Abstract
Nutlin-3a is a preclinical drug that stabilizes p53 by blocking the interaction between p53 and MDM2. In our previous study, Nutlin-3a promoted a tetraploid G1 arrest in two p53 wild-type cell lines (HCT116 and U2OS), and both cell lines underwent endoreduplication after Nutlin-3a removal. Endoreduplication gave rise to stable tetraploid clones resistant to therapy-induced apoptosis. Prior knowledge of whether cells are susceptible to Nutlin-induced endoreduplication and therapy resistance could help direct Nutlin-3a-based therapies. In the present study, Nutlin-3a promoted a tetraploid G1 arrest in multiple p53 wild-type cell lines. However, some cell lines underwent endoreduplication to relatively high extents after Nutlin-3a removal whereas other cell lines did not. The resistance to endoreduplication observed in some cell lines was associated with a prolonged 4N arrest after Nutlin-3a removal. Knockdown of either p53 or p21 immediately after Nutlin-3a removal could drive endoreduplication in otherwise resistant 4N cells. Finally, 4N-arrested cells retained persistent p21 expression; expressed senescence-associated β-galactosidase; displayed an enlarged, flattened phenotype; and underwent a proliferation block that lasted at least 2 weeks after Nutlin-3a removal. These findings demonstrate that transient Nutlin-3a treatment can promote an apparently permanent proliferative block in 4N cells of certain cell lines associated with persistent p21 expression and resistance to endoreduplication.
Keywords: Anticancer Drug, Cancer Therapy, Cell Cycle, DNA Replication, p53, Nutlin, Endoreduplication, p21, Senescence
Introduction
Inactivation of the p53 tumor suppressor pathway is essential for the development of most or all human cancers. In >50% of cancers, p53 is inactivated by missense mutations in the P53 gene that destroy the proteins function. Cancers in which the P53 gene is not mutated often contain alterations in the p53-signaling pathway that compromise its function (1). The levels and activity of wild-type p53 are controlled in large part by MDM2, an E3 ubiquitin-ligase enzyme that binds p53 and promotes its degradation through the ubiquitin-proteasome pathway (2, 3). There has been considerable interest in restoring wild-type p53 function in cancer as a therapeutic approach. This goal has led to the development of small molecule MDM2 antagonists, the Nutlins, of which Nutlin-3a is highly effective (4). Nutlin-3a occupies the p53-binding pocket in MDM2 and effectively blocks the interaction between MDM2 and p53. This results in the stabilization and activation of p53 and subsequent p53-dependent growth arrest and/or apoptosis (4, 5).
Several reports have supported the potential use of Nutlin-3a in cancer therapy. For example, Nutlin-3a inhibited growth of p53 wild-type human tumors grown as xenografts in nude mice (4, 6), and multiple cancer cell lines that express wild-type p53 have been shown to undergo G1 and G2 phase growth arrest or apoptosis in response to Nutlin-3a (5, 6). Notably, most hematologic cancer cells that express wild-type p53 undergo apoptosis as their primary response to Nutlin-3a (7–10), whereas most nonhematologic cancers (sarcomas, carcinomas) undergo growth arrest as their primary response (5, 6). Nutlin-3a was reported to cause permanent, irreversible arrest (senescence) in fibroblasts and fibrosarcoma cells that was dependent in some cases on the length of Nutlin-3a exposure (11, 12). However, recent studies have reported that cell cycle arrest induced by prolonged Nutlin-3a treatment in p53 wild-type cells is largely if not entirely reversible (13, 14). Specifically, exposure to 10 μm Nutlin-3a for up to 6 days induced a senescent-like arrest that included expression of senescence-associated β-galactosidase in multiple p53 wild-type cancer cell lines. Despite this arrest, nearly 100% of Nutlin-3a-treated cells resumed cycling after Nutlin removal, as determined by BrdUrd2 incorporation. This suggested that Nutlin-3a-mediated cell cycle arrest is not permanent and provided a rationale for the potential use of Nutlin-3a against p53-null or mutant cancers. In this rationale, pretreatment of p53-null or mutant tumors is predicted to arrest normal tissues and cells surrounding the tumor but allow the tumor cells to continue proliferation. Subsequent exposure to drugs that target proliferating cells would then selectively kill the tumor cells while leaving the normal surrounding cells unaffected (15).
Cell cycle checkpoints ensure that individual phases of the cell cycle are not initiated unless conditions are favorable and previous phases have been successfully completed. Under normal conditions, S phase entry requires the prior completion of mitosis and activation of G1 phase Cyclin·CDK complexes. DNA damage and other stresses can induce endoreduplication, a process in which 4N (G2 or M phase) cells enter S phase prematurely and replicate their DNA before completing mitosis (16). At least two things are thought to be required for endoreduplication to occur. First, 4N (G2 or M phase) cells must assume a G1-like state from which they can enter S phase, referred to as tetraploid G1. Typically, tetraploid G1 is characterized by depletion/loss of G2/M marker proteins (e.g. Cyclins A/B, CDC2) and increased expression of G1 phase markers in 4N cells. Second, DNA replication origins must be licensed in 4N cells and then fire to initiate DNA synthesis, a process that requires activation of Cyclin E·CDK2 (17, 18). In our recent study (19), Nutlin-3a promoted a tetraploid G1 arrest in two different cancer cell lines that express wild-type p53 (HCT116 and U2OS), and both cell lines underwent endoreduplication after Nutlin-3a removal. Stable tetraploid clones could be isolated from Nutlin 3a-treated cells, and these tetraploid clones were more resistant to ionizing radiation and cisplatin-induced apoptosis than diploid counterparts. These results suggested potentially adverse side effects of Nutlin-3a-based therapy are endoreduplication and the generation of therapy-resistant tetraploid cells. Prior knowledge of whether cells are susceptible to Nutlin-induced tetraploidy and therapy resistance could help direct Nutlin-3a-based therapies. In the present study we have extended these studies to a larger panel of p53 wild-type cell lines. Our data indicate that Nutlin-3a promotes a tetraploid G1 arrest in each of the cell lines tested. However, although some cell lines undergo endoreduplication to relatively high extents after Nutlin-3a removal, other cell lines do not. The resistance to endoreduplication observed in some cell lines is associated with a prolonged 4N arrest after Nutlin-3a removal. Knockdown of either p53 or p21 immediately after Nutlin-3a removal could drive endoreduplication in 4N cells that are otherwise resistant. Finally, 4N-arrested cells retained persistent p21 expression and displayed an enlarged, flattened phenotype and proliferation block that lasted at least 2 weeks after Nutlin-3a removal. These findings demonstrate that transient Nutlin-3a treatment can promote an apparently permanent proliferative block in 4N cells of certain cell lines associated with persistent p21 expression and resistance to endoreduplication.
EXPERIMENTAL PROCEDURES
Cell Lines and Culture Conditions
AG01522 was purchased from CORIELL. RKO cells were purchased from ATCC. U2OS cells were from Dr. Peter Howley (Harvard Medical School). HCT116 cells were from Dr. Bert Vogelstein (John Hopkins University). A549 and LNCaP cells were from Dr. Ralph Weichselbaum (University of Chicago). MCF7 cells were from Dr. Victor Levenson (Rush University Medical Center). AG01522, U2OS, and A549 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. RKO and LNCaP cells were grown in RPMI 1640 medium with 10% fetal bovine serum. HCT116 cells were grown in McCoy's 5A medium with 10% fetal bovine serum. MCF7 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and insulin (6 μg/ml). Cells were plated 24 h before being treated with Nutlin-3 (N6287; Sigma) at the indicated concentration. Dimethyl sulfoxide was used as vehicle control.
Immunoblotting
Whole cell extracts were prepared by resuspending cell pellets in lysis buffer (160 mm NaCl, 5 mm EDTA, 0.5% Nonidet P-40, 50 mm Tris, pH 7.5), resolved by SDS-PAGE, and transferred to polyvinylidene difluoride membranes (NEN Life Science Products). Antibodies to p21 (187), Cyclin A (H432), Geminin (FL-309), and cdk2 (M2) were from Santa Cruz Biotechnology; antibodies to Cyclin B1 (V152) and CDC2 (POH1) were from Cell Signaling; antibodies to p53 (AB-2) and pRb (Ab-5) were from Calbiochem. Antibody to Cyclin E (HE-2) was from BD Pharmingen. Primary antibodies were detected with goat anti-mouse or goat anti-rabbit secondary antibodies conjugated to horseradish peroxidase (Jackson ImmunoResearch), using enhanced chemiluminescence (PerkinElmer Life Sciences).
Flow Cytometry Analysis and Live Cell Sorting
For cell cycle analysis, cells were harvested and fixed in 25% ethanol overnight. The cells were then stained with propidium iodide (25 μg/ml; Calbiochem). For the BrdUrd incorporation assay, cells were incubated with 20 μm BrdUrd (BD Pharmingen) for the indicated time. Cells were then treated according to the manufacturer's guidelines. Fluorescein isothiocyanate-conjugated mouse anti-BrdUrd monoclonal antibody (BD Pharmingen) and propidium iodide were used to stain cells. Antibodies to p53 (DO-1; Santa Cruz Biotechnology) and p21 (F-5; Santa Cruz Biotechnology) were used for flow cytometry analysis to monitor p53 and p21 levels in treated cells. Flow cytometry analysis was performed on FACS Canto (Becton Dickinson) and analyzed with CellQuest (Becton Dickinson) and FlowJo 8.7 (Treestar, Inc). For each sample, 10,000 events were collected. FSC width versus SSC width was used to eliminate doublets. For live cell sorting, cells were incubated with Hoechst 33342 (2 μmol/liter; Invitrogen) for 90 min at 37 °C and then harvested. Cell sorting was performed based on DNA content using a MoFlo cytometer equipped with a UV excitation laser. The sorted cells were plated at low density.
siRNA-mediated Transient Knockdown
p53, p21 RNA interference (On-target plus smart pool) and control RNA interference (On-target plus siControl nontargeting pool) were purchased from Dharmacon. They were transfected according to the manufacturer's guidelines using DharmaFECT I reagent.
Colony Formation Assay
The colony-forming ability of sorted 2N and 4N cells was determined by seeding 200 cells in 10-cm dishes. After 12 days of incubation, cells were washed with phosphate-buffered saline, and colonies were stained with 0.5% crystal violet. Senescence-associated β-galactosidase assay was performed using a Senescence Colorimetric Assay kit (Sigma).
Immunofluorescence
A549 cells were treated with Nutlin for 24 h. Cells were sorted for 2N and 4N populations, and cells were plated on glass coverslips in 6-well dishes. 12 days after plating, cells were fixed in 4% paraformaldehyde. Coverslips were next blocked and permeabilized in 1% bovine serum albumin, 0.1% Triton X-100 in phosphate-buffered saline and then incubated with primary antibodies (1:100 dilution in phosphate-buffered saline, 1% bovine serum albumin for all antibodies) for 1.5 h. Cells were incubated with Alexa Fluor 488-conjugated goat anti-rabbit IgG (Invitrogen) for 1 h with DAPI. Primary antibodies used were specific against p21 (H164) (Santa Cruz Biotechnology) and BrdUrd (560210; BD Bioscience, San Jose, CA). Fluorescent staining was visualized using an Axiovert 200 fluorescence microscope (Zeiss, Göttingen, Germany).
RESULTS
Transient Nutlin Treatment Promotes Endoreduplication in p53 Wild-type Cells
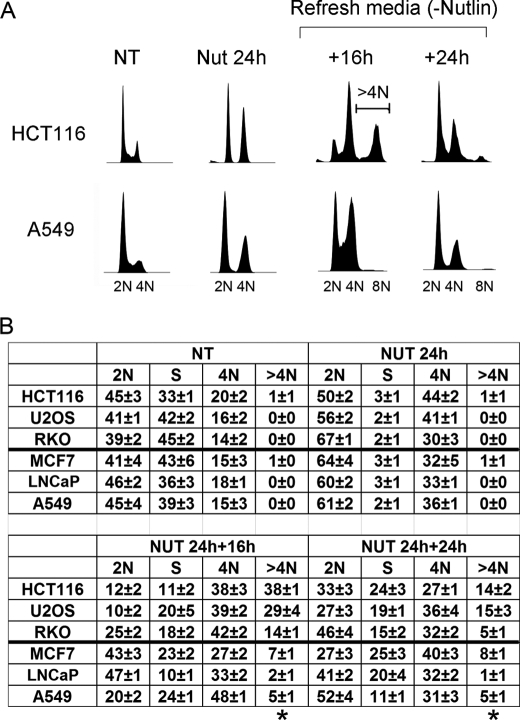
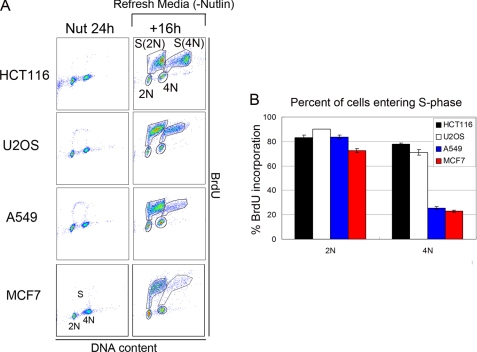
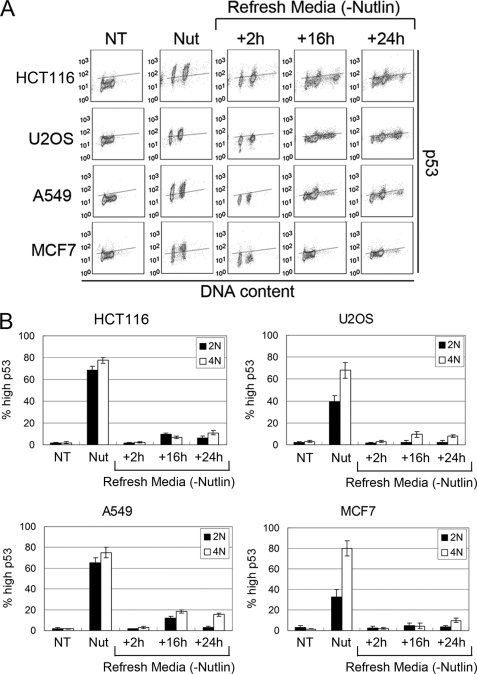
Nutlin-3a (Nutlin) is a preclinical drug that stabilizes p53 by blocking its interaction with MDM2 (4). We reported that two p53 wild-type cancer cell lines (U2OS and HCT116) treated with Nutlin for 24 h accumulated with 2N and 4N DNA content and that both cell lines underwent endoreduplication after Nutlin removal (19). This endoreduplication gave rise to stable tetraploid clones that displayed resistance to radiation and cisplatin-induced apoptosis. Based on these results, we proposed that potentially adverse side effects associated with Nutlin are endoreduplication and the generation of therapy-resistant tetraploid cells. In the current report, we expanded these studies to a panel of six different p53 wild-type cancer cell lines as well as a normal human fibroblast line (AG01522). Each cell line was exposed to Nutlin (10 μm) for 24 h followed by Nutlin removal for different periods, and cell cycle profiles were monitored by flow cytometry (Fig. 1). We used 10 μm because preliminary BrdUrd incorporation experiments showed that this was the minimal dose that caused complete cell cycle arrest in all the cell lines tested (data not shown). 24 h Nutlin treatment caused cell cycle arrest in all six cell lines, demonstrated by the depletion of S phase cells and accumulation of cells with 2N and 4N DNA content (Fig. 1B). In three of the six cancer cell lines (HCT116, U2OS, and RKO) we observed a pronounced emergence of >4N cells (cells with more than 4N DNA content) after Nutlin removal and their transient accumulation in an 8N peak, indicative of endoreduplication (Fig. 1). AG01522 normal human fibroblasts also underwent relatively high levels of endoreduplication after Nutlin removal (supplemental Fig. 1). In contrast, the remaining three cancer cell lines (A549, LNCaP, and MCF7) showed little or no emergence of >4N cells after Nutlin removal, indicating a lack of endoreduplication (Fig. 1 and supplemental Fig. 1). Representative data in Fig. 1A show endoreduplication by HCT116 cells upon Nutlin removal and lack of endoreduplication in A549 cells. We used a BrdUrd incorporation assay to confirm the >4N cells that emerged after Nutlin removal resulted from 4N cells initiating DNA synthesis (Fig. 2). Briefly, HCT116, U2OS, A549, and MCF7 cells were Nutlin-treated for 24 h. The Nutlin was then removed, and the cells were incubated in BrdUrd-containing medium for 16 h. The percentage of 2N- and 4N-arrested cells that incorporated BrdUrd after Nutlin removal was then quantified (Fig. 2B). In HCT116 and U2OS cells, a high percentage (70–90%) of both 2N and 4N cells incorporated BrdUrd after Nutlin removal. The incorporation of BrdUrd by 4N cells is consistent with these cells undergoing endoreduplication. In contrast, for A549 and MCF7 cells, a high percentage (70–80%) of 2N cells incorporated BrdUrd after Nutlin removal whereas only a small percentage (∼20%) of 4N cells incorporated BrdUrd.
FIGURE 1.
Transient Nutlin treatment induces the appearance of cells with >4N DNA content. HCT116, U2OS, RKO, A549, MCF7, and LNCaP cells were either untreated (NT) or treated with Nutlin (Nut; 10 μmol/liter) for 24 h followed by Nutlin removal. The cells were harvested at the indicated time points after Nutlin removal. Fixed cells were stained with propidium iodide (25 μg/ml) and subjected to flow cytometry analysis. A, representative DNA profile histograms for HCT116 and A549 cells, analyzed using FlowJo (cell count versus propidium iodide/DNA content). The positions of 2N, 4N, and 8N cells are indicated. B, cell cycle distribution determined from DNA profiles using FlowJo. The numbers represent averages from three independent experiments (mean ± S.E.; n = 3). Cells with >4N DNA content (>4N) are gated as shown in A (HCT116 +16h) and also marked with and asterisk in B.
FIGURE 2.
Nutlin-arrested 2N and 4N cells enter S phase after Nutlin removal. A, HCT116, U2OS, A549, and MCF7 cells were either treated with Nutlin (Nut; 10 μmol/liter) for 24 h or treated with Nutlin for 24 h followed by Nutlin removal for an additional 16 h (+16h). BrdUrd incorporation by cells treated with Nutlin for 24 h was determined by adding BrdUrd (20 μm) to the culture medium 2 h before harvesting. BrdUrd incorporation after Nutlin removal was determined by culturing cells in medium containing BrdUrd (−Nutlin) for 16 h. Cells were harvested and stained with fluorescein isothiocyanate-conjugated anti-BrdUrd antibody and propidium iodide and analyzed by two-dimensional flow cytometry (BrdUrd versus propidium iodide/DNA content). Cells entering S phase from 2N state are labeled as S(2N); cells entering S phase from 4N state are labeled as S(4N). B, the percentage of 2N and 4N cells entering S phase after Nutlin removal was determined for all four cell lines. The percentage of 2N cells entering S phase was calculated by dividing the number of cells entering S phase S(2N) by the total number of 2N cells (S(2N)+2N). The percentage of 4N cells entering S phase was calculated by dividing the number of cells entering S phase S(4N) by the total number of 4N cells (S(4N)+4N). The mean of three independent experiments is shown ± S.E. (error bars).
Nutlin Promotes a Tetraploid G1 Arrest in p53 Wild-type Cells
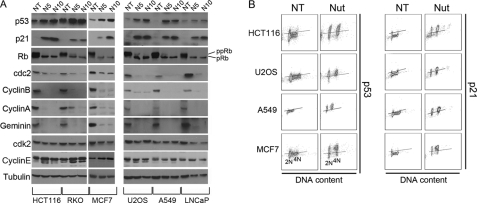
We wished to examine the basis for these different cellular responses to Nutlin. Two things are thought to be required for endoreduplication to occur: First, 4N cells must exit G2 or M phase and enter a G1-like state (tetraploid G1). Tetraploid G1 arrest is characterized by decreased expression of G2/M markers in 4N cells (Cyclin A, Cyclin B, CDC2) and increased expression of G1 arrest markers in these cells (p53, p21, hypophosphorylated pRb) (17, 18, 20, 21). Second, DNA replication origins must be inappropriately licensed in 4N cells and then fire to drive 4N cells into S phase. Origin firing and S phase initiation are believed to require activation of Cyclin E·CDK2 complexes. Two approaches were taken to test whether Nutlin caused a tetraploid G1 arrest in cells, including those that resisted endoreduplication after Nutlin removal. First, we monitored levels of various G2/M regulators and G1 arrest proteins in response to Nutlin by immunoblotting. As shown in Fig. 3A, each Nutlin-treated cell line showed complete or near-complete depletion of Cyclins A/B and CDC2, increased expression of p53 and p21, and hypophosphorylation of pRb. We also monitored expression of Geminin, a DNA replication inhibitor that is absent during G1 phase and accumulates through S, G2, and M phases (22). As shown in Fig. 3A, Geminin was depleted to undetectable levels in Nutlin-treated cells, consistent with both the 2N and 4N cells being arrested in a G1-like state. Notably, the effects of Nutlin on Cyclin B1, Cyclin A, CDC2, and Geminin were specific because Cyclin E and cdk2 levels were not depleted in Nutlin-treated cells. Second, p53 and p21 levels were monitored in 4N cells using flow cytometry and antibodies against both proteins. As shown in Fig. 3B, p53 and p21 were induced by Nutlin to high levels in both 2N and 4N cells. The depletion of Cyclins A/B, CDC2, and Geminin, combined with the induction of p53 and p21 in 4N cells, supports the idea that Nutlin promotes a tetraploid G1 arrest in each cell line, including those that resist endoreduplication after Nutlin removal.
FIGURE 3.
Nutlin treatment promotes a tetraploid G1 arrest in p53 wild-type cancer cell lines. A, HCT116, U2OS, RKO, A549, MCF7, and LNCaP cells were either untreated (NT) or treated with 5 μm (N5) or 10 μm (N10) Nutlin for 24 h. Cell lysates were analyzed by immunoblotting with the indicated antibodies. Tubulin was used as a loading control. pRb, hypophosphorylated Rb; ppRb, hyperphosphorylated Rb. B, cells were either untreated or treated with 10 μm Nutlin (Nut) for 24 h. The expression of p53 and p21 was monitored using flow cytometry (p53/p21 expression versus DNA content). Lines were drawn in the untreated condition to demarcate basal expression (below the line) and induced expression (above the line). The lines were drawn in the same position for each cell line under both untreated and Nutlin-treated conditions, and analysis was done with same instrumental setting. 2N and 4N indicate the positions of cells with 2N and 4N DNA content.
Transient Nutlin Treatment Promotes a Prolonged 4N Arrest in A549 and MCF7 Cells
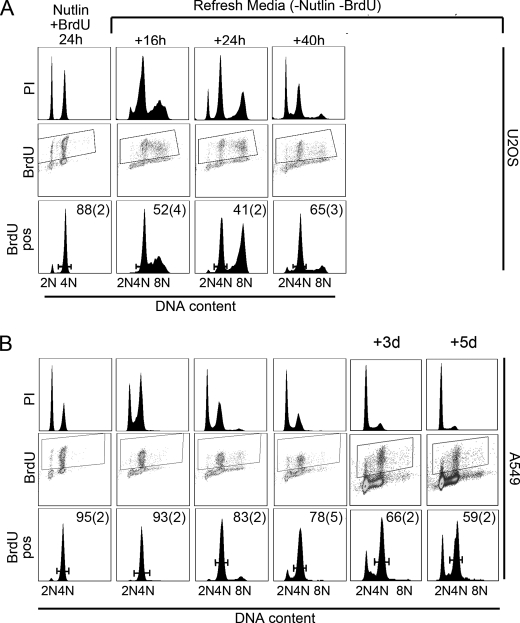
To examine further why some cell lines resist Nutlin-induced endoreduplication, we established a cell labeling protocol to monitor the fate of 4N cells after Nutlin removal. Briefly, cells are incubated with Nutlin for 6 h to initiate 2N and 4N arrest. BrdUrd is then added in the continued presence of Nutlin and cells incubated for an additional 18 h. In this way, S phase cells incorporate BrdUrd before arresting with 4N DNA content, and the BrdUrd signal ends up almost entirely in arrested 4N cells (Fig. 4). Using this method, 80–90% of the BrdUrd-labeled cells are arrested with 4N DNA content, with the remaining 10–20% of BrdUrd-labeled cells arresting with 2N DNA content (Fig. 4 and supplemental Fig. 2). Nutlin and BrdUrd are then removed, and cells are subsequently monitored by flow cytometry with propidium iodide for DNA content and anti-BrdUrd antibody to follow labeled 4N cells. In U2OS and HCT116, 2N cells entered S phase within 16 h after Nutlin removal (visualized by propidium iodide staining), and BrdUrd-labeled 4N cells appeared with >4N DNA content also within 16 h after Nutlin removal, indicative of endoreduplication (Fig. 4A and supplemental Fig. 2). In contrast, in A549 and MCF7, the majority of BrdUrd-labeled 4N cells remained arrested in a 4N state for up to 40 h after Nutlin removal, whereas the 2N populations reentered the cell cycle and entered S phase within 16 h after Nutlin removal (visualized by propidium iodide staining; Fig. 4B and supplemental Fig. 2). BrdUrd-labeled A549 cells were followed for 3–5 days after Nutlin removal to determine whether these cells remain arrested with 4N DNA content for an extended period. Nearly 60% of BrdUrd-labeled A549 cells retained 4N DNA content 5 days after Nutlin removal, indicative of a prolonged 4N arrest.
FIGURE 4.
Transient Nutlin treatment promotes a prolonged 4N arrest in A549 cells. U2OS (A) and A549 (B) cells were treated with 10 μm Nutlin for 24 h. BrdUrd (20 μm) was added 6 h after the Nutlin addition. At the 24-h time point, cells were rinsed with phosphate-buffered saline and refed with normal medium (−Nutlin and BrdUrd). Cells were harvested at the indicated time points and analyzed by flow cytometry. The top panels for each cell line (labeled PI) show the DNA profile for the total population determined by propidium iodide staining. Middle panels (labeled BrdU) show the distribution of BrdUrd signaling for the whole population. BrdUrd-positive cells were gated, shown by the boxed regions in the middle panels. Bottom panels (labeled BrdU pos) show the DNA profile of BrdUrd-positive cells as gated in the middle panels. The numbers represent the percentage of BrdUrd-positive cells with 4N DNA content at each time point. The numbers are the average from three independent experiments, and the S.E. is shown in parentheses.
Persistent p21 Expression after Nutlin Removal Limits Endoreduplication by 4N Cells
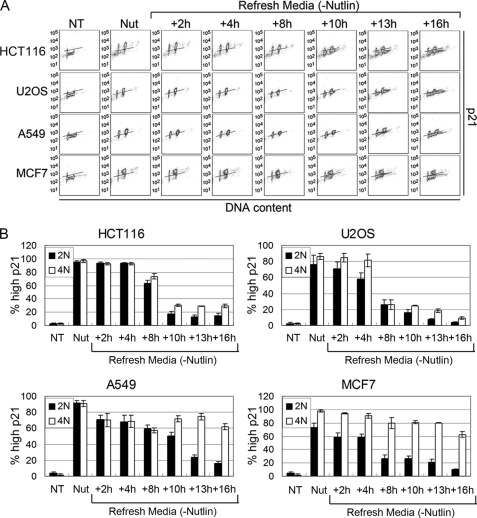
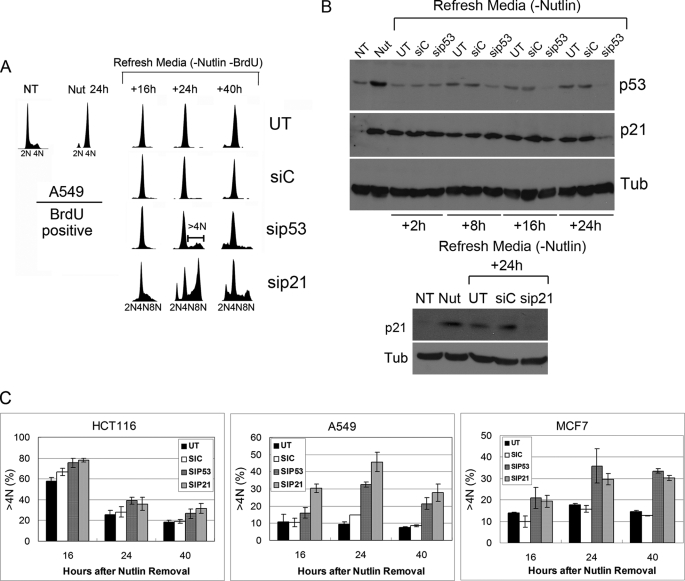
We showed previously that tetraploid G1 arrest induced by Nutlin is dependent on p53 and p21 (19). We speculated, therefore, that the prolonged 4N arrest observed in A549 and MCF7 cells could result if p53 and p21 remain elevated in 4N cells. To investigate this possibility, we used flow cytometry to quantify the percentage of 2N and 4N cells that retained elevated p53 and p21 protein expression at multiple time points after Nutlin removal. p53 and p21 levels were increased by 24-h Nutlin treatment in both 2N and 4N populations of HCT116, U2OS, A549, and MCF7 cells (Figs. 5 and 6), consistent with the results of Fig. 3B. In HCT116 and U2OS, p53 decreased rapidly after Nutlin removal (within 2 h) to background levels in 2N and 4N cells (Fig. 5). p53 remained at low levels in the vast majority of cells for 16–24 h after Nutlin removal, although a small percentage (10–15%) of 2N and 4N cells appeared to have slightly elevated p53 by 16–24 h after Nutlin removal. p21 also decreased rapidly (within 8–10 h) after Nutlin removal in the vast majority of 2N and 4N HCT116 and U2OS cells (Fig. 6). p21 remained low in 2N and 4N HCT116 and U2OS cells for up to 16 h after Nutlin removal, and both cell populations initiated DNA synthesis (Fig. 6). In A549 and MCF7, p53 also decreased rapidly after Nutlin removal (within 2 h) in 2N and 4N cells (Fig. 5). p53 remained low in the vast majority of these cells for 16–24 h after Nutlin removal, although, similar to HCT116 and U2OS, a small percentage of cells appeared to have elevated p53 levels at the 16–24 h time points. p21 decreased rapidly (within 8–13 h) after Nutlin removal in the majority of 2N A549 and MCF7 cells (Fig. 6). However, p21 remained elevated for up to 16 h after Nutlin removal in a high percentage of 4N A549 and MCF7 cells (Fig. 6). Based on this, we speculated that persistent p21 expression in 4N A549 and MCF7 cells might be blocking these cells from initiating DNA synthesis and undergoing endoreduplication. To test this possibility, A549 cells were Nutlin-treated and BrdUrd-labeled as described above and then transfected with p53 or p21 siRNA (or control siRNA) after Nutlin and BrdUrd removal. Immunoblotting of A549 cells demonstrated that p53 and p21 were induced by 24-h Nutlin treatment (Fig. 7B). Consistent with the results from Fig. 5, p53 decreased rapidly after Nutlin removal to near basal levels in A549 cells either untransfected or transfected with control siRNA and was depleted further by p53 siRNA at the 16- and 24-h time points (Fig. 7B, upper). In contrast, and consistent with results from Fig. 6, p21 remained elevated for up to 24 h after Nutlin removal in A549 cells either untransfected or transfected with control siRNA, but was depleted in cells transfected with either p53 or p21 siRNA at the 24-h time point (Fig. 7B). Flow cytometric analysis demonstrated that BrdUrd-labeled 4N cells remained in a 4N state (arrested) after Nutlin removal when untransfected or transfected with control siRNA (Fig. 7A). In contrast, BrdUrd-labeled 4N cells appeared with >4N DNA content after Nutlin removal in both p53 siRNA and p21 siRNA transfectants (Fig. 7A). Similar experiments were carried out in HCT116 and MCF7 cells, and the amount of endoreduplication was quantified by determining the percentage of BrdUrd-labeled cells with >4N DNA content (Fig. 7C). HCT116 cells displayed abundant endoreduplication indicated by >4N cells in both untransfected and control siRNA conditions, and a modest increase after transfection with p53 or p21 siRNA. In contrast, A549 and MCF7 cells displayed relatively little endoreduplication indicated by >4N cells in control conditions, but abundant endoeduplication after transfection with p21 or p53 siRNA (Fig. 7C). p21 siRNA appeared to drive slightly more endoreduplication after Nutlin removal in A549 cells than did p53 siRNA. Taken together, the results suggest that persistent p21 expression in 4N cells, mediated by p53, prevents endoreduplication after Nutlin removal in A549 and MCF7 cell lines.
FIGURE 5.
p53 decreases rapidly in both 2N and 4N populations after Nutlin removal. HCT116, U2OS, A549, and MCF7 cells were treated with 10 μm Nutlin (Nut) for 24 h followed by Nutlin removal. A, p53 expression was monitored using flow cytometry at each indicated time point. Lines were drawn in the untreated (NT) condition to demarcate basal expression (below the line) and induced expression (above the line). The lines were drawn at the same position for all time points for each cell line. Representative flow cytometry histograms are shown. B, the percentage of 2N and 4N cells with high p53 was calculated by dividing the number of cells above the line by the total number of cells in either 2N or 4N state. The mean of three independent experiments is shown ± S.E. (error bars).
FIGURE 6.
p21 remains elevated in 4N populations after Nutlin removal in A549 and MCF7 cells. HCT116, U2OS, A549, and MCF7 cells were treated with 10 μm Nutlin (Nut) for 24 h followed by Nutlin removal. p21 expression was monitored using flow cytometry at each indicated time point. The analysis was done as described in Fig. 5.
FIGURE 7.
Knockdown of p53 or p21 after Nutlin removal can promote endoreduplication. A, A549 cells were either untreated (NT) or treated with Nutlin (Nut; 10 μmol/liter) for 24 h. BrdUrd (20 μm) was added at 6 h after Nutlin addition. At the 24-h time point, cells were rinsed with phosphate-buffered saline and refed with normal medium (−Nutlin and BrdUrd). The cells were then either untransfected (UT) or transfected with siControl (siC), sip53, or sip21. Cells were harvested at the indicated time points and subjected to flow cytometry analysis. Representative DNA profiles of the BrdUrd-positive population are shown. B, A549 cells were treated as in A. Cell lysates were collected at the indicated time points and subjected to immunoblot analysis for p53 and p21. Tubulin expression was determined as a loading control. C, HCT116, A549, and MCF7 cells were Nutlin-treated and BrdUrd-labeled as described in A. After Nutlin and BrdUrd removal, cells were either untransfected or transfected with siControl, sip53, or sip21. The percentage of BrdUrd-positive cells with >4N DNA content (>4N) was quantified using FlowJo. The mean of three independent experiments is shown ± S.E. (error bars).
Transient Nutlin Treatment Promotes a Senescent-like Proliferation Arrest in 4N A549 and MCF7 Cells
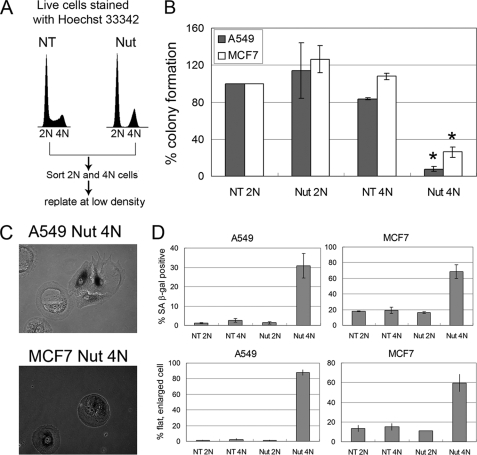
It was recently reported for multiple p53 wild-type cell lines that cell cycle arrest caused by prolonged (6 days) Nutlin treatment is reversible, with nearly 100% of cells reentering the cell cycle and proliferating after Nutlin removal (13, 14). The results presented above (Fig. 4 and supplemental Fig. 2) indicate that Nutlin arrests cells in a 2N and 4N state and that in certain cell lines (A549 and MCF7) the 4N cells remain arrested in a 4N state for a prolonged period of at least 40 h after Nutlin removal. We wished to determine whether these 4N cells remain permanently arrested or are able to recover at later time points and resume proliferation. To this end, A549 and MCF7 cells were untreated or exposed to Nutlin for 24 h. We then carried out live-cell staining with Hoechst dye and flow cytometry to isolate the 2N and 4N populations (Fig. 8A). The isolated 2N and 4N cells were replated in the absence of Nutlin and examined over a 2-week period for colony-forming ability, morphology, and expression of senescence-associated β-galactosidase. 2N populations from untreated and Nutlin-treated A549 and MCF7 cells were able to grow into colonies with comparable efficiencies, indicating that Nutlin treatment did not permanently arrest these cells. Strikingly, however, 4N populations of A549 and MCF7 cells that had been exposed to Nutlin for 24 h had a greatly diminished colony-forming ability compared with 4N populations from untreated cells (Fig. 8B). In addition to lacking proliferative ability, 4N cells from Nutlin-treated A549 and MCF7 cells displayed an enlarged, flattened phenotype 12 days after Nutlin removal, and many of them expressed senescence-associated β-galactosidase (Fig. 8, C and D). The block in their proliferation, flattened phenotype, and expression of senescence-associated β-galactosidase suggest that these cells are senescent.
FIGURE 8.
Transient Nutlin treatment promotes a senescent-like proliferation arrest in 4N A549 and MCF7 cells. A, A549 and MCF7 cells were either untreated (NT) or treated with 10 μm Nutlin (Nut) for 24 h. The cells were then live-stained with Hoechst 33342 (5 μg/ml). Cell sorting was performed on a MoFlo cytometer equipped with a UV excitation wavelength laser. Sorted 2N and 4N cells were plated at low density in normal medium (minus Nutlin). B, colony formation was determined for each sorted population (NT 2N, NT 4N, Nut 2N, and Nut 4N) for A549 and MCF7 cells. The plating efficiency for NT 2N population was set at 100%. The mean of three independent experiments is shown ± S.E. (error bars). *, p < 0.05 (comparing Nut 4N versus Nut 2N or Nut 4N versus NT 4N for both A549 and MCF7). C, senescence-associated β-galactosidase senescence assay was performed on each sorted population (NT 2N, NT 4N, Nut 2N, and Nut 4N) for A549 and MCF7 cells 12 days after replating. Shown are representative images of senescence-associated β-galactosidase-positive cells from sorted Nut 4N populations of A549 and MCF7 cells. D, The percentage of senescence-associated β-galactosidase-positive cells and the percentage of flat, enlarged cells were determined. The mean of three independent experiments is shown ± S.E. (error bars).
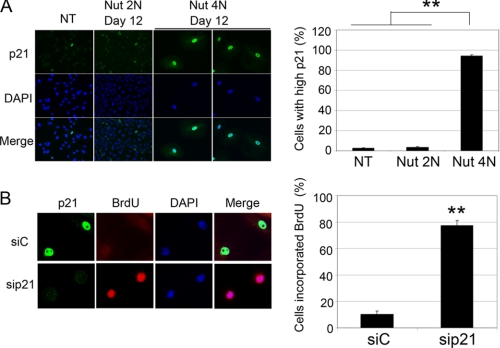
Next, we asked whether these apparently senescent cells retained high p21 expression 12 days after Nutlin removal. For these experiments, 2N and 4N populations from Nutlin-treated A549 cells were isolated and plated on glass coverslips. The cells were examined 12 days after plating by immunofluorescence staining with anti-p21 antibody (Fig. 9A). The 2N cells proliferated during the 12-day period after Nutlin removal, and immunofluorescence showed that they had low p21 expression similar to untreated, control cells (Fig. 9A). In contrast, the majority of 4N cells did not proliferate and retained high p21 expression at this 12-day time point after Nutlin removal (Fig. 9A). Finally, we tested whether this high p21 expression contributes to the proliferation arrest in these cells. To this end, p21 was knocked down by siRNA in 4N cells at the 12-day time point after Nutlin removal. BrdUrd was then added to the medium, and BrdUrd incorporation was monitored by immunofluorescence with anti-BrdUrd antibody. p21 remained high in the 4N population transfected with control siRNA, and the cells did not incorporate BrdUrd. In contrast, p21 was depleted in the 4N population transfected with p21 siRNA, and cells incorporated BrdUrd (Fig. 9B). Taken together, these results indicated that 4N cells retained elevated p21 levels up to 12 days after Nutlin removal, and this elevated p21 contributed to their senescent-like, proliferation arrest.
FIGURE 9.
Persistent p21 expression in 4N cells after Nutlin removal. A, A549 cells were treated with 10 μm Nutlin (Nut) for 24 h and sorted as described in Fig. 8. Sorted 2N and 4N cells were plated at low density in normal medium (minus Nutlin) on glass coverslips. Cells were fixed with 4% formaldehyde at 12 days after plating and subjected to anti-p21 immunofluorescence analysis. p21 antibody was detected using an Alexa Fluor 488-conjugated goat anti-rabbit IgG. Cell nuclei were counterstained with DAPI (blue). Images were captured at ×40 magnification. Representative images are shown. Untransfected (NT) control was plated 24 h before fixing. Percentage of cells with high p21 expression was determined, and the mean of three independent experiments is shown ± S.E. (error bars) with 200 cells counted per experiment. B, A549 cells were treated as described in A. At 12 days after plating, cells were either transfected with siControl (siC) or sip21. BrdUrd was added at 24 h after transfection and kept on for another 24 h. Cells were then fixed with 4% formaldehyde and subject to anti-p21 and anti-BrdUrd immunofluorescence analysis. p21 antibody was detected using an Alexa Fluor 488-conjugated goat anti-rabbit IgG. BrdUrd was detected using Alexa Fluor 555 anti-BrdUrd. Images were taken as described in A. Percentage of cells incorporating BrdUrd was determined, and the mean of three independent experiments is shown ± S.E. (error bars) with 200 cells counted per experiment. **, p < 0.01.
DISCUSSION
Our recent study (19) demonstrated that Nutlin treatment promoted a tetraploid G1 arrest in two different cancer cell lines that express wild-type p53 (U2OS and HCT116). Endoreduplication was observed in both cell lines after Nutlin removal. Tetraploid clones could be isolated from endoreduplicating cells, and these clones proved to be resistant to ionizing radiation and cisplatin-induced apoptosis compared with diploid clones. The current study was initiated to determine whether all or only select p53 wild-type cell lines are susceptible to these effects of Nutlin. Prior knowledge of whether cells are susceptible to Nutlin-induced tetraploidy and therapy resistance may help direct Nutlin-based therapies. Nutlin-3a promotes a tetraploid G1 arrest in each of the six cell lines tested. However, although some cell lines undergo endoreduplication to relatively high extents after Nutlin removal, other cell lines do not. The resistance to endoreduplication observed in some cell lines is associated with a prolonged 4N arrest after Nutlin removal. Knockdown of either p53 or p21 immediately after Nutlin removal could drive endoreduplication in otherwise resistant 4N cells. Finally, 4N-arrested cells that resisted endoreduplication after Nutlin removal retain elevated p21 expression; express senescence-associated β-galactosidase; display an enlarged, flattened phenotype; and are blocked from proliferating for at least 2 weeks after Nutlin removal. These findings demonstrate that transient Nutlin-3a treatment promotes a permanent senescence-like arrest in 4N cells of certain cell lines that is associated with persistent p21 expression and resistance to endoreduplication.
Endoreduplication requires that 4N cells first enter a G1 state (tetraploid G1) from which they can enter S phase and replicate their DNA. Tetraploid G1 arrest is characterized by loss of G2/M regulators in 4N cells (Cyclins A/B, CDC2) and increased expression of G1 arrest markers in these cells (p53 and p21). Immunoblotting demonstrates that Nutlin treatment causes complete or near complete depletion of Cyclin A, Cyclin B, and CDC2 in all cell lines tested, including those that resist endoreduplication after Nutlin removal. Moreover, p53 and p21 levels are increased by Nutlin treatment in both 2N and 4N cells. This suggests that all cell lines respond to Nutlin treatment by undergoing a tetraploid G1 arrest in addition to a normal 2N G1 arrest. DNA replication origins must be licensed in 4N cells for endoreduplication to occur. Origin licensing involves the sequential binding of the origin recognition complex, CDC6, and Cdt1, and the replicative helicase MCM2–7 to replication origins to form what is called the prereplication complex (23). Subsequent origin firing/S phase entry occurs upon recruitment of the DNA synthesis machinery and activation of Cyclin E·CDK2. Under normal conditions, Cyclin A/B·CDC2 complexes prevent inappropriate origin licensing in 4N cells through inhibitory phosphorylation of origin recognition complex, MCM4, CDC6, and others (24–28). Thus, depletion of CDC2, Cyclin B, and Cyclin A can prime 4N cells for S phase entry by allowing prereplication complex formation. We find that knockdown of either p53 or p21 promotes endoreduplication after Nutlin removal in cells that otherwise resist endoreduplication. This suggests that replication origins are inappropriately licensed in 4N populations from these cells but that the p53-p21 pathway prevents origin firing and/or DNA synthesis after Nutlin removal. Notably, high levels of p21 may inhibit DNA synthesis by 4N cells (endoreduplication) through at least two mechanisms. First, p21 binds to and inhibits Cyclin·CDK complexes (29). Cyclin E·CDK2 complexes drive DNA synthesis through phosphorylation and activation of factors such as NPAT and Cdc45 (30–32). Accordingly, high p21 levels may block origin firing and S phase entry (endoreduplication) through its ability to inhibit Cyclin E·CDK2. Second, p21 can inhibit DNA synthesis directly through binding the processivity factor proliferating cell nuclear antigen (33). Inhibition of proliferating cell nuclear antigen by p21 might also contribute to the inhibition of endoreduplication observed in some cell lines.
p53 promotes G1 cell cycle arrest through its ability to induce p21 expression, and p53 and p21 levels are increased by Nutlin treatment in both 2N and 4N cells for HCT116, U2OS, A549, and MCF7. In cell lines susceptible to endoreduplication (HCT116 and U2OS), p53 and p21 levels decrease in both 2N and 4N cells after Nutlin removal, and both populations progress into S phase. In cell lines resistant to endoreduplication (A549 and MCF7), p53 and p21 levels decrease only in 2N cells after Nutlin removal, and these 2N cells enter S phase. However, whereas p53 levels decrease in 4N populations of A549 and MCF7 cells after Nutlin removal, p21 levels remain elevated, and these cells remain arrested in a 4N state. The elevated level of p21 in these cells is p53-dependent because knockdown of p53 by siRNA causes p21 depletion. Moreover, siRNA-mediated knockdown of p21 or p53 immediately after Nutlin removal could drive endoreduplication in these otherwise resistant 4N cells. These data indicate that p21 is maintained at high levels after Nutlin removal, at least in part, through transcriptional activation by p53, and the initial 4N arrest and endoreduplication block results from p53-mediated expression of p21.
It was recently reported that cell cycle arrest following prolonged Nutlin treatment is largely if not entirely reversible. Specifically, exposure to 10 μm Nutlin-3a for up to 6 days induced a senescent-like arrest that included expression of senescence-associated β-galactosidase in multiple p53 wild-type cancer cell lines (13, 14). Despite this arrest, nearly 100% of Nutlin-3a-treated cells were reported to resume cycling after Nutlin removal, as determined by their ability to incorporate BrdUrd. We found that 24-h Nutlin treatment promotes a 2N and 4N arrest in multiple p53 wild-type cell lines. The 4N cells appear to be arrested by Nutlin in a tetraploid G1 state, as described above. Upon Nutlin removal, both 2N and 4N populations of HCT116 and U2OS cells enter S phase, indicating that their arrest is only transient. Similarly, 2N populations of A549 and MCF7 also enter S phase after Nutlin removal, indicating that their arrest in this 2N state is also transient. However, 4N populations of A549 and MCF7 remain arrested after Nutlin removal. Visual inspection shows that many of these 4N cells assume a flattened, enlarged phenotype, and analysis of their colony-forming ability demonstrates a failure to proliferate that last for at least 12 days. Many of these 4N-arrested cells express senescence-associated β-galactosidase. Furthermore, p21 was maintained at an elevated level in the majority of these 4N cells, and knockdown of p21 allowed these cells to incorporate BrdUrd, suggestive of cell cycle reentry. In summary, our results demonstrate that transient Nutlin-3a treatment can promote a permanent, senescence-like arrest in 4N cells in certain cell lines that is associated with persistent p21 expression and resistance to endoreduplication.
Supplementary Material

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
- BrdUrd
- bromodeoxyuridine
- CDK
- cyclin-dependent kinase
- PBS
- phosphate-buffered saline
- siRNA
- small interfering RNA.
REFERENCES
- 1.Vogelstein B., Lane D., Levine A. J. (2000) Nature 408, 307–310 [DOI] [PubMed] [Google Scholar]
- 2.Kubbutat M. H., Jones S. N., Vousden K. H. (1997) Nature 387, 299–303 [DOI] [PubMed] [Google Scholar]
- 3.Haupt Y., Maya R., Kazaz A., Oren M. (1997) Nature 387, 296–299 [DOI] [PubMed] [Google Scholar]
- 4.Vassilev L. T., Vu B. T., Graves B., Carvajal D., Podlaski F., Filipovic Z., Kong N., Kammlott U., Lukacs C., Klein C., Fotouhi N., Liu E. A. (2004) Science 303, 844–848 [DOI] [PubMed] [Google Scholar]
- 5.Tovar C., Rosinski J., Filipovic Z., Higgins B., Kolinsky K., Hilton H., Zhao X., Vu B. T., Qing W., Packman K., Myklebost O., Heimbrook D. C., Vassilev L. T. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 1888–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitagawa M., Aonuma M., Lee S. H., Fukutake S., McCormick F. (2008) Oncogene 27, 5303–5314 [DOI] [PubMed] [Google Scholar]
- 7.Kojima K., Konopleva M., Samudio I. J., Shikami M., Cabreira-Hansen M., McQueen T., Ruvolo V., Tsao T., Zeng Z., Vassilev L. T., Andreeff M. (2005) Blood 106, 3150–3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Secchiero P., Barbarotto E., Tiribelli M., Zerbinati C., di Iasio M. G., Gonelli A., Cavazzini F., Campioni D., Fanin R., Cuneo A., Zauli G. (2006) Blood 107, 4122–4129 [DOI] [PubMed] [Google Scholar]
- 9.Drakos E., Atsaves V., Schlette E., Li J., Papanastasi I., Rassidakis G. Z., Medeiros L. J. (2009) Leukemia 23, 2290–2299 [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa H., Yamada Y., Iha H., Tsukasaki K., Nagai K., Atogami S., Sugahara K., Tsuruda K., Ishizaki A., Kamihira S. (2009) Leukemia 23, 2090–2101 [DOI] [PubMed] [Google Scholar]
- 11.Efeyan A., Ortega-Molina A., Velasco-Miguel S., Herranz D., Vassilev L. T., Serrano M. (2007) Cancer Res. 67, 7350–7357 [DOI] [PubMed] [Google Scholar]
- 12.Kumamoto K., Spillare E. A., Fujita K., Horikawa I., Yamashita T., Appella E., Nagashima M., Takenoshita S., Yokota J., Harris C. C. (2008) Cancer Res. 68, 3193–3203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang B., Deo D., Xia M., Vassilev L. T. (2009) Mol. Cancer Res. 7, 1497–1509 [DOI] [PubMed] [Google Scholar]
- 14.Korotchkina L. G., Demidenko Z. N., Gudkov A. V., Blagosklonny M. V. (2009) Cell Cycle 8, 3777–3781 [DOI] [PubMed] [Google Scholar]
- 15.Steelman L. S., McCubrey J. A. (2009) Cell Cycle 8, 3634–3635 [PubMed] [Google Scholar]
- 16.Lilly M. A., Duronio R. J. (2005) Oncogene 24, 2765–2775 [DOI] [PubMed] [Google Scholar]
- 17.Ganem N. J., Pellman D. (2007) Cell 131, 437–440 [DOI] [PubMed] [Google Scholar]
- 18.Ganem N. J., Storchova Z., Pellman D. (2007) Curr. Opin. Genet. Dev. 17, 157–162 [DOI] [PubMed] [Google Scholar]
- 19.Shen H., Moran D. M., Maki C. G. (2008) Cancer Res. 68, 8260–8268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang B. D., Broude E. V., Fang J., Kalinichenko T. V., Abdryashitov R., Poole J. C., Roninson I. B. (2000) Oncogene 19, 2165–2170 [DOI] [PubMed] [Google Scholar]
- 21.Badie C., Itzhaki J. E., Sullivan M. J., Carpenter A. J., Porter A. C. (2000) Mol. Cell. Biol. 20, 2358–2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wohlschlegel J. A., Dwyer B. T., Dhar S. K., Cvetic C., Walter J. C., Dutta A. (2000) Science 290, 2309–2312 [DOI] [PubMed] [Google Scholar]
- 23.Fujita M. (2006) Cell Division 1, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujita M., Yamada C., Tsurumi T., Hanaoka F., Matsuzawa K., Inagaki M. (1998) J. Biol. Chem. 273, 17095–17101 [DOI] [PubMed] [Google Scholar]
- 25.Jiang W., Wells N. J., Hunter T. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 6193–6198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujita M., Yamada C., Goto H., Yokoyama N., Kuzushima K., Inagaki M., Tsurumi T. (1999) J. Biol. Chem. 274, 25927–25932 [DOI] [PubMed] [Google Scholar]
- 27.Hendrickson M., Madine M., Dalton S., Gautier J. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 12223–12228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen V. Q., Co C., Li J. J. (2001) Nature 411, 1068–1073 [DOI] [PubMed] [Google Scholar]
- 29.Xiong Y., Hannon G. J., Zhang H., Casso D., Kobayashi R., Beach D. (1993) Nature 366, 701–704 [DOI] [PubMed] [Google Scholar]
- 30.Zhao J., Kennedy B. K., Lawrence B. D., Barbie D. A., Matera A. G., Fletcher J. A., Harlow E. (2000) Genes Dev. 14, 2283–2297 [PMC free article] [PubMed] [Google Scholar]
- 31.Walter J., Newport J. (2000) Mol. Cell 5, 617–627 [DOI] [PubMed] [Google Scholar]
- 32.Zou L., Stillman B. (1998) Science 280, 593–596 [DOI] [PubMed] [Google Scholar]
- 33.Chen J., Peters R., Saha P., Lee P., Theodoras A., Pagano M., Wagner G., Dutta A. (1996) Nucleic Acids Res. 24, 1727–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.