurons, demonstrated a localization of WSP-1 at the presynaptic termini of the neuromuscular junction, and showed that wsp-1 is epistatic to other genes involved in multiple steps of the synaptic vesicle cycling pathway. These results show that WSP-1 plays an essential role regulating neuromuscular activity by stabilizing the actin cytoskeleton at the nerve ending in the adult nematode. Further studies are required to determine in more detail how WSP-1 regulates actin dynamics to coordinate vesicle targeting and exocytosis.
Acknowledgments
We thank Dr. Mei Zhen (Mount Sinai Hospital, Toronto, Canada) for the pJH1045 plasmid and the Caenorhabditis Genetics Center for worm strains.
This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada.
- WASP
- Wiskott-Aldrich syndrome protein
- CRIB
- Cdc42/Rac-interactive binding
- GFP
- green fluorescent protein
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- MBP
- maltose-binding protein.
REFERENCES
- 1.Ho H.-Y., Rohatgi R., Lebensohn A. M., Ma L., Li J., Gygi S. P., Kirschner M. W. (2004) Cell 118, 203–216 [DOI] [PubMed] [Google Scholar]
- 2.Etienne-Manneville S., Hall A. (2002) Nature 420, 629–635 [DOI] [PubMed] [Google Scholar]
- 3.Heasman S. J., Ridley A. J. (2008) Nat. Rev. Mol. Cell Biol. 9, 690–701 [DOI] [PubMed] [Google Scholar]
- 4.Ramesh N., Geha R. (2009) Immunol. Res. 44, 99–111 [DOI] [PubMed] [Google Scholar]
- 5.Soderling S. H. (2009) Sci. Signal. 2, pe5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richmond J. (2007) WormBook, doi/10.1895/wormbook.1.69.1 [Google Scholar]
- 7.Gracheva E. O., Burdina A. O., Holgado A. M., Berthelot-Grosjean M., Ackley B. D., Hadwiger G., Nonet M. L., Weimer R. M., Richmond J. E. (2006) PLos Biol. 4, e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brenner S. (1974) Genetics 77, 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu T., Orita S., Han M. (1998) Mol. Cell. Biol. 18, 4556–4564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colavita A., Krishna S., Zheng H., Padgett R. W., Culotti J. G. (1998) Science 281, 706–709 [DOI] [PubMed] [Google Scholar]
- 11.Johnstone I. L., Barry J. D. (1996) EMBO J. 15, 3633–3639 [PMC free article] [PubMed] [Google Scholar]
- 12.Klassen M. P., Shen K. (2007) Cell 130, 704–716 [DOI] [PubMed] [Google Scholar]
- 13.Locke C., Berry K., Kautu B., Lee K., Caldwell K., Caldwell G. (2008) J. Vis. Exp. 18, 837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Linden A. M., Simmer F., Cuppen E., Plasterk R. H. A. (2001) Genetics 158, 221–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahoney T. R., Luo S., Nonet M. L. (2006) Nat. Protoc. 1, 1772–1777 [DOI] [PubMed] [Google Scholar]
- 16.Miki H., Sasaki T., Takai Y., Takenawa T. (1998) Nature 391, 93–96 [DOI] [PubMed] [Google Scholar]
- 17.Gracheva E. O., Burdina A. O., Touroutine D., Berthelot-Grosjean M., Parekh H., Richmond J. E. (2007) J. Physiol. 585, 705–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sieburth D., Ch'ng Q., Dybbs M., Tavazoie M., Kennedy S., Wang D., Dupuy D., Rual J.-F., Hill D. E., Vidal M., Ruvkun G., Kaplan J. M. (2005) Nature 436, 510–517 [DOI] [PubMed] [Google Scholar]
- 19.Richmond J. E., Davis W. S., Jorgensen E. M. (1999) Nat. Neurosci. 2, 959–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nonet M. L., Holgado A. M., Brewer F., Serpe C. J., Norbeck B. A., Holleran J., Wei L., Hartwieg E., Jorgensen E. M., Alfonso A. (1999) Mol. Biol. Cell 10, 2343–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jorgensen E. M., Hartwieg E., Schuske K., Nonet M. L., Jin Y., Horvitz H. R. (1995) Nature 378, 196–199 [DOI] [PubMed] [Google Scholar]
- 22.Withee J., Galligan B., Hawkins N., Garriga G. (2004) Genetics 167, 1165–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shakir M. A., Jiang K., Struckhoff E. C., Demarco R. S., Patel F. B., Soto M. C., Lundquist E. A. (2008) Genetics 179, 1957–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rohatgi R., Ma L., Miki H., Lopez M., Kirchhausen T., Takenawa T., Kirschner M. W. (1999) Cell 97, 221–231 [DOI] [PubMed] [Google Scholar]
- 25.Tal T., Vaizel-Ohayon D., Schejter E. D. (2002) Dev. Biol. 243, 260–271 [DOI] [PubMed] [Google Scholar]
- 26.Wang X. H., Zheng J. Q., Poo M. M. (1996) J. Physiol. 491, 187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernstein B. W., DeWit M., Bamburg J. R. (1998) Brain Res. Mol. Brain Res. 53, 236–251 [DOI] [PubMed] [Google Scholar]
- 28.Morales M., Colicos M. A., Goda Y. (2000) Neuron 27, 539–550 [DOI] [PubMed] [Google Scholar]
- 29.Halpain S. (2003) Nat. Neurosci. 6, 101–102 [DOI] [PubMed] [Google Scholar]
- 30.Malacombe M., Bader M.-F., Gasman S. (2006) Biochim. Biophys. Acta 1763, 1175–1183 [DOI] [PubMed] [Google Scholar]
- 31.Gasman S., Chasserot-Golaz S., Malacombe M., Way M., Bader M.-F. (2004) Mol. Biol. Cell 15, 520–531 [DOI] [PMC free article] [PubMed] [Google Scholar]