Abstract
Streptococcus pneumoniae, a human pathogen, recruits complement regulator factor H to its bacterial cell surface. The bacterial PspC protein binds Factor H via short consensus repeats (SCR) 8–11 and SCR19–20. In this study, we define how bacterially bound Factor H promotes pneumococcal adherence to and uptake by epithelial cells or human polymorphonuclear leukocytes (PMNs) via a two-step process. First, pneumococcal adherence to epithelial cells was significantly reduced by heparin and dermatan sulfate. However, none of the glycosaminoglycans affected binding of Factor H to pneumococci. Adherence of pneumococci to human epithelial cells was inhibited by monoclonal antibodies recognizing SCR19–20 of Factor H suggesting that the C-terminal glycosaminoglycan-binding region of Factor H mediates the contact between pneumococci and human cells. Blocking of the integrin CR3 receptor, i.e. CD11b and CD18, of PMNs or CR3-expressing epithelial cells reduced significantly the interaction of pneumococci with both cell types. Similarly, an additional CR3 ligand, Pra1, derived from Candida albicans, blocked the interaction of pneumococci with PMNs. Strikingly, Pra1 inhibited also pneumococcal uptake by lung epithelial cells but not adherence. In addition, invasion of Factor H-coated pneumococci required the dynamics of host-cell actin microfilaments and was affected by inhibitors of protein-tyrosine kinases and phosphatidylinositol 3-kinase. In conclusion, pneumococcal entry into host cells via Factor H is based on a two-step mechanism. The first and initial contact of Factor H-coated pneumococci is mediated by glycosaminoglycans expressed on the surface of human cells, and the second step, pneumococcal uptake, is integrin-mediated and depends on host signaling molecules such as phosphatidylinositol 3-kinase.
Keywords: Adhesion, Cell Surface Receptor, Complement, Heparin, Integrin, Pneumococci
Introduction
Streptococcus pneumoniae (pneumococci) colonize as harmless commensals the mucosal epithelium of the human upper respiratory tract. However, pneumococci are also harmful pathogens causing severe infections in humans that are associated with high mortality rates and death. In addition to their ability to cause severe local infections such as otitis media and sinusitis, pneumococci cause life-threatening invasive diseases, including community-acquired pneumonia, sepsis, and meningitis (1–4). Pneumococci have evolved several strategies to adhere to host cells and to evade the host complement and immune attack, both representing prerequisites for pneumococci to disseminate into the lungs and bloodstream or to survive in various host niches. The key bacterial players are virulence determinants that are, with the exception of the toxin pneumolysin, displayed on the pneumococcal cell wall (3, 5, 6). To avoid complement-mediated bacterial lysis, pneumococci recruit, similar to other pathogens, the central complement regulators Factor H and C4b-binding protein (7, 8). The major Factor H-binding protein of Streptococcus pneumoniae is the choline-binding protein PspC (pneumococcal surface protein C), which represents a polymorphic surface protein and is termed Hic (factor H-binding inhibitor of complement) in another subset of strains (9–15). The C-terminal choline-binding domain of PspC anchors the protein noncovalently to the phosphorylcholine of the cell wall, whereas the PspC-like Hic (PspC11.4) is covalently anchored to the peptidoglycan of pneumococci after transpeptidase cleavage of the LPXTG motif (16). Both PspC and Hic share in their N-terminal regions a binding domain for the human complement inhibitor Factor H (12, 17, 18). The classical PspC was initially identified as an adhesin mediating adherence to host cells via hexameric motifs located in repeated domains of PspC. These repeats, referred to as R domains, are only present in the classical PspC proteins and interact in a human-specific manner with the ectodomain of the polymeric immunoglobulin receptor (pIgR)3 of mucosal epithelial cells (9, 19–21). As PspC interacts via two different epitopes with Factor H and the ectodomain of pIgR, this adhesin is able to execute both functions in parallel when displayed on the pneumococcal cell surface (10, 17).
Factor H, which consists of 20 domains referred to as short consensus repeats (SCR), is a single chain plasma glycoprotein and an important fluid-phase regulator of the alternative complement pathway (22). Factor H regulates the alternative pathway of complement activation in the fluid phase as well as on host cellular surfaces. Factor H binds to and inactivates C3b in fluid phase; however, Factor H can also bind to eukaryotic cells (23). Factor H surface binding is mediated by the host cellular surface, and molecules such as sialic acids, sulfated polysaccharides (heparins), or glycosaminoglycans. Factor H simultaneously binds both polyanionic surface molecules and surface-bound C3b molecule (24–26).
Factor H has three different C3b- and polyanion-binding sites, which are located in SCR1–4, SCR12–14, and SCR19–20 for C3b and SCR7, SCR13, and SCR19–20 for heparin, respectively (27–31). Factor H also binds dermatan sulfate (DS), which is a sulfated glycosaminoglycan and constituent of various proteoglycans that are present on eukaryotic cell surfaces and in the extracellular matrix (32–35). Moreover, Factor H and Factor H-like protein 1 (FHL-1), which is encoded by an alternatively spliced transcript and consists of the first seven SCR, bind via an RGD sequence in SCR4 to host cells (36). The integrin CR3, also referred to as CD11b/CD18 or αM/β2, of human polymorphonuclear leukocytes (PMNs) was identified as the cellular receptor recognizing Factor H (37).
We have previously demonstrated that Factor H binds to the pneumococcal PspC via two regions that are localized in SCR8–11 and SCR19–20 of Factor H (17). In addition, we indicated that pneumococcal-bound Factor H promotes bacterial adhesion and facilitates uptake thereby increasing the numbers of intracellular pneumococci. Although the function of Factor H as a bridging molecule is intriguing, the host cellular receptor(s) and induced signal cascades have not yet been addressed. In this study, we explored the molecular mechanisms that facilitate ingestion of pneumococci by epithelial cells via the Factor H mechanism. We suggest a two-step mechanism for Factor H-mediated pneumococcal invasion into host cells. First, Factor H binds to pneumococci, and bound Factor H is oriented in a way that it can interact with polyanionic molecules on the surface of host cells. This facilitates pneumococcal adhesion, and second, pneumococci exploit a Factor H-integrin complex for invasion into epithelial cells. This invasion process via Factor H requires the dynamics of the actin cytoskeleton and kinase activities of signal transduction molecules.
EXPERIMENTAL PROCEDURES
Cultivation of Pneumococci
S. pneumoniae (NCTC10319, serotype 35A, PspC3.3) were cultured on blood agar plates (Oxoid, Wesel, Germany) at 37 °C and 5% CO2 or in Todd-Hewitt broth (Roth, Karlsruhe, Germany) supplemented with 0.5% yeast extract (THY) to a density of 5 × 108 colony-forming units ml−1 (A600 of ∼0.5). Binding of Factor H to pneumococci is a general phenomenon, and previous data showed that NCTC10319 can be used to demonstrate the effects (17). NCTC10319 is a low encapsulated, pneumolysin-positive strain and a model strain for in vitro cell culture infection studies (17, 38, 39). Cytolytic effects due to pneumolysin are avoided in infections up to 3 h as described earlier (40).
Cell Lines and Culture Conditions
Cultivation of host cell lines was performed as described previously (17). Briefly, human A549 cells (lung alveolar epithelial cells, type II pneumocytes; ATCC CCL-185) were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mm glutamine, penicillin G (100 units ml−1), and streptomycin (0.1 mg ml−1) (all from PAA, Germany) at 37 °C and 5% CO2. A549 cells synthesize heparan sulfate, dermatan sulfate, or chondroitin sulfates but not the polymeric Ig receptor (19, 41). CHO-K1 wild-type cells (ATCC CCL-61, a hamster fibroblast cell line) and stably transfected CHO-CD11b/CD18 (CHO-K1 were stably transfected with cDNA for full-length human integrin complement receptor CR3 (CD11b/CD18)) (42) were cultivated in Ham's F-12 medium (Invitrogen) supplemented with 10% FBS and 2 mm glutamine (PAA). The medium for CHO-CR3 medium was further supplemented with 1 mg ml−1 of the antibiotic G418.
Factor H, Antibodies, and Other Reagents
Human Factor H and polyclonal anti-factor H antibodies were purchased from Calbiochem; mouse anti human CD11b antibodies and mouse anti-human CD18 antibodies were purchased from Invitrogen, and monoclonal mouse IgG1 and IgG2 isotype control antibodies were purchased from Ancell (Loerrach, Germany). Purification of rabbit polyclonal anti-pneumococcal IgG (19) was performed by protein A-Sepharose 4B affinity chromatography. Monoclonal antibodies (mAbs) M14, CO2, and C18 employed in blocking experiments were previously mapped to the middle region (M14), SCR19 (CO2), and SCR19–20 (C18), respectively (43, 44). Dermatan sulfate and FITC-heparin were purchased from ICN and Invitrogen, respectively, and heparin and heparinase III were purchased from Sigma. Candida albicans pH-regulated antigen 1 (Pra1) was expressed as described previously (45, 46). Cytochalasin D was purchased from MP Biomedicals; nocodazole was obtained from Sigma, and wortmannin, genistein, NSC23766, as well as Y276322 were obtained from Calbiochem. Secramine A, a specific inhibitor of Cdc42, was a kind gift of Tomas Kirchhausen, Immune Disease Institute, Harvard Medical School, Boston, and used as described previously (47–49). Clostridium difficile toxins TcdB10463 were kindly provided by Klaus Aktorius and Gudula Schmidt, Institute of Experimental and Clinical Pharmacology and Toxicology, University of Freiburg, Germany (50). The amounts of inhibitors used in this study are not toxic to pneumococci and A549 cells as reported recently (38, 47).
Pneumococcal Host Cell Adherence and Invasion Assay
Pneumococcal host cell adherence and invasion assays were performed as described previously (17). Briefly, A549 cells were seeded at a density of 2.5 × 104 in plain medium either on 24-well tissue culture plates (Greiner, Germany) or on glass coverslips (diameter, 12 mm) when assayed by immunofluorescence and cultivated for 48 h. Confluent monolayers were washed thoroughly and infected for 3 h with pneumococci in 500 μl of Dulbecco's minimal essential medium/HEPES (DMEM/HEPES; PAA) supplemented with 1% FBS at 37 °C using a multiplicity of infection of 50 bacteria per cell. The role of human Factor H for adherence was analyzed by incubating (1 × 107) pneumococci for 20 min with 2 μg of Factor H in a total volume of 100 μl of DMEM/HEPES with 1% FBS at 37 °C prior to host cell infections, and the infection assays were carried out in a total volume of 500 μl after adding the preincubated bacteria, without washing off the unbound Factor H. Post-infection cells were washed three times with PBS to remove unbound bacteria. The total number of adherent and invasive bacteria was monitored after detachment and lysis of cells with saponin (1% w/v) and plating the bacteria on blood agar. The number of viable intracellular bacteria was quantified by employing the antibiotic protection assay (40). Briefly, epithelial cells were infected with pneumococci (multiplicity of infection of 50), and thereafter, the infected and washed host cells were incubated for 1 h with DMEM/HEPES containing 100 μg ml−1 gentamicin and 100 units ml−1 penicillin G at 37 °C and 5% CO2 to kill extracellular and nonadherent pneumococci. Invasive and viable pneumococci were recovered from the intracellular compartments of the host cells by a saponin-mediated host cell lysis (1.0% w/v), and the total number of invasive pneumococci was monitored after plating sample aliquots on blood agar plates, followed by colony formation and enumeration. In inhibition experiments, infection assays were carried out in the presence of soluble heparin, dermatan sulfate, and antibodies or after pretreatment of host cells with 10 milliunits ml−1 heparinase III or with various pharmacological inhibitors as described recently (38, 47). The cells were pretreated for 3 h with heparinase III, which cleaves heparan sulfate but does not cleave unfractionated heparin or low molecular weight heparins. Thereafter, the cells were thoroughly washed prior to bacterial infections as described recently (39). Each experiment was repeated at least three times, and results were expressed as mean ± S.D.
Fluorescence Microscopy
Pneumococci attached to host epithelial cells were stained using a polyclonal pneumococcal antibodies (IgG) in combination with a secondary goat anti-rabbit IgG coupled with Alexa Fluor 488 (green) or Alexa Fluor 568 (red) (MoBiTec) (47). Post-infection nonspecific binding sites were blocked with 10% FBS, and before incubating the cells with pneumococcal antibodies (1:100), the infected cell layer was thoroughly washed with PBS. Bound antibodies were detected with an Alexa Fluor-labeled goat anti-rabbit Ig conjugate (MoBiTec, Göttingen, Germany). The glass coverslips were embedded “upside down” in Mowiol, sealed with nail polish, and stored at 4 °C. A confocal laser scanning microscope (Leica TCS SP5 AOBS) and the appropriate CLSM software (LAS AF SP5) were used for the image acquisition.
Flow Cytometric Analysis of Factor H Binding to Pneumococci
Binding of Factor H to viable pneumococci was measured using flow cytometry. Bacteria (1 × 107), cultivated in THY, were incubated in 100 μl of PBS with Factor H in the absence or presence of heparin, which was used as competitor. The suspensions were incubated at 37 °C, and after 30 min the bacteria were washed three times. Factor H bound to pneumococci was then detected by incubating the washed bacteria for 30 min at 37 °C with anti-Factor H antibodies followed by a FITC-conjugated anti-goat Ig antibody (MoBiTec) resulting in fluorescent bacteria. Finally, bacteria were washed, and the fluorescence intensity was analyzed by flow cytometry using a FACSCanto (BD Biosciences). FITC-labeled heparin was used to examine binding of heparin to pneumococci or to Factor H-coated pneumococci. Bacteria were detected using log-forward and log-side scatter dot plot, and a gating region was set to exclude debris and larger aggregates of bacteria. 10,000 bacteria (events) were analyzed for fluorescence using log scale amplifications. The mean fluorescence intensity multiplied by the percentage of PMNs in complex with fluorescent-labeled bacteria was recorded as a measure for binding activity.
Flow Cytometric Analysis of Pneumococcal Association with PMNs
Human PMNs were isolated from peripheral blood of healthy volunteers (Department of Transfusion Medicine, University of Greifswald; oral informed consent was obtained) as described previously (39). Briefly, 1 × 105 PMNs were incubated for 30 min in 100 μl of PBS, 1% FBS with 1 × 106 pneumococci pretreated with or without Factor H. Following bacterial incubations, PMNs were washed once with ice-cold PBS, 0.5% FBS by centrifugation at 280 × g for 5 min. The PMNs were then resuspended in 1:100 dilution of rabbit polyclonal anti-pneumococcal IgG and then kept on ice for 30 min. The suspension was washed once with ice-cold PBS, 0.5% FBS and incubated with Alexa Fluor 488-conjugated anti-rabbit Ig antibody (Invitrogen) for 30 min on ice. Finally, PMNs were fixed with 200 μl of PBS, 0.5% FBS, 1% paraformaldehyde. Binding of bacteria (PMN-pneumococci associates) was assessed by flow cytometry using a FACSCanto flow cytometer (BD Biosciences). The geometric mean fluorescence intensity multiplied with the percentage of gated labeled cells was recorded as a measure for pneumococcal binding and uptake.
Statistical Analysis
The binding experiments and infection experiments were performed at least three times, each in duplicate, and the data were expressed as mean ± S.D. Differences in adherence or invasion were analyzed by the two-tailed unpaired Student's t test. In all analyses, a p value of <0.05 was considered statistically significant.
RESULTS
Epithelial Cell Surface Glycosaminoglycans Promote Factor H-mediated Adherence of Pneumococci
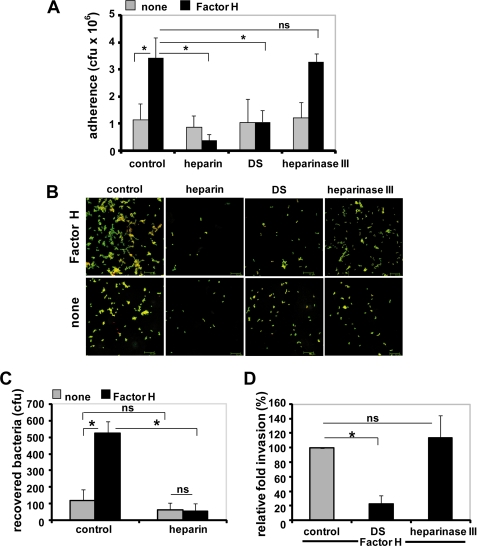
As Factor H binds heparin and DS, we investigated the role of the glycosaminoglycans heparin and DS on Factor H-mediated adherence of pneumococci to human lung epithelial cells A549. Competitive inhibition experiments were performed in the presence of soluble heparin (50 units/ml), DS (100 μg/ml), or after pretreatment of epithelial cells with heparinase III. Heparin and also DS inhibited Factor H-mediated pneumococcal adherence to A549 cells significantly, although the basal level of adherence in the absence of Factor H was not altered by these two inhibitors (Fig. 1A). In contrast, treatment of the lung epithelial cells with heparinase III, which specifically cleaves heparin sulfate without affecting the low molecular weight heparins, did not affect Factor H-mediated pneumococcal adherence (Fig. 1A). Immunofluorescence microscopy of adherent pneumococci confirmed the inhibitory effect of heparin and DS (Fig. 1B).
FIGURE 1.
Glycosaminoglycans inhibit Factor H-mediated pneumococcal adhesion to human lung epithelial cells. A, adherence of pneumococci via Factor H to lung epithelial A549 cells in the absence (control) or the presence of heparin (50 units/ml), dermatan sulfate (100 μg/ml), or after pretreatment with heparinase III (10 milliunits/ml) was estimated by quantifying the colony-forming units (cfu) per well obtained from plating onto blood agar plates. The infection assays were conducted with or without (none) pretreatment of pneumococci with Factor H. *, p < 0.02. B, immunofluorescence microscopy of Factor H-mediated pneumococcal adhesion after treatment of host cells with glycosaminoglycans or heparinase III. Adherent bacteria appear green/yellow (Alexa Fluor 488/568), and intracellular bacteria were stained red (Alexa Fluor 568). C and D, pneumococcal invasion into epithelial cells via Factor H is diminished in the presence of glycosaminoglycans. Pneumococcal invasion was determined after conducting infection assays in the absence (control) or presence of glycosaminoglycans by employing the antibiotic protection assay. *, p < 0.001 relative to infections carried out with Factor H but in the absence of inhibitor; ns, not significant.
Binding of Pneumococcus-bound Factor H to Glycosaminoglycans Interferes with Bacterial Invasion into Epithelial Cells
In addition to impairing pneumococcal adherence, both heparin and DS significantly reduced Factor H-initiated pneumococcal invasion into epithelial cells (Fig. 1, C and D). Again, treatment of epithelial cells with heparinase III had, similar to adherence, no effect on pneumococcal invasion (Fig. 1D). These data imply that heparin and DS interfered with Factor H-mediated pneumococcal invasion into epithelial cells. However, this effect is not directly linked with invasion as the degree of reduction is the consequence of the diminished ability of pneumococci to adhere to epithelial cells when glycosaminoglycans were added to the infection assays.
SCR19–20 of Pneumococcal-bound Factor H Mediates the Contact with Host Epithelial Cells
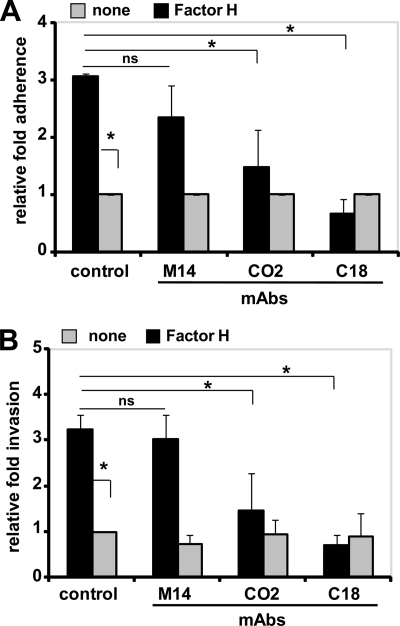
To identify the region or SCR of Factor H that mediates pneumococcal attachment to the surface of eukaryotic cells, domain mapped mAbs for specific regions of Factor H were used as blocking antibodies. The mAbs CO2 and C18 employed as competitors bind within the C terminus of Factor H to SCR19 and SCR19–20, respectively (43, 44). The presence of CO2 and C18 caused 42 and 85%, respectively, reduction of Factor H-mediated pneumococcal adherence to A549 epithelial cells compared with bacterial adherence in the absence of competitors but presence of Factor H (Fig. 2A). In contrast, mAb M14, which binds to the middle region of Factor H, showed no effect (Fig. 2A). None of the three mAbs, however, affected the basal level of pneumococcal adherence in the absence of Factor H. Similar to adherence, the C-terminal binding mAbs CO2 and C18 reduced Factor H-mediated pneumococcal invasion into epithelial cells (Fig. 2B). In conclusion, the heparin-binding site at the C terminus of Factor H, namely SCR19–20, mediates the contact between pneumococcus-bound Factor H and the surface of epithelial cells. By blocking the C-terminal domain of Factor H attached to pneumococci, Factor H-mediated bacterial adherence to human epithelial cells is reduced. Consequently, pneumococcal ingestion by epithelial cells is also reduced to basal levels.
FIGURE 2.
C-terminal SCR19–20 of Factor H is the key co-factor in Factor H-mediated pneumococcal adherence to and invasion into host cells. A, adherence of Factor H-coated pneumococci to A549 cells was determined in the presence of mAbs M14, CO2, or C18 (each 2 μg ml−1 per well) or absence of a mAb (control). The antibodies bind to the middle region (M14), SCR19 (CO2), or SCR19–20 (C18) of Factor H. Results are shown as relative adherence of Factor H-bound pneumococci compared with untreated pneumococci. *, p < 0.02; ns, not significant. B, invasion and intracellular survival of Factor H-coated pneumococci in the presence of monoclonal antibodies M14, CO2, or C18 (each 2 μg ml−1) were determined by the antibiotic protection assay. Results show the relative invasion of pneumococci into A549 cells compared with Factor H-treated bacteria and in the absence (control) of monoclonal antibodies. *, p < 0.02; ns, not significant.
Acquisition of Factor H by Pneumococci Is Not Influenced by Soluble Heparin
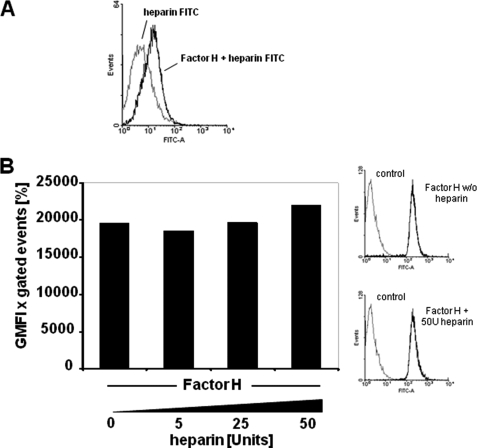
The effect of heparin on the interaction between pneumococci and Factor H was analyzed in competitive binding experiments to elucidate whether heparin competes with Factor H for binding to the bacteria. Binding of FITC-labeled heparin was investigated to untreated and Factor H-pretreated pneumococci, respectively. The flow cytometric analyses showed no binding of FITC-heparin to the surface of untreated pneumococci, whereas FITC-heparin bound to Factor H-pretreated and consequently Factor H-coated pneumococci (Fig. 3A). Importantly, Factor H acquisition by pneumococci was not affected by heparin (Fig. 3B). However, when Factor H is bound to the pneumococcal cell surface, heparin binding is enhanced as it binds to the accessible heparin-binding sites of Factor H (Fig. 3A). In conclusion, the presence of heparin did not interfere with pneumococcal acquisition of Factor H but blocks the C-terminal heparin-binding site of Factor H, which is involved in Factor H-mediated adherence.
FIGURE 3.
Effect of heparin on Factor H recruitment by pneumococci. A, binding of FITC-heparin (2 μg) to pneumococci or to Factor H-pretreated pneumococci was determined by flow cytometry. The histogram shows the log fluorescence intensity (FITC-A) on the x axis, and the y axis shows the number of events. B, binding of heparin-pretreated Factor H to pneumococci. The effect of heparin on pneumococcal recruitment of Factor H was analyzed by flow cytometry after preincubation of 2 μg of purified Factor H with the indicated amounts of heparin per reaction. Bacterial bound Factor H was determined by flow cytometry, and results were expressed as mean fluorescence intensity multiplied with the percentage of FITC-labeled bacteria. The graph shows a representative experiment. The results are also represented as histograms, where the x axis represents fluorescence of pneumococcus-associated Factor H, in the absence (w/o) or presence of 50 units of heparin, on a log10 scale, and the y axis represents the number of events.
Factor H Promotes Pneumococcal Binding to PMNs via Integrin CR3
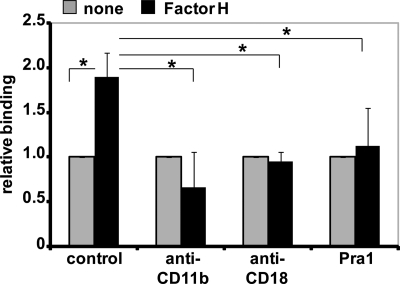
Binding of Factor H by pneumococci resulted in reduced complement activation on the pneumococcal surface and diminished complement-mediated opsonophagocytosis (8, 13, 14). As Factor H binds to the integrin CR3 of PMNs (37), we assessed whether CR3 acts as a receptor on PMNs and thereby promotes adhesion of Factor H-coated pneumococci. Pretreatment of pneumococci with Factor H resulted in increased association of pneumococci with PMNs as determined by flow cytometry (Fig. 4). However, mAbs that block specifically CD11b and CD18, respectively, and also the CR3 ligand Pra1 (pH-regulated antigen 1) of Candida albicans (45) abolished binding of Factor H-coated pneumococci to human PMNs (Fig. 4). In contrast, the corresponding isotype control antibodies IgG1 and IgG2, respectively, showed no effect (data not shown). These data suggest that integrin CR3 of PMNs recognizes Factor H-coated pneumococci and that Factor H bound to pneumococci promotes pneumococcal binding to PMNs.
FIGURE 4.
Factor H promotes pneumococcal binding to PMNs via integrin CR3. Pneumococci were incubated with PMNs for 30 min, in the absence (control) or presence of anti-CD11b (2 μg), anti-CD18 (2 μg), or integrin CR3 (CD11b/CD18) ligand Pra1 (2 μg). Pneumococcal binding to PMNs was investigated in the absence (none) or presence of bacteria-bound Factor H by flow cytometry. The results were calculated (mean fluorescence intensity × percentage of gated positive events), and the data show the relative binding ratios compared with Factor H untreated pneumococci. *, p < 0.02.
CR3 Facilitates Factor H-mediated Pneumococcal Invasion into Eukaryotic Cells
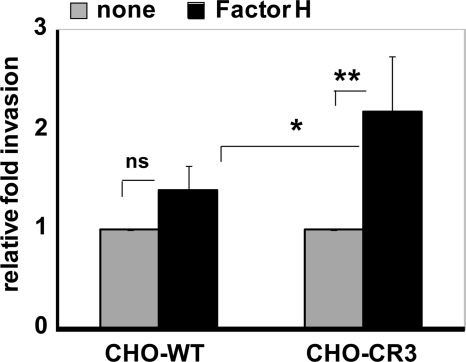
To assess the role of CR3 as host cell receptor facilitating ingestion of Factor H-coated pneumococci by epithelial cells, CHO cells expressing human CR3 (42) were infected with Factor H-coated pneumococci; thereafter, bacterial invasion was assayed and compared with wild-type CHO cells lacking the CR3 receptor. Invasion of Factor H-coated pneumococci into CR3-expressing CHO cells was >2-fold increased compared with untreated pneumococci. However, invasion of Factor H-coated pneumococci into wild-type CHO-K1 cells that lack CR3 was also increased but at a relatively low level, and the Factor H effect in wild-type CHO cells was statistically not significant (Fig. 5A). Adherence of pneumococci to CR3-expressing and wild-type CHO cells was similar as visualized by immunofluorescence microscopy (data not shown). However, adherence of pneumococci to CHO-CR3 cells via Factor H was significantly decreased when blocking antibodies to the subunits of CR3, namely CD11b and CD18, respectively, or Pra1 was used as a competitive inhibitor in the infections (data not shown).
FIGURE 5.
Integrin CR3 (CD11b/CD18) promotes invasion of Factor H-coated pneumococci into epithelial cells. Invasion and intracellular survival of pneumococci in CHO-WT (CHO-K1 cells) and CHO-CR3 cells were determined by the antibiotic protection assay. The results are shown relative to infections conducted with untreated pneumococci. *, p < 0.05; **, p < 0.01; ns, not significant.
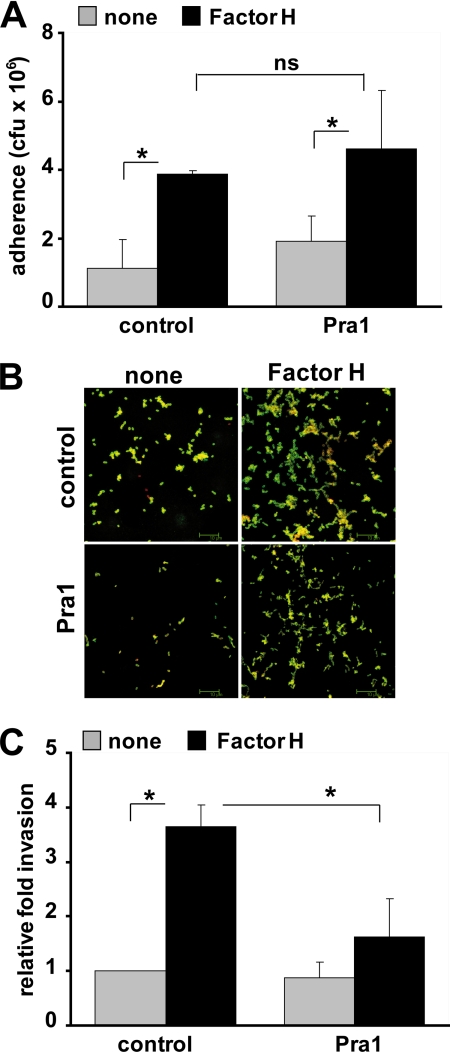
Factor H facilitates pneumococcal invasion into human lung epithelial cells A549 and also into CHO-K1 wild-type cells, although the latter at relatively low levels (Figs. 2 and 5). Consequently, we assumed the existence of an additional receptor on epithelial cells that may contribute to Factor H-mediated pneumococcal uptake by these host cells. Moreover, A549 lung epithelial cells are not known to express the CR3 receptor (51). Therefore, the fungal Pra1 protein, which is a CR3 ligand and which, however, binds also to non-CR3-expressing endothelial cells,4 was used as a competitor to investigate whether Pra1 can also inhibit infections of Factor H-coated pneumococci to A549 cells. Pra1 did not affect Factor H-mediated pneumococcal adhesion to A549 epithelial cells (Fig. 6, A and B). However, Pra1 inhibited pneumococcal invasion of lung epithelial cells via the Factor H mechanism by ∼45% (Fig. 6C). Taken together, pneumococcal adherence via Factor H is mediated by heparin and DS, and bacterial invasion into host cells is receptor-mediated. Moreover, Factor H-mediated invasion can be blocked efficiently by the integrin-ligand Pra1.
FIGURE 6.
Factor H-mediated pneumococcal internalization by epithelial cells is inhibited by the integrin-binding protein Pra1. A, pneumococcal adherence to A549 cells was determined in the absence (control) or presence of integrin CR3 ligand Pra1 (2 μg ml−1). The infection assays were conducted with or without (none) pretreatment of pneumococci with Factor H. *, p < 0.03; ns, not significant, relative to infections conducted in the absence of Factor H. B, immunofluorescence microscopy of pneumococcal adherence via Factor H to A549 cells in the absence (control) or presence of Pra1. C, invasion and intracellular survival of pneumococci in A549 cells were monitored in the absence (control) or the presence of Pra1 (2 μg ml−1) by the antibiotic protection assay. The results are shown relative to infections conducted in the absence of Pra1 and Factor H. *, p < 0.02.
Actin Cytoskeleton Dynamics Are Essential for Factor H-mediated Pneumococcal Internalization by Epithelial Cells
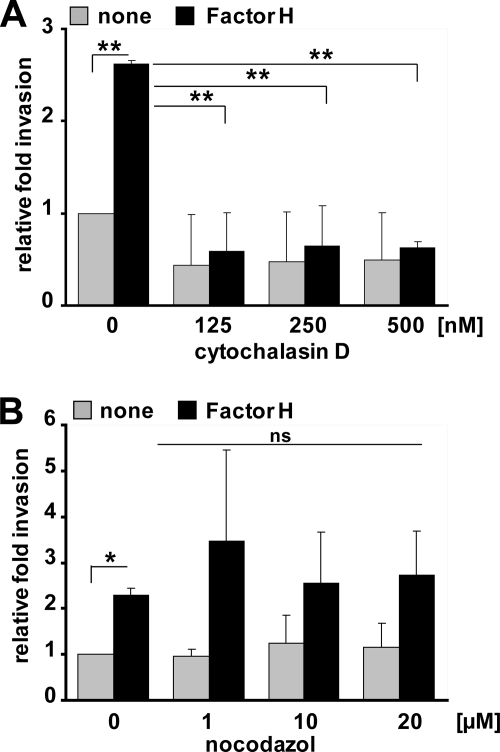
The impact of the actin cytoskeleton and microtubules on pneumococcal internalization by host cells via bacterially bound Factor H was investigated by employing the pharmacological inhibitors cytochalasin D and nocodazole. Cytochalasin D, which inhibits actin polymerization, inhibited significantly Factor H-mediated pneumococcal invasion into A549 cells indicating the important role of the actin dynamics for pneumococcal uptake (Fig. 7A). In contrast, inhibition of microtubule polymerization by nocodazole did not affect internalization of Factor H-coated pneumococci by host cells (Fig. 7B).
FIGURE 7.
Factor H-mediated pneumococcal ingestion by epithelial cells requires actin cytoskeleton dynamics. A and B, pneumococcal invasion into A549 was determined in the presence of cytochalasin D (A) or nocodazole (B) by the antibiotic protection assay as described previously (47). Pneumococcal invasion indicates the relative number of intracellular bacteria (Factor H pretreated or untreated (none)) in the presence of an inhibitor compared with pneumococcal invasion in the absence of an inhibitor. *, p < 0.05; **, p < 0.001; ns, not significant.
Factor H-mediated Pneumococcal Invasion Relies on Protein-Tyrosine Kinase and PI3K Activities
Protein-tyrosine kinases, PI3K, and the activities of the small GTPases of Rho family are essential for uptake of various pathogenic bacteria by host cells. Phosphatidylinositol 3-kinase activity is critical for the invasion of host cells by pathogenic bacteria such as Listeria monocytogenes, Helicobacter pylori, and Escherichia coli (52–54). In addition, the small GTPase RhoA has been demonstrated to be important for uptake of Mycobacterium avium and Pseudomonas aeruginosa (55, 56), whereas Rac1 and Cdc42 play a crucial role in host cell invasion of Salmonella enterica, Shigella flexneri, and Campylobacter jejuni (57–59). Finally, Rho family GTPases RhoA, Rac1, and Cdc42 are required for efficient invasion of HeLa cells by group B streptococci (60).
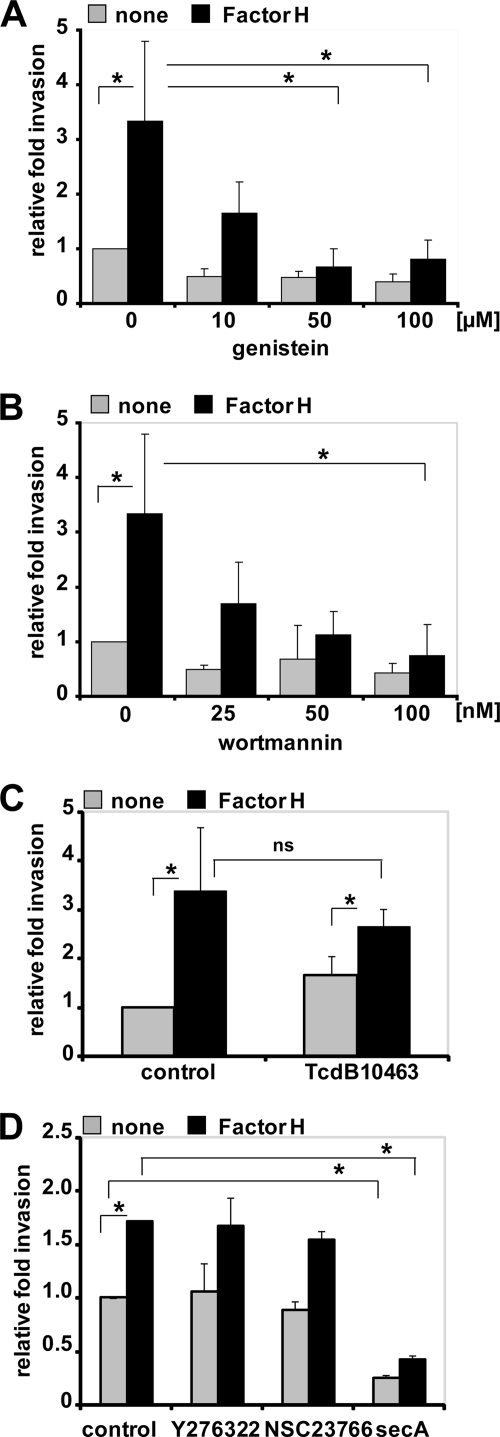
To explore the role of protein-tyrosine kinases and PI3K for ingestion of Factor H-coated pneumococci by epithelial cells, bacterial invasion into A549 cells was determined in the presence of protein-tyrosine kinase inhibitors genistein or PI3K-specific inhibitor wortmannin. The results revealed that both inhibitors blocked Factor H-mediated pneumococcal internalization in a dose-dependent manner (Fig. 8, A and B). To elucidate the role of small GTPases (RhoA, Rac1, and Cdc42), C. difficile toxin B TcdB10463, which glucosylates and inactivates the Rho family of small GTPases Rho (A/B/C), Rac1, and Cdc42, was employed. In addition, Y27632, a specific Rho-associated protein kinase inhibitor, NSC23766, a specific Rac1 inhibitor, or secramine A, which is a potent inhibitor of Cdc42 activation, were also used. Pretreatment of A549 cells with TcdB10463 did not affect Factor H-mediated pneumococcal uptake by host cells (Fig. 8C). Similarly, treatment of the host cells with Y27632 or NSC23766 did not affect pneumococcal invasion (Fig. 8D). Although secramine A pretreatment of A549 cells resulted in significant reduction in Factor H-initiated pneumococcal invasion, a similar level of reduction was also observed for Factor H untreated pneumococci (Fig. 8D), suggesting that Cdc42 is not involved in pneumococcal invasion via Factor H. The concentration of the inhibitors used did not affect the increase in Factor H-mediated pneumococcal adherence (data not shown). Taken together, the data indicated the essential roles of host cell protein-tyrosine kinases and PI3Ks for invasion of Factor H-coated pneumococci.
FIGURE 8.
Protein-tyrosine kinase activities and PI3K but not small Rho family GTPases are essential for Factor H-mediated pneumococcal ingestion by epithelial cells. The number of invasive pneumococci was determined in the presence of genistein (A), which is a phosphotyrosine kinase inhibitor, PI3K inhibitor wortmannin (B), C. difficile toxin B, TcdB10463 (30 ng ml−1) (C), or specific individual inhibitors of Rho family GTPases such as Y27632 (50 μm), Rac1 inhibitor NSC23766 (50 μm), or Cdc42 inhibitor secramine A (10 μm) (D) by employing the antibiotic protection assay. Shown is the relative invasion of Factor H pretreated or untreated (none) pneumococci in the presence of an inhibitor compared with pneumococcal invasion in the absence of an inhibitor. *, p < 0.05; ns, not significant, relative to infections in the presence of Factor H but absence of inhibitors.
DISCUSSION
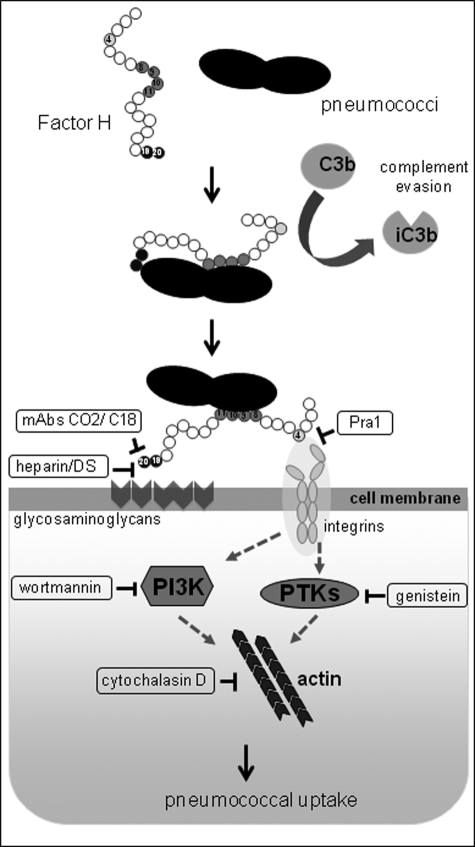
The complement system is part of the host innate immune system and pivotal for the host defense mechanisms. To establish an infection, the pathogen has to overcome this first line of immune defense. Hence, pathogens have evolved various efficient immune evasion strategies to counteract host complement attacks (61–65). A strategy that has attracted particular interest is the ability of pathogenic microorganisms to acquire fluid phase soluble complement regulators to their surface. Factor H, a fluid phase regulator of the alternative complement pathway, is a central host protein that is acquired by pathogens and attached to the pathogen surface it aids in immune evasion and avoids complement-mediated killing. Pneumococci, serious pathogenic bacteria causing life-threatening infections in humans, recruit Factor H to their surface (9–15). Recently, we demonstrated that Factor H bound to pneumococci via an interaction with PspC increased in a cell type unspecific manner bacterial attachment to and invasion into host cells (17). Here, we demonstrate that Factor H-promoted pneumococcal invasion is at least a two-step process that requires a concerted action of host epithelial cell surface glycosaminoglycans, integrin receptors, as well as host cell-signaling molecules of different pathways. Moreover, the interaction of bacterial bound Factor H with glycosaminoglycans and integrins suggests a critical role for the C terminus of the protein, i.e. SCR19–20, as well as the RGD-containing SCR4 of Factor H that may interact with the integrins for the infectious process (Fig. 9).
FIGURE 9.
Schematic model of the two-step Factor H-integrin complex-mediated pneumococcal epithelial cell invasion mechanism. PTK, protein-tyrosine kinase.
Factor H is an abundant human plasma protein that binds to the surface of host cells and other self-surface moieties via recognition of polyanionic components such as glycosaminoglycans and sialic acids (24, 26). The cell surface of the used A549 lung epithelial cells is decorated with polyanionic components such as heparan sulfate, dermatan sulfate, or chondroitin sulfate (41). The impact of host cell glycosaminoglycans for adherence of Factor H-coated pneumococci was demonstrated in inhibition experiments with heparin or dermatan sulfate as competitors. Both glycosaminoglycans significantly reduced adherence and also invasion of Factor H-coated pneumococci into human epithelial cells, whereas treatment of host cells with heparinase III, an enzyme that cleaves exclusively heparan sulfate on cell surfaces, showed no inhibitory effect. This is in contrast to pneumococcal adherence via human thrombospondin-1, which was significantly reduced by host cell treatment with heparinase III (39). Taken together, these data point to a critical role of the heparin/glycosaminoglycan-binding sites within the Factor H molecule. In addition, the competitive binding assays demonstrated that heparin did not compete with Factor H for binding to pneumococci, whereas it can interact with Factor H attached to the pneumococcal surface. These results seem not to be in agreement with the in vitro effect of heparin to block Factor H binding to PspC protein as shown by surface plasmon resonance studies (17). However, the assays conducted here represent a closer in vivo scenario. Under physiological conditions, the SCR8–11 of Factor H, which acts as the major binding region for PspC and lacks a heparin-binding site, represent the binding region required for recruitment of Factor H to the pneumococcal surface. The major heparin/glycosaminoglycan-binding site of Factor H is located in the C terminus, in SCR19–20, and mediates binding of Factor H to endothelial cells (43, 66, 67). This region is also pivotal and makes the first contact of pneumococci to host cells as only mAbs, which bind to this particular region, block Factor H-mediated bacterial adherence to host cells. In contrast, mAbs that bind to the middle region of Factor H had no effect.
Factor H is also an adhesion molecule for human PMNs, and the integrin CR3 was identified as its cellular receptor on PMNs (37). Moreover, adherence of PMNs to immobilized glycosaminoglycans such as heparin and chondroitin was enhanced in the presence of Factor H, suggesting that in this scenario Factor H promotes adhesion of PMNs to tissues (37). Therefore, we investigated the effect of Factor H on pneumococcal attachment to PMNs and aimed to identify the receptor(s) engaged by pneumococcus-bound Factor H. Apparently, Factor H-enhanced attachment of pneumococci to PMNs and CR3 was identified as a cellular receptor required for augmented bacterial attachment and probably also for enhanced phagocytosis. Inhibition experiments conducted with mAbs blocking each subunit of the dimeric CR3 receptor (i.e. anti-CD11b and anti-CD18) and also the fungal CR3-ligand Pra1 of C. albicans (45) confirmed an essential role of this cellular receptor for attachment of Factor H-coated pneumococci. Infection assays performed with CHO cells expressing CR3 further demonstrated that this integrin can also act as a cellular receptor facilitating Factor H-dependent invasion of pneumococci into epithelial cells. Moreover, pneumococcus-bound Factor H promoted bacterial invasion into A549 cells, and this effect was dramatically blocked by Pra1. Strikingly, Pra1 had no inhibitory effect on the increased adherence of Factor H-coated pneumococci to lung epithelial cells, whereas a significant reduction was observed when using CHO-CR3 cells (data now shown). These results suggested that host cells use a different repertoire of Factor H receptors and also that the Pra1 protein interacts with several distinct receptors expressed on the surface of human cells that allow Factor H-mediated uptake of pneumococci by host cells. Similar to adhesive glycoproteins such as fibronectin, the Factor H molecule contains in SCR4 an Arg-Gly-Asp sequence. These tripeptides bind to different integrin receptors as has been shown for fibronectin, for example (68). Factor H binding to CR3 and its likely interaction via Arg-Gly-Asp to an additional integrin receptor suggest that integrin(s) acts as receptors and mediators of Factor H-promoted pneumococcal and bacterial invasion. This hypothesis is strengthened by the fact that the Pra1 protein binds directly to CR3 and also to an additional cellular receptor as Pra1 inhibited Factor H-mediated invasion of pneumococci into A549 cells.
This study demonstrates the importance and the impact of host cell cytoskeleton and signaling molecules for the Factor H-mediated bacterial internalization into eukaryotic cells. This Factor H mechanism requires the dynamics of the actin cytoskeleton but not microtubules, and this effect is in contrast to PspC-pIgR-mediated pneumococcal internalization into human cells as shown recently (47). Cytochalasin D significantly inhibited Factor H-mediated pneumococcal invasion into human lung epithelial cells, and inhibition of microtubule polymerization did not interfere with Factor H-dependent pneumococcal uptake. Moreover, inhibition assays showed that the activities of protein-tyrosine kinases and PI3K but not small GTPases of the Rho family (RhoA, Rac1, and Cdc42) are essential for Factor H-mediated pneumococcal ingestion by host epithelial cells. Pretreatment of A549 lung epithelial cells with genistein, which is a general protein-tyrosine kinase inhibitor, or with wortmannin, a specific PI3K inhibitor, blocked pneumococcal internalization in a dose-dependent manner. These effects are similar to another integrin-mediated pneumococcal uptake mechanism (38). However, in contrast to PspC-pIgR-mediated pneumococcal uptake that requires the activity of small GTPase Cdc42, pneumococcal internalization via the bridging molecule Factor H does not rely on small GTPases. Inhibition of endogenous Rho family members by C. difficile toxin B TcdB-10463 did not affect Factor H-dependent pneumococcal uptake by host cells. Similarly, specific inhibition of Rac1 using NSC23766 or blocking of Rho-associated protein kinase using the inhibitory substance Y27632 had no effect on Factor H-coated pneumococcal uptake by host cells. Apparently, inhibition of Cdc42 activity by secramine A resulted in a significant reduction of pneumococcal uptake by Factor H mechanism. Similar, secramine A inhibited invasion of Factor H-untreated pneumococci, suggesting that Cdc42 also plays no significant role in the Factor H-mediated pneumococcal invasion mechanism.
Further investigations are required to identify the individual kinases that are activated during Factor H-mediated pneumococcal uptake by host cells and to delineate the outside-inside and inside-outside signaling events during Factor H-mediated pneumococcal infection of eukaryotic host cells.
In conclusion, Factor H acquisition by pneumococci endows the bacteria with an adhesive host-derived molecule that on mucosal epithelial surfaces promotes pneumococcal adherence to and invasion into host cells. This two-step mechanism requires a concerted action of glycosaminoglycans expressed on the surface of host epithelial cells and of integrin receptors and also host cell signaling proteins and pathways.
Acknowledgments
We are grateful to Tomas Kirchhausen (Harvard Medical School, Boston) and Bo Xu and Gerald B. Hammond (University of Louisville, Louisville) for providing secramine A and to Gudula Schmidt and Klaus Aktories (University of Freiburg, Germany) for kindly providing toxins TcdB-10463. CHO-K1 and CHO-CR3 cells were kindly provided by Rainer Haas, Max von Pettenkofer Institute, Munich, Germany.
This work was supported in part by Deutsche Forschungsgemeinschaft Grants DFG Ha 3125/2-1 and 3125/4-1.
S. Luo and P. F. Zipfel, manuscript in preparation.
- pIgR
- polymeric immunoglobulin receptor
- PI3K
- phosphatidylinositol 3-kinase
- SCR
- short consensus repeat
- mAb
- monoclonal antibody
- DMEM
- Dulbecco's modified Eagle's medium
- FBS
- fetal bovine serum
- PBS
- phosphate-buffered saline
- FITC
- fluorescein isothiocyanate
- DS
- dermatan sulfate
- CHO
- Chinese hamster ovary.
REFERENCES
- 1.Austrian R. (1986) J. Antimicrob. Chemother. 18, Suppl. A, 35–45 [DOI] [PubMed] [Google Scholar]
- 2.Cartwright K. (2002) Eur. J. Pediatr. 161, 188–195 [DOI] [PubMed] [Google Scholar]
- 3.Hammerschmidt S. (2006) Curr. Opin. Microbiol. 9, 12–20 [DOI] [PubMed] [Google Scholar]
- 4.Musher D. M. (1992) Clin. Infect. Dis. 14, 801–807 [DOI] [PubMed] [Google Scholar]
- 5.Bergmann S., Hammerschmidt S. (2006) Microbiology 152, 295–303 [DOI] [PubMed] [Google Scholar]
- 6.Kadioglu A., Weiser J. N., Paton J. C., Andrew P. W. (2008) Nat. Rev. Microbiol. 6, 288–301 [DOI] [PubMed] [Google Scholar]
- 7.Dieudonné-Vatran A., Krentz S., Blom A. M., Meri S., Henriques-Normark B., Riesbeck K., Albiger B. (2009) J. Immunol. 182, 7865–7877 [DOI] [PubMed] [Google Scholar]
- 8.Zipfel P. F., Hallström T., Hammerschmidt S., Skerka C. (2008) Vaccine 26, Suppl. 8, I67–74 [DOI] [PubMed] [Google Scholar]
- 9.Dave S., Brooks-Walter A., Pangburn M. K., McDaniel L. S. (2001) Infect. Immun. 69, 3435–3437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dave S., Carmicle S., Hammerschmidt S., Pangburn M. K., McDaniel L. S. (2004) J. Immunol. 173, 471–477 [DOI] [PubMed] [Google Scholar]
- 11.Duthy T. G., Ormsby R. J., Giannakis E., Ogunniyi A. D., Stroeher U. H., Paton J. C., Gordon D. L. (2002) Infect. Immun. 70, 5604–5611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janulczyk R., Iannelli F., Sjoholm A. G., Pozzi G., Bjorck L. (2000) J. Biol. Chem. 275, 37257–37263 [DOI] [PubMed] [Google Scholar]
- 13.Jarva H., Hellwage J., Jokiranta T. S., Lehtinen M. J., Zipfel P. F., Meri S. (2004) J. Immunol. 172, 3111–3118 [DOI] [PubMed] [Google Scholar]
- 14.Jarva H., Janulczyk R., Hellwage J., Zipfel P. F., Björck L., Meri S. (2002) J. Immunol. 168, 1886–1894 [DOI] [PubMed] [Google Scholar]
- 15.Neeleman C., Geelen S. P., Aerts P. C., Daha M. R., Mollnes T. E., Roord J. J., Posthuma G., van Dijk H., Fleer A. (1999) Infect. Immun. 67, 4517–4524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iannelli F., Oggioni M. R., Pozzi G. (2002) Gene 284, 63–71 [DOI] [PubMed] [Google Scholar]
- 17.Hammerschmidt S., Agarwal V., Kunert A., Haelbich S., Skerka C., Zipfel P. F. (2007) J. Immunol. 178, 5848–5858 [DOI] [PubMed] [Google Scholar]
- 18.Lu L., Ma Y., Zhang J. R. (2006) J. Biol. Chem. 281, 15464–15474 [DOI] [PubMed] [Google Scholar]
- 19.Elm C., Braathen R., Bergmann S., Frank R., Vaerman J. P., Kaetzel C. S., Chhatwal G. S., Johansen F. E., Hammerschmidt S. (2004) J. Biol. Chem. 279, 6296–6304 [DOI] [PubMed] [Google Scholar]
- 20.Hammerschmidt S., Tillig M. P., Wolff S., Vaerman J. P., Chhatwal G. S. (2000) Mol. Microbiol. 36, 726–736 [DOI] [PubMed] [Google Scholar]
- 21.Zhang J. R., Mostov K. E., Lamm M. E., Nanno M., Shimida S., Ohwaki M., Tuomanen E. (2000) Cell 102, 827–837 [DOI] [PubMed] [Google Scholar]
- 22.Ripoche J., Day A. J., Willis A. C., Belt K. T., Campbell R. D., Sim R. B. (1986) Biosci. Rep. 6, 65–72 [DOI] [PubMed] [Google Scholar]
- 23.Zipfel P. F., Skerka C. (2009) Nat. Rev. Immunol. 9, 729–740 [DOI] [PubMed] [Google Scholar]
- 24.Jokiranta T. S., Jaakola V. P., Lehtinen M. J., Pärepalo M., Meri S., Goldman A. (2006) EMBO J. 25, 1784–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manuelian T., Hellwage J., Meri S., Caprioli J., Noris M., Heinen S., Jozsi M., Neumann H. P., Remuzzi G., Zipfel P. F. (2003) J. Clin. Invest. 111, 1181–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meri S., Pangburn M. K. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 3982–3986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blackmore T. K., Hellwage J., Sadlon T. A., Higgs N., Zipfel P. F., Ward H. M., Gordon D. L. (1998) J. Immunol. 160, 3342–3348 [PubMed] [Google Scholar]
- 28.Blackmore T. K., Sadlon T. A., Ward H. M., Lublin D. M., Gordon D. L. (1996) J. Immunol. 157, 5422–5427 [PubMed] [Google Scholar]
- 29.Hellwage J., Jokiranta T. S., Friese M. A., Wolk T. U., Kampen E., Zipfel P. F., Meri S. (2002) J. Immunol. 169, 6935–6944 [DOI] [PubMed] [Google Scholar]
- 30.Jokiranta T. S., Hellwage J., Koistinen V., Zipfel P. F., Meri S. (2000) J. Biol. Chem. 275, 27657–27662 [DOI] [PubMed] [Google Scholar]
- 31.Pangburn M. K., Atkinson M. A., Meri S. (1991) J. Biol. Chem. 266, 16847–16853 [PubMed] [Google Scholar]
- 32.Iozzo R. V. (1998) Annu. Rev. Biochem. 67, 609–652 [DOI] [PubMed] [Google Scholar]
- 33.Iozzo R. V., Murdoch A. D. (1996) FASEB J. 10, 598–614 [PubMed] [Google Scholar]
- 34.Kjellén L., Lindahl U. (1991) Annu. Rev. Biochem. 60, 443–475 [DOI] [PubMed] [Google Scholar]
- 35.Saito A., Munakata H. (2005) J. Biochem. 137, 225–233 [DOI] [PubMed] [Google Scholar]
- 36.Hellwage J., Kühn S., Zipfel P. F. (1997) Biochem. J. 326, 321–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DiScipio R. G., Daffern P. J., Schraufstätter I. U., Sriramarao P. (1998) J. Immunol. 160, 4057–4066 [PubMed] [Google Scholar]
- 38.Bergmann S., Lang A., Rohde M., Agarwal V., Rennemeier C., Grashoff C., Preissner K. T., Hammerschmidt S. (2009) J. Cell Sci. 122, 256–267 [DOI] [PubMed] [Google Scholar]
- 39.Rennemeier C., Hammerschmidt S., Niemann S., Inamura S., Zähringer U., Kehrel B. E. (2007) FASEB J. 21, 3118–3132 [DOI] [PubMed] [Google Scholar]
- 40.Pracht D., Elm C., Gerber J., Bergmann S., Rohde M., Seiler M., Kim K. S., Jenkinson H. F., Nau R., Hammerschmidt S. (2005) Infect. Immun. 73, 2680–2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berry L., Andrew M., Post M., Ofosu F., O'Brodovich H. (1991) Am. J. Respir. Cell Mol. Biol. 4, 338–346 [DOI] [PubMed] [Google Scholar]
- 42.Ingalls R. R., Arnaout M. A., Golenbock D. T. (1997) J. Immunol. 159, 433–438 [PubMed] [Google Scholar]
- 43.Józsi M., Heinen S., Hartmann A., Ostrowicz C. W., Hälbich S., Richter H., Kunert A., Licht C., Saunders R. E., Perkins S. J., Zipfel P. F., Skerka C. (2006) J. Am. Soc. Nephrol. 17, 170–177 [DOI] [PubMed] [Google Scholar]
- 44.Oppermann M., Manuelian T., Józsi M., Brandt E., Jokiranta T. S., Heinen S., Meri S., Skerka C., Götze O., Zipfel P. F. (2006) Clin. Exp. Immunol. 144, 342–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soloviev D. A., Fonzi W. A., Sentandreu R., Pluskota E., Forsyth C. B., Yadav S., Plow E. F. (2007) J. Immunol. 178, 2038–2046 [DOI] [PubMed] [Google Scholar]
- 46.Luo S., Poltermann S., Kunert A., Rupp S., Zipfel P. F. (2009) Mol. Immunol. 47, 541–550 [DOI] [PubMed] [Google Scholar]
- 47.Agarwal V., Hammerschmidt S. (2009) J. Biol. Chem. 284, 19427–19436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pelish H. E., Peterson J. R., Salvarezza S. B., Rodriguez-Boulan E., Chen J. L., Stamnes M., Macia E., Feng Y., Shair M. D., Kirchhausen T. (2006) Nat. Chem. Biol. 2, 39–46 [DOI] [PubMed] [Google Scholar]
- 49.Xu B., Pelish H., Kirchhausen T., Hammond G. B. (2006) Org. Biomol. Chem. 4, 4149–4157 [DOI] [PubMed] [Google Scholar]
- 50.Genth H., Dreger S. C., Huelsenbeck J., Just I. (2008) Int. J. Biochem. Cell Biol. 40, 592–597 [DOI] [PubMed] [Google Scholar]
- 51.Bermudez L. E., Goodman J., Petrofsky M. (1999) Infect. Immun. 67, 4912–4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ireton K., Payrastre B., Chap H., Ogawa W., Sakaue H., Kasuga M., Cossart P. (1996) Science 274, 780–782 [DOI] [PubMed] [Google Scholar]
- 53.Kwok T., Backert S., Schwarz H., Berger J., Meyer T. F. (2002) Infect. Immun. 70, 2108–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reddy M. A., Prasadarao N. V., Wass C. A., Kim K. S. (2000) J. Biol. Chem. 275, 36769–36774 [DOI] [PubMed] [Google Scholar]
- 55.Kazmierczak B. I., Jou T. S., Mostov K., Engel J. N. (2001) Cell. Microbiol. 3, 85–98 [DOI] [PubMed] [Google Scholar]
- 56.Sangari F. J., Goodman J., Bermudez L. E. (2000) Cell. Microbiol. 2, 561–568 [DOI] [PubMed] [Google Scholar]
- 57.Hardt W. D., Chen L. M., Schuebel K. E., Bustelo X. R., Galán J. E. (1998) Cell 93, 815–826 [DOI] [PubMed] [Google Scholar]
- 58.Krause-Gruszczynska M., Rohde M., Hartig R., Genth H., Schmidt G., Keo T., König W., Miller W. G., Konkel M. E., Backert S. (2007) Cell. Microbiol. 9, 2431–2444 [DOI] [PubMed] [Google Scholar]
- 59.Tran Van Nhieu G., Caron E., Hall A., Sansonetti P. J. (1999) EMBO J. 18, 3249–3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burnham C. A., Shokoples S. E., Tyrrell G. J. (2007) FEMS Microbiol. Lett. 272, 8–14 [DOI] [PubMed] [Google Scholar]
- 61.Kraiczy P., Würzner R. (2006) Mol. Immunol. 43, 31–44 [DOI] [PubMed] [Google Scholar]
- 62.Lambris J. D., Ricklin D., Geisbrecht B. V. (2008) Nat. Rev. Microbiol. 6, 132–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rooijakkers S. H., van Strijp J. A. (2007) Mol. Immunol. 44, 23–32 [DOI] [PubMed] [Google Scholar]
- 64.Zipfel P. F., Skerka C., Hellwage J., Jokiranta S. T., Meri S., Brade V., Kraiczy P., Noris M., Remuzzi G. (2002) Biochem. Soc. Trans. 30, 971–978 [DOI] [PubMed] [Google Scholar]
- 65.Zipfel P. F., Würzner R., Skerka C. (2007) Mol. Immunol. 44, 3850–3857 [DOI] [PubMed] [Google Scholar]
- 66.Cheng Z. Z., Hellwage J., Seeberger H., Zipfel P. F., Meri S., Jokiranta T. S. (2006) Mol. Immunol. 43, 972–979 [DOI] [PubMed] [Google Scholar]
- 67.Jokiranta T. S., Cheng Z. Z., Seeberger H., Jòzsi M., Heinen S., Noris M., Remuzzi G., Ormsby R., Gordon D. L., Meri S., Hellwage J., Zipfel P. F. (2005) Am. J. Pathol. 167, 1173–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ruoslahti E., Pierschbacher M. D. (1987) Science 238, 491–497 [DOI] [PubMed] [Google Scholar]