Abstract
Despite a lack of definitive evidence, it is frequently proposed that the basal ganglia (BG) motor circuit plays a critical role in the storage and execution of movement sequences (or motor habits). To test this hypothesis directly, we inactivated the sensorimotor territory of the globus pallidus internus (sGPi, the main BG motor output) in two monkeys trained to perform overlearned and random sequences of four out-and-back reaching movements directed to visual targets. Infusion of muscimol (a GABAA agonist) into sGPi caused dysmetria and slowing of individual movements, but these impairments were virtually identical for overlearned and random sequences. The fluid predictive execution of learned sequences and the animals' tendency to reproduce the sequence pattern in random trials was preserved following pallidal blockade. These results suggest that the BG motor circuit contributes to motor execution, but not to motor sequencing or the storage of overlearned serial skills.
Introduction
A role for the basal ganglia (BG) in the storage and expression of learned sequential skills is advocated widely based on a variety of experimental evidence (Hikosaka et al., 2002; Graybiel, 2008; Doyon et al., 2009). Several imaging studies report that movement-related activation of striatum is greater when learned motor sequences are performed (Hazeltine et al., 1997; Doyon et al., 2002; Seidler et al., 2005). During learning, neural activity shifts progressively from the associative to the sensorimotor territories of the striatopallidal complex (Lehéricy et al., 2005; Coynel et al., 2010). Striatal dysfunctions perturb skillful performance of learned motor sequences (Benecke et al., 1987; Agostino et al., 1992). In healthy animals, neuronal activity in the sensorimotor BG loop encodes specific aspects of already-learned sequences (Kimura, 1990; Mushiake and Strick, 1995; Jog et al., 1999). Also, reversible inactivations in the posterior (sensorimotor) striatum disrupt the execution of well learned sequences (Miyachi et al., 1997).
Although accepted widely, a role for the BG in the storage and execution of motor sequences is not without controversy. Several imaging studies have failed to find increased activity in the BG following extensive training (Rijntjes et al., 1999; Jansma et al., 2001; Wu et al., 2004). At the same time, clinical evaluations have shown that therapeutic ablation of the sensorimotor territory of the globus pallidus internus [sGPi, main output nucleus of the sensorimotor BG circuit (Alexander et al., 1990)] has few deleterious motor effects (Green et al., 2002; Bastian et al., 2003) and actually improves some aspects of motor sequencing in humans (Kimber et al., 1999; Obeso et al., 2009). Similar disconnection of the BG motor circuit in neurologically normal animals affects specific kinematic parameters of individual movements (Horak and Anderson, 1984; Mink and Thach, 1991; Kato and Kimura, 1992; Inase et al., 1996; Desmurget and Turner, 2008), without degrading the fluid coordination of simple reach-grasp-and-retrieve sequences (Wenger et al., 1999). Although birdsong bears many similarities to the sequential behaviors of mammals (Doupe and Kuhl, 1999), lesions of the bird's BG homolog have little impact on the execution of well learned songs (Bottjer et al., 1984; Brainard, 2004). Similar negative results have been found for monkeys performing well learned arm sequences following systemic administration of dopamine antagonists (Levesque et al., 2007; Tremblay et al., 2009).
In the present study, we reasoned that if the BG sensorimotor circuit contributes to the execution and/or storage of learned motor sequences, then pharmacological inactivation of the main output of this circuit (sGpi; Alexander et al., 1990) should impair aspects of motor performance that are unique to the performance of already-learned sequences.
Materials and Methods
The experiments were approved by the University of California, San Francisco Animal Institutional Review Board. Two monkeys (Macaca mulatta; C, female ∼7.5 kg; H, male ∼12 kg) participated in the study. The apparatus has been described previously (Desmurget and Turner, 2008). In brief, animals moved a vertically oriented joystick with the right hand, thereby controlling the position of a cursor on an liquid crystal display monitor. Empty circles on the monitor marked one central target zone and four peripheral zones (radius 14 cm) (Fig. 1). Trials progressed as follows (supplemental Fig. 1, available at www.jneurosci.org as supplemental material): (1) A filled “instruction” cue appeared within the central target, instructing the animal to move the cursor to that position and hold there for a random period (1–2 s). (2) The instruction cue jumped to one of the peripheral targets. (3) The animal was allowed 0.8 s to move the cursor to the indicated target. (4) Immediately upon entry of the cursor into the peripheral target, the instruction cue jumped back to the central target, prompting a return-to-center movement of the cursor. (5) A bolus of food was delivered when the still-moving cursor entered the central target. Stages 2–5 were repeated four times in quick succession. For the second-to-fourth repetition, the cue jumped to a peripheral target 230 ms after stage 5 of the previous out-and-back movement. Because of this, return movements were completed at a variable time point following reward delivery (165 ± 42 ms, mean ± SD) and preceding onset of the next peripheral cue (65 ± 42 ms).
Figure 1.
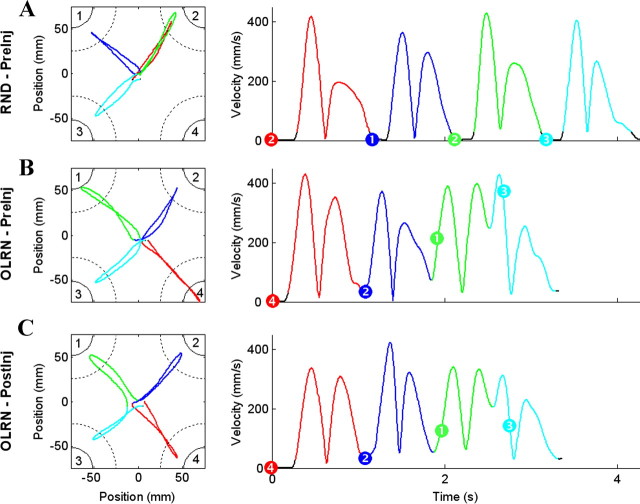
Representative sequences performed by monkey H in the RND preinjection (A), OLRN preinjection (B), and OLRN postinjection (C) conditions. The left and right panels show position and velocity data, respectively. Each sequence component is identified by a different color. Black sections of the velocity curves indicate periods of immobility (velocity <25 mm/s). Left, Continuous arcs in corners indicate positions of the instruction cues. Dotted arcs indicate the peripheral target zones for cursor movements. Right, Dots on the velocity curves indicate the instant of presentation of the instruction cue. Numbers define targets (left) and which target was indicated by each instruction cue (right). The figures are scaled to show the central region of the workspace.
The degree to which successive peripheral targets could be predicted, and thus learned as a sequence, was manipulated under two task conditions presented in alternating blocks of 10–20 trials. In the random (RND) condition, four targets were chosen at random with replacement, yielding 44 = 256 possible permutations of target order. Trial-to-trial randomization of target order made it impossible to predict accurately which peripheral target would be presented next at any time under the RND condition. In the overlearned (OLRN) condition, target presentation followed an immutable, completely predictable, sequence. One animal performed one OLRN sequence for all sessions (targets 1→2→3→4) (Fig. 1). The second animal performed two different sequences during early and late experiments (1→2→3→4 and then 4→2→1→3). Animals practiced both tasks, including the specific OLRN sequences, >6 months (>50,000 trials) before the injection experiments. During early training (∼3 months), OLRN trials were three times more frequent (>18,750 trials) than RND trials (>6250). During late training (3–6 months) OLRN and RND trials were equally frequent (>12,500 trials). Given the 256 possible orderings of targets under the RND condition, each permutation was performed >75 times during training. Following this training, animals switched easily between RND and OLRN blocks without explicit cues, after a single transition trial (i.e., when a RND trial followed a series of OLRN trials or an OLRN trial followed a series of RND trials; see Results). Transition (first) trials of all blocks were excluded from analyses.
Surgery, mapping, microinjections, and histology.
These methods have been described previously (Desmurget and Turner, 2008). After implantation of a recording chamber, the sGPi was delineated according to anatomical boundaries and neuronal responses to movements of contralateral limbs. For subsequent microinjection experiments, the GABAA agonist muscimol hydrobromide was dissolved in artificial CSF (1 μg/μl) and infused at sites in the sGPi (0.5–2.0 μl at 0.2 μl/min via fused-silica or 30 ga stainless-steel cannula). Behavioral data were collected before and starting 10 min after completion of each injection.
Injection locations were reconstructed after the last microinjection experiment using standard methods. Animals were killed by transcardial perfusion (saline then 10% formalin). The brains were blocked, cryoprotected, frozen, cut (50 μm), and stained (cresyl violet). Injection locations were estimated by comparing chamber positions, electrophysiologically derived maps, and positions of marking lesions.
Data analyses.
Joystick position signals were digitized (200 Hz), low-pass filtered (10 Hz cutoff), and differentiated to obtain velocity and acceleration. Periods of immobility during sequence execution were defined as periods with hand velocity <25 mm/s. When velocity between two movement components did not drop below this threshold, the local velocity minimum was taken as the onset of the second outward movement (Fig. 1B,C). To encourage animals to continue working despite hypometric reaches (a principal effect of injections), the control software was adjusted during an experiment to reward movements that did not reach the target zone (2 cm tolerance).
Whether OLRN trials were performed as a series of independent movements or as an integrated motor sequence was determined primarily by intercomponent reaction times (RTs). There is universal agreement from the rodent (Berridge and Whishaw, 1992; Jay and Dunnett, 2007), nonhuman primate (Hikosaka et al., 1995; Procyk et al., 2000), and human (Nissen and Bullemer, 1987; Keele et al., 2003) literature that RT is an appropriate objective measure of whether a sequence has been learned. Extensive training can yield intercomponent RTs <100 ms and even negative (Matsuzaka et al., 2007), thereby indicating that sequence components are being selected and initiated predictively. Thus, component movements with RTs <100 ms were classified as “predictive.”
Results
Preinjection
Figure 1, A and B, shows exemplar trials performed under RND and OLRN conditions. Under both, outward movements to a peripheral target were followed immediately by a return-to-center movement, thus yielding a bilobed velocity profile for each of the four out-and-back component movements (hereafter termed “components”) (Fig. 1, coded by color). For RND trials, each cue presentation was followed by a typical RT such that periods of immobility intervened between movement components (Fig. 1A, black segments of velocity trace). Periods of immobility were often absent, however, for components 2–4 of OLRN trials (52% of all OLRN trials studied) such that one return-to-center movement was followed immediately, without stopping, by movement outward to the next peripheral target (Fig. 1B). In agreement with these observations, the total time spent in immobility between components was significantly shorter for OLRN than RND sequences [two-way between (animals) × within (condition) ANOVA; condition effect, F(1,17) = 121, p < 0.0001] (Fig. 2Aii). This decrease led to an overall shortening of sequence durations in OLRN as compared to RND (F(1,17) = 170, p < 0.0001) (Fig. 2Ai).
Figure 2.
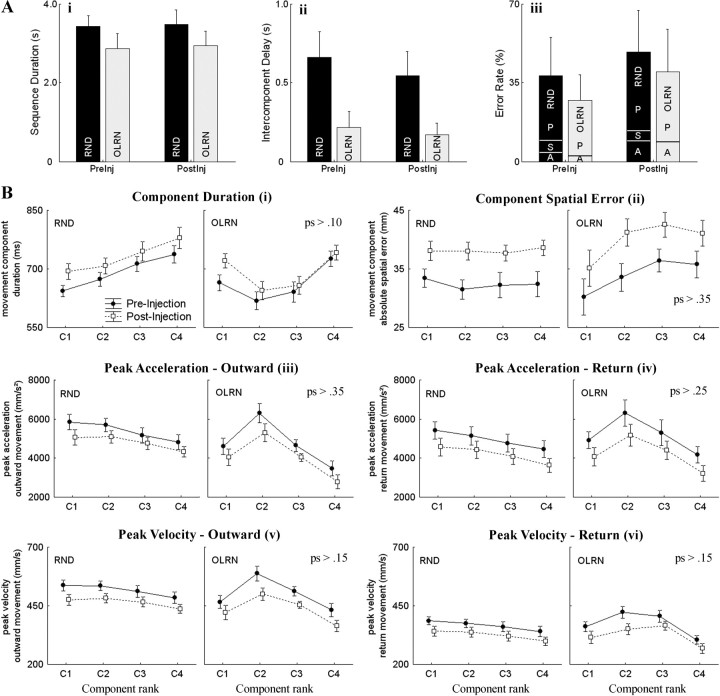
A, The effects of GPi inactivation on general measures of task performance did not differ between RND and OLRN conditions. Errors rates (iii) are subdivided into the following: P, performance errors; S, OLRN-consistent misdirections in the RND condition; and A, aborted trials. B, Movement kinematics differed across component ranks (C1–C4) and between conditions (RND or OLRN) due to idiosyncratic differences in performance. Inactivation effects did not interact with any rank or condition effects, however. Shown are means (±SD) for 19 injection sessions in two animals. p values summarize results for injection × rank, injection × condition, and injection × rank × condition interactions from a four-way between (animals) × within (injection, condition, rank) ANOVA.
Consistent with the view that individual components of OLRN sequences were initiated and guided from memory, 78% of OLRN components 2–4 had predictive RTs and 51% started before target presentation. On average, RT was negative for these components (−0.015 s). For components 2–4 of RND trials, outward movements started at a typical RT interval following cue onset (mean 0.160 s). For the first component movement, the hand started moving after a typical RT in both conditions, but prior knowledge of target position caused RT to be slightly shorter in OLRN than in RND (means: 0.175 vs 0.188 s, F(1,17) = 10, p < 0.01).
Spatial aspects of task performance were very similar under RND and OLRN sequences. In particular, extent and direction errors were not statistically different across conditions for a given target [three-way between (animals) × within (condition, target location); condition effect, F(1,17) values <3.8, p values >0.6 (supplemental Fig. 2, available at www.jneurosci.org as supplemental material)].
Specific analyses were performed to confirm that the animals were able to switch easily between RND and OLRN blocks after a single transition trial (see Materials and Methods). Mean kinematic characteristics of the first posttransition trial were compared with the characteristics of trials occurring early (average of trials 3–5) and late (average of the last three trials) in a block. No significant effects were found (supplemental material, available at www.jneurosci.org).
Postinjection
A total of 19 muscimol injections were performed at 14 sites in sGPi (supplemental Fig. 3, Table 1, available at www.jneurosci.org as supplemental material).
As observed in previous studies (see Discussion), sGPi inactivation caused the animals to move more slowly (Fig. 2B). Statistically, muscimol injection increased component-movement duration [four-way between (animals) × within (injection, condition, rank) ANOVA; injection effect, F(1,17) = 7.0, p < 0.02] while decreasing component mean velocity (F(1,17) = 6.4, p < 0.03) and maximum extent (F(1,17) = 17, p < 0.001). Peak acceleration and velocity were also reduced for both outward and return phases of movement (F(1,17) values > 12, p values < 0.005). These effects were very robust across sessions (supplemental Fig. 4, available at www.jneurosci.org as supplemental material). Injections did not interfere with the animals' ability to switch between RND and OLRN blocks (supplemental material, available at www.jneurosci.org).
Figure 2B summarizes the main kinematic features of each of the four out-and-back components. It can be seen that movement characteristics were affected by component rank (C1–C4) and condition (RND vs OLRN). Rank-related variations were due to target-dependent effects (target order was randomized trial to trial for the RND condition but fixed across all OLRN trials). Of particular interest, the influence of muscimol injections on kinematics did not vary across component rank or between task conditions (no significant injection × rank, injection × condition, or injection × rank × condition interactions for the main kinematic markers of the sequence) (Fig. 2B). In other words, the effects of sGPi blockade on kinematics were not statistically different for the OLRN and RND sequences, and they were not larger toward the end of a sequence, as would be expected if BG output was important for the smooth unfolding of the OLRN sequence.
At the temporal level, the differences in task timing that distinguished OLRN from RND trials were preserved following muscimol injections (Fig. 1C). In particular, the effect of sGPi blockade on sequence duration and intercomponent delay was similar in both conditions [three-way between (animals) × within (injection, condition) ANOVA; injection × condition interaction, sequence duration: F(1,17) = 0.3, p > 0.55 (Fig. 2Ai); intercomponent delay: F(1,17) = 3.5, p > 0.075 (Fig. 2Aii)]. Strikingly, intercomponent periods of immobility were not lengthened following injections, but if anything, were slightly decreased (Fig. 2Aii). The time required to reverse directions at peripheral targets between outward and return movements also remained unaffected following injections (0.004 vs 0.007 s; F(1,17) = 3.7, p > 0.07). The proportions of OLRN trials with “predictive” (78% vs 70%) and negative (51% vs 59%) RTs in components 2–4 were preserved as well (difference proportion tests, p values > 0.55). Sequence duration became more variable following sGPi blockade (SD averaged across subjects and sessions, 0.162 vs 0.252 s). However, this effect was similar for both conditions (injection × condition interaction, F(1,17) = 0.01, p > 0.90), suggesting that the increased variability did not reflect an alteration of movement sequencing, but an increase of execution noise following muscimol injection.
At a more general level, sGPi blockade did not interfere with an animal's ability to complete the four-element sequence. Figure 2Aiii shows that execution errors were more common after injections (F(1,17) = 7.6, p < 0.02). However, this increase was similar in both conditions as shown by the absence of a condition-by-injection interaction (F(1,17) = 0.2, p > 0.65). The postinjection increase in errors had a dual origin: (1) degradation of motor performance (Fig. 2Aiii, P), making it more difficult for animals to reach the target zone and bring the hand back to the starting area within the allotted time; and (2) self-aborted trials (Fig. 2Aiii, A) in which the animal simply stopped moving became more frequent.
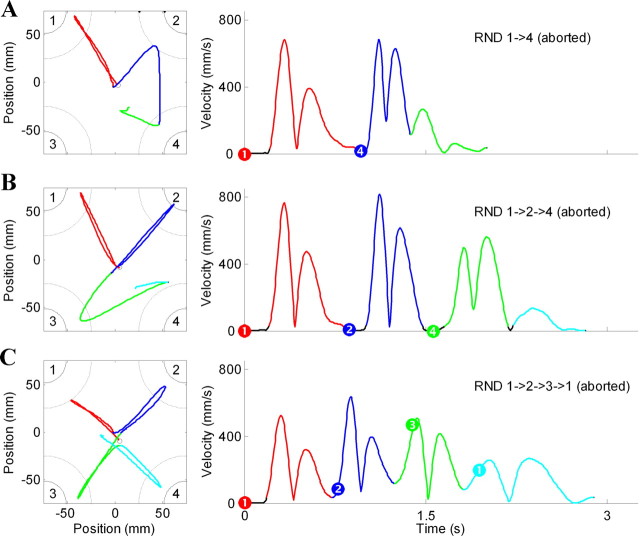
Figure 2Aiii also shows that errors were more frequent under the RND than the OLRN condition (F(1,17) = 11.0, p < 0.01). This was due to the fact that the animals tended erroneously to reproduce the OLRN sequence when, by happenstance, the first or more components of a RND trial followed the order of the OLRN sequence. For example, Figure 3A shows kinematic data from a RND trial in which the first component movement was directed to target #1, the first target of this animal's OLRN sequence. The second component of the RND trial began with presentation of target #4, but at the same time, the animal had already initiated a misdirected movement to capture target #2 (component 2 of the OLRN sequence). After reaching target #2, the animal made a rapid corrective movement to capture target #4, but the trial was aborted by the task-control program. This behavior suggested that the animals were strongly biased toward performing OLRN sequences. Interestingly, this bias persisted after injection at comparable rates (pre-inj: 25%; post-inj: 19%; difference proportion test, p > 0.65). As expected, an animal's tendency to fall into the OLRN sequence increased as the number of concurring component movements increased. Figure 3, B and C, illustrates examples in which the first two and the first three cues of the RND trial concurred with the OLRN sequence. Across all postinjection periods in both animals, 201 trials were found in which the first target of a RND trial concurred with the first OLRN target. Within these, 24 OLRN-consistent misdirected movements were found (12%). The rate of OLRN-consistent misdirections jumped to 43% and 30%, respectively, for trials in which the initial two and three components concurred with the OLRN sequence (19/44 trials and 5/17 trials, respectively).
Figure 3.
Representative examples of erroneous predictive responses performed by monkey C during RND trials after one (A), two (B), or three (C) targets followed, by coincidence, the order of the OLRN sequence (1→2→3→4). All three examples are taken from postinjection epochs. The figure follows conventions from Figure 1.
Discussion
Our results are consistent with previous evidence that the BG motor circuit contributes to movement execution, but they fail to support the concept that this circuit is involved in the storage or execution of well learned motor habits. When the main output of this circuit was inactivated, movement duration was increased, motor responses were slowed, and movement extent was reduced. These impairments were equally present when four discrete movements were performed in quick succession (RND) and when a four-component overlearned sequence was executed predictively (OLRN). Similar impairments in kinematics have been reported in previous studies, in the context of isolated limb movements (Horak and Anderson, 1984; Mink and Thach, 1991; Kato and Kimura, 1992; Inase et al., 1996) and reach-grasp-and-retrieve responses (Wenger et al., 1999).
Aside from the motor deficits described above, task performance was fully preserved. OLRN trials unfolded as a rapid arpeggio of component movements. The second through fourth component movements of this condition were initiated predictively in >75% of the trials. We were unable to identify significant changes in this proportion or other selective impairments in the production of OLRN sequences following inactivation of sGPi. sGPi blockade did not perturb the fluid unfolding of the OLRN sequence, nor did it degrade motor execution differentially for OLRN trials as a whole or as a function of the rank-order of a movement in the sequence. Also, the animals' tendency to reproduce the learned habit erroneously when a RND trial started like the OLRN sequence was not weakened following muscimol injection. Regarding these “negative” results, one potential concern is that small infusion volumes may not have affected a large enough fraction of sGPi to test the hypothesis adequately. Additional motor impairments might have been evoked if larger injection volumes had been used. In the same vein, other deficits might have been produced if injections had been placed in subregions of sGPi that we did not explore. Although these are valid concerns, we do not think they can explain the preservation of movement sequencing in the present study. Marked and consistent kinematic impairments were produced by focal inactivations distributed across a significant fraction of the posterior GPi. These impairments were similar to ones reported in numerous previous studies in which sGPi was inactivated (Horak and Anderson, 1984; Mink and Thach, 1991; Kato and Kimura, 1992; Inase et al., 1996; Wenger et al., 1999). Their existence shows that sGPi was inactivated reliably in the present study, even if not totally. Given this result, it appears unlikely that the failure of GPi inactivation to degrade motor sequencing can be explained by a lack of sensitivity of the injection procedure or by misdirected anatomic targeting. At minimum, our data warrant the conclusion that the BG skeletomotor circuit is not necessary for the generation of well learned motor sequences. This conclusion does not rule out the possible importance of associative and limbic BG circuits for this type of behavior.
How do we harmonize the present results with those of previous studies that implicate BG motor circuits in sequential behaviors (see Introduction)? First, sequencing deficits observed in cases of striatal pathology in humans [e.g., Parkinson's disease (Benecke et al., 1987; Agostino et al., 1992)] and following experimental manipulations of the striatum (Miyachi et al., 1997) may well reflect secondary disruption of remote cortical functions rather than a function of the BG per se (Ayalon et al., 2004; Shin et al., 2005). Second, the fact that sequence-related information is encoded at several stages through the BG (Mushiake and Strick, 1995; Doyon et al., 2002; Lehéricy et al., 2005; Seidler et al., 2005) does not prove that the BG supports sequence retention or generation. BG encoding of sequence-related information may be used for functions such as reward anticipation, behavior optimization, forward modeling, and learning. Similar arguments can be made for the shifts in neuronal encoding during the course of sequence learning (Jog et al., 1999; Lehéricy et al., 2005; Coynel et al., 2010). Such shifts are not unexpected considering that learning causes major changes in motor execution (Schmidt and Lee, 1999). Although these shifts provide intriguing information about learning-related alterations in task encoding, they offer little insight into how this information is used by the motor control regions modulated by BG output.
In summary, the present study shows that transient pharmacological inactivations of the sensorimotor portion of GPi cause dysmetria and slowing of individual movements, while preserving the fluid automatized integration of these movements into a learned sequence. This finding suggests that the BG contributes to motor execution but not to the production or storage of well learned serial skills.
Footnotes
This work was supported by National Institutes of Health Grants R01NS39146, R01NS44551, and P01NS044393 (to R.S.T.). We thank Kevin McCairn and Donn Simmons for their contribution to data collection and Caroline Tricot for her assistance with data analyses. We thank Dr. Jonathan Horton for assistance with histologic processing.
References
- Agostino R, Berardelli A, Formica A, Accornero N, Manfredi M. Sequential arm movements in patients with Parkinson's disease, Huntington's disease and dystonia. Brain. 1992;115:1481–1495. doi: 10.1093/brain/115.5.1481. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia thalamo-cortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- Ayalon L, Doron R, Weiner I, Joel D. Amelioration of behavioral deficits in a rat model of Huntington's disease by excitotoxic lesions of globus pallidus. Exp Neurol. 2004;186:46–58. doi: 10.1016/S0014-4886(03)00312-1. [DOI] [PubMed] [Google Scholar]
- Bastian AJ, Kelly VE, Perlmutter JS, Mink JW. Effects of pallidotomy on walking and reaching movements in Parkinson's disease. Mov Disord. 2003;18:1008–1017. doi: 10.1002/mds.10494. [DOI] [PubMed] [Google Scholar]
- Benecke R, Rothwell JC, Dick JP, Day BL, Marsden CD. Disturbance of sequential movements in patients with Parkinson's disease. Brain. 1987;110:361–379. doi: 10.1093/brain/110.2.361. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Whishaw IQ. Cortex, striatum, and cerebellum: control of serial order in a grooming sequence. Exp Brain Res. 1992;90:275–290. doi: 10.1007/BF00227239. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224:901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- Brainard MS. Contributions of the anterior forebrain pathway to vocal plasticity. Ann N Y Acad Sci. 2004;1016:377–394. doi: 10.1196/annals.1298.042. [DOI] [PubMed] [Google Scholar]
- Coynel D, Marrelec G, Perlbarg V, Pélégrini-Issac M, Van de Moortele PF, Ugurbil K, Doyon J, Benali H, Lehéricy S. Dynamics of motor-related integration during motor sequence learning. Neuroimage. 2010;49:759–766. doi: 10.1016/j.neuroimage.2009.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmurget M, Turner RS. Testing basal ganglia motor functions through reversible inactivations in the posterior internal globus pallidus. J Neurophysiol. 2008;99:1057–1076. doi: 10.1152/jn.01010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annu Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- Doyon J, Song AW, Karni A, Lalonde F, Adams MM, Ungerleider LG. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc Natl Acad Sci U S A. 2002;99:1017–1022. doi: 10.1073/pnas.022615199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J, Bellec P, Amsel R, Penhune V, Monchi O, Carrier J, Lehéricy S, Benali H. Contributions of the basal ganglia and functionally related structures to motor learning. Behav Brain Res. 2009;199:61–75. doi: 10.1016/j.bbr.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Green J, McDonald WM, Vitek JL, Haber M, Barnhart H, Bakay RA, Evatt M, Freeman A, Wahlay N, Triche S, Sirockman B, DeLong MR. Neuropsychological and psychiatric sequelae of pallidotomy for PD: clinical trial findings. Neurology. 2002;58:858–865. doi: 10.1212/wnl.58.6.858. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Grafton ST, Ivry R. Attention and stimulus characteristics determine the locus of motor-sequence encoding. A PET study. Brain. 1997;120:123–140. doi: 10.1093/brain/120.1.123. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Rand MK, Miyachi S, Miyashita K. Learning of sequential movements in monkey: process of learning and retention of memory. J Neurophysiol. 1995;74:1652–1661. doi: 10.1152/jn.1995.74.4.1652. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Sakai K, Nakahara H. Central mechanisms of motor skill learning. Curr Opin Neurobiol. 2002;12:217–222. doi: 10.1016/s0959-4388(02)00307-0. [DOI] [PubMed] [Google Scholar]
- Horak FB, Anderson ME. Influence of globus pallidus on arm movements in monkeys. I. Effects of kainic acid-induced lesions. J Neurophysiol. 1984;52:290–304. doi: 10.1152/jn.1984.52.2.290. [DOI] [PubMed] [Google Scholar]
- Inase M, Buford JA, Anderson ME. Changes in the control of arm position, movement, and thalamic discharge during local inactivation in the globus pallidus of the monkey. J Neurophysiol. 1996;75:1087–1104. doi: 10.1152/jn.1996.75.3.1087. [DOI] [PubMed] [Google Scholar]
- Jansma JM, Ramsey NF, Slagter HA, Kahn RS. Functional anatomical correlates of controlled and automatic processing. J Cogn Neurosci. 2001;13:730–743. doi: 10.1162/08989290152541403. [DOI] [PubMed] [Google Scholar]
- Jay JR, Dunnett SB. An operant serial implicit learning task in rats: acquisition, performance and the effects of striatal lesions. J Neurosci Methods. 2007;163:235–244. doi: 10.1016/j.jneumeth.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. Building neural representations of habits. Science. 1999;286:1745–1749. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- Kato M, Kimura M. Effects of reversible blockade of basal ganglia on a voluntary arm movement. J Neurophysiol. 1992;68:1516–1534. doi: 10.1152/jn.1992.68.5.1516. [DOI] [PubMed] [Google Scholar]
- Keele SW, Ivry R, Mayr U, Hazeltine E, Heuer H. The cognitive and neural architecture of sequence representation. Psychol Rev. 2003;110:316–339. doi: 10.1037/0033-295x.110.2.316. [DOI] [PubMed] [Google Scholar]
- Kimber TE, Tsai CS, Semmler J, Brophy BP, Thompson PD. Voluntary movement after pallidotomy in severe Parkinson's disease. Brain. 1999;122:895–906. doi: 10.1093/brain/122.5.895. [DOI] [PubMed] [Google Scholar]
- Kimura M. Behaviorally contingent property of movement-related activity of the primate putamen. J Neurophysiol. 1990;63:1277–1296. doi: 10.1152/jn.1990.63.6.1277. [DOI] [PubMed] [Google Scholar]
- Lehéricy S, Benali H, Van de Moortele PF, Pélégrini-Issac M, Waechter T, Ugurbil K, Doyon J. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci U S A. 2005;102:12566–12571. doi: 10.1073/pnas.0502762102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque M, Bedard MA, Courtemanche R, Tremblay PL, Scherzer P, Blanchet PJ. Raclopride-induced motor consolidation impairment in primates: role of dopamine type-2 receptor in movement chunking into integrated sequences. Exp Brain Res. 2007;182:499–508. doi: 10.1007/s00221-007-1010-4. [DOI] [PubMed] [Google Scholar]
- Matsuzaka Y, Picard N, Strick PL. Skill representation in the primary motor cortex after long-term practice. J Neurophysiol. 2007;97:1819–1832. doi: 10.1152/jn.00784.2006. [DOI] [PubMed] [Google Scholar]
- Mink JW, Thach WT. Basal ganglia motor control. III. Pallidal ablation: normal reaction time, muscle cocontraction, and slow movement. J Neurophysiol. 1991;65:330–351. doi: 10.1152/jn.1991.65.2.330. [DOI] [PubMed] [Google Scholar]
- Miyachi S, Hikosaka O, Miyashita K, Kárádi Z, Rand MK. Differential roles of monkey striatum in learning of sequential hand movement. Exp Brain Res. 1997;115:1–5. doi: 10.1007/pl00005669. [DOI] [PubMed] [Google Scholar]
- Mushiake H, Strick PL. Pallidal neuron activity during sequential arm movements. J Neurophysiol. 1995;74:2754–2758. doi: 10.1152/jn.1995.74.6.2754. [DOI] [PubMed] [Google Scholar]
- Nissen MJ, Bullemer P. Attentional requirements of learning: evidence from performance measures. Cogn Psychol. 1987;19:1–32. [Google Scholar]
- Obeso JA, Jahanshahi M, Alvarez L, Macias R, Pedroso I, Wilkinson L, Pavon N, Day B, Pinto S, Rodríguez-Oroz MC, Tejeiro J, Artieda J, Talelli P, Swayne O, Rodríguez R, Bhatia K, Rodriguez-Diaz M, Lopez G, Guridi J, Rothwell JC. What can man do without basal ganglia motor output? The effect of combined unilateral subthalamotomy and pallidotomy in a patient with Parkinson's disease. Exp Neurol. 2009;220:283–292. doi: 10.1016/j.expneurol.2009.08.030. [DOI] [PubMed] [Google Scholar]
- Procyk E, Tanaka YL, Joseph JP. Anterior cingulate activity during routine and non-routine sequential behaviors in macaques. Nat Neurosci. 2000;3:502–508. doi: 10.1038/74880. [DOI] [PubMed] [Google Scholar]
- Rijntjes M, Dettmers C, Büchel C, Kiebel S, Frackowiak RS, Weiller C. A blueprint for movement: functional and anatomical representations in the human motor system. J Neurosci. 1999;19:8043–8048. doi: 10.1523/JNEUROSCI.19-18-08043.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RA, Lee TD. Motor control and learning. Champaign, IL: Human Kinetics; 1999. [Google Scholar]
- Seidler RD, Purushotham A, Kim SG, Ugurbil K, Willingham D, Ashe J. Neural correlates of encoding and expression in implicit sequence learning. Exp Brain Res. 2005;165:114–124. doi: 10.1007/s00221-005-2284-z. [DOI] [PubMed] [Google Scholar]
- Shin JC, Aparicio P, Ivry RB. Multidimensional sequence learning in patients with focal basal ganglia lesions. Brain Cogn. 2005;58:75–83. doi: 10.1016/j.bandc.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Tremblay PL, Bedard MA, Levesque M, Chebli M, Parent M, Courtemanche R, Blanchet PJ. Motor sequence learning in primate: role of the D2 receptor in movement chunking during consolidation. Behav Brain Res. 2009;198:231–239. doi: 10.1016/j.bbr.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Wenger KK, Musch KL, Mink JW. Impaired reaching and grasping after focal inactivation of globus pallidus pars interna in the monkey. J Neurophysiol. 1999;82:2049–2060. doi: 10.1152/jn.1999.82.5.2049. [DOI] [PubMed] [Google Scholar]
- Wu T, Kansaku K, Hallett M. How self-initiated memorized movements become automatic: a functional MRI study. J Neurophysiol. 2004;91:1690–1698. doi: 10.1152/jn.01052.2003. [DOI] [PubMed] [Google Scholar]