Abstract
Purpose
Chronic epilepsy frequently develops after brain injury, but prediction of which individual patient will develop spontaneous recurrent seizures (i.e., epilepsy) is not currently possible. Here, we use continuous radiotelemetric electroencephalogram (EEG) and video monitoring along with automated computer detection of EEG spikes and seizures to test the hypothesis that EEG spikes precede and are correlated with subsequent spontaneous recurrent seizures.
Methods
The presence and pattern of EEG spikes was studied during long recording epochs between the end of kainate-induced status epilepticus (SE) and the onset of chronic epilepsy.
Results
The presence of spikes, and later spike clusters, over several days after SE before the first spontaneous seizure were consistently associated with the development of chronic epilepsy. The rate of development of epilepsy (i.e., increase in seizure frequency) was strongly correlated with the frequency of EEG spikes and the cumulative number of EEG spikes after SE.
Conclusions
The temporal features of EEG spikes (i.e., their presence, frequency and pattern [clusters]), when analyzed over prolonged periods, may be a predictive biomarker for the development of chronic epilepsy after brain injury. Future clinical trials using prolonged EEG recordings may reveal the diagnostic utility of EEG spikes as predictors of subsequent epilepsy in brain-injured humans.
Keywords: seizures, temporal lobe, EEG, kainic acid, epileptogenesis
Introduction
Chronic epilepsy is a late complication of a variety of brain injuries, including civilian (Frey, 2003) and military (Salazar et al., 1985) head trauma, cerebrovascular accidents (Lossius et al., 2005), hypoxic-ischemic injury (Tekgul et al., 2006), and status epilepticus (SE) (Shovron, 2002). For example, the incidence of epilepsy after traumatic brain injury is 15–50% (Salazar et al., 1985; Annegers et al., 1996). The interval between brain injury and the first spontaneous seizure can range from months to years (Annegers et al. 1996). Although several epidemiologically defined risk factors for chronic epilepsy after traumatic brain injury have been identified (Frey, 2003; Englander et al., 2003), no clinically useful methods are available for predicting which brain-injured patients will develop epilepsy.
An expert consensus panel recently identified the development of biomarkers to predict epilepsy after brain injury as a key research goal (Kelley et al., 2009). These biomarkers could provide clinically useful prognostic information during the latent period. Biomarkers would also accelerate research regarding the processes underlying the development of epilepsy, or epileptogenesis, as well as the development of therapies designed to prevent this process. In the models of experimental epilepsy studied to date, seizure frequency increases continuously over months from very low initial rates (Williams et al., 2009). These low seizure frequencies can only be measured using very prolonged and intensive monitoring. This monitoring has become a rate-limiting factor in many areas of epilepsy research, and could be supplanted by sufficiently accurate biomarkers.
Monitoring the EEG after brain injury is a logical means of predicting future epilepsy, but early attempts at prediction based on EEG findings were inconclusive (Roseman and Woodhall, 1946; Jennett and Van De Sande, 1975). These early studies necessarily used brief EEG recordings that were stored on paper and thus not readily analyzed quantitatively. However these influential studies have led to the widely-held understanding that EEG recordings during the latent period are not useful predictors of the development of epilepsy (Lowenstein, 2009). The current widespread availability of digital EEG equipment (Brenner and Scheuer, 1998) and epilepsy monitoring units makes feasible the extraction of new types of quantitative information from the EEG using computerized analysis methods of larger EEG datasets (White et al., 2006).
Rodent models of chronic epilepsy based on chemoconvulsant-induced SE capture many of the key features of acquired human epilepsy. The development of chronic epilepsy in human patients has similarities to what has been reported after experimental kainate-induced SE (Williams et al., 2009). For example, experimental animals subjected to SE with resultant brain injury demonstrate a characteristic delay between the time of injury and the onset of clinical seizures, which is similar although shorter (Williams et al. 2009) than what is reported for brain-injured human patients (Annegers et al., 1996). The salient features of the neuropathology of medically intractable temporal lobe epilepsy in human tissue from resective surgeries include neuronal loss and sprouting of recurrent axons (De Lanerolle et al., 1989; Houser et al., 1990; Babb et al., 1991; Mathern et al., 1995a,b) in the hilus and dentate gyrus, respectively. Similar changes have been found in rats subjected to SE induced by systemic kainic acid or pilocarpine, or by electrical stimulation (Ben-Ari, 1985; Buckmaster and Dudek, 1997; Gorter et al., 2001; Nadler, 1991; Turski et al., 1983; Williams et al., 2002)
As a first step in the development of quantitative assessments of the risk of epilepsy after SE, we examined the EEG of rats whose brains had undergone mild versus robust SE from systemic injection of kainic acid. In our analyses, we focused on the EEG spike. We provide evidence that in this model of status epilepticus, EEG spikes precede spontaneous recurrent seizures, and several features of EEG spikes recorded prior to the first spontaneous seizure are strongly predictive of the development of chronic epilepsy.
Methods
Terminology
In these experiments, all of the spikes and seizures were recorded electrographically with radiotelemetry (see below). The seizures were also recorded behaviorally on video tapes. The “spikes” described here were defined as events in the electrographic (EEG) recordings that had rapid positive and/or negative components, were mono-, bi- or multi-phasic, were <200 msec (Gastaut and Broughton, 1972; Niedermyer and Silva, 2004), and included but were not limited to interictal spikes and sharp waves, but not the spike components of electrographically recorded seizures. These “EEG spikes” occurred during and between the seizures associated with status epilepticus, after status epilepticus but before the onset of spontaneous recurrent seizures (i.e., epileptic seizures), and between the spontaneous seizures. EEG spikes are to be differentiated from “single-unit” spikes, which are recorded extracellulary, but are far shorter in duration (i.e., a few milliseconds) and represent the electrical activity (i.e., action potentials) of individual neurons. Electrographic (EEG) seizures in this study refer to any seizure that was recorded electrically, which was essentially all of the seizures. These electrographically recorded seizures were either non-convulsive seizures or convulsive motor seizures. The Racine scale (Racine, 1972; Ben-Ari, 1985) was modified to describe the severity of convulsive motor seizures: Class III seizures had forelimb clonus with a lordotic posture; Class IV seizures showed both forelimb clonus and rearing; Class V seizures were Class IV seizures, but with loss of the righting reflex. Convulsive seizures were always associated with electrographic (EEG) seizure activity and are probably similar to generalized motor or grand mal seizures in human patients. The behaviors during non-convulsive seizures were those generally described as Class I or Class II seizures (Racine, 1972); because characteristic electrographic seizures (e.g., Fig. 2d and 3c) were used to identify these non-convulsive seizures, they are frequently referred to here as electrographic non-convulsive seizures, and may be a model for complex partial seizures. The EEG-seizure latent period was the time from kainate treatment to the first EEG-recorded seizure, which was typically a non-convulsive seizure (Williams et al., 2009). The motor-seizure latent period was the time to the first convulsive motor seizure, which was usually longer than the EEG-seizure latent period (Williams et al., 2009).
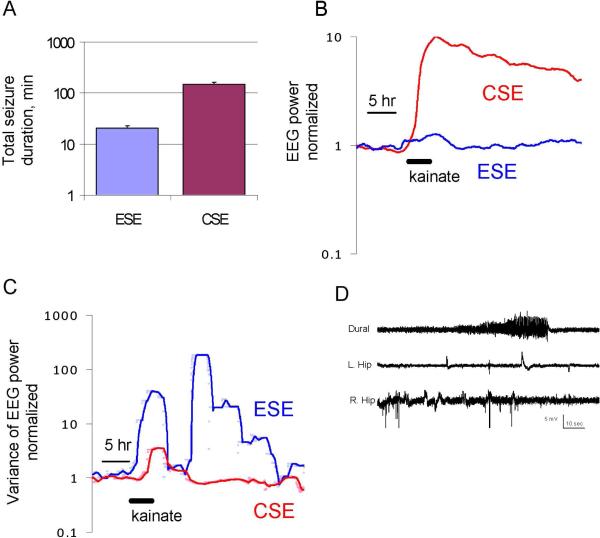
Figure 2.
Electrographic seizures induced by high- and low-dose kainate. A: Total seizure duration in minutes for rats administered low-dose kainate (ESE; mean dose 6.5 mg / kg) and high-dose kainate (CSE; mean dose 13 mg / kg). B: Broad-band EEG power for rats treated with high and low-dose kainate. EEG power was computed over 1 minute epochs for 8 hours before and 30 hours after kainate. The ratio of each group's median EEG power to the pre-kainate baseline power is plotted on a log scale. Plots were smoothed using a 3-hour moving square window. The low-dose kainate seizures were brief and followed by postictal depression (panel D), so that the net increase in EEG power in each 1-minute epoch is much smaller than the EEG power in high-dose kainate, where seizures were longer and epochs of postictal depression were much less prominent. C: The group variance of EEG power (computed as in B) is plotted as individual values (dots) as well as a 1-hour moving average (solid line) to illustrate the intermittent nature of seizures in low-dose kainate rats (ESE). D: Examples of seizures recorded after low-dose kainate. Dural refers to the EEG electrode on the brain surface and L. and R. hip refer to the hippocampal leads.
Figure 3.
Seizures induced by kainate (A,B) and a spontaneous seizure (C). A, B: Kainate-induced seizures during CSE. Electrographic spikes were continuous, but decreased in frequency from 1 h (A) to 8 h (B) following kainate treatment. C: Spontaneous seizure recorded 12 weeks after CSE in the same rat as A, B. For each panel, top trace = dural EEG record; the second and third traces = left and right hippocampi, respectively. Boxed areas are illustrated at higher temporal resolution in lower traces with corresponding lettering.
Surgery
To record continuous EEG data, male Sprague-Dawley rats (175-350 g, Harlan, Indianapolis, IN) were implanted with teflon-coated stainless-steel depth-recording electrodes as previously described (White et al,.2006; Williams et al., 2006). A total of three channels were recorded. Electrodes were positioned in the granule cell layer of both the right and left dentate gyrus. The stereotaxic coordinates used were 2.5 mm lateral and 4.0 mm caudal from the bregma. An audio amplifier was used to detect action potentials and ensure correct electrode placement in the dentate granule cell layer. A diagram of the electrode placement can be found in Williams, et al., 2006. An EEG-recording electrode was positioned on the dura of the left hemisphere and ground electrodes were located on both hemispheres anterior to bregma.
Radiotelemetry system and video monitoring
The EEG was recorded via radiotelemetry as previously described (White et al., 2006; Williams et al., 2006). The analog output signal was digitized at 250 Hz using a 64-channel PCI-DAS board (ComputerBoards, Inc, Middleboro, MA), and the digital signal was then recorded using routines written in Visual Basic 6.0 (Microsoft, Seattle, WA). After implantation, baseline EEG was recorded 1 week prior to kainate treatment. Rats were video monitored continuously. Video behavioral data were evaluated by trained personnel to determine the time, severity (Racine, 1972), and frequency of behavioral seizures.
Kainate treatment
Kainate was administered to induce an episode of SE as previously described (Hellier et al., 1998; Hellier et al., 1999; Hellier et al., 2005). One to 2 weeks after electrode implantation, rats were given hourly injections of kainate (5 mg/kg, intraperitoneal, Sigma, St. Louis, MO) or saline, and seizure activity was rated according to a modified Racine's scale (i.e., class III/ IV/ V seizures) (Racine, 1972). For 9 rats, kainate injections were performed hourly until ≥10 convulsive seizures per hour were recorded for ≥3 continuous hours (i.e., convulsive SE, or CSE). These rats received up to 5 kainate injections, using a status epilepticus induction protocol we have previously described (Hellier et al., 1998). In 5 rats, electrographic SE (ESE) was induced by 5 mg/kg/hr of kainate until the rats experienced at least 10 EEG (nonconvulsive) seizures per hour for 1.5 h. All rats implanted and subjected to kainite treatment did survive the acute seizure period. The control group was injected with 0.5 cc/h of saline for 3 h (n=7). At the end of either electrographic or convulsive SE (i.e., ESE vs. CSE), the kainate-treated rats were injected with lactated Ringer's (3-5 ml, SC), and given fresh fruit for the first week after treatment.
Histology
The brains of 17 of the 21 rats implanted with chronic recording electrodes were available for histological examination (the other brains were damaged at necropsy). Timm staining was used to confirm mossy fiber reorganization in the inner molecular layer of the dentate gyrus, and cresyl violet stain was used to confirm depth electrode positions. The EEG leads were connected subcutaneously to the radiotelemetry transmitter so it was not feasible to inject current to mark the electrode tip locations. We therefore serially sectioned the hippocampi to locate the gliosis associated with the tips of the chronically implanted Teflon coated, stainless steel electrodes (Figure 1). The hippocampi were removed and emersion fixed with 0.37% sodium sulfide (Sigma), and then with 4% paraformaldehyde (Sigma), and post-fixed overnight at 4°C. The hippocampi were saturated in 30% sucrose and then sectioned transversely (30 m) with a sliding microtome (Leica, Nussloch, Germany), and sections were mounted on slides and allowed to air-dry overnight. Alternating sections, creating a section interval of 60 um, were processed with a modified Timm staining protocol and counterstained with cresyl violet, and with cresyl violet alone (Buckmaster and Dudek, 1997; Babb et al., 1991).
Figure 1.
A: Hippocampal electrode location in a kainate animal. Glial proliferations and distortions of the dentate blade mark the electrode track, which is highlighted by a white elipse. Scale bar = 200 um. B: Assessment of kainate-induced sprouting by Timm score. Timm staining of rats sacrificed 3.5 months after injection of either kainate at high (CSE) or low (ESE) doses, or saline. Timm scores of CSE animals were typical of scores obtained by other investigators at this time interval after status epilepticus (Waurin and Dudek 2001).
Spike detection
EEG spikes were identified based on a commonly used definition of human EEG spikes: a transient that is clearly distinguished from background activity lasting between 20 and 70 ms (Chartrian et al., 1974). Amplitude and duration criteria (rectangular window) were thus used to identify spikes (White, et al., 2006). The amplitude criterion was set such that the sum of the rise and decay components of the spike was greater than 12 times the average upslope of background EEG transients. Spikes whose amplitude saturated the amplifier inputs were not considered. Amplifier saturation was rare and almost always associated with artifact such as removal of the cage from the receiver plate. Transients of <20 ms or >0.8 sec duration were eliminated. For validation purposes, 24 h of data were analyzed (Rat B5, 09/03/03) using the algorithm and the output was then analyzed by one of the authors (AMW) (White et al., 2006). Using this analysis as the standard, 63% of the spikes detected were confirmed as epileptiform spikes. Reasons for rejection of spikes identified by the algorithm include jaw motion artifact, which was easily identifiable on the basis of the characteristically high frequency; and external interference, which was identified both by the unique regularity of the signal and by video review. Video review identified events such as removal of rats from the cages, which increased the distance between the EEG transmitter and the receivers located under the cages, resulting in noise. The algorithm identified 90% of spikes that were identified by one of the authors (AMW). There was approximately 80% agreement obtained when spikes identified by a second author (JLH) were compared to the first to determine inter-rater reliability. We note that this result is similar to that found in one study of correlation and reliability of spike detection among human experts, where the average inter-reader correlation was 0.79 (Wilson et al., 1996). Spike detection was performed on 24-48 h of EEG data per week using automated methods (White et al., 2006).
Spike clusters were noted in the analysis in all CSE animals. These were defined as groups of spikes with relatively short, uniform interspike intervals (frequencies ranging from 0.15 to 0.7 Hz) and morphology. No characteristic cluster duration or interval between clusters was detected.
Seizure Detection
A combination of automated detection algorithms and expert inspection of the record, as previously described, were used to detect kainate-induced and spontaneous seizures (White et al., 2006). The sensitivity and specificity of the various seizure detections are described in White et al. (2006). The seizure detection techniques use the presence of highly correlated ictal spike characteristics (amplitude and interval) to detect seizures. An operational definition of seizure used for this study was the presence of greater than 20 detected spikes in 10 sec (White et al., 2006). To determine convulsive seizure duration following kainate injection, 40 convulsive seizures were identified from a time window that extended from the time of kainate administration to the time of the last convulsive seizure. The average seizure duration was computed and multiplied by the number of total seizures for each animal. Spontaneous seizure detection was performed on all EEG data, which was available for over 75% of the duration of the experiments.
Statistics
Data are presented as mean ± the standard deviation. Fisher's exact test was used to compare proportions. For comparison between variables such as total number of spikes per day per rat, a Gaussian distribution was assumed (White et al., 2006). In the test of statistical significance of the correlation coefficient, the relation
where r is the correlation coefficient, and n is the number of degrees of freedom, was used to determine the t value (Bobko, 2001). One-tailed t-tests were performed to test the significance of directional correlations. Statistical tests that compared three groups or more used an ANOVA with the Student-Newman-Keuls (SNK) multiple comparison test to determine significance. Results were considered statistically significant when P<0.05.
To characterize seizure progression, Boltzmann sigmoidal distributions were used to fit the seizure probability as a function of time following kainate injection (Williams et al., 2009). The Boltzman distribution is characterized as
where SF(t) denotes the seizure frequency at time t, SF(p) denotes the plateau seizure frequency (maximal seizure frequency), F50 denotes the time to half maximum, and λ is the time constant. Specifically, for each rat, a time to half-maximum was determined by fitting seizure frequency vs. time elapsed from kainate treatment to the Boltzman equation (GraphPad Prism). These parameters are described in much more detail in (Williams et al., 2009). This measure was used for correlations between spike parameters and seizure progression. In our analyses we used F50 as a measure of the time elapsed between SE and the onset of frequent seizures. For one of the rats, there was insufficient plateau phase data to fit to the Boltzman equation, so the criteria of two consecutive days with ten or greater seizures was used to approximate the half-maximum value. When applied to the other rats, this criterion yielded results that were very close to the half-maximum method.
The Kolmogorov-Smirnov test was used to compare two distributions. The following expression was used to establish significance at the P<0.05 level:
where m and n are the number of points in the two cumulative distribution functions and F and G are the discrete cumulative distribution functions.
To compute random spike cumulative distribution functions, a Monte Carlo technique was employed. Specifically, random numbers were generated in the range from 0 to 1. These were then multiplied by the number of seconds in a day, producing a specific second for each random spike. From these spike times, interspike intervals were computed and a cumulative distribution function was derived.
Results
Electrode placement
To determine electrode locations, 17 rat brains, including 7 rats injected with saline, 7 rats that underwent convulsive status epilepticus (CSE), and 2 rats that underwent electrical but not convulsive episodes of status epilepticus (ESE), were examined histologically. Because radiotelemetry precluded elecrocautery as a means to locate electrode tips, we relied on histological evidence for gliosis near around the electrode tip. In 8 of 17 brains, definitive evidence of focal gliosis established the location of the electrode tip in the dentate gyrus (Fig 1B) (Williams, et al., 2009). In 9 brains, a gliotic scar could not be definitively identified, so the tip location could not be confirmed. There was no evidence for electrode placement outside of the dentate gyrus. Status epilepticus-induced cell loss (Buckmaster and Dudek, 1997) and consequent tissue shrinkage could have affected electrode positioning, but we found no evidence for malpositioned electrodes in brains with clear evidence of seizure-induced synaptic reorganization (Figure 1C). Timm staining revealed evidence of axonal sprouting in the CSE group, but not in the ESE or saline groups, consistent with earlier studies (Tauck and Nadler 1985; Buckmaster and Dudek 1997; Wuarin and Dudek 2001; Sutula 2002).
Kainate treatment
Kainate treatment sufficient to establish 10 motor seizures per hour for 3 h induced convulsive status epilpticus (CSE) in 9 of 9 rats. The duration of convulsive seizures in the CSE rats was 67.4 ± 13.3 min (mean ± standard error). The mean dosage of kainate used to induce status epilepticus in the CSE rats was 13 ± 6.5 mg/kg. In 5 of 5 rats treated with only the number of kainate doses sufficient to induce ≥10 seizures per hour for 1.5 h, electrical status epilepticus (ESE) was elicited without CSE. The mean dosage of kainate administered to ESE rats was 6.5 mg/kg. No ESE or saline-treated rats developed convulsive seizures.
EEG record on the day of kainate treatment
The average summed duration of electrical seizures in a CSE rat was 148.0 ± 14.5 min. For the ESE rats, the average summed duration of electrical seizures was 20.7 ± 2.78 min (Table 1; Figure 2) (Lowenstein et al., 1999). No electrical seizures were recorded in the saline control rats.
Table 1.
Summary table of results (Value +/- SEM)
| Parameter | CSE rats | ESE rats | Control rats |
|---|---|---|---|
| Convulsive seizures (treatment) | 67 ± 13 min | 0 | 0 |
| Electrical seizures (treatment) | 148 ± 15 min | 21 ± 3 min | 0 |
| Clusters/day (day 8) | 9.2 ± 2.3 | 0 | 0 |
| Clusters/day (day 16) | 6.9 ± 3.2 | 0 | 0 |
| Spike Count (day 8) | 16224 ±1673 | 3990 ± 1526 | 1964 ± 369 |
| Spike Count (day 16) | 21897 ± 4359 | 4012 ± 1298 | 2450 ± 855 |
| Plateau seizure frequency | 20.5 sz/day | 0 | 0 |
Prior to kainate treatment, the frequency of spikes in the EEGs of the CSE rats was 0.02 ± 0.01 Hz. On the day of kainate treatment, all rats with CSE developed regular 1.5 Hz to 2.5 Hz EEG discharges similar to periodic lateralizing epileptiform discharges (PLEDs) recorded in the human EEG after severe brain injury (Chatrian et al., 1964) (Fig. 3A). These discharges occurred between seizures and persisted after acute seizure activity subsided. Mean duration of the PLED-like regular spiking was 10.6 ± 1.4 h. The spike frequency averaged over the first 10 h was 1.93 ± 0.13 Hz, which was significantly lower than the spike discharge frequency observed during seizures (8.6 ± 2.5 Hz, n = 68 seizures). The frequency of these PLED-like EEG discharges decreased steadily over the first 10 h following kainate treatment and their amplitude became more variable (Figs. 3B). The regular discharges gradually transitioned to EEG spikes that had an irregular interval. There was not an identifiable transition from regular to irregular spiking. Interictial spikes were identifiable in 9 of 9 CSE rats and 5 of 5 ESE rats. In the ESE animals, non-convulsive seizures and interictal EEG spikes were evident on the EEG on the day of kainate treatment (Fig. 2D) but PLED-like periodic interictal EEG discharges did not occur. None of the saline-treated rats developed the PLED-like activity described above.
Chronic spontaneous electrographic seizures
All rats that experienced CSE developed chronic spontaneous electrographic and convulsive seizures days to months after kainate treatment. Analysis of all EEG recordings indicated that the mean seizure-free period between kainate-induced SE and the first spontaneous EEG seizure was 11.33 ± 9.33 days (n=9), which was followed by a steep increase in seizure frequency with a time-to-half-maximum seizure frequency (F50) of 72 ± 52 days after kainate-induced CSE (Figure 4A; Williams et al., 2009). In 1 of 9 of the rats with CSE, a single electrographic seizure was detected on day 36 after treatment (Table 1). The variance in time to first seizure was congruent with epidemiological studies of the onset of epilepsy after human brain injury (Annegers et al., 1998). None of the saline-treated rats developed EEG or behavioral seizures.
Figure 4.
The natural history of spikes and seizures after kainate-induced status epilepticus. A: Time course of interictal spikes vs. seizures after kainate injection. Data are presented as mean normalized frequencies of spikes or seizures vs. time since kainate injection, normalized to the time of maximum seizure frequency. The data were normalized to illustrate that interictal spike frequency is high and increases before seizure frequency begins to increase, and the precedence of the increase in spike vs. seizure frequency is independent of the time course of seizure frequency. B: The total number of EEG spikes per day and the fraction of time spent seizing are plotted in this figure for CSE rats with transition periods greater than 3 weeks. Each rat is represented by a different color. Closed circles represent seizure time (left Y axis) and open circles represent the number of spikes per day (right Y axis). The rapid rise in seizure time was preceded by a similar rise in spikes in 6 out of 7 rats.
Figure 4 plots the fraction of time spent seizing (electrographic seizures) as well as the total number of EEG spikes per day as a function of time after kainate treatment in 7 CSE rats with latent periods of at least 2 weeks. These rats were selected to correspond to the clinical situation of brain-injured patients who do not develop epilepsy immediately after an injury, but are at risk to develop epilepsy (Frey, 2003; Annegers et al., 1998). The number of EEG spikes increased approximately 2 weeks prior to the rise in seizure time (seizure minutes/day; Figure 4B). An overall schematic of both spike and spontaneous seizure behavior is given in Figure 5.
Figure 5.
Timeline for seizure and spike progression for rats with CSE (convulsive status epilepticus). Times given in the figure represent ranges for the duration of each interval. Both seizures and spikes were recorded during CSE, after which EEG spikes decreased in frequency (top timeline). In all rats with CSE (n=9), spike clusters occurred prior to the end of the seizure-free interval. Spike clusters, once present, persisted for the life of the animal. Convulsive seizures appeared during kainate treatment for rats with CSE, and resolved within 24 h (bottom timeline). Spontaneous seizures occurred after a seizure-free interval, with seizure frequency increasing continuously from a low initial frequency to a high-frequency plateau (Williams et al. 2009). The transition phase was defined as the time from the first kainate treatment until 10 seizures were observed per day for 2 consecutive days. This parameter was used to assess possible correlations between spiking and epileptogenesis.
EEG spikes
During a 90–day observation period after kainate treatment, EEG spikes were observed in 9 of 9 rats with kainate-induced CSE (Fig. 6A-C). EEG spikes were generally recorded as positive monophasic or biphasic events (Figs. 6A vs. 6B), which varied in amplitude and duration. After CSE, EEG spikes could be recorded in all three leads as opposed to control rats and rats that had experienced ESE, in whom EEG spikes were rarely recorded in all three leads (Figs. 6A-C vs. 6D). The computer algorithms detected EEG spikes in all control rats and rats that experienced ESE, but spike clusters were never observed in these groups. The frequency of EEG spikes in control and with ESE rats was well within the error rate of our spike detection algorithms (see Methods), and on expert review represented physiological dentate sharp waves (Bragin et al., 1995) and artifacts. These events were included in the analysis because sharp waves and artifacts that cannot always be differentiated from EEG activity also occur in human EEG recordings (Wilson et al., 2002), and a realistic epilepsy prediction algorithm based on EEG spikes must take these events into account.
Figure 6.
EEG spikes before and after the onset of spontaneous seizures. A: A single EEG spike recorded 2 weeks after kainate but before the onset of seizures. B: Two more complex EEG spikes recorded in the same animal after the onset of spontaneous seizures. Same voltage and time scale as A. C: A cluster of EEG spikes recorded in the same animal 1 week after kainate but before the onset of spontaneous seizures. D: An EEG spike from a control animal. Note that it was only present in a single lead, suggesting that it represented either a physiological dentate sharp wave or artifact. For each panel, top trace = dural EEG record; the second and third traces = left and right hippocampi, respectively.
Interspike interval probability distributions demonstrate clustering
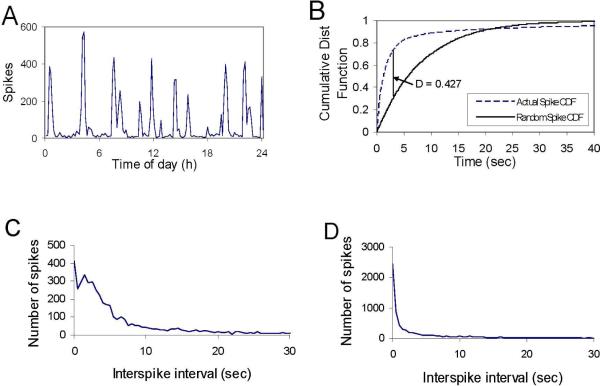
The intervals between spikes are plotted over 24 h in Figure 7A. To differentiate rats that were likely to have seizures from those that were not, we examined the distribution of interspike intervals after SE for rats that developed chronic epilepsy (CSE rats) versus those that did not (control and rats with ESE). The cumulative probability distribution functions (CDFs) of the interspike interval were compared in rats after CSE with the expected random distribution. Figure 7B shows a significant difference between the two distributions (P<0.001). The probabilistic distribution functions for the interspike interval for 2 individual rats after CSE vs. ESE are shown in Figures 7C and D. The CDF of rats that developed chronic epilepsy showed a large peak in spikes with interspike intervals of 1.5–7 sec, corresponding to the interspike intervals observed during the regular-spiking epochs termed spike clusters. To demonstrate that this effect was significant when analyzed for all animals in each group, we compared the fraction of spikes which fell into this frequency range after CSE and ESE. At day 8, the fractions were 0.34 ± 0.083 after CSE and 0.16 ± 0.07 after ESE rats (P=0.0008). At day 16, the fractions were 0.40 ± 0.045 for the CSE group and 0.18 ± 0.072 for the ESE group (P= 0.0004).
Figure 7.
EEG spike rate vs. time. A: Plot of the number of spikes per 10-min interval across time for a day. The peaks correspond to the presence of spike clusters, as shown in Figure 7C and 9. B: The cumulative distribution function of spike intervals after CSE was compared with a random (i.e., Monte Carlo generated) distribution. A K-S test showed a significant difference between the two distributions (P<1.E-6), indicating that the clustering of spikes was not a stochastic sequence of short spike intervals. C and D: The probabilistic distribution functions (PDF) of the interspike intervals after CSE (C) and saline treatment (D) show that the number of spikes declined exponentially in control animals (or after ESE), consistent with random timing, while for CSE animals the EEG spike interval distribution had a peak between 2 and 6 sec.
Spike Clusters
Clusters of spikes developed 89 ± 30 h (3.7 ± 1.3 days) after kainate-induced CSE. The mean number of spike clusters per 24 h was 9.2 ± 2.3 at 8 days after kainate-induced SE and 6.9 ± 3.2 at 16 days after kainate in the 9 rats that developed chronic epilepsy. The animals were usually in the waking state at the onset of spike clusters, but could have been asleep. During spike clusters, regularly spaced and distinct EEG spikes were evident early in the cluster (frequency range of 0.15 – 0.6 Hz). As the cluster evolved, spike morphology remained consistent but the amplitude and frequency became more variable (Fig. 8A). At the end of one cluster, another spike cluster could begin (Figs. 8B, D), so that the EEG record alternated between regular, distinct spikes and variable spikes (Fig. 8C). The spike clusters occurred intermittently throughout the recording period, lasting from 1 min to several hours (Fig 8A). During spike clusters, no convulsive activity or other behavioral correlate was observed. In all rats that developed chronic epilepsy, the appearance of EEG spikes and spiking clusters preceded the onset of epilepsy.
Figure 8.
EEG spike clusters occurred in all chronically epileptic rats after CSE (n=9), days to months after kainate-induced SE until euthanasia. A: Spike clusters appeared randomly and could last tens of seconds to hours. In this example from the right hippocampus, the duration of the spike cluster was 50 min. B: In long-lasting spike clusters, well-defined EEG spikes continued for minutes, although amplitude and frequency gradually became less distinct (see arrows in A and C). After the period of less distinct spiking, the spike cluster could end or spikes could once again develop well-defined frequency and amplitude. The polarity of all spikes was similar to the lead spike found in seizures for the same rat. D: Expanded time-scale of well-defined spikes in the spike cluster.
EEG spike frequency and epileptogenesis
EEG spike frequency increased above baseline in all rats that became epileptic. Because the spike rate did not change in the ESE or saline animals, the cumulative plot of the total number of spikes vs. time demonstrates differences that become progressively more obvious with time after injury (Figure 9A; group mean difference, CSE vs. [ESE + saline]). The differences in cumulative spike number became more significant with time: ANOVA at 24 hours: p < 1.5 × 10-5; at 8 days, p < 6.3 × 10-7; at 16 days, p < 6.2 × 10-8. To determine if the frequency of EEG spikes is a useful biomarker for predicting individuals who will develop chronic epilepsy, we analyzed the number of EEG spikes in two standardized time intervals, each lasting 24 h, at 8 and 16 days following kainate administration.
Figure 9.
Cumulative plot of the total number of EEG spikes in excess of the daily baseline rate (observed prior to kainate treatment) vs. time following status epilepticus. A: Group means and standard deviations for 9 epileptic (CSE) and 12 nonepileptic (ESE and saline control) animals. B: The same data and color scheme, plotted for Individual animals. The individual plots demonstrate that the increase in variance in epileptic rats is due to inter-animal variation in frequency of spiking, corresponding to differences in rates of epileptogenesis (Willams et al. 2009), rather than intra-animal variation.
On day 8 after kainate-induced CSE, rats that developed chronic epilepsy had significantly more spikes compared to rats that did not (P<0.001, ANOVA, SNK; Fig. 10A). The spike rate after ESE was not significantly different from the spike rate after saline treatment (P>0.05, ANOVA, SNK; Fig. 10A). The same correlations between spike frequency and the subsequent occurrence of chronic seizures were observed at day 16 after treatment (P <0.01, ANOVA, SNK; Fig. 10B). Spike frequency after ESE was not significantly different from saline treatment (P>0.05, ANOVA, SNK; Fig. 10B). The number of spikes in rats that did not develop epilepsy did not change significantly from day 8 to 16 to 30 (P>0.05, ANOVA, repeated measures), indicating that there were no progressive changes in excitability as would be expected during epileptogenesis (Fig. 10A vs B).
Figure 10.
The average EEG spike frequency (spikes/day) on days 8 (A) and 16 (B) after CSE was significantly greater than after ESE or control treatment (P<0.001 and P<0.001, respectively, ANOVA). Asterisks = significant.
Rats that developed chronic epilepsy could be distinguished from the rats that did not develop chronic epilepsy (ESE and control groups combined) based on spike frequency at day 8 or 16. On day 8, 9 of 9 rats that exceeded a threshold spike frequency of 8000 spikes per day developed chronic epilepsy, whereas only 1 of 12 rats that did not develop chronic epilepsy had a spike frequency above this threshold (P= 0.02). On day 16, 9 of 9 rats that exceeded a threshold spike frequency of 8000 spikes per day 16 developed chronic epilepsy, whereas 0 of 12 rats with subthreshold spike frequencies developed chronic epilepsy (P=0.004).
To test whether spike frequency shortly after SE reflects the rate of epileptogenesis, spike frequency on days 8 and 16 after kainate treatment was compared to the time to reach half the eventual steady-state seizure frequency (F50) in the rats that developed chronic epilepsy (n=9; Fig. 11A, B). The mean F50 for these rats was 76 ± 52 days. The number of EEG spikes on day 16 was significantly correlated to the time to develop frequent seizures, which was estimated by the F50 (R = -0.66 respectively with directional P = 0.027, Fig. 11B). There was a similar but non-significant trend on day 8 (R = -0.55, directional P = 0.061, Fig. 11A). The time to onset of spike clusters showed a significant positive correlation to the F50 (r = 0.66, directional P = 0.026; Fig. 11C). These data support the hypothesis that the temporal pattern of EEG spike activity is related to the rate of epileptogenesis, which may also provide useful clinical prognostic information.
Figure 11.
A and B:The time to the end of the transition phase was inversely correlated with frequency of EEG spikes at 8 (A) and 16 (B) days after SE. Although the correlation at 8 days after kainate treatment was not significant (R = 0.55, P = 0.06), the correlation was significant 16 days after treatment (R = -0.66, P = 0.027). C: After kainate-induced CSE (n=9), seizure progression (seizures/day) could be modeled with a Boltzman sigmoid curve (Williams et al. 2009), and the time from kainate treatment to the time of half-maximum for this curve (i.e., time to frequency seizures) was correlated with the time of the appearance of EEG spiking clusters; R = 0.66, directional P = 0.027).
Sentinel EEG spikes
Most seizures began with a spike that appeared similar to an interictal spike. This has previously been described as a sentinel EEG spike (Baldauf et al., 2003; Cukiert et al., 2003). In the first 5 spontaneous seizures of each CSE rat, 82% of seizures (37 out of 45) exhibited a sentinel spike. Subsequently, seizure discharges began as high-frequency, low-amplitude spikes and gradually evolved to low-frequency, high-amplitude spikes. EEG spikes that occurred in spike clusters had the same polarity as the sentinel spike (if present) in a seizure (Cukiert et al., 2001). In all cases, the polarity of the sentinel spike was the same as the polarity of EEG spikes in the spiking clusters, and the amplitudes were also similar.
Discussion
These data indicate that those rats that developed chronic epilepsy after status epilepticus had significantly increased EEG spike frequencies compared to the rats that did not develop chronic epilepsy (i.e., CSE vs. ESE or saline treatment). The development of epilepsy and the rate of epileptogenesis was predictable shortly after SE based on the frequency of spikes, which could be detected by computer algorithms. Before the first electrographic seizure, the temporal pattern of spikes in a 24-h epoch (clustering) was also predictive of the rate of epileptogenesis.
Limitations of this study
In this study, EEG spikes were detected in a very large dataset using computer algorithms. The positive predictive value of the algorithms were limited by physiological and noise sources that have spike-like waveforms (White et al., 2006). As a consequence, EEG spikes were recorded in control rats as well as rats treated with kainate. The sharp transients recorded in the dentate gyrus of control animals most likely represent physiological dentate spikes (Bragin et al., 1995). Extracranial noise sources may also have been detected as spikes. Gradual increases in the noise levels of the recordings over the several months of the experiment might have exacerbated these problems (White et al., 2006; Williams et al., 2006), although the stable spike frequency in the control and ESE rats suggest that changes in the fidelity of the recording were not a major source of error. It is possible that some of the EEG spikes detected in control animals represented EEG epileptiform activity due to electrode-induced brain damage, but the similarity of many of these waveforms to those described in acute recordings (Bragin et al., 1995), the lack of seizures in these rats, the limited spatial distribution of the spikes, and the lack of evolution of spike frequency over time all make electrode injury a less likely source of these EEG transients.
In designing this study, we did not know a priori what level of injury (i.e. duration of status epilepticus) would result in chronic epilepsy. The dosages of convuslants used in this study resulted in a bimodal distribution of SE duration that correlated strongly with the subsequent development of epilepsy. During SE, total seizure durations of 20 min were not followed by chronic epilepsy, suggesting that this duration of seizure activity is not sufficient to induce epileptogenesis. On the other hand, SE episodes with mean total EEG seizure durations of 140 min were consistently associated with subsequent epilepsy. These data are congruent with earlier findings regarding the duration of seizures necessary to induce neuronal loss (Meldrum 2002), and suggest that the duration of SE may be a robust predictor of epileptogenesis. However, this is not a useful clinical biomarker because the duration of electrographic SE is almost never known in clinical situations. As an experimental biomarker, the duration of SE has only limited utility because anti-epileptogenic interventions are designed to be administered after brain injury.
We did not attempt to determine the anatomical origin of the EEG spikes detected by the dentate and epidural electrodes in this study, because the electrodes were not designed to do so. Spikes were often detected in all three EEG leads, including those in both hemispheres, because the inter-electrode distances were exceptionally short compared to human recordings. Gliosis around electrodes (Williams, et al., 2009) and shifts in electrode position due to brain injury and skull growth may have altered the morphology of EEG spikes, but the fact that the same spikes could be detected in multiple electrode locations indicates that electrode shifts were not a major determinant of the change in spike frequency over time (Figure 6).
When considering the relevance of these observations to human epilepsy, differences between human and experimental epileptogenesis must be kept in mind. For example, the kainate model appears to have a shorter latent period and a higher seizure frequency than most acquired human epilepsies (Hellier et al., 1998), although human patients are treated with anticonvulsants and the rats were not. The correlation between EEG spike frequency and epilepsy severity (Rosati et al., 2003) suggests that the threshold spike frequency predictive of future epilepsy in human patients would likely be much lower than what was observed here (Fig. 9). A lower spike frequency threshold would also be consistent with the lower interictal spike frequencies reported in human patients (Martins da Silva et al., 1984) vs. the rats with kainate-induced epilepsy in this study (Fig. 9).
EEG spikes before the first spontaneous seizure: a biomarker of epileptogenesis?
EEG spike frequency is not an accurate short-term predictor of acute seizures; human studies have reported reductions in spiking shortly before a seizure (Gotman, 1991). This study investigated a different relationship: the correlation of EEG spikes and subsequent epilepsy. In human patients, EEG spikes are correlated with the presence of epilepsy as well as ictogenic location (Hufnagel et al., 2000). The presence or absence of spikes after epilepsy surgery are accurate predictors of recrudescent epilepsy (Hildebrandt et al., 2005), and EEG spike frequency is correlated with the severity of epilepsy (Rosati et al., 2003). Further, the EEG spikes detected in this study had the same amplitude and polarity as ictal EEG discharges (Fig. 8). These findings support the idea that EEG spikes and seizures are both generated by the same epileptic neural network. Spikes and seizures both reflect the synchronous, paroxysmal activation of neurons (Gastaut and Broughton, 1972; Niedermyer and Silva, 2004; Dichter and Spencer, 1969), so that it is reasonable to ask whether early post-injury EEG spike patterns reflect the probability that the injured brain regions will eventually generate other synchronous activity such as seizures.
The frequency and temporal clustering of EEG spikes recorded prior to the first spontaneous seizure were strongly correlated with subsequent epilepsy in this experimental paradigm. This finding represents a departure from the current understanding of the relationship of EEG spikes to epileptogenesis. Although a relationship between epilepsy due to brain injury and EEG abnormalities has long been known (Roseman and Woodhall, 1946; Jabbari et al. 1986), and routine EEG studies can be used to identify patients at increased risk for future epilepsy (Angeleri et al. 1999), EEG spikes are not considered to be useful biomarkers in individual patients (Jennett and Van De Sande, 1975). However, in these studies the injuries, post-injury intervals, and sleep-wake states of the patients were not homogeneous. Perhaps more importantly, the data were derived from standard-length (~60 min) EEG samples. Our data strongly suggests that the predictive value of the EEG is directly proportional to the duration of monitoring (Fig. 9). As shown in Figure 7A, the rate of spiking varies widely over the course of a day (Martins da Silva et al., 1984), and random 60-min samples of EEG spikes from the distribution shown in Figure 7A would have obscured any relationship between EEG spikes and subsequent epilepsy. The availability of digital EEG and spike-detection algorithms have now made it possible to sample EEG spike rates over much larger periods of time, which increases the sensitivity of the EEG (Majchrzak et al., 1992) and provides a more accurate picture of the relationship between spikes and subsequent experimental epilepsy (Figure 9).
Spike clusters were also predictive of epilepsy. Clustering of seizures over periods of weeks has recently been described in experimental epilepsy (Williams et al., 2009). Our data indicate that clustering of spikes also occur prior to the onset of spontaneous seizures. Clustering may help differentiate pathological spikes from physiological spikes and noise, although we have not determined whether clustering is simply a manifestation of periods of more frequent EEG spiking in subjects undergoing state-dependent changes in spike frequency (Gotman, 1991; Buzsaki et al., 1989). The regularity of spiking during the clusters (Figure 8 and (Holmes et al., 1998)), however, argue that while spike clusters are most likely to be observed during sleep and immobility (Buzsaki et al., 1989), the clustering per se is more than a stochastic manifestation of overall spike frequency. Rather, spike clusters may represent the activity of the injured limbic circuits during states that normally produce physiological sharp waves (Buzsaki et al., 1989). Clusters of spikes are most easily seen in multi-hour blocks of data, suggesting that use of this phenomenon as a predictor of post-injury epilepsy will entail more datasets that are longer than routine outpatient EEG recordings.
Other possible biomarkers
Earlier studies have investigated the correlation between the severity of experimental seizures and the induced brain damage (Holmes et al., 1998; Ben-Ari et al., 1980). It is reasonable to assume that the degree of brain damage correlates with the risk of subsequent epilepsy, as has been demonstrated in epidemiological studies of post-traumatic epilepsy (Frey, 2003; Salazar et al., 1985; Englander et al., 2003; Annegers et al., 1998). Cell loss and subsequent axonal sprouting are consequences of brain injury that are also related to epileptogenesis. The degree of injury, the circuits affected, and the degree of subsequent circuit alterations due to sprouting and homeostatic alterations are not easy to quantify in human patients or experimental subjects, although continued advances in MRI imaging may eventually make such quantification feasible in the future. For these reasons our study was not designed to test correlations between measures of the severity of brain injury and epileptogenesis. On the other hand, digital EEG data are readily available, and these EEG recordings are amenable to automated spike detection. We therefore studied the correlation between EEG spikes recorded shortly after injury and the subsequent development of epilepsy.
Changes in the dose of convulsant that induces seizures, the latency to onset of such seizures, and the fraction of animals who seize in response to a fixed dose of a convulsant are frequently used in experimental studies to estimate the severity of an epileptogenic insult or the response to an anti-epileptogenic intervention. However, the clinical relevance of these surrogate markers and their correlation with the later development of epilepsy have not been defined. Further, these measures are generally assessed months after injury, adding substantially to vivarium costs. The strong early correlation of interictal spiking and epileptogenesis suggests that EEG spiking may be a robust early biomarker of the subsequent development of spontaneous seizures.
EEG spikes and possible mechanisms of epileptogenesis
Our study was not designed to test whether EEG spikes have a causal role in epileptogenesis. However, the presence of EEG spikes during the period preceding chronic spontaneous seizures raises that possibility (Staley et al., 2005). Gowers proposed that “seizures beget seizures” as a mechanism of progressive epilepsy. This explanation cannot underlie the earliest stages of epileptogenesis, because no seizures occur immediately after brain injury. In contrast, EEG spikes have been detected in experimental epilepsy during the period between SE and the first spontaneous seizure (Hellier et al., 1998 and Fig. 6). Interictal spikes are due to short bursts of synchronous neural activity (Gastaut and Broughton, 1972; Dichter and Spencer, 1969). This activity resembles the synchronous bursts of network activity that are known to be important in strengthening synaptic connections between neurons (Bains et al., 1999) and guiding the wiring of neural circuits in the developing nervous system (Khazipov et al., 2004). Synchronous activity has been shown to be necessary for sprouting in subcortical brain regions (Carmichael and Chesselet, 2002). Thus, we hypothesize that EEG spikes may guide the development of recurrent axon collaterals (i.e., sprouting (Wuarin and Dudek, 2001; Sutula, 2002)) as well as strengthen the synapses that are formed by these collaterals. These processes are important components of the positive feedback that underlies seizure activity (Staley et al., 2005). However, the current study was not designed to test the role of spikes in epileptogenesis; rather, we tested their utility as a biomarker for future epilepsy.
Future studies
Biomarkers of epileptogenesis are critically needed to accelerate research into the causes and therapies of epilepsy, which is currently limited by the prolonged monitoring required to determine whether an animal has become epileptic. To extend the current correlational findings, the predictive power of EEG spiking could be tested in a prospective trial. The utility of EEG spikes as a biomarker could be increased by using different kainate doses and/or studying animals over a broad range of SE durations in order to determine the predictive power of this test in animals whose SE duration is just above and just below the threshold for development of subsequent epilepsy. Future studies can also test whether clustering of spikes is an independent predictor of epilepsy, as suggested by the current findings.
The utility of EEG spikes as a predictor of human epilepsy after brain injury could be definitively answered by a prospective study of EEG spikes after various forms of human brain injury such as stroke and head trauma. The optimal timing of the EEG sampling in such a study would need to be determined empirically. For example, although our data suggest that EEG spikes are predictive in the first days after injury, this early predictive utility may be related to the nature of the experimental injury: prolonged seizures that gradually evolved into periodic discharges and then interictal spikes. Another question that will need to be answered by clinical studies is the duration of EEG recordings required to accurately predict future epilepsy. Figure 10 indicates that 24h recordings are sufficient, although shorter recordings may also be useful. As shown in Figure 7A, clustering of EEG spikes in this study would have severely limited the predictive power of 1-h EEG samples, but this issue needs to be explored in human patients with other forms of brain injury. Unfortunately there is no shortage of either brain-injured patients or post-injury epilepsy. Now that digital EEG recorders and epilepsy monitoring units are widely available, it is time to update the early pioneering studies of EEG as a noninvasive predictor of epilepsy after brain injury.
Acknowledgements
This research was supported by NIH grants NS045144 (F.E.D.) and NS34360 (K.J.S.). We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
Disclosure of Conflicts of Interest
None of the authors has any conflict of interest to disclose.
References
- Angeleri F, Majkowski J, Cacchiò G, Sobieszek A, D'Acunto S, Gesuita R, Bachleda A, Polonara G, Królicki L, Signorino M, Salvolini U. Posttraumatic epilepsy risk factors: one-year prospective study after head injury. Epilepsia. 1999;40:1222–30. doi: 10.1111/j.1528-1157.1999.tb00850.x. [DOI] [PubMed] [Google Scholar]
- Annegers JF, Rocca WA, Hauser WA. Causes of epilepsy: contributions of the Rochester epidemiology project. Mayo Clin Proc. 1996;71:570–575. doi: 10.4065/71.6.570. [DOI] [PubMed] [Google Scholar]
- Annegers JF, Hauser WA, Coan SP, Rocca WA. A population-based study of seizures after traumatic brain injuries. N Engl J Med. 1998;338:20–4. doi: 10.1056/NEJM199801013380104. [DOI] [PubMed] [Google Scholar]
- Babb TL, Kupfer WR, Pretorius JK, Crandall PH, Levesque MF. Synaptic reorganization by mossy fibers in human epileptic fascia dentata. Neuroscience. 1991;42:351–363. doi: 10.1016/0306-4522(91)90380-7. [DOI] [PubMed] [Google Scholar]
- Bains JS, Longacher JM, Staley KJ. Reciprocal interactions between CA3 network activity and strength of recurrent collateral synapses. Nat Neurosci. 1999;2:720–6. doi: 10.1038/11184. [DOI] [PubMed] [Google Scholar]
- Baldauf C, Mariani P, Cukiert A, Burattini J, Vieira J, Brandao R, Ceda L, Forster C, Baise C, Frayman L, Mello V, Argentoni M. Generalized sentinel spike in patients with refractory frontal lobe epilepsy. Epilepsia. 2003;44s8:189. [Google Scholar]
- Ben-Ari Y, Tremblay E, Ottersen O, Meldrum B. The role of epileptic activity in hippocampal and “remote” cerebral lesions induced by kainic acid. Brain Res. 1980;191:79–97. doi: 10.1016/0006-8993(80)90316-9. [DOI] [PubMed] [Google Scholar]
- Bobko P. Correlation and regression. 2nd edition Sage Publications; Thousand Oaks, CA: 2001. [Google Scholar]
- Bragin A, Jando G, Nadasdy Z, van Landeghem M, Buzsaki G. Dentate EEG spikes and associated interneuronal population bursts in the hippocampal hilar region of the rat. J Neurophysiol. 1995;73:1691–1705. doi: 10.1152/jn.1995.73.4.1691. [DOI] [PubMed] [Google Scholar]
- Brenner RP, Scheuer MLJ. Cross-country digital EEG survey 1998. Clin Neurophysiol. 1998;15:485–488. doi: 10.1097/00004691-199811000-00006. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Jongen-Relo AL. Highly specific neuron loss preserves lateral inhibitory circuits in the dentate gyrus of kainate-induced epileptic rats. J Neurosci. 1999;19:9519–29. doi: 10.1523/JNEUROSCI.19-21-09519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Dudek FE. Neuron loss, granule cell reorganization, and functional changes in the dentate gyrus of epileptic kainatetreated rats. J Comp Neurol. 1997;385:385–404. [PubMed] [Google Scholar]
- Buzsaki G, Ponomareff GL, Bayardo F, Ruiz R, Gage FH. Neuronal activity in the subcortically denervated hippocampus: a chronic model for epilepsy. Neuroscience. 1989;28:527–38. doi: 10.1016/0306-4522(89)90002-x. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Chesselet MF. Synchronous neuronal activity is a signal for axonal sprouting after cortical lesions in the adult. J Neurosci. 2002;22:6062–70. doi: 10.1523/JNEUROSCI.22-14-06062.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartrian G, Bergamini L, Dondey M, Klass D, Lennox-Buchthal M, Peterson I. Glossary of terms most commonly used by clinical electroencephalographers. Electroenceph. Clin. Neurophysiol. 1974;37:538–548. doi: 10.1016/0013-4694(74)90099-6. [DOI] [PubMed] [Google Scholar]
- Chatrian GE, Shaw C-M, Leffman H. The significance of periodic lateralized epileptiform discharges in EEG: An electrographic, clinical and pathological study. Electroenceph Clin Neurophysiol. 1964;17:177–193. doi: 10.1016/0013-4694(64)90149-x. [DOI] [PubMed] [Google Scholar]
- Coppola G, Licciardi F, Sciscio N, Russo F, Carotenuto M, Pascotto A. Lamotrigine as first-line drug in childhood absence epilepsy: a clinical and neurophysiological study. Brain Dev. 2004;26:26–29. doi: 10.1016/s0387-7604(03)00090-1. [DOI] [PubMed] [Google Scholar]
- Cukiert A, Argentoni M, Burattini J, Mariani P, Vieira J, Brandao R, Ceda L, Baldauf C, Baise C, Forster C, Frayman L, Mello V. Sentinel spike in patients with frontal lobe epilepsy (FLE). Epilepsia. 2003;44s9:145. [Google Scholar]
- Cukiert A, Buratini JA, Machado E, Sousa A, Vieira JO, Argentoni M, Forster C, Baldauf C. Results of surgery in patients with refractory extratemporal epilepsy with normal or nonlocalizing magnetic resonance findings investigated with subdural grids. Epilepsia. 2001;42:889–894. doi: 10.1046/j.1528-1157.2001.00201.x. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio R, Fairbanks JP, Fender JS, Born DE, Doyle DL, Miller JW. Post-traumatic epilepsy following fluid percussion injury in the rat. Brain. 2004;127:304–314. doi: 10.1093/brain/awh038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport CJ, Brown WJ, Babb TL. Sprouting of GABAergic and mossy fiber axons in dentate gyrus following intrahippocampal kainate in the rat. Exp Neurol. 1990;109:180–190. doi: 10.1016/0014-4886(90)90072-z. [DOI] [PubMed] [Google Scholar]
- De Lanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989;495:387–395. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- Dichter M, Spencer WA. Penicillin-induced interictal discharges from the cat hippocampus. I. Characteristics and topographical features. J Neurophysiol. 1969;32:649–662. doi: 10.1152/jn.1969.32.5.649. [DOI] [PubMed] [Google Scholar]
- Dinocourt C, Petanjek Z, Freund TF, Ben-Ari Y, Esclapez M. Loss of interneurons innervating pyramidal cell dendrites and axon initial segments in the CA1 region of the hippocampus following pilocarpine-induced seizures. J Comp Neurol. 2003;439:407–25. doi: 10.1002/cne.10622. [DOI] [PubMed] [Google Scholar]
- Englander J, Bushnik T, Duong TT, Cifu DX, Zafonte R, Wright J, Hughes R, Bergman W. Analyzing risk factors for late posttraumatic seizures: a prospective, multicenter investigation. Arch Phys Med Rehabil. 2003;84:365–373. doi: 10.1053/apmr.2003.50022. [DOI] [PubMed] [Google Scholar]
- Frey L. Epidemiology of posttraumatic epilepsy: A Critical Review. Epilepsia. 2003;44s10:11. doi: 10.1046/j.1528-1157.44.s10.4.x. [DOI] [PubMed] [Google Scholar]
- Gastaut H, Broughton R. Epileptic seizures: clinical and electrographic features, diagnosis and treatment. Thomas; Springfield (IL): 1972. [Google Scholar]
- Gotman J. Relationships between interictal spiking and seizures: Human and experimental evidence. Can J Neuro Sci. 1991;18:573–6. doi: 10.1017/s031716710003273x. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Patrylo PR, Buckmaster PS, Dudek FE. Recurrent spontaneous convulsive seizures after repeated low-dose systemic treatment with kainate: assessment of a rat model of temporal lobe epilepsy. Epilepsy Res. 1998;31:73–84. doi: 10.1016/s0920-1211(98)00017-5. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Patrylo PR, Dou P, Nett M, Rose GM, Dudek FE. Assessment of inhibition and epileptiform activity in the septal dentate gyrus of freely-behaving rats during the first week after kainate treatment. J Neurosci. 1999;19:10053–10064. doi: 10.1523/JNEUROSCI.19-22-10053.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellier JL, Dudek FE. Current Protocols in Neuroscience. Vol. 31. John Wiley & Sons, Inc.; 2005. Chemoconvulsant model of chronic spontaneous seizures. pp. 9.19.1–9.19.12. [DOI] [PubMed] [Google Scholar]
- Hildebrandt M, Schulz R, Hoppe M, May T, Ebner A. Postoperative routine EEG correlates with long-term seizure outcome after epilepsy surgery. Seizure. 2005;14:446–51. doi: 10.1016/j.seizure.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Holmes G, Gairsa J, Chevassus-Au-Louis N, Ben-Ari Y. Consequences of neonatal seizures in the rat; morphological and behavioral effects. Ann Neurol. 1998;44:845–57. doi: 10.1002/ana.410440602. [DOI] [PubMed] [Google Scholar]
- Houser CR, Miyashiro JE, Swartz BE, Walsh GO, Rich JR, Delgado-Escueta AV. Altered patterns of dynorphin immunoreactivity suggest mossy fiber reorganization in human hippocampal epilepsy. J. Neurosci. 1990;10:267–282. doi: 10.1523/JNEUROSCI.10-01-00267.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufnagel A, Dumpelmann M, Zentner J, Schijns O, Elger CE. Clinical relevance of quantified intracranial interictal spike activity in presurgical evaluation of epilepsy. Epilepsia. 2000;41:467–478. doi: 10.1111/j.1528-1157.2000.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Jabbari B, Vengrow MI, Salazar AM, Harper MG, Smutok MA, Amin D. Clinical and radiological correlates of EEG in the late phase of head injury: a study of 515 Vietnam veterans. Electroencephalogr Clin Neurophysiol. 1986;64:285–93. doi: 10.1016/0013-4694(86)90151-3. [DOI] [PubMed] [Google Scholar]
- Jennett B, Van De Sande J. EEG prediction of post-traumatic epilepsy. Epilepsia. 1975;16:251–6. doi: 10.1111/j.1528-1157.1975.tb06055.x. [DOI] [PubMed] [Google Scholar]
- Kelley MS, Jacobs MP, Lowenstein DH. The NINDS epilepsy research benchmarks. Epilepsia. 2009;50:579–82. doi: 10.1111/j.1528-1167.2008.01813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsaki G. Early motor activity drives spindle bursts in the developing cortex. Nature. 2004;432:758–61. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- Klitgaard H, Pitkanen A. Antiepileptogenesis, neuroprotection, and disease modification in the treatment of epilepsy: focus on levetiracetam. Epileptic Disord. 2003;5(Suppl1):S9–16. [PubMed] [Google Scholar]
- Kobayashi M, Buckmaster PS. Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. J Neurosci. 2003;23:2440–2452. doi: 10.1523/JNEUROSCI.23-06-02440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossius MI, Ronning OM, Slapo GD, Mowinckel P, Gjerstad L. Poststroke epilepsy: occurrence and predictors – a long-term prospective controlled study (Akershus Stroke Study). Epilepsia. 2005;46:1246–51. doi: 10.1111/j.1528-1167.2005.57904.x. [DOI] [PubMed] [Google Scholar]
- Lowenstein DH, Bleck T, Macdonald RL. It's time to revise the definition of status epilepticus. Epilepsia. 1999;40:120–122. doi: 10.1111/j.1528-1157.1999.tb02000.x. [DOI] [PubMed] [Google Scholar]
- Lowenstein DH. Epilepsy after head injury: an overview. Epilepsia. 2009;50(Suppl. 2):4–9. doi: 10.1111/j.1528-1167.2008.02004.x. [DOI] [PubMed] [Google Scholar]
- Majchrzak R, Stelmach-Wawrzyczek M, Kazibutowska Z, Grudzińska B. 24-hour monitoring of bioelectric activity of the brain in patients after cranial injuries. Neurol Neurochir Pol. 1992;26:172–7. [PubMed] [Google Scholar]
- Mathern GW, Pretorius JK, Babb TL. Quantified patterns of mossy fiber sprouting and neuron densities in hippocampal and lesional seizures. J Neurosurg. 1995;82:211–219. doi: 10.3171/jns.1995.82.2.0211. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Babb TL, Pretorius JK, Leite JP. Reactive synaptogenesis and neuron densities for neuropeptide Y, somatostatin, and glutamate decarboxylase immunoreactivity in the epileptogenic human fascia dentata. J Neurosci. 1995;15:3990–4004. doi: 10.1523/JNEUROSCI.15-05-03990.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins da Silva A, Aarts JH, Binnie CD, Laxminarayan R, Lopes da Silva FH, Meijer JW, Nagelkerke N. The circadian distribution of interictal epileptiform EEG activity. Electroencephalogr Clin Neurophysiol. 1984;58:1–13. doi: 10.1016/0013-4694(84)90195-0. [DOI] [PubMed] [Google Scholar]
- Meldrum BS. Concept of activity-induced cell death in epilepsy: historical and contemporary perspectives. Prog Brain Res. 2002;135:3–11. doi: 10.1016/S0079-6123(02)35003-9. [DOI] [PubMed] [Google Scholar]
- Niedermyer E, Silva L, editors. Electroencephalography: basis, principles, clinical applications and related fields. 5th ed. Williams & Wilkins; Baltimore: 2004. [Google Scholar]
- Placidi F, Tombini M, Romigi A, Bianchi L, Izzi F, Sperli F, Mattia D, Cervellino A, Marciani MG. Topiramate: effect on EEG interictal abnormalities and background activity in patients affected by focal epilepsy. Epilepsy Res. 2004;58:43–52. doi: 10.1016/j.eplepsyres.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation: II. motor seizures. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rosati A, Aghakhani Y, Bernasconi A, Oivier A, Andermann F, Gotman J, Dubeau F. Intractable temporal lobe epilepsy with rare spikes is less severe than with frequent spikes. Neurology. 2003;60:1292–1295. doi: 10.1212/01.wnl.0000058761.12715.0e. [DOI] [PubMed] [Google Scholar]
- Roseman E, Woodhall B. Assoc. Res Nerv Mental Dis Proc. 1946:201–19. [Google Scholar]
- Salazar AM, Jabbari B, Vance SC, Grafman J, Amin D, Dillon JD. Epilepsy after penetrating head injury. I. Clinical correlates: a report of the Vietnam Head Injury Study. Neurology. 1985;35:1406–1414. doi: 10.1212/wnl.35.10.1406. [DOI] [PubMed] [Google Scholar]
- Sayin U, Osting S, Hagen J, Rutecki P, Sutula T. Spontaneous seizures and loss of axoaxonic and axosomatic inhibition induced by repeated brief seizures in kindled rats. J Neurosci. 2003;23:2759–2768. doi: 10.1523/JNEUROSCI.23-07-02759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shovron S. Does convulsive status epilepticus (SE) result in cerebral damage or affect the course of epilepsy – the epidemiological and clinical evidence? Prog Brain Res. 2002;135:85–93. doi: 10.1016/S0079-6123(02)35009-X. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Hellier JL, Dudek FE. Do interictal spikes drive epileptogenesis? Neuroscientist. 2005;11:272–276. doi: 10.1177/1073858405278239. [DOI] [PubMed] [Google Scholar]
- Sutula T. Seizure-induced axonal sprouting: assessing connections between injury, local Circuits, and epileptogenesis. Epilepsy Curr. 2002;2:86–91. doi: 10.1046/j.1535-7597.2002.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauck DL, Nadler JV. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J Neurosci. 1985;5:1016–22. doi: 10.1523/JNEUROSCI.05-04-01016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekgul H, Gauvreau K, Soul J, Murphy L, Robertson R, Stewart J, Volpe J, Bourgeois B, du Plessis AJ. The current etiologic profile and neurodevelopmental outcome of seizures in term newborn infants. Pediatrics. 2006;117:1270–1280. doi: 10.1542/peds.2005-1178. [DOI] [PubMed] [Google Scholar]
- White AM, Williams PA, Ferraro DJ, Clark S, Kadam SD, Dudek FE, Staley KJ. Efficient unsupervised algorithms for the detection of seizures in continuous EEG recordings from rats after brain injury. J Neurosci Methods. 2006;152:255–266. doi: 10.1016/j.jneumeth.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Williams PA, White AM, Clark S, Ferraro DJ, Swiercz W, Staley KJ, Dudek FE. The latent period and the progression of spontaneous seizures in rats with kainate-induced epilepsy. J Neurosci. 2009;29:2103–2112. doi: 10.1523/JNEUROSCI.0980-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, Dou P, Dudek FE. Epilepsy and synaptic reorganization in a perinatal rat model of hypoxia-ischemia. Epilepsia. 2004;45:1210–8. doi: 10.1111/j.0013-9580.2004.60403.x. [DOI] [PubMed] [Google Scholar]
- Williams PA, White AM, Ferraro DJ, Clark S, Staley K, Dudek FE. The use of radiotelemetry to evaluate electrographic seizures in rats with kainate-induced epilepsy. J Neurosci Methods. 2006;155:39–48. doi: 10.1016/j.jneumeth.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Wilson S, Harner R, Duffy F, Tharp B, Nuwer M, Sperling M. Spike detection I. Correlation and reliability of human experts. Electroenceph. Clin Neurophysiol. 1996;98:186–198. doi: 10.1016/0013-4694(95)00221-9. [DOI] [PubMed] [Google Scholar]
- Wilson SB, Emerson R. Spike detection: a review and comparison of algorithms. Clin Neurophysiol. 2002;113:1873–81. doi: 10.1016/s1388-2457(02)00297-3. [DOI] [PubMed] [Google Scholar]
- Wuarin JP, Dudek FE. Excitatory synaptic input to granule cells increases with time after kainate treatment. J Neurosci. 2001;85:1067–1077. doi: 10.1152/jn.2001.85.3.1067. [DOI] [PubMed] [Google Scholar]