Abstract
ABCG2, a member of the ATP binding cassette (ABC) transporters, has been identified as a molecular determinant for bone marrow stem cells and proposed as a universal marker for stem cells. This study investigates ABCG2 expression and its potential as a marker that identifies human limbal epithelial stem cells. ABCG2 expression was evaluated by immunofluorescent and immunohistochemical staining, laser scanning confocal microscopy, flow cytometry, and semiquantitative reverse transcription–polymerase chain reaction. Cells selected from primary limbal epithelial cultures by flow cytometry with ABCG2 monoclonal antibody (mAb) or Hoechst 33342 dye staining were evaluated for their gene expression and colony-forming efficiency (CFE). ABCG2 protein was mainly located in the basal cells of limbal epithelia but not in the limbal suprabasal and corneal epithelia. ABCG2 staining was also observed in primary limbal epithelial cultures. Limbal epithelia express higher levels of ABCG2 and ΔNp63 mRNAs than corneal epithelia. Labeling with ABCG2 mAb yielded 2.5%–3.0% positive cells by flow cytometry. The ABCG2-positive cells exhibited greater CFE on a 3T3 fibroblast feeder layer than ABCG2-negative cells. A side population (SP) was detected by the Hoechst 33342 exclusion assay. SP cells displayed stronger expression of ABCG2 and ΔNp63 mRNA and greater CFE than the non-SP cells. In conclusion, these findings demonstrate that ABCG2 transporter was exclusively expressed by limbal basal cells and that the ABCG2-positive and SP cells possess enriched stem cell properties, suggesting for the first time that ABCG2 could serve as a marker to identify the putative limbal epithelial stem cells.
Keywords: ABCG2 transporter, Stem cell, Side population, Cornea, Limbus, Epithelium
Introduction
The tissue-specific stem cells residing in certain adult tissues have been recognized to possess the ability to regenerate tissues, and they offer great therapeutic potential for treating diseased and damaged tissues and serving as gene delivery vehicles [1, 2]. Widely accepted criteria for defining adult stem cells are their slow cycling or long cell cycle time during homeostasis in vivo; poor differentiation with primitive cytoplasm; high capacity for error-free self–renewal; and high proliferative potential after wounding or placement in culture [3-7]. The easily visualized well-defined tissues of the ocular surface (i.e., conjunctiva, limbus, and cornea) make it an ideal region to study epithelial stem cell biology, and the compartmentalization of the corneal epithelial stem cells within the limbus provides a valuable opportunity to study the behavior of adult stem cells [4, 8, 9]. Although the concept that corneal epithelial stem cells reside in the limbus is widely accepted, there is no direct method to identify the corneal epithelial stem cells to date because of the lack of unique molecular markers.
A variety of stem cell markers have been proposed to identify the stem cells. The major markers proposed for epithelial stem cells in the past decade can be categorized into at least three groups: nuclear proteins, such as the transcription factor p63; cell membrane or transmembrane proteins, including integrins (integrin ß1, α6, and α9), receptors (epidermal growth factor [EGF] receptor and transferrin receptor CD71), and drug-resistance transporters (ABCG2); and cytoplasmic proteins such as cytokeratins (cytokeratin 19) and α-enolase. We have recently evaluated these proposed stem cell markers to characterize a putative stem cell phenotype in human limbal epithelia [10]. That study demonstrated for the first time that the ABCG2 transporter was exclusively expressed by the limbal epithelium on the ocular surface.
ABCG2, formally known as breast cancer resistance protein 1, is a member of the ATP-binding cassette (ABC) family of cell surface transport proteins, which includes more than 50 members and mediates the transfer of a diverse array of substrates across cellular membranes. ABCG2 expression occurs in a variety of normal tissues and is relatively limited to primitive stem cells [11]. ABCG2 expression is associated with the side population (SP) phenotype based on the ability to efflux Hoechst 33342 dye [12]. Dye efflux studies with Hoechst 33342 have identified a minor fraction of CD34– hematopoietic stem cells termed SP cells on the basis of unique fluorescent properties [13]. SP is highly enriched for hematopoietic stem cells with long-term repopulation activity after transplantation, and it is present in multiple mammalian species [14, 15]. Inhibition of the SP phenotype by verapamil, presumably by blocking dye efflux, suggests that the SP phenotype may result from the expression of certain membrane pumps. The expression of ABCG2 was recently reported to be upregulated in SP cells isolated from mouse, rhesus monkey, and human bone marrow, and it has been identified as a molecular determinant for the SP phenotype. Furthermore, it was suggested that expression of ABCG2 transporter gene is a conserved feature of stem cells from a wide variety of tissues [15-17].
The purpose of this study was to evaluate the expression of this novel ABCG2 transporter by human limbal epithelial cells and to determine whether the limbal SP cells and ABCG2– cells possess stem cell properties, with the intention to determine whether ABCG2 could serve as a new marker for limbal epithelial stem cells.
Materials and Methods
Materials and Reagents
Mouse monoclonal antibodies (mAb) against ABCG2 were purchased from Calbiochem (San Diego; clone BXP-21) and from eBioscience (San Diego; clone 5D3). Alexa-fluor 488 goat anti-mouse antibody was from Molecular Probes (Eugene, OR). Vectastain Elite Kits were from Vector Laboratories (Burlingame, CA). Fetal bovine serum (FBS) was from Hyclone (Logan, UT). Dulbecco’s modified Eagle’s medium (DMEM), Ham F-12, amphotericin B, gentamicin, 0.25% trypsin/0.03% EDTA solution, and phenol were from Invitrogen-GIBCO BRL (Grand Island, NY). Dispase II was from Roche (Indianapolis). Cholera toxin subunit A, dimethyl sulfoxide (DMSO), hydrocortisone, insulin-transferrin-sodium selenite media supplement, human recombinant EGF, mitomycin C, Hoechst 33342, verapamil, propidium iodide (PI), and rhodamine were from Sigma (St. Louis). Mouse NIH 3T3 fibroblasts were from American Type Culture Collection (Rockville, MD) and used at passages 129 through 132. GeneAmp RNA-PCR kit was from Applied Biosystems (Foster City, CA). All plastic ware was from Becton, Dickinson (Lincoln Park, NJ).
Human Corneal and Limbal Tissue
Fresh human corneoscleral tissues (less than 72 hour postmortem) that were not suitable for clinical use, from donors aged 19–64 years, were obtained from the Lions Eye Bank of Texas (Houston) and from the National Disease Research Interchange (Philadelphia). They were cut through horizontal meridian, frozen, and sectioned for immunostaining, whole mount for laser-scanning confocal microscopy, or prepared for single epithelial cells, which were used for primary limbal epithelial cultures and ABCG2 or Hoechst 33342 staining assays by flow cytometry. Fresh corneas less than 48 hours postmortem were used for gene expression analysis. An 8-mm trephine was used to isolate the cornea from the limbus, and the epithelia in both areas, the central button (K) and the limbal rim (L), were scraped and collected for RNA extraction and reverse transcription–polymerase chain reaction (RT-PCR).
Primary Human Limbal Epithelial Cell Culture
Limbal epithelial cell cultures were established from single-cell suspensions that were isolated from limbal tissues and cocultured on a mitomycin C (MMC)–treated 3T3 fibroblast feeder layer using a previously reported method with modification [18-20]. In brief, each corneoscleral rim was trimmed, and the endothelial layer and iris remnants were removed. The limbal rim was incubated with dispase II (5 mg/mL) at 37°C for 1 hour. The limbal epithelial sheets were then collected and treated with 0.25% trypsin/0.03% EDTA at 37°C for 10 minutes to isolate single cells. Mouse NIH 3T3 fibroblasts, grown in DMEM containing 10% FBS to confluence, were treated with MMC (5 μg/mL) for 2 hours and then trypsinized and plated at a density of 2 × 104 cells/cm2 in 35-mm dishes or six-well plates. The isolated limbal epithelial single cells were seeded at a density of 1 × 103 cells/cm2 on a 3T3 feeder layer in supplemented hormonal epidermal medium (SHEM) containing equal amounts of DMEM and Ham’s F12 medium supplemented with 5% FBS, 5 ng/mL EGF, 5 μg/mL insulin, 5 μg/mL transferrin, 5 ng/mL sodium selenite, 0.5 μg/mL hydrocortisone, 30 ng/mL cholera toxin A, 0.5% DMSO, 50 μg/mL gentamicin, and 1.25 μg/mL amphotericin B. Cultures were incubated at 37°C under 5% CO2 and 95% humidity, and the media were changed every 2–3 days. Confluent cultures (10–14 days) were used for experiments.
Immunofluorescent Staining
Fresh human corneas were embedded in a mixture of 75% (vol) OCT compound (Sakura Finetek USA Inc., Torrance, CA) and 25% (vol) Immu-Mount (Thermo-Shandon, Pittsburgh) and then flash frozen in liquid nitrogen. Sections (10-μm thick) were cut with a cryostat (HM 500; Micron, Waldorf, Germany) and were stored at −80°C until they were used. Immunofluorescent staining was performed by a previously reported method [10, 21, 22]. In brief, corneal and limbal frozen sections and primary limbal epithelial cultures had fixation with 2% paraformaldehyde at 4°C for 10 minutes. After blocking with 5% normal goat serum in phosphate-buffered saline (PBS) for 30 minutes, a primary mAb, clone BXP-21, reactive with the 70-kD transmembrane ABCG2 transporter protein, was applied at a 1:50 dilution (5 μg/mL) for 1 hour at room temperature. After washing with PBS, Alexafluor-488 conjugate secondary antibody (1:300) was applied for 1 hour in a dark incubation chamber. After washing with PBS, Antifade Gel/Mount (Fisher, Atlanta) containing 1 μg/mL Hoechst 33342 dye and a cover slip were applied. Staining patterns were examined and photographed with a Nikon Eclipse 400 epifluorescent microscope with a DMX 1200 digital camera (Garden City, NY).
Immunohistochemical Staining
Immunohistochemical staining was performed by a previously reported method [10] to evaluate the expression of ABCG2. In brief, the corneal frozen sections were fixed in 2% paraformaldehyde in PBS at 4°C for 10 minutes. The endogenous peroxidases were quenched with 0.3% H2O2 in PBS containing 0.5% goat serum and incubated with 5% goat serum to block the nonspecific sites. ABCG2 mAb (clone BXP-21, 1:50) was applied and incubated for 1 hour at room temperature, followed by incubation with biotinylated anti-mouse second antibody using a Vectastain Elite ABC kit according to the manufacturer’s protocol. The samples were finally incubated with diaminobenzidine peroxidase substrate to give a brown stain and counterstained with hematoxylin. The samples were mounted with cover slips and examined with a Nikon Eclipse 400 microscope.
Laser Scanning Confocal Microscopy
Whole fresh human corneas were fixed in fresh 2% paraformaldehyde in PBS for 10 minutes at room temperature. After fixation, they were rinsed extensively with PBS containing 0.1% Tween 20 (TPBS). The corneas were bisected and then blocked with 20% goat serum in TPBS from 2 hours to overnight to reduce nonspecific labeling. The tissues were then incubated with ABCG2 mAb (clone BXP-1, 1:50 dilution) in TPBS containing 20% goat serum at 4°C overnight. Tissues without primary antibody were used as negative controls. After extensive washing with TPBS, Alexa Fluor-488 conjugated goat anti-mouse antibody (1:300) was applied for 1 hour. Tissues were rinsed and counterstained with DNA binding dye propidium iodide (1 μg/mL in PBS) for 5 minutes. After washing with PBS, corneal tissues were flatted on slides and mounted with antifade Gel/Mount (Fisher) and cover slips. The samples were observed with a laser-scanning confocal microscope (LSM 510, Zeiss with Krypton-argon and He-Ne laser, Thornwood, NY) with 488- and 543-nm excitation and emission filters, LP505 and LP560, respectively. Images were acquired with × 40 oil-immersion objective and processed using Zeiss LSM-PC software and Adobe Photoshop 6.0.
RNA Isolation and Semiquantitative RT-PCR
Total RNA was isolated from cornea and limbal epithelia in fresh human tissues and from selected populations of primary cultured limbal epithelial cells sorted by flow cytometry with ABCG2 mAb or Hoechst 33342 staining, using acid guanidium thiocyanate-phenol-chloroform extraction [21]. The RNA was quantified by its absorption at 260 nm and stored at −80°C before use. Using a housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), as an internal control, the mRNA expression of different molecular markers was analyzed by semiquantitative RT-PCR as previously described [21, 23]. Briefly, first-strand cDNAs were synthesized from 0.5 mg of total RNA with murine leukemia virus reverse transcriptase. PCR amplification was performed with specific primer pairs designed from published human gene sequences (Table 1) for different markers using a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA). Semiquantitative RT-PCR was established by terminating reactions at intervals of 20, 24, 28, 32, 36, and 40 cycles for each primer pair to ensure that the PCR products formed were within the linear portion of the amplification curve. The fidelity of the RT-PCR products was verified by comparing their size with the expected cDNA bands and by sequencing the PCR products.
Table 1.
Human primer sequences used for semiquantitative RT-PCR
| Gene | Accession | Sense primer | Antisense primer | PCR Product |
|---|---|---|---|---|
| ΔNp63 | XM_036421 | CAGACTCAATTTAGTGAG | AGCTCATGGTTGGGGCAC | 440 bp |
| ABCG2 | AY017168 | AGTTCCATGGCACTGGCCATA | TCAGGTAGGCAATTGTGAGG | 379 bp |
| GAPDH | M33197 | GCCAAGGTCATCCATGACAAC | GTCCACCACCCTGTTGCTGTA | 498 bp |
Abbreviation: RT–PCR, reverse transcription–polymerase chain reaction
Flow Cytometry Analysis and Fluorescence-Activated Cell Sorting with ABCG2 mAb Staining
Freshly harvested limbal epithelial cells were used for ABCG2 staining analysis, and primary cultured human limbal epithelial cells were used for ABCG2 staining analysis and cell sorting by flow cytometry using a functional-grade purified anti-human ABCG2 mAb clone 5D3. This antibody reacts with the extracellular portion of the human ABCG2 protein and has been used for flow cytometry analysis of living cells such as acute myeloid leukemia cells [24], cell lines overexpressing ABCG2 [25], and human limbal epithelial cells [26]. Briefly, limbal epithelial cells at 0.5 × 106 cells/100 μl were incubated with ABCG2 mAb antibody (clone 5D3, 1:25 dilution, 40 μg/ml) for 30 minutes on ice, washed with PBS containing 1% FBS, incubated with Alexafluor-488 conjugate goat anti-mouse secondary antibody (1:100 dilution) for 30 minutes, washed with PBS/1% FBS, and then resuspended in SHEM. The cells were kept on ice until flow cytometry was performed.
Fluorescence-activated cell sorting (FACS) was performed at the Baylor College of Medicine Core Facility using a triple-laser Beckman-Coulter Altra high-pressure, high-speed cell sorter (Beckman-Coulter Altra, Hialeah, FL). A 488-nm argon laser excited fluorescein isothiocyanate, and a band-pass filter of 525/20 was used to measure emitted light. Gates in the right angle scatter versus forward scatter diagrams were used to exclude debris. At least 100,000 events were collected before analysis. All flow cytometric data was analyzed with Expo 32 software (Beckman-Coulter). The primary cultured limbal epithelial cells were sorted into subpopulations differing in their expression of ABCG2. The sorted ABCG2-positive and -negative cell populations were used to evaluate their colony-forming efficiency (CFE) and gene expression of stem cell markers.
Hoechst 33342 Exclusion Assay and FACS for SP
Hoechst 33342 exclusion assay and FACS for SP were performed according to a previously described method for isolating an SP for hematopoietic stem cells [13] using Hoechst 33342, which is a DNA binding dye that allows identification of cell cycle. Single cells isolated from fresh human limbus or from their primary cultures were used for the Hoechst 33342 exclusion assay to identify SP. FACS for SP was performed using primary cultured cells but not limbal epithelial cells from human tissues because of the limited number of cells that were available for sorting. The SP is characterized by low blue and low red fluorescence intensity on a dot-plot displaying dual wavelength of Hoechst blue versus red. Briefly, the cells were resuspended at 1 ×106 cells/mL in SHEM containing 5 μg/mL of Hoechst 33342 and incubated at 37°C for 120 minutes with shaking. The cell suspension was centrifuged and resuspended in cold SHEM with HEPES containing PI (2 μg/mL) to identify dead cells. To identify the SP, 50 μM of verapamil, a transporter inhibitor, was preadded for 30 minutes before adding Hoechst 33342. To ensure that the SP was sorted, a sorting gate was chosen in which the inhibition of Hoechst exclusion by verapamil was the highest. The non-SP cells were sorted from cells that were in the G0/G1 stage of cell cycle. Sorted cell populations were used to evaluate CFE and gene expression for stem cell markers.
These experiments were performed at the Baylor College of Medicine Core Facility using a triple-laser instrument (Beckman-Coulter Altra) equipped with the autoclone feature. Two of the lasers are water-cooled argon lasers, tunable to 350, 457, 488, and 514 nm, and the third is a smaller air-cooled helium-neon laser that emits light with a wavelength of 632 nm. Hoechst and PI dyes were excited with 350 nm UV (100 mW power) and 488 argon (50 mW power) lasers, respectively. The 450/20 and 675 band-pass filters, in combination with a 640 long-pass dichroic filter, were used for detection of Hoechst blue and red emission, respectively. Concomitant PI fluorescence was read using a 610/20 band-pass filter. At least 100,000 events were collected before analysis. All flow cytometric data were analyzed with Expo 32 software (Beckman-Coulter). Cells were displayed in a Hoechst blue versus Hoechst red dot-plot to visualize the SP.
CFE
To evaluate CFE, selected populations of ABCG2-positive and -negative cells and SP and non-SP cells sorted by FACS were seeded, at least in triplicate, at 1 × 103 cells/cm2 into six-well culture plates containing a mitomycin C–treated 3T3 fibroblast feeder layer. The colonies were counted at days 5 through 8, and CFE was calculated as a percentage of the number of colonies at days 5 through 8, generated by the number of epithelial cells plated in a well. The growth capacity was evaluated on day 14, when cultured cells were stained with 1% rhodamine.
Results
ABCG2 Expression and Growth Potential in Human Corneal and Limbal Epithelial Cells
Immunofluorescent (Figs. 1A–1D) and immunohistochemical (Fig. 1E) stainings of histologic sections of human cornea and limbus showed that ABCG2 transporter was expressed in the membrane and cytoplasm of certain basal cells in the human limbal epithelia (Figs. 1D, 1E) but not in the suprabasal layers of the limbal epithelium or in any layer of the corneal epithelium (Fig. 1B). This staining pattern is similar to that observed for nuclear protein p63, a proposed keratinocyte stem cell marker [27]. Laser scanning confocal microscopy was also used to evaluate ABCG2 expression in all layers of the corneal and limbal epithelia. ABCG2 staining was observed only in the cell membranes of certain basal epithelial cells in the human limbus, forming a net-like pattern (Figs. 1H, 1I). In contrast, no ABCG2 staining could be visualized in the entire corneal epithelia, even in its basal layer (Figs. 1F, 1G).
Figure 1.
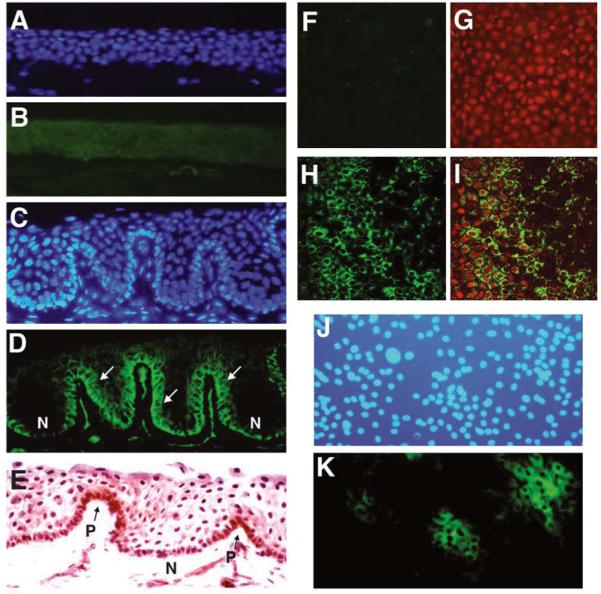
Immunofluorescent (A–D, J, K) and immunohistochemical (E) stainings and laser scanning confocal microscopy (F–I) for ABCG2 transporter in human cornea (A, B, F, G), limbus (C–E, H, I), and primary cultured human limbal epithelial cells (J–K). (A, C, J): Hoechst 33342 counterstaining. (B): Cornea showing no staining for ABCG2. (D): Limbus showing brighter membrane staining for ABCG2 on certain basal cells (arrows), whereas other basal cells are negative (N). (E): Limbus showing cytoplasmic staining in some of basal cells (P). (K): Cultured limbal epithelial cells showing positive ABCG2 staining in clusters of small cells. Confocal microscopy showing ABCG2 stained on basal layer of whole-mounted human limbus (H, I) but not corneal basal layer (F, G). (G, I): Merged color images for ABCG2 (green) and propidium iodide (red). Original magnification × 400.
In primary cultured human limbal epithelial cells, the ABCG2 antibody mainly stained the membranes of clusters of small cells, and minimal or no staining was observed in the large cells. ABCG2– cells accounted for 10.62 ± 4.04% (mean ± standard deviation [SD]) of cells in limbal primary cultures (n = 6) (Fig. 1K).
Semiquantitative RT-PCR analysis revealed that ABCG2 mRNA was expressed at low levels by the corneal epithelia and at higher levels by the limbal epithelia (Fig. 2). A similar pattern was observed for the expression of ΔNp63 by the corneal and limbal epithelia. ΔNp63, a truncated dominant-negative isoform of p63, is the predominant species in epithelial stem cells [28]. No difference in the levels of GAPDH, a housekeeping gene used as an internal control, was observed between the corneal and limbal epithelia.
Figure 2.
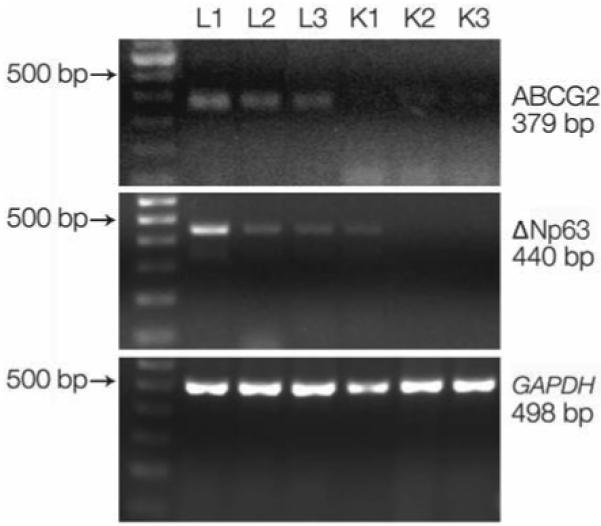
Semiquantitative reverse transcription–polymerase chain reaction showing expression of ABCG2 (379 bp), ΔNp63 (440 bp), and GAPDH (498 bp) mRNAs by corneal (K1, K2, and K3) and limbal (L1, L2, and L3) epithelia. A 100-bp DNA ladder is shown in the first left lane.
ABCG2 Expression and CFE of ABCG2− Cells Sorted by FACS
The expression of ABCG2 in freshly harvested limbal epithelial cells and primary cultured limbal epithelial cells (Fig. 3) was also evaluated by flow cytometry using an anti-human ABCG2 mAb clone 5D3. These experiments were repeated several times, and the results averaged. Cells positively stained with ABCG2 accounted for 3.02 ± 0.83% (mean ± SD, n = 3) of freshly harvested limbal epithelial cells and 2.31 ± 1.83% (n = 6) of primary limbal epithelial cultures. One of the representative experiments using primary cultured limbal epithelial cells is shown in Figure 3A.
Figure 3.
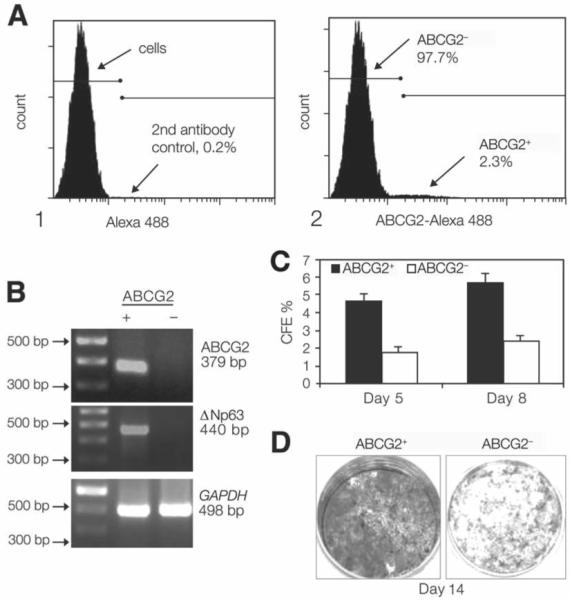
(A): Flow cytometry analysis of cultured human limbal epithelial cells showing a distinctive small population of ABCG2+ cells (2.31%). (B): Semiquantitative reverse transcription–polymerase chain reaction showing higher expression of ABCG2 mRNA (379 bp) in the ABCG2+ cells sorted by fluorescence–activated cell sorting. A 100-bp DNA ladder is shown in the first left lane. GAPDH (498 bp), a housekeeping gene, was used as an internal control. (C): CFE of ABCG2-selected populations showing a greater colony-forming efficiency generated by the ABCG2+ cells at days 5 and 8 than that by ABCG2− cells (p < .005 and p < .001, respectively). (D): Growth capacity of ABCG2+ cells and ABCG2− cells, evaluated by 1% rhodamine staining at day 14, showing that ABCG2+ cells were confluent, whereas ABCG2− cells had fewer colonies. Abbreviation: CFE, colony-forming efficiency.
ABCG2 expression in the positive population sorted by FACS was confirmed by comparing the levels of ABCG2 mRNA detected by RT-PCR in positive and negative populations. The ABCG2 and p63 transcripts were highly expressed in the ABCG2– population, whereas they were barely detectable in ABCG2– population. There was no difference in GAPDH expression between these two groups (Fig. 3B).
The CFE of the ABCG2-positive and -negative cells was evaluated at days 5 and 8 after they were seeded on a 3T3 feeder layer. By day 5, wells seeded with ABCG2– cells showed greater CFE than the wells with ABCG2– cells (4.65 ± 0.42% versus 1.79 ± 0.29%; p < .005; n = 3). By day 8, ABCG2– cells continued to show superior growth, with a greater CFE (5.67 ± 0.54% versus 2.39 ± 0.33%; p < .001; n = 3) and larger sized colonies than the ABCG2– cell wells (Fig. 3C). The ABCG2– grew to confluence by day 14, whereas the ABCG2– never reached confluence (Fig. 3D).
ABCG2 Expression and CFE of the SP Sorted by FACS Using the Hoechst 33342 Exclusion Assay
To further evaluate the expression and function of ABCG2 transporter in limbal epithelial cells, the Hoechst exclusion assay was performed to detect the SP, defined as a distinct cell population that displays lowest fluorescence intensities of both Hoechst blue and Hoechst red. To confirm the SP, the cells were preincubated with verapamil for 30 minutes before staining with Hoechst 33342. Verapamil is a transporter inhibitor that is able to block Hoechst dye exclusion from bone marrow SP [13]. We could demonstrate the inhibitory effect of verapamil in fresh epithelial cells isolated from limbal tissues and in primarily cultured limbal epithelial cells with a similar fashion to the ability of this agent to block bone marrow SP (Fig. 4). The formation of the SP from human limbal epithelial cells was blocked by verapamil (50–200 μM) in a concentration-dependent manner (data not shown). The percentage of the cells that were in the SP gate dramatically decreased from 2.90 ± 1.14% to 0.78 ± 1.15% (p < .01, n = 4) with 50 μM of verapamil.
Figure 4.
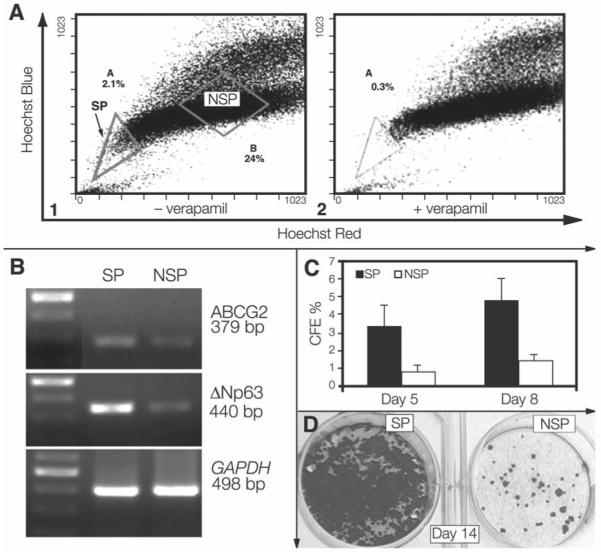
(A1): Flow cytometry analysis of cultured limbal epithelial cells showing SP (gate A) effluxing the Hoechst 33342 dye. Corneal SP cells were sorted from gate A, and NSP limbal cells were sorted from gate B. (A2): Dye efflux from limbal SP cells is inhibited in the presence of 50 μM verapamil, evidenced by decreased limbal SP from 2.1% in A1 to 0.3% in A2. (B): Semiquantitative reverse transcription–polymerase chain reaction showing that limbal SP cells sorted by Hoechst 33342 assay express higher levels of ABCG2 (379 bp) and p63 (440 bp) than NSP cells. A 100–bp DNA ladder is shown in the first left lane. GAPDH (498 bp) was used as an internal control. (C): CFE of SP and NSP cells sorted by fluorescence–activated cell sorting showing a greater colony-forming efficiency generated by SP than NSP at days 5 (p < .05) and 8 (p < .01). (D): Growth capacity of SP and NSP cells evaluated by 1% rhodamine staining at day 14 showing that an SP well was confluent, whereas few colonies were in the NSP well. Abbreviations: CFE, colony–forming efficiency; NSP, non–side population; SP, side population.
SP from primary cultured human limbal epithelial cells was detected by Hoechst exclusion assay and sorted by FACS. One representative experiment is shown in Figure 4. Using the Hoechst exclusion assay, 2.1% of all cells were observed in the SP region (region A, Fig. 4A1). Non-SP cells (in region B) corresponded to cells that are in the G0/G1 stage of the cell cycle (Fig. 4A1). The sorted cells from SP and non-SP were used to evaluate their gene expression and CFE.
Using the housekeeping gene GAPDH as an internal control, semiquantitative RT-PCR disclosed a differential expression pattern of ABCG2 in SP and non-SP limbal epithelial cells (Fig. 4B). ABCG2 mRNA was much more abundantly expressed by the SP cells than by non-SP cells. The limbal SP cells also showed higher expression of ΔNp63 than the non-SP cells. The differential patterns for ABCG2 and ΔNp63 expression in SP and non-SP populations resembled the differences seen between human limbal and corneal epithelia (Fig. 2).
The CFE was evaluated at days 5 and 8 (Fig. 4C). The SP-containing wells showed a greater number of colonies than the non-SP wells (CFE, 3.38 ± 1.18% versus 0.83 ± 0.29%; p < .05; n = 3) at day 5. On day 8, the colonies in SP-containing wells increased in size and number, whereas little increase in colony size and number was observed in the non–SP-containing wells (CFE, 4.84 ± 1.56% versus 1.44 ± 0.33%; p < .01; n = 3). The limbal SP cells grew to confluence at day 14, whereas the non-SP cells never reached confluence (Fig. 4D).
Discussion
Most studies of ABCG2 have focused on its potential role in producing the multidrug resistance phenotype in cancer cells and its expression in primitive stem cells. Different from most other transporters, ABCG2 expression is present in the plasma membrane and is relatively limited to primitive stem cells [11]. ABCG2 transporter has been identified as a molecular determinant for bone marrow stem cells, and it has been proposed as a universal marker for stem cells from a variety of sources [12, 15]. Although ABCG2 expression occurs in a variety of normal tissues, its expression and functional role in corneal epithelial cells was only recently reported [10, 26]. This present study confirmed the exclusive expression pattern of ABCG2 transporter by the basal cells of human limbal epithelia and its potential role in identifying human corneal epithelial stem cells.
ABCG2 Transporter Was Uniquely Expressed in Human Limbal Epithelia and Their Primary Cultures
The results of this study indicate that ABCG2 protein is uniquely expressed by certain cells of basal layers in the limbal epithelium, whereas it is absent in the central cornea, as shown by immunofluorescent and immunohistochemical staining on frozen sections (Figs. 1A–1E) as well as laser scanning confocal microscopy on whole mount corneas (Figs. 1F-1I). The staining pattern agrees with previous reports that ABCG2 protein is primarily localized in the plasma membrane; however, some cytoplasmic staining has been observed [29]. ABCG2 protein was also expressed by primary cultures of human limbal epithelial cells and preferentially by clusters of small cells (Figs. 1J, 1K). ABCG2 transcripts were also found to be much more abundant in the limbus than in the cornea, a pattern similar to the expression of ΔNp63, a truncated dominant-negative isoform of p63. Nuclear protein p63, a member of the p53 family, was proposed to be an epithelial stem cell marker [27]. p63 is highly expressed by the basal cells of many human epithelial tissues, and ΔNp63 is the predominant species in these stem cells [28]. Analyzed by flow cytometry with ABCG2 mAb staining, the ABCG2− cells accounted for 2%–3% of the population of epithelial cells isolated from limbal epithelial tissues or their primary cultures. These results support the widely accepted concept that the corneal stem cells reside at limbus [5, 9].
ABCG2 Expression Was Correlated to Growth Capacity and SP Selected by FACS
To evaluate whether ABGC2 expression identifies a population of limbal epithelial cells with stem cell features, we used FACS to separate human limbal epithelial cells based on their immunoreactivity to an ABCG2 mAb (clone 5D3). A small but distinct population of ABCG2− epithelial cells expressed higher levels of ABCG2 transcripts and exhibited greater CFE in culture than did the ABCG2− cells (Fig. 3).
ABCG2 expression is associated with the SP phenotype of Hoechst 33342 efflux [12]. Human hematopoietic stem cells isolated as an SP express high levels of ABCG2 [12], and its expression dropped sharply in committed progenitor cells [16]. Using an ABCG2 knockout mouse model, Zhou et al. [30] demonstrated a significant decrease in the number of bone marrow SP cells and loss of repopulation capability of these cells when sorted. A population of cells with an SP phenotype has also been found in other non–bone marrow–derived cells, including muscle [31, 32], brain [33], pancreas [34], mammary gland [35, 36], lung [37], testis [38], heart [39], and human limbus [26], and the SP may identify primitive progenitor cells in those tissues. It was reported that the bromodeoxyurindine label–retaining cells (stem cells) isolated from primary mouse mammary cultures were enriched for both stem cell antigen-1 and Hoechst dye–effluxing SP properties [35].
A controversy around the SP cells representing the stem cells of the epidermis has been reported very recently. Terunuma et al. [40] found that the SP epidermal keratinocytes from human newborn foreskins have a different cell surface phenotype (low ß1 integrin and low α6 integrin expression) than label-retaining keratinocytes, and they represent a unique population of keratinocytes that are distinctly different from the traditional keratinocyte stem cell candidate. Triel et al. [41] found that the SP cells in human and mouse epidermis lack stem cell characteristics. The SP cells from 44 human skin samples, at frequencies ranging from 0.01%–5.39%, did not express particular high levels of ß1 integrin. Mouse epidermis SP cells are not identical to the label-retaining population but are cycling cells.
We performed FACS using Hoechst exclusion assay on the limbal epithelium. Similarly to the bone marrow, human limbal epithelial cells showed decreased SP formation in the presence of the blocker verapamil. The sorted limbal SP cells expressed higher levels of ABCG2 as well as the stem cell–associated marker, ΔNp63. Limbal SP cells sorted by FACS exhibited higher CFE in culture than the non-SP cells, and they reached confluence by 14 days. Consistent with a recent report by Watanabe et al. [26] that human limbal epithelium contains SP cells expressing the ATP-binding cassette transporter ABCG2, our results further suggest that ABCG2 expression is correlated to limbal SP, with greater proliferative capacity and higher ΔNp63 expression.
In conclusion, our findings demonstrate that ABCG2 transporter is exclusively expressed by the basal cells of human limbal epithelia. The ABCG2− population and SP showed higher expression of ABCG2 and ΔNp63 and greater proliferative capacity. Taken together, it suggests that ABCG2 transporter could serve as a marker to identify the putative limbal epithelial stem cells.
Acknowledgments
We thank Dr. Margaret A. Goodell for her knowledgeable advice and assistance with the methods, especially the SP protocol. We thank the Lions Eye Bank of Texas for their great support in providing human corneoscleral tissues and Jeffrey M. Scott for his technical help in performing FACS. This study was supported by NIH Grants, EY014553 (DQL) and EY11915 (SCP), National Eye Institute, Bethesda, MD, Lions Eye Bank Foundation 2004 grant award (ZC), a post-doctoral research fellowship from Fight For Sight (CSP), an unrestricted grant from Research to Prevent Blindness, the Oshman Foundation, and the William Stamps Farish Fund.
Footnotes
Presented in part as an abstract at the annual meeting of the Association for Research in Vision and Ophthalmology, May 4–8, 2003, Fort Lauderdale, Florida.
References
- 1.Hall PA, Watt FM. Stem cells: the generation and maintenance of cellular diversity. Development. 1989;106:619–633. doi: 10.1242/dev.106.4.619. [DOI] [PubMed] [Google Scholar]
- 2.Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- 3.Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: entity or function? Cell. 2001;105:829–841. doi: 10.1016/s0092-8674(01)00409-3. [DOI] [PubMed] [Google Scholar]
- 4.Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 5.Lavker RM, Sun TT. Epidermal stem cells: properties, markers, and location. Proc Natl Acad Sci U S A. 2000;97:13473–13475. doi: 10.1073/pnas.250380097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watt FM. Epidermal stem cells as targets for gene transfer. Hum Gene Ther. 2000;11:2261–2266. doi: 10.1089/104303400750035799. [DOI] [PubMed] [Google Scholar]
- 7.Cotsarelis G, Kaur P, Dhouailly D, et al. Epithelial stem cells in the skin: definition, markers, localization and functions. Exp Dermatol. 1999;8:80–88. doi: 10.1111/j.1600-0625.1999.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 8.Dua HS, Azuara-Blanco A. Limbal stem cells of the corneal epithelium. Surv Ophthalmol. 2000;44:415–425. doi: 10.1016/s0039-6257(00)00109-0. [DOI] [PubMed] [Google Scholar]
- 9.Tseng SC. Concept and application of limbal stem cells. Eye. 1989;3(pt 2):141–157. doi: 10.1038/eye.1989.22. [DOI] [PubMed] [Google Scholar]
- 10.Chen Z, de Paiva CS, Luo L, et al. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22:355–366. doi: 10.1634/stemcells.22-3-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trosko JE, Ruch RJ. Cell-cell communication in carcinogenesis. Front Biosci. 1998;3:D208–D236. doi: 10.2741/a275. [DOI] [PubMed] [Google Scholar]
- 12.Kim M, Turnquist H, Jackson J, et al. The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes Hoechst 33342 and is overexpressed in hematopoietic stem cells. Clin Cancer Res. 2002;8:22–28. [PubMed] [Google Scholar]
- 13.Goodell MA, Brose K, Paradis G, et al. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodell MA, Rosenzweig M, Kim H, et al. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997;3:1337–1345. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- 15.Zhou S, Schuetz JD, Bunting KD, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 16.Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99:507–512. doi: 10.1182/blood.v99.2.507. [DOI] [PubMed] [Google Scholar]
- 17.Cooray HC, Blackmore CG, Maskell L, et al. Localisation of breast cancer resistance protein in microvessel endothelium of human brain. Neuroreport. 2002;13:2059–2063. doi: 10.1097/00001756-200211150-00014. [DOI] [PubMed] [Google Scholar]
- 18.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 19.Tseng SC, Kruse FE, Merritt J, et al. Comparison between serum-free and fibroblast-cocultured single-cell clonal culture systems: evidence showing that epithelial anti-apoptotic activity is present in 3T3 fibroblast-conditioned media. Curr Eye Res. 1996;15:973–984. doi: 10.3109/02713689609017643. [DOI] [PubMed] [Google Scholar]
- 20.Kim HS, Jun S X, de Paiva CS, et al. Phenotypic characterization of human corneal epithelial cells expanded ex vivo from limbal explant and single cell cultures. Exp Eye Res. 2004;79:41–49. doi: 10.1016/j.exer.2004.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li DQ, Tseng SC. Three patterns of cytokine expression potentially involved in epithelial-fibroblast interactions of human ocular surface. J Cell Physiol. 1995;163:61–79. doi: 10.1002/jcp.1041630108. [DOI] [PubMed] [Google Scholar]
- 22.Liu Z, Carvajal M, Carraway CA, et al. Expression of the receptor tyrosine kinases, epidermal growth factor receptor, ErbB2, and ErbB3, in human ocular surface epithelia. Cornea. 2001;20:81–85. doi: 10.1097/00003226-200101000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Li DQ, Lokeshwar BL, Solomon A, et al. Regulation of MMP-9 production by human corneal epithelial cells. Exp Eye Res. 2001;73:449–459. doi: 10.1006/exer.2001.1054. [DOI] [PubMed] [Google Scholar]
- 24.Abbott BL, Colapietro AM, Barnes Y, et al. Low levels of ABCG2 expression in adult AML blast samples. Blood. 2002;100:4594–4601. doi: 10.1182/blood-2002-01-0271. [DOI] [PubMed] [Google Scholar]
- 25.Robey RW, Steadman K, Polgar O, et al. Pheophorbide a is a specific probe for ABCG2 function and inhibition. Cancer Res. 2004;64:1242–1246. doi: 10.1158/0008-5472.can-03-3298. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe K, Nishida K, Yamato M, et al. Human limbal epithelium contains side population cells expressing the ATP-binding cassette transporter ABCG2. FEBS Lett. 2004;565:6–10. doi: 10.1016/j.febslet.2004.03.064. [DOI] [PubMed] [Google Scholar]
- 27.Pellegrini G, Dellambra E, Golisano O, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang A, Schweitzer R, Sun D, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- 29.Maliepaard M, Scheffer GL, Faneyte IF, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–3464. [PubMed] [Google Scholar]
- 30.Zhou S, Morris JJ, Barnes Y, et al. Bcrp1 gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivo. Proc Natl Acad Sci U S A. 2002;99:12339–12344. doi: 10.1073/pnas.192276999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Majka SM, Jackson KA, Kienstra KA, et al. Distinct progenitor populations in skeletal muscle are bone marrow derived and exhibit different cell fates during vascular regeneration. J Clin Invest. 2003;111:71–79. doi: 10.1172/JCI16157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asakura A. Stem cells in adult skeletal muscle. Trends Cardiovasc Med. 2003;13:123–128. doi: 10.1016/s1050-1738(03)00024-0. [DOI] [PubMed] [Google Scholar]
- 33.Hulspas R, Quesenberry PJ. Characterization of neurosphere cell phenotypes by flow cytometry. Cytometry. 2000;40:245–250. [PubMed] [Google Scholar]
- 34.Lechner A, Leech CA, Abraham EJ, et al. Nestin-positive progenitor cells derived from adult human pancreatic islets of Langerhans contain side population (SP) cells defined by expression of the ABCG2 (BCRP1) ATP-binding cassette transporter. Biochem Biophys Res Commun. 2002;293:670–674. doi: 10.1016/S0006-291X(02)00275-9. [DOI] [PubMed] [Google Scholar]
- 35.Welm BE, Tepera SB, Venezia T, et al. Sca-1(pos) cells in the mouse mammary gland represents an enriched progenitor cell population. Dev Biol. 2002;245:42–56. doi: 10.1006/dbio.2002.0625. [DOI] [PubMed] [Google Scholar]
- 36.Alvi AJ, Clayton H, Joshi C, et al. Functional and molecular characterisation of mammary side population cells. Breast Cancer Res. 2003;5:R1–R8. doi: 10.1186/bcr547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Summer R, Kotton DN, Sun X, et al. Side population cells and Bcrp1 expression in lung. Am J Physiol Lung Cell Mol Physiol. 2003;285:L97–L104. doi: 10.1152/ajplung.00009.2003. [DOI] [PubMed] [Google Scholar]
- 38.Lassalle B, Bastos H, Louis JP, et al. “Side population” cells in adult mouse testis express Bcrp1 gene and are enriched in spermatogonia and germinal stem cells. Development. 2004;131:479–487. doi: 10.1242/dev.00918. [DOI] [PubMed] [Google Scholar]
- 39.Martin CM, Meeson AP, Robertson SM, et al. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265:262–275. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 40.Terunuma A, Jackson KL, Kapoor V, et al. Side population keratinocytes resembling bone marrow side population stem cells is distinct from label-retaining keratinocyte stem cells. J Invest Dermatol. 2003;121:1095–1103. doi: 10.1046/j.1523-1747.2003.12531.x. [DOI] [PubMed] [Google Scholar]
- 41.Triel C, Vestergaard ME, Bolund L, et al. Side population cells in human and mouse epidermis lack stem cell characteristics. Exp Cell Res. 2004;295:79–90. doi: 10.1016/j.yexcr.2003.11.032. [DOI] [PubMed] [Google Scholar]


