Abstract
Aim
To evaluate the expression pattern of glial cell line-derived neurotrophic factor (GDNF) with its receptors GDNF family receptor alpha-1 (GFRα-1) and Ret in the human corneal and limbal tissues, as well as in the primary human limbal epithelial cultures (PHLEC).
Methods
Expression of GDNF and its receptors, and the co-localisation with stem cell associated and differentiation markers were evaluated by immunofluorescent staining, western blot analysis and real-time PCR in the fresh human corneoscleral tissues, as well as in the PHLEC. Single cell colony-forming and wound-healing assays were also evaluated in PHLEC.
Results
GDNF and GFRα-1 were found to be expressed by a subset of basal cells and co-localised with ATP-binding cassette, subfamily G (WHITE), member 2 (ABCG2) and p63, but not with cytokeratin 3 in the human limbal basal epithelium. In PHLEC, they were expressed by a small population of cells in the less differentiated stage. The GDNF and GFRα-1-positive subpopulations were enriched for the expression of ABCG2 and p63 (p<0.01). Recombinant human GDNF promoted the proliferation and wound healing of epithelial cells in the PHLEC. In contrast, Ret was abundantly located in the human corneal epithelium except for the basal cells of the limbal epithelium.
Conclusion
These findings indicate that GDNF and GFRα-1 may represent a property for the phenotype of human corneal epithelial precursor cells. GDNF may signal independently of Ret through GFRα-1 in the stem cell-containing limbal epithelium.
Glial cell line-derived neurotrophic factor (GDNF) is a member of the GDNF family of neurotrophic factors, which also includes neurturin, artemin and persephin.1 GDNF exerts its effects through a multicomponent receptor system consisting of the GDNF family receptor alpha-1 (GFRα-1), Ret receptor tyrosine kinase (Ret) and neural cell adhesion molecule (NCAM).2 3 GDNF has been shown to support the growth, maintenance and differentiation of a wide variety of neuronal systems. In recent years, GDNF was identified as an essential growth factor maintaining mouse spermatogonial stem cells,4 and it was found to increase motility and survival of cultured mesenchymal stem cells and ameliorate acute kidney injury,5 whereas its potential role in regulating corneal epithelial stem cells (SCs) is still not elucidated.
Corneal epithelial homeostasis is governed by a small subpopulation of corneal epithelial SCs located in the basal layer of the limbus.6–8 However, no direct method has been found to identify the corneal epithelial SCs to date because of the lack of a unique molecular marker. In recent years, the functional role and the possible signal-transduction pathways induced by GDNF in the corneal epithelium have been investigated.9 10 GDNF may play an important role in corneal regeneration and wound healing. In the present study, we evaluated the expression pattern of GDNF with its receptors in the human corneal and limbal epithelia, as well as in the primary limbal epithelial cell cultures (PHLEC), with the intention of exploring the potential role of GDNF in the human stem cell niche.
MATERIALS AND METHODS
Materials and reagents
Progenitor cell targeted corneal epithelium medium (CnT-20) was purchased from Chemicon International (Temecula, CA). Recombinant human GDNF (rhGDNF) was from R&D Systems (Minneapolis, MN). Recombinant human epidermal growth factor (rhEGF) and mitomycin C were from Sigma (St. Louis, MO). Rabbit polyclonal antibody (pAb) against GFRα-1 (H-70), Ret (C-19) and their antibody-specific blocking peptides were from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit pAb against GDNF and mouse monoclonal antibody (mAb) against human p63 (4A4, which recognises all known isotypes of p63, including the ΔNp63 isoform) were from Lab Vision (Fremont, CA). Human ATP-binding cassette, subfamily G (WHITE), member 2 (ABCG2) mAb (BXP-21) was from Calbiochem (San Diego, CA). Human AE5 mAb for cytokeratin 3 (K3) was from ICN Pharmaceuticals (Costa Mesa, CA). Anti-β-actin rabbit pAb was from Cell Signaling Technology (Danvers, MA). GeneAmp RNA-polymerase chain reaction (PCR) kit, Tagman Universal PCR master mix, TaqMan primers for GDNF (Hs00181185_m1) and GFRα-1 (Hs00237133_m1) were from Applied Biosystems (Foster City, CA). Ready-To-Go You-Prime First-Strand Beads was from Amersham Biosciences (Piscataway, NJ). The BCA protein assay kit was from Pierce Chemical (Rockford, IL).
Human corneal and limbal tissue preparation
Fresh normal human corneal tissues (less than 48 h post-mortem) for immunostaining were obtained from the National Disease Research Interchange (NDRI, Philadelphia, PA) for this study. The corneal and limbal specimens were prepared using a previously described method.11
Primary human limbal epithelial cultures
Fresh human corneoscleral tissues were obtained from the Lions Eye Bank of Texas (LEBT, Houston, TX). Primary human limbal epithelial cultures (PHLEC) were established from limbal explants using a previously described method12 with modification. In brief, each limbal rim was cultured in CnT-20 media. For immunofluorescent staining, confluent primary cultures at days 18–21 were trypsinised and seeded at 2×104 cells/chamber in eight-chamber culture slides (VWR International, West Chester, PA).
Immunofluorescent staining and laser scanning confocal microscopy
Immunofluorescent staining was performed to evaluate the expression and location of GDNF (1:100), GFRα-1 (1:50) and Ret (1:100) in the human corneal and limbal frozen sections, as well as in the PHLEC, using a previously reported method.11 12 The immunopositive cells were assessed by point counting, using a previously reported method.13
For double-staining of GDNF and GFRα-1 with stem cell and differentiation associated markers, one of the pAbs against GDNF or GFRα-1 and one of the mAbs against human p63 (1:600), ABCG2 (1:40) or K3 (1:200) were used in combination. Sections without primary antibodies applied or receiving the primary antibody preneutralised with fivefold excess of antibody-specific blocking peptide (GFRα-1 or Ret) for 2 h were used as negative controls.
Western blot analysis
Western blot analysis was performed with specific antibodies to GDNF (1:200) and GFRα-1 (1:100) using a previously described method.14 Corneal epithelia were collected from the central corneal button with an 8 mm diameter trephine, and limbal epithelia were collected from the limbal ring outside the 8 mm central button. β-Actin was used as an internal standard.
RNA isolation and relatively quantitative real-time PCR
Total RNA was isolated by acid guanidium thiocyanate-phenol-chloroform extraction using our previously described method.11 Relatively quantitative real-time PCR was performed as previously described13 using a Mx3005P QPCR System (Stratagene, LA Jolla, CA). A non-template control was included to detect DNA contamination. The GAPDH gene was used as an endogenous reference for each reaction to correct differences in the amount of total RNA added.
Single-cell colony-forming and in vitro wound healing assay
A single-cell suspension isolated from human limbal tissue and cocultured on an mitomcycine C-treated 3T3 fibroblast feeder layer (ATCC, Rockville, MD) was established as previously report.12 To evaluate the effect of GDNF on the proliferative potential of limbal epithelial cells, 50 ng/ml of rhGDNF or 5 ng/ml of rhEGF was used. The colony-forming efficiency (CFE) was determination by the number of colonies per dish at day 6.
In vitro alkali burn model in PHLEC (cultured in SHEM12) was used to investigate the wound-healing assay. The subconfluent cell layer was injured by application of filter paper with 1 mm diameter soaked 1 N NaOH for 10 s. After injury, cells were washed and incubated in SHEM with or without rhGDNF (50 ng/ml) or rhEGF (5 ng/ml). The images of the injured area were photographed with a stereoscopic zoom microscope (model SMZ 1500; Nikon, Melville, NY). For each condition, six representative areas were evaluated within three dishes. The mean area of the alkali burn in each representative image was set as 100% at the beginning of the experiment. Twenty hours later, the mean area of the same wound was recalculated and expressed as a percentage of the area at the beginning of the experiment. The inhibition of GDNF signaling by its neutralising antibody was performed, in which rhGDNF (50 ng/ml) was preneutralised with GDNF antibody (5 µg/ml) for 2 h as a negative control.
Statistical analysis
The Student t test was used to compare difference between two groups. One-way ANOVA test was used to make comparisons among three groups, and the Dunnett test was further used to compare each treated group with the control group. A p value of ≤0.05 was considered significant.
RESULTS
Expression of GDNF and its receptors in the human corneal-limbal epithelial tissue and in the PHLEC
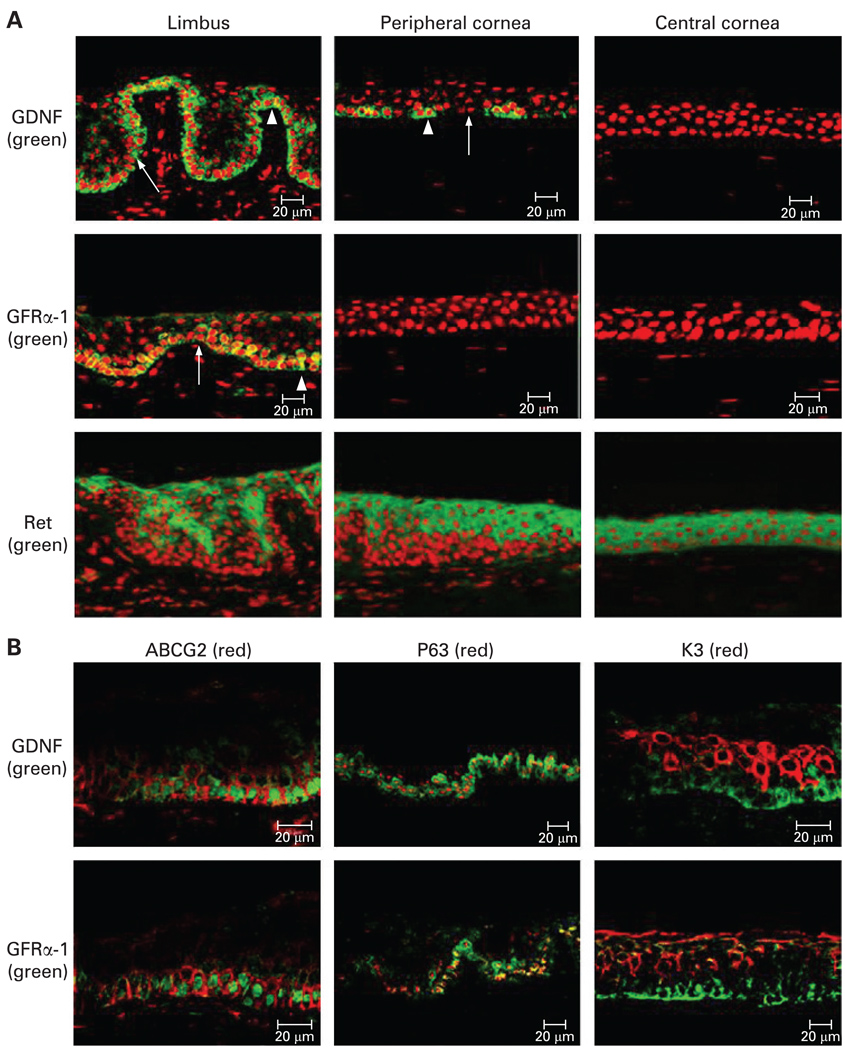
As shown in fig 1A, GDNF immunoreactivity was found to be exclusively localised in the cytoplasm of a subset of human limbal basal epithelial cells, extending to the small part of the basal cells of the peripheral corneal epithelium. Clusters of GDNF-positive cells were interspersed between negative basal cells. GFRα-1 shared the same expression pattern as its ligand with its expression only limited to the limbal basal epithelium. In contrast, Ret immunoreactivity was found to be localised to the suprabasal cells of the limbal epithelium and to the entire corneal epithelium. The basal cells of the limbal epithelium were totally negative. The specific immunoreactivities to GFRα-1 and Ret were abolished where the antibodies were neutralised by their antibody-specific blocking peptides. The presence of GDNF and GFRα-1 in human corneal and limbal epithelial tissues was confirmed by western blot analysis (fig 2). The reported molecular weight of GDNF is 21 kDa 15 and its glycosylated, disulfide-bonded homodimer protein has been noted to be about 35 kDa.16 A 35 kDa band of glycosylated GDNF was detected in both the limbal and the corneal epithelia, while the 21 kDa band was only detected in the limbal epithelium. A 53 kDa GFRα-1 band15 was detected only in the limbal epithelium. Real-time PCR showed that the levels of GDNF and GFRα-1 mRNA transcripts were four- to 10-fold higher in the limbal than in the corneal epithelia (fig 3A1, Student t test, p<0.01).
Figure 1.
Immunofluorescent staining (A) of glial cell line-derived neurotrophic factor (GDNF), GDNF family receptor alpha-1 (GFRα-1) and Ret (green) with propidium iodide nuclear counterstaining (red) and dual immunofluorescent staining (B) of GDNF and GFRα-1 (green) with ATP-binding cassette, subfamily G (WHITE), member 2 (ABCG2), p63 and K3 (red) in the human corneoscleral tissue sections. GDNF immunoreactivity was found to be localised to the cytoplasm of a subset of human limbal basal epithelial cells, extending to the small part of the basal cells of the peripheral corneal epithelium (▲). GFRα-1 shared the same expression pattern as its ligand with its expression only limited to limbal basal epithelium. In contrast, Ret immunoreactivity was found to be localised to the clusters of suprabasal cells of the limbal epithelium, to the suprabasal cells of the peripheral corneal epithelium and to the entire corneal epithelium. The basal cells of the limbal epithelium were totally negative (A). ABCG2 (B) was primarily immunodetected in the cell membrane of limbal basal epithelial cells. p63 was immunodetected primarily in the nuclei of basal limbal epithelium, and of some suprabasal limbal epithelial cells. K3 was strongly expressed by the superficial limbal epithelia. GDNF and GFRα-1 were co-expressed by ABCG2 and p63-positive cells (red) in the limbal basal layer. All GDNF and GFRα-1-positive cells were K3-negative (red). Scale bars, 20 µm.
Figure 2.
Western blot analysis of glial cell line-derived neurotrophic factor (GDNF) and GDNF family receptor alpha-1 (GFRα-1) in the human corneal (C) and limbal (L) epithelial tissues with β-actin (45 kDa) as a control. GFRα-1 (53 kDa) and a 21 kDa band of GDNF were detected only in the limbal epithelium, but not in the cornea. A 35 kDa band of glycosylated GDNF was detected in both the limbal and the corneal epithelia.
Figure 3.
Expression of glial cell line-derived neurotrophic factor (GDNF) and GDNF family receptor alpha-1 (GFRα-1) mRNA (A) evaluated by relative quantitative real-time PCR and the effect of recombinant human GDNF (rhGDNF, 50 ng/ml) in the primary human limbal epithelial cultures (B). Levels of GDNF and GFRα-1 mRNA transcripts were four- to 10-fold higher in limbal than in corneal epithelia (A1, n=8, compared with the corneal epithelium); both transcripts decreased more than 70% in the 90% confluent cultures and in the airlifted stratified limbal epithelial cultures (A2, n=5, compared with the 40% confluent cells). The number of colonies per dish at day 6 increased sixfold on the addition of 50 ng/ml rhGDNF (B1, n = 8, compared with the control group). Twenty hours after alkali burn was made in vitro (B2), GDNF significantly increased in vitro wound healing (B2, n = 8, compared with the control group). Although the effect of GDNF was smaller than that of epidermal growth factor (EGF), there was no significant difference between the two groups (Dunnett test, p>0.05). However, this effect of GDNF was blocked in the presence of GDNF neutralising antibody. *p<0.05; **p<0.01.
In the PHLEC (fig 4A), 24.6 (SD 6.3)% of cells were GDNF-positive, and 15.2 (4.7)% of cells were GFRα-1-positive (table 1). Evaluated by real-time PCR, the expression levels of GDNF and GFRα-1 mRNA were significantly different among the cells in three stages of growth and differentiation (one-way ANOVA, GDNF, p<0.01; GFRα-1, p<0.0001). Compared with the 40% confluent limbal epithelium cultures, the levels of GDNF and GFRα-1 mRNAs were found to decrease more than 70% in both the 90% confluent and in the airlifted stratified cultures (fig 3A2, Dunnett test, p<0.01).
Figure 4.
Single immunofluorescent staining of glial cell line-derived neurotrophic factor (GDNF) and GDNF family receptor alpha-1 (GFRα-1) (A), double staining of GDNF and GFRα-1 with p63 (B) and ATP-binding cassette, subfamily G (WHITE), member 2 (ABCG2) (C) in the primary human limbal epithelial cultures established from limbal explants with Hoechst 33342 nuclear counterstaining (blue). GDNF and GFRα-1 were expressed by a small population of cells (A, red). The GDNF and GFRα-1 immunopositive subpopulations (B and C1, red) expressed higher levels of p63 (B, green) and ABCG2 (C2, green). Scale bars, 25 µm. These images are representative of the data summarised in table 1. (D) GDNF and GFRα-1 (green) were expressed by cells at front edge of growing colonies on 3T3 feeder layer on day 10 with propidium iodide nuclear counterstaining (red).
Table 1.
Percentage of ABCG2/p63 and GDNF/GFRα-1-positive cells in the entire cell population and in the subpopulations of the primary human limbal epithelial cultures
| Cell populations | ABCG2 (%) | p63 (%) |
|---|---|---|
| Entire cells | 10.6 (3.4) | 35.3 (10.4) |
| GDNF-positive | 39.0 (15.5)* | 91.8 (2.4)** |
| GFRα-1-positive, mean (SD) | 53.9 (14.4)** | 73.0 (7.2)** |
| GDNF (%) | GFRα-1 (%) | |
| Entire cells | 24.6 (6.3) | 15.2 (4.7) |
| ABCG2-positive | 71.1 (12.5)** | 71.4 (28.6)** |
| p63-positive | 64.5 (14.8)** | 45.9 (14.5)** |
The numbers in the table show the mean (SD).
p<0.05;
p<0.01 (n=3, compared with the entire cell population).
ABCG2, ATP-binding cassette, subfamily G (WHITE), Member 2; GDNF, glial-cell-line derived neurotrophic factor; GFRα-1, GDNF family receptor alpha-1.
Co-expression of GDNF and GFRα-1 with stem-cell-associated and differentiation markers in the human limbal epithelium and their cultures
As shown in fig 1B, the staining patterns for ABCG2, p63 and K3 in the frozen sections of human limbal tissues were consistent with our previously report.11 12 17 GDNF and GFRα-1 were coexpressed by ABCG2 and p63-positive cells in the limbal basal layer. All GDNF and GFRα-1-positive cells were K3-negative.
In the PHLEC (fig 4), 10.6 (3.4)% of cells were ABCG2-positive, and 35.3 (10.4)% of cells were p63-positive (table 1). Double immunofluorescent staining showed that the GDNF and GFRα-1-positive subpopulations contained more than twofold p63-positive cells (fig 4B and table 1, Dunnett test, p<0.01) and more than three- to fivefold ABCG2-positive cells (fig 4C and table 1, Dunnett test, GDNF, p<0.05; GFRα-1, p<0.01). The numbers of GDNF and GFRα-1-positive cells were also two- to fourfold more in the p63 and ABCG2 immunopositive subpopulations than in the entire cell population (table 1, one-way ANOVA, p<0.0001 in each group).
Functional role of rhGDNF in the PHLEC
As shown in Figure 3B1, compared with the control group (no growth factor), the number of colonies per dish in the PHLEC increased sixfold with the addition of GDNF (Dunnett test, p<0.0001). The effect of GDNF was slightly but not significantly smaller than that of EGF (Dunnett test, p>0.05). This effect of GDNF was blocked in the presence of GDNF neutralising antibody (Dunnett test, p>0.05, compared with the control group; p<0.0001, compared with the GDNF group). Interestingly, the GDNF and GFRα-1 immunopositive cells were found mainly at the front edge of the colonies grown to day 10 (Figure 4D).
As shown in Figure 3B2, in vitro wound healing after alkali burn was also significantly different among these four groups (one-way ANOVA, p<0.05). The wound gap was filled 45 (26)% in the control group (SHEM without growth factor) 20 h after the alkali burn. In the presence of 50 ng/ml GDNF, 74 (18)% of the gap was filled, similar to the EGF group, 78 (25)% (Dunnett test, both p<0.05, compared with control). The effect of GDNF was blocked by its neutralising antibody (Dunnett test, p>0.05, compared with the control group; p<0.05, compared with the GDNF group).
DISCUSSION
GDNF and GFRα-1 may represent a property for the phenotype of human corneal epithelial precursor cells
Corneal epithelial SCs are believed to be located at the basal limbus.18–21 SCs are believed to represent less than 10% of the total limbal basal epithelial cell population.22 Using immunofluorescent staining, GDNF and GFRα-1 were detected only in a subset of basal cells in the human limbal epithelium. Two GDNF bands, a 21 kDa mature form and 35 kDa glycosylated, disulfide-bonded homodimer, were expressed by limbal epithelia. We also detected a weaker band of 35 kDa glycosylated GDNF in corneal epithelium by western blot analysis; it might be due to a small portion of the peripheral corneal epithelium included in the corneal epithelial samples which were collected with an 8 mm diameter trephine. There was no mature GDNF band detected in corneal epithelia. The expression of GFRα-1 was detected only in the limbal epithelium by western blot analysis. The levels of GDNF and GFRα-1 transcripts were also found to be much more abundant in limbal epithelia than in corneal tissues by real-time PCR.
Furthermore, GDNF and GFRα-1-positive cells were co-expressed with stem-cell-associated markers ABCG2 and p63, but not with differentiation marker K3. In the PHLEC, GDNF and GFRα-1 were expressed by a small population of cells in the proliferative and less differentiated stage. We previously reported12 17 that ABCG2 and p63 antibodies mainly stained a subpopulation of small cells, 10.62 (4.04)% and 18.3 (4.3)%, respectively, in SHEM. Limbal epithelial SCs were considered to be included among these-positive cells. Our current study found roughly the same percentage of ABCG2-positive cells and a higher percentage of p63-positive cells in CnT-20 cultured cells compared with the SHEM cultured cells. This indicates that CnT-20 media may keep these progenitor cells in a primitive undifferentiated state. Under the same conditions, fewer cells expressed GDNF (24.6 (6.3)%) and GFRα-1(15.2 (4.7)%) than p63 (35.3 (10.4)%). Dual immunofluorescent staining showed that the GDNF and GFRα-1 immunopositive subpopulations contain ABCG2 and p63-positive cells several times higher than the entire cell population (p<0.05); and the ABCG2 and p63 immunopositive subpopulations also contain GDNF and GFRα-1-positive cells at a frequency several times higher than the entire cell population (p<0.01). Interestingly, the GDNF and GFRα-1 immunopositive cells were found mainly at the front edge of the growing colonies, indicating the potential correlation of GDNF and cell proliferation. The unique expression pattern of GDNF and GFRα-1, their expression levels inversely correlated with cell differentiation conditions, their co-expression with ABCG2 and p63 suggest that GDNF and GFRα-1 may represent a property for the phenotype of human corneal epithelial precursor cells.
GDNF may signal independently of Ret through GFRα-1 in the human limbal stem cell niche
The cellular responses to GDNF are usually mediated by a multicomponent receptor complex consisting of Ret receptor tyrosine kinase and a glycosyl phosphatidylinositol (GPI)-linked ligand-binding subunit known as GFRα-1.23 GDNF signalling may require heparan sulfate glycosaminoglycans in addition to the known receptors GFRα-1 and RET.24 In addition, GDNF also signal independently of Ret, and in particular through NCAM. In cells lacking Ret, GDNF binds with a high affinity to the NCAM and GFRα-1 complex, which activates Fyn and FAK.25
In this study, we verified the function of GDNF in promoting the proliferation and wound healing of human corneal epithelium in vitro, and this effect was tested in the presence of an inhibitor of GDNF signaling. This finding suggests that GDNF could be used to enhance corneal wound healing. Furthermore, an in vivo study using laboratory animals is necessary, since the in vitro alkali injury model might not be extrapolated to the conditions of wound healing in vivo. In addition, our immunofluorescent staining revealed that in contrast to GDNF and GFRα-1 exclusively locating in the basal cells of human limbal epithelium, Ret was abundantly located in the human corneal epithelium except for the basal cells of the limbus. Western blot analysis also showed that GFRα-1 and the non-glycosylated GDNF protein were only produced by the limbal epithelium. This suggests that GDNF may signal independently of Ret through GFRα-1 in the stem cell-containing limbal epithelium as it does in the other cell types.2 The signal-transduction pathways induced by GDNF in the human limbal epithelium need to be further studied.
Acknowledgements
The authors thank the Lions Eye Bank of Texas for kindly providing human corneoscleral tissues.
Funding: This study was supported by NIH Grants, EY11915 (SCP) and EY014553 (DQL), National Institutes of Health, Bethesda, MD; Department of Defense Congressionally Directed Medical Research Programs (CDMRP) grant FY06 PR064719 (DQL); a grant from Lions Eye Bank Foundation (HQ); an unrestricted grant from Research to Prevent Blindness, the Oshman Foundation and the William Stamps Farish Fund.
Footnotes
This study was presented in part as abstract at the Annual Meeting of the Association for Research in Vision and Ophthalmology, 6–10 May 2007, Fort Lauderdale, Florida.
Competing interests: None.
REFERENCES
- 1.Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- 2.Sariola H, Saarma M. Novel functions and signalling pathways for GDNF. J Cell Sci. 2003;116:3855–3862. doi: 10.1242/jcs.00786. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi M. The GDNF/RET signaling pathway and human diseases. Cytokine Growth Factor Rev. 2001;12:361–373. doi: 10.1016/s1359-6101(01)00012-0. [DOI] [PubMed] [Google Scholar]
- 4.Sariola H, Immonen T. GDNF maintains mouse spermatogonial stem cells in vivo and in vitro. Methods Mol Biol. 2008;450:127–135. doi: 10.1007/978-1-60327-214-8_9. [DOI] [PubMed] [Google Scholar]
- 5.Shi H, Patschan D, Dietz GP, et al. Glial cell line-derived neurotrophic growth factor increases motility and survival of cultured mesenchymal stem cells and ameliorates acute kidney injury. Am J Physiol Renal Physiol. 2008;294:F229–F235. doi: 10.1152/ajprenal.00386.2007. [DOI] [PubMed] [Google Scholar]
- 6.Dua HS, Azuara-Blanco A. Limbal stem cells of the corneal epithelium. Surv Ophthalmol. 2000;44:415–425. doi: 10.1016/s0039-6257(00)00109-0. [DOI] [PubMed] [Google Scholar]
- 7.Tseng SC. Concept and application of limbal stem cells. Eye. 1989;3:141–157. doi: 10.1038/eye.1989.22. [DOI] [PubMed] [Google Scholar]
- 8.Lavker RM, Sun TT. Epidermal stem cells: properties, markers, and location. Proc Natl Acad Sci U S A. 2000;97:13473–13475. doi: 10.1073/pnas.250380097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.You L, Kruse FE, Volcker HE. Neurotrophic factors in the human cornea. Invest Ophthalmol Vis Sci. 2000;41:692–702. [PubMed] [Google Scholar]
- 10.You L, Ebner S, Kruse FE. Glial cell-derived neurotrophic factor (GDNF)-induced migration and signal transduction in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2001;42:2496–2504. [PubMed] [Google Scholar]
- 11.Chen Z, de Paiva CS, Luo L, et al. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22:355–366. doi: 10.1634/stemcells.22-3-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HS, Jun SX, de Paiva CS, et al. Phenotypic characterization of human corneal epithelial cells expanded ex vivo from limbal explant and single cell cultures. Exp Eye Res. 2004;79:41–49. doi: 10.1016/j.exer.2004.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Z, Evans WH, Pflugfelder SC, et al. Gap junction protein connexin 43 serves as a negative marker for a stem cell-containing population of human limbal epithelial cells. Stem Cells. 2006;24:1265–1273. doi: 10.1634/stemcells.2005-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim HS, Luo L, Pflugfelder SC, et al. Doxycycline inhibits TGF-{beta}1-induced MMP-9 via Smad and MAPK pathways in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:840–848. doi: 10.1167/iovs.04-0929. [DOI] [PubMed] [Google Scholar]
- 15.Kretz A, Jacob AM, Tausch S, et al. Regulation of GDNF and its receptor components GFR-alpha1, -alpha2 and Ret during development and in the mature retino-collicular pathway. Brain Res. 2006;1090:1–14. doi: 10.1016/j.brainres.2006.01.131. [DOI] [PubMed] [Google Scholar]
- 16.Shibata SB, Osumi Y, Yagi M, et al. Administration of amitriptyline attenuates noise-induced hearing loss via glial cell line-derived neurotrophic factor (GDNF) induction. Brain Res. 2007;1144:74–81. doi: 10.1016/j.brainres.2007.01.090. [DOI] [PubMed] [Google Scholar]
- 17.de Paiva CS, Chen Z, Corrales RM, et al. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells. 2005;23:63–73. doi: 10.1634/stemcells.2004-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cotsarelis G, Cheng SZ, Dong G, et al. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- 19.Schermer A, Galvin S, Sun T-T. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tseng SCG. Regulation and clinical implications of corneal epithelial stem cells. Mol Biol Rep. 1996;23:47–58. doi: 10.1007/BF00357072. [DOI] [PubMed] [Google Scholar]
- 21.Lehrer MS, Sun TT, Lavker RM. Strategies of epithelial repair: modulation of stem cell and transit amplifying cell proliferation. J Cell Sci. 1998;111:2867–2875. doi: 10.1242/jcs.111.19.2867. [DOI] [PubMed] [Google Scholar]
- 22.Lavker RM, Dong G, Cheng SZ, et al. Relative proliferative rates of limbal and corneal epithelia. Implications of corneal epithelial migration, circadian rhythm, and suprabasally located DNA-synthesizing keratinocytes. Invest Ophthalmol Vis Sci. 1991;32:1864–1875. [PubMed] [Google Scholar]
- 23.Nozaki C, Asai N, Murakami H, et al. Calcium-dependent Ret activation by GDNF and neurturin. Oncogene. 1998;16:293–299. doi: 10.1038/sj.onc.1201548. [DOI] [PubMed] [Google Scholar]
- 24.Barnett MW, Fisher CE, Perona-Wright G, et al. Signalling by glial cell line-derived neurotrophic factor (GDNF) requires heparan sulphate glycosaminoglycan. J Cell Sci. 2002;115:4495–4503. doi: 10.1242/jcs.00114. [DOI] [PubMed] [Google Scholar]
- 25.Paratcha G, Ledda F, Ibanez CF. The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell. 2003;113:867–879. doi: 10.1016/s0092-8674(03)00435-5. [DOI] [PubMed] [Google Scholar]