Abstract
The development of vectors and techniques to transfer therapeutic genes to corneal epithelium has broad clinical applications. To determine if adenoviral (Ad5) vectors could be tailored to increase transduction of corneal epithelial progenitor cells expressing epidermal growth factor receptor (EGFR), the feasibility of targeting gene therapy vectors to genetically modify primary cultured human corneal epithelial cells (PHCEC) was evaluated. PHCECs were cultured from human limbal explants and transduced with Ad5 vectors containing the enhanced green fluorescent protein (GFP) reporter cassette to mediate gene transfer. The efficiencies of transduction with different Ad5 dosages and different time periods of exposure were compared. Metabolically biotinylated Ad5 vectors were retargeted to PHCECs using biotinylated epidermal growth factor (EGF) as a cell-targeting ligand. Phenotypes and function assays of transduced cells were determined by real-time PCR and BrdU incorporation. Ad5 vectors transduced approximately 50–93% of PHCEC at 10–100 PFU/cell in a dose-dependent manner and the transgene persisted for more than 2 weeks in vitro. Retargeting of biotinylated Ad5 with EGF increased transduction of EGFR and ABCG2-expressing corneal epithelial progenitor cells up to nine-fold and reduced transduction of K12 and involucrin-expressing differentiated corneal epithelial cells and had higher BrdU incorporation indexes. These data provide proof of principle that ligand-bearing modified Ad5 vectors can target a population of corneal epithelial progenitor cells for corneal gene therapy.
Keywords: corneal epithelial cells, progenitor cells, gene therapy, adenovirus 5, cell targeting
1. Introduction
The corneal surface is covered by a non-keratinized stratified squamous epithelium approximately five cell layers thick. The cornea is an excellent target for gene therapy, since it has well-defined anatomy, is transparent, and is easily accessible. Furthermore, unlike other transplantable tissues, the cornea can be maintained in standard culture conditions for extended periods of up to 1 month prior to transplantation, which allows a window of opportunity for genetic modification prior to reintroduction into patients. There are many potential applications for corneal gene therapy, including those aimed at reducing inflammation and autoimmune reactions after allogeneic corneal transplantation or maintaining stem like properties and proliferative potential of corneal epithelial progenitor cells containing stem cells (SC) and transient amplifying cells (TAC).
While gene delivery to differentiated corneal epithelial cells will likely have utility for a number of ocular surface diseases, genetic modification of more primitive epithelial progenitor cells (PCs) may have greater utility by enabling the production of gene products in the whole lineage of their progeny or by providing bioactive proteins to the epithelium early in the repair process. The epithelial PCs in the limbal region of the cornea supply the regenerative capacity of the cornea by replacing the differentiated post-mitotic corneal epithelial cells as they naturally slough off the surface of the eye. Three functionally different cell populations reside at the limbus: putative SC, TACs, and terminally differentiated cells (DCs) (Cotsarelis et al., 1989; Schermer et al., 1986). Although corneal epithelial SCs have been long known to reside in the limbus (see review articles by Dua and Azuara-Blanco, 2000; Lavker and Sun, 2000; Tseng, 1989), a marker identifying these putative SCs has remained elusive. Recently, we characterized a phenotype of corneal epithelial PCs in the basal epithelia of the human limbus (Chen et al., 2004b). These cells bear a number of unique markers including p63 and the ABCG2 and high levels of epidermal growth factor receptor (EGFR). In contrast to other cells in the limbal and corneal epithelium, these PCs lack expression of involucrin, keratins K3 and K12.
Given the promise of corneal gene therapy, we have tested short-term gene delivery methods that could be used to treat ocular surface diseases needing transient correction (i.e. inflammation, corneal ulcers, wound healing, limbal epithelial deficiency and expressed specific growth factor or cytokine, etc.). In addition, we tested vectors that avoid integration of DNA into the genomes of corneal cells to avoid the potential for insertional mutagenesis (Ritchie et al., 2004). Previous work in the cornea has demonstrated functional delivery of genes by non-integrating methods into corneal cells by gene gun (Zagon et al., 2005), electroporation (Chen et al., 2004a; Oshima et al., 1998), liposomes (Lipofectamine) (Dean et al., 1999); and with adenoviral serotype 5 (Ad5) vectors (Danjo and Gipson, 2002; Fehervari et al., 1997; Larkin et al., 1996; Oral et al., 1997; Tsubota et al., 1998). But these data were limited to the corneal endothelial cells of rat, rabbit or mice, an SV-40 transformed human corneal epithelial cell line and human conjunctival epithelial cells. Recently Xiao et al. reported gene delivery on human primary cultured corneal epithelial cells by adenovirus 19 (Xiao et al., 2005). However, this report did not determine which lineages of cells in the corneal cells were modified by this approach.
In this study, we transferred genes to PHCEC using Ad5 of different titers and found that the transducing efficiency reached as high as 93%, depending on the dosage of Ad5 used, and noted that expression of transduced genes lasted more than 2 weeks in vitro. We also evaluated if transduction into the epithelial PCs could be increased and delivery into epithelial DCs could be decreased by retargeting Ad5. To do this, we retargeted metabolically biotinylated Ad5 (Parrott et al., 2003) by conjugating this vector to the biotinylated EGF ligand using tetrameric avidin as a bridge (Fig. 1). Using this approach, we demonstrated that the EGF-targeted Ad5 vector enhanced gene delivery into epithelial PCs.
Fig. 1.
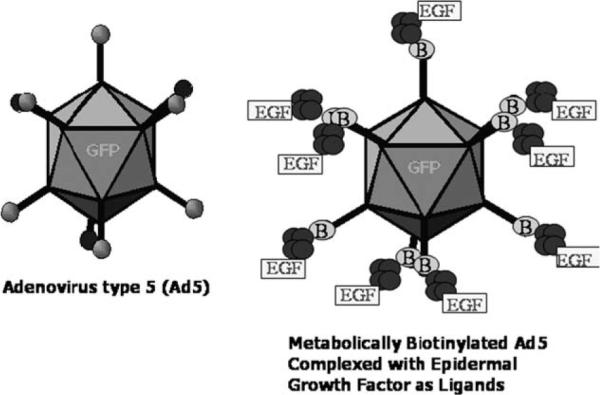
Cartoons of Adenovirus 5 (Ad5), biotinylated adenovirus with EGF complex (Ad-Fiber-BAP/EGF) carrying EGF reporter cassette.
2. Materials and methods
2.1. Generation of biotinylated Adenovirus (Ad-Fiber-BAP)
First generation replication incompetent (E1 deleted) Ad5 vectors carrying a green fluorescent protein (GFP) reporter cassette, together with a biotinylation domain added to the C-terminus of the fiber, were produced in 293 cells as previously described (Parrott et al., 2003). Briefly, 293 cells were harvested 3 days after infection from seed viral stock (Ad-Fiber-BAP). The cells were pelleted by centrifugation and freeze/thawed three times. The resulting viral lysate supernatant was then banded twice on a CsCl gradient. The viral band was subsequently collected, desalted into HEPES buffer (10 mM HEPES, 150 mM NaCl, 1 mM MgCl2, 5% w/v sucrose, pH 7.8) and stored at −80 °C (Fig. 1). Viral concentrations were then determined by measuring absorbance at 260 nm and real-time polymerase chain reaction (PCR).
2.2. Formation of epidermal growth factor (EGF) targeted complex (Ad-Fiber-BAP/EGF)
5 × 1012 viral particles of purified Ad-Fiber-BAP were incubated with a 10-fold molar excess of neutravidin (Pierce, Rockville, IL) on ice for 30 min to saturate the biotins on the vector. These viral complexes were then banded on a CsCl gradient to remove excess avidin. The viral band was isolated and incubated with a 100-fold molar excess of biotinylated EGF for 30 min on ice, followed by banding on another CsCl gradient. The band was then isolated and desalted in HEPES buffer. Viral concentrations were measured by real-time PCR.
2.3. PHCEC preparation and transduction
Fresh human corneoscleral tissues from donors aged 19–67 years (less than 72 h post mortem) that were not suitable for clinical use, were obtained from the Lions Eye Bank of Texas (LEBT, Houston, TX). Human corneal epithelial cells were cultured from fresh limbal explants by a previously described method (Kim et al., 2004). Briefly, after carefully removing the central cornea, excess sclera, iris, corneal endothelium, conjunctiva and Tenon's capsule, the remaining limbal rim was cut in 12 pieces and cultured in a 6-well plate with the epithelial side up in SHEM medium for explant culture. Twenty-four hours before transduction, confluent cultured cells were trypsinized with 0.25% trypsin/0.03% EDTA solution for 10–15 min. After the epithelial sheet detached from the culture well, 10% fetal bovine serum with DMEM was added to neutralize the trypsin. The cell suspension was then harvested by centrifugation and resuspended in Keratinocyte-SFM (KSFM) (Invitrogene–GIBCO BRL, Grand Island, NY) and seeded at 4 × 104 cells/cm2 into 12-well plates.
Prior to transduction, the culture media was changed to remove dead cells, the HPCEC density was around 3 × 104 cells/cm2 into 12-well plates, different doses of Ad5 (500, 1000 and 5000 viral particles per cell) were added to the PHCEC. Cells were incubated in KSFM for 1 h at 37 °C. Cells were then washed twice with phosphate-buffered saline (PBS) to remove unbound viruses before the addition of SHEM. Fresh medium was added the next day, and the cells were harvested after 48 h for analysis.
2.4. Flow cytometry analysis of receptor density measurements on HPCEC
PHCEC were evaluated for expressions of Coxsackie and adenovirus receptor (CAR) and EGFR by flow cytometry. Briefly, 0.5 × 106 cells were allowed to incubate with 1:100 dilution of anti-CAR antibodies (ATCC, Manassas, VA; CRL-2379, 1:40, 0.4 mg/ml) or anti-EGFR antibodies (Lab Vision, Fremont, CA; Clone H11, 1:100, 1 mg/ml) for 20 min at 4 °C. The cells were then washed twice with PBS/1% FBS, and 1:500 dilution Alexa-Fluor 488-conjugated goat anti-mouse antiserum was then applied to the cells for 20 min at 4 °C. The cells were washed again and resuspended in SHEM media. The receptor densities on these cells were then analyzed by flow cytometry (Beckman Coulter, Hialeah, FL) at the Baylor College of Medicine Flow Cytometry Core Facility.
2.5. FACS of transduced cells
EGFR expression by PHCEC and GFP expression in Ad5 transduced PHCEC were evaluated by fluorescence activated cell sorting (FACS). The cell populations were then sorted into subpopulations according to their relative level of EGFR expression (EGFR-positive versus EGFR-negative) or GFP expression (GFP-positive versus GFP-negative). FACS was performed at the Baylor College of Medicine Core Facility using a triple-laser Beckman Coulter Altra high-pressure, high-speed cell sorter (Beckman Coulter). A 488 nm Argon laser excited fluorescein isothiocyanate and a band-pass filter of 525/20 were used to measure emitted light. Gates in the right angle scatter versus forward scatter diagrams were used to exclude debris. All FACS data was analyzed with Expo 32 software.
2.6. Immunofluorescent staining
Immunofluorescent staining was performed by a previously reported method (Kim et al., 2004) to evaluate protein expression in GFP-positive and GFP-negative cell populations after Ad5 transduction. Briefly, sorted cells were seeded into wells of 8 chamber slides and incubated overnight at 37 °C with 4% CO2. They were then fixed in cold methanol at 4 °C for 10 min, and then blocked with 10% normal goat serum in PBS for 1 h to decrease non-specific background. Primary monoclonal antibodies against EGFR (Lab Vision; Clone H11, 1:20, 10 μg/ml), ABCG2 (Calbiochem, San Diego, CA; Clone BXP-21, 1:100, 2.5 μg/ml), K3/K12 (Chemicon, Temecula, CA; Clone AE5, 1:50, 20 μg/ml) or involucrin (Lab Vision; SY5, 1:40, 5 μg/ml) were applied and incubated for 1 h at room temperature. After extensive washing, secondary antibodies, Alexa-Fluor 594 conjugated goat anti-mouse (Molecular Probes, Eugene, OR; 1:400), were then applied and incubated in a dark chamber for 1 h, followed by extensive washing and counterstaining with Hoechst 33342 DNA binding dye (1μg/ml in PBS) for 2 min. After washing with PBS, antifade Gel/Mount (Fisher, Atlanta, GA) and a coverslip were applied. Cells were then examined and photographed with an epifluorescent microscope (Eclipse 400, Nikon, Japan) with a digital camera (model DMX 1200, Nikon) with 1 s exposure times.
2.7. BrdU incorporation
A BrdU incorporation assay was performed to measure DNA synthesis and cell proliferation. All groups (n = 3 every group) including unfractionated cells (control), GFP-positive and -negative cells transduced by Ad-Fiber-BAP, and EGF-positive and -negative cells transduced by Ad-Fiber-BAP/EGF were incubated in fresh medium containing 10 μM BrdU for 30 min and then BrdU immunofluorescent staining and label index counting were performed. The BrdU labeling index was assessed by point counting through a Nikon TE200 inverted microscope using a 40× objective lens. A total of 500–951 nuclei were counted in 6–8 representative fields because this number was considered as a minimum requirement to obtain representative figures (Selvamurugan et al., 2004). The labeling index was expressed as the number of positively labeled nuclei/total number of nuclei × 100%.
2.8. Relative quantitative real-time PCR
Total RNA was isolated by guanidinine–isothiocyanate ethanol and silica-gel-based membrane extraction using a Qiagen (Valencia, CA) RNA extraction kit. The RNA was quantified by its absorption at 260 nm and stored at −80 °C before use. Relatively quantitative real-time PCR was performed by the following method. Briefly, the first-strand cDNA was synthesized from 1 μg of total RNA with random hexamer and M-MuLV reverse transcriptase using Ready-To-Go You-Prime First-Strand Beads. Real-time PCR was performed in a Smart Cycler (Cepheid, Sunnyvale, CA) with a 25 μL reaction volume containing cDNA, TaqMan® primers and reporter probes for EGFR, ABCG2, involucrin, K12 (TaqMan Gene Expression Assays, Applied Biosystems) and TaqMan Universal PCR Master Mix. Assays were performed in duplicate. A non-template control was included in all the experiments to evaluate DNA contamination of the reagent used. The results of were analyzed by the comparative threshold cycle (CT) method and normalized by GAPDH as an internal control.
2.9. Colony forming efficiency (CFE) and growth capacity
To evaluate growth capacity of the EGFR selected cell populations, a mitomycin C (MMC) treated 3T3 fibroblast feeder layer was used as previously described (Schlotzer-Schrehardt and Kruse, 2005; Tseng et al., 1996). Each cell population was seeded at 1 × 103 cells/cm2 into 6-well culture plates at least in triplicate. The CFE was calculated as a percentage of the number of colonies generated at day 6 by the number of epithelial cells plated in a well.
2.10. Statistical analyses
Based on the normality of the data distribution, the Student t-test was used for statistical comparison of assay results between groups of CFE (Fig. 3), and real-time PCR relative folds (Fig. 5).
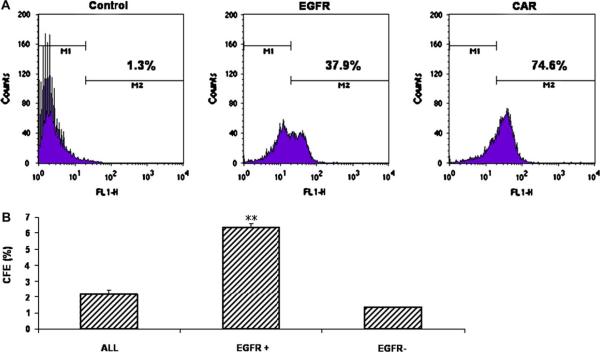
Fig. 3.
(A) Flow cytometry for EGFR (37.9 ± 9.1%) and CAR (74.6 ± 4.7%) expressions in the PHCEC. (B) The CFE on day 6 of EGFR-positive cells and EGFR-negative cells sorted by FACS based on EGFR expression compared to (ALL). **P < 0.01, compared with unfractionated cells (ALL).
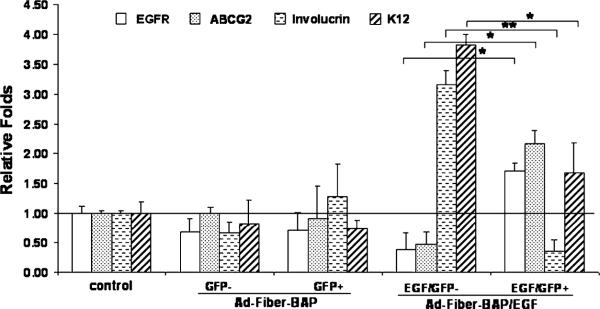
Fig. 5.
Representative relative-quantitative real-time PCR showing mRNA expressions of EGFR, ABCG2, involucrin, K3 and GAPDH of unfractionated cells (control, ALL) GFP-positive and GFP cells transduced by Ad-Fiber-BAP, EGF/GFP-positive and EGF/GFP cells transduced by Ad-Fiber-BAP/EGF sorted by flow cytometry based on levels of GFP expression of PHCEC. *P < 0.05, **P < 0.01.
3. Results
3.1. Ad5 transduction
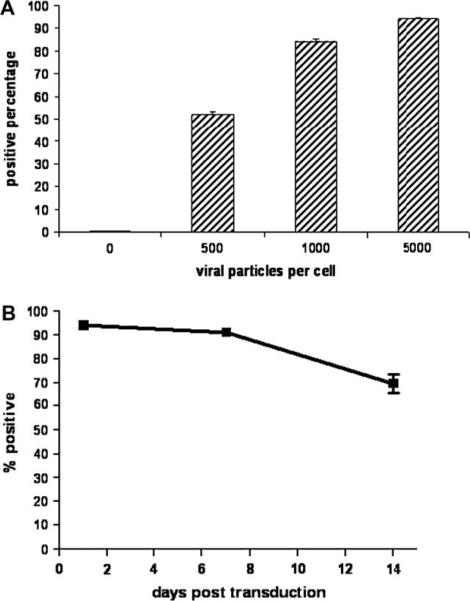
PHCECs were incubated with 500 Ad5 particles/cell (10 PFU/cell) and were examined after 48 h by fluorescence microscopy and flow cytometry. About 52 ± 1.5% of PHCECs expressed the GFP transgene (Fig. 2A). When the amount of vector was titrated, higher concentrations of Ad5 increased transduction efficiency to a maximum of 93% when 5000 viral particles/cell (100 PFU/cell) was used (Fig. 2A). Toxicity was not observed with any of the concentrations of Ad5 tested.
Fig. 2.
Ad5 transduction of PHCEC in a dose-dependent manner (A); transduction can be maintained in PHCEC at 70% after 14 days (B).
Persistence of the transferred gene in the cultured cells was evaluated by monitoring them for 2 weeks after transduction. Seventy percent of cells remained transduced after 2 weeks (Fig. 2B); however, the mean fluorescence intensity (MFI) of the cell population decreased over time to 25% of the original value (1 day post transduction).
3.2. Surface markers expression and growth potential of PHCECs
Flow cytometry was used to detect expression of surface markers by PHCEC. The assay showed that 74.6 ± 4.7% of PHCECs expressed CAR, while only 37.9 ± 9.1% of the cells expressed EGFR (Fig. 3A). To evaluate the growth capacity of the EGFR-positive and EGFR-negative populations, CFE was compared. CFE on day 6 from three separate experiments showed that EGFR-positive cells isolated from PHCEC by FACS had a greater growth potential than EGFR-negative cells. EGFR-positive cells produced almost 5-fold higher CFE than EGFR-negative cells (6.35 ± 0.21% versus 1.33 ± 0.28% Fig. 3B, n = 3, P < 0.001).
3.3. Transduction of PHCECs with biotinylated adenovirus targeted with EGF
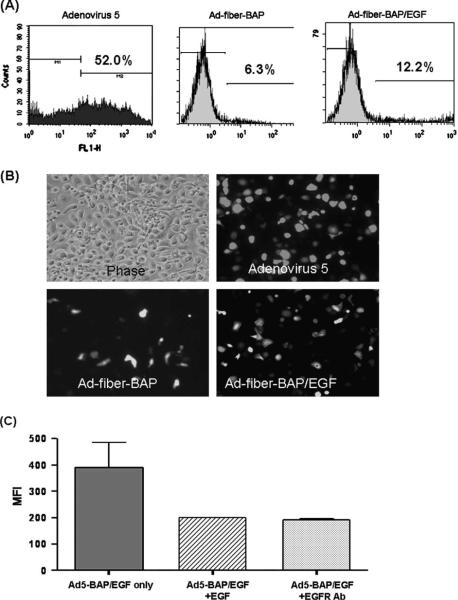
PHCEC were incubated with either Ad5, untargeted biotinylated Adenovirus (Ad-Fiber-BAP, a modified virus with reduced CAR-mediated transduction [Parrott et al., 2003]), or EGF-targeted Ad-Fiber-BAP (Ad-Fiber-BAP/EGF) and their transduction efficiencies were examined after 48 h. Ad-Fiber-BAP without EGF produced an 8-fold lower transduction than the wild-type Ad5 vector (Fig. 4A,B) consistent with previous results on other CAR-expressing cells (Parrott et al., 2003). When transduction by Ad-Fiber-BAP and Ad-Fiber-BAP/EGF complexes were compared, the addition of EGF to the vector doubled the number of GFP-positive PHCECs and increased the mean fluorescent intensify (MFI) of the population by 9 times (272.6 in cells transduced with Ad-Fiber-BAP/EGF compared to 30.8 in cells transduced with untargeted Ad-Fiber-BAP) (Fig. 4A,B). To determine if EGF was mediating this increase, competition experiments were performed with excess EGF or EGFR antibodies. When either EGF or EGFR antibody were used as competitor, the MFI was reduced by half in the Ad-Fiber-BAP/EGF group (Fig. 4C), while no difference was observed in Ad-Fiber-BAP group (data not shown).
Fig. 4.
Transduction of PHCECs by Ad5, targeted and untargeted Ad-Fiber-BAP. (A) Flow cytometry analysis of transduction efficiency and mean fluorescent intensity (MFI) of PHCEC transduced with different Ad5. (B). Fluorescent images of transduction by different adenovirus vectors at 500 particle Ad5/PHCEC. (C) EGF and anti-EGFR competitively inhibit the Ad-Fiber-BAP/EGF transduction efficiency.
3.4. Phenotype of Ad-Fiber-BAP/EGF transduced cells
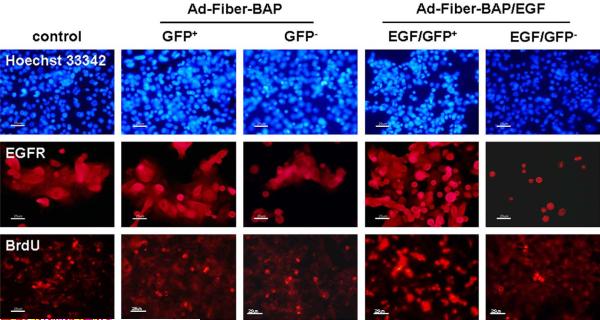
Cells transduced by Ad-Fiber-BAP and Ad-Fiber-BAP/EGF were sorted by FACS for GFP-positive and -negative cells and were analyzed for phenotypic expression of specific cellular markers by real-time PCR and by immunofluorescent staining. GFP-positive cells after Ad-Fiber-BAP/EGF transduction expressed 6-fold higher levels of EGFR mRNA than their untransduced counterparts by real-time PCR (Fig. 5, P < 0.05) and by immunofluorescence (Fig. 6). In contrast, there was no difference in EGFR mRNA expression between the GFP-positive and -negative cells modified by the untargeted Ad-Fiber-BAP (Fig. 5, P > 0.05 and Fig. 6). When the cells were analyzed for phenotypic markers, the GFP-positive cells produced by transduction with Ad-Fiber-BAP/EGF expressed 4 times more ABCG2 mRNA than the GFP-negative population (Fig. 5, n = 3, P < 0.05) and were more strongly stained with ABCG2 antibody (data not shown). The same GFP-positive cells from Ad-Fiber-BAP/EGF expressed 8 and 2.5 lower levels of involucrin and keratin K12 than the GFP-negative cell population (Fig. 5, n = 3, P < 0.01 and P < 0.05, respectively) and were more weakly stained by keratin and involucrin antibodies (data not shown). Analysis of cells transduced with the untargeted Ad-Fiber-BAP vector revealed that both GFP-positive and GFP-negative cells expressed similar levels of ABCG2, involucrin and K12 mRNAs (Fig. 5, n = 3, P > 0.05) and were stained similarly by immunofluorescence (data not shown). These data suggest that re-targeting Ad5 with EGF enhanced transduction of EGFR and ABCG2-expressing cells and decreased transduction of involucrin and K12-expressing epithelial cells.
Fig. 6.
Immunofluorescent staining of EGFR, BrdU incorporation in unfractionated cells (control), GFP-positive cells and GFP-negative cells transduced by Ad-Fiber-BAP, and EGF/GFP-positive cells and EGF/GFP-negative cells transduced by Ad-Fiber-BAP/EGF. Hoechst 33342 was used as a counterstain.
Immunofluorescent staining confirmed that the GFP-positive Ad-Fiber-BAP/EGF transduced cells expressed significantly more EGFR on their surface than EGF/GFP-negative cells (Fig. 6). When unmodified and transduced cells were analyzed for the proliferative capacity by BrdU incorporation, notable differences were observed in the populations. In bulk, unmodified PHCEC cells, 24.6 ± 3.5% of the cells were positive for BrdU (Fig. 6). In contrast in Ad-Fiber-BAP/EGF transduced cells, 45.3 ± 5.1% of the GFP-positive cells were positive for BrdU, whereas only 7.5 ± 2.3% of the GFP-negative cells were positive. When compared to the unmodified PHCEC cells, this indicated that the cells transduced with the EGF-targeted vector were enriched for proliferating cells, whereas the GFP-negative, untransduced cells were depleted for proliferating cells. When untargeted Ad-Fiber-BAP transduced cells were analyzed, BrdU incorporation was essentially identical between the GFP-positive and -negative cells (27.7 ± 2.9 and 22.6 ± 3.3%, respectively) and the unmodified bulk PHCEC cells (24.6 ± 3.5%). These data suggest that the EGF-targeted vector transduced a subpopulation of PHCECs with higher ABCG2 expression and higher proliferative capacity.
4. Discussion
4.1. Ad5 efficiently transduced PHCECs in a dose-dependent manner
Donated human corneoscleral tissues are an excellent source for whole or lamellar corneal transplantation and for isolation of PHCECs for corneal cell therapy or tissue engineering. Previous gene delivery studies were limited to animal corneal endothelial cells or SV40 transformed human corneal epithelial cells (Chen et al., 2004a; Danjo and Gipson, 2002; Dean et al., 1999; Fehervari et al., 1997; Larkin et al., 1996; Oral et al., 1997; Oshima et al., 1998; Tsubota et al., 1998; Zagon et al., 2005). To the best of our knowledge, this is the first reported attempt to transduce PHCECs with Adenovirus 5. Compared to other vectors, adenoviral vector are clearly safer than integrating vectors such as adeno-associated virus, retroviruses, or lentiviruses.
To test the feasibility of directly genetically modifying these cells, PHCECs cultured from limbal tissue explants were used as gene delivery targets. The transduction efficiency of Ad5 reached a maximum of 93% without observed toxicity. Due to episomal loss of GFP transgene through cell division, the MFI of the cell population decreased over time, but the expression of the transgene was still observed 2 weeks after transduction in vitro. Ad5 gene transfer can provide long-term expression in mitotically quiescent tissues; however, transgene expression in dividing cells is only transient due to the non-integrated status of the adenoviral genome (Benihoud et al., 1999). Under homeostatic conditions in vivo, the proliferative rate of PHCECs is low (Kim et al., 2004). Therefore, it is likely that expression of this gene vector could be sustained for months when these cells are maintained under conditions where their proliferation is suppressed.
4.2. Ad-Fiber-BAP/EGF retargets transduction to cells EGFR-positive PHCECs
We have previously shown that phenotypes of corneal epithelial cells, ranging from basal undifferentiated to superficial differentiated cells, are maintained in both single cell and explant cultures ex vivo (Kim et al., 2004). One of these cell populations has a smaller, more compact morphology and expresses the limbal stem cell (SC)-associated markers, ABCG2, p63, EGFR, K19 and integrin β1. Furthermore, expression of EGFR has been noted to markedly decrease in terminally differentiated corneal epithelial cells (Liu et al., 2001). EGFR-positive cells isolated from PHCEC by FACS showed greater growth potential with 5-fold higher CFE than EGFR-negative cells.
To determine if transduction of these PCs could be enhanced, we utilized metabolically-biotinylated Ad5 vectors (Parrott et al., 2003). These vectors are biologically biotinylated at a specific biotin acceptor peptide (BAP) on the fiber protein of the virus during vector production and they can be easily retargeted to new receptors by conjugation of a biotinylated ligand to the vector using tetrameric avidin as a bridge (Parrott et al., 2003). Given the higher expression of EGFR on the limbal epithelial PCs, we complexed biotinylated Ad5 (Ad-Fiber-BAP) to biotinylated EGF to test if this could increase transduction of these PCs.
Ad5 normally utilizes CAR for cell binding. Our data showed that the level of CAR expression in PHCECs is double that of EGFR expression. Ad-Fiber-BAP maintains this CAR binding function, but this interaction is reduced approximately 3- to 9-fold due to steric hindrance of the BAP on the c-terminus of the fiber protein (Parrott et al., 2003). Therefore, Ad-Fiber-BAP targeted with EGF could preferentially bind via EGFR on the cell surface of the PCs, but retain some residual transduction via CAR.
PHCEC that were incubated with Ad-Fiber-BAP without EGF showed an 8-fold lower transduction than those incubated with untargeted wild-type Ad5 vector. Thus Ad-Fiber-BAP can be considered to be partially detargeted from its native CAR receptor and provides a lower background of transduction of PHCECs. When Ad-Fiber-BAP and Ad-Fiber-BAP/EGF complexes were compared, the addition of EGF to the vector increased the number of GFP-positive PHCECs 2-fold and the MFI of the population by 9 times.
Competition experiments showed the specificity of Ad-Fiber-BAP/EGF for EGFR-positive cells. The specificity was also confirmed again by real-time PCR and immunofluorescent staining. The EGF-targeted vector mediated 6-fold higher selectivity of transduction into EGFR-positive cells relative to EGFR-negative cells by real-time PCR. As expected, in Ad-Fiber-BAP/EGF transduced cells, EGF/GFP-positive cells expressed significantly more EGFR on their surface than EGF/GFP-negative cells.
4.3. Ad-Fiber-BAP/EGF transduced cells are corneal epithelial PCs that express SC associated markers and have higher proliferative capacity
ABCG2 is a putative marker for stem cells in bone marrow, corneal epithelium and cancers. Basal limbal epithelial cells expressing ABCG2 have been found to have greater proliferative potential in culture (de Paiva et al., 2005). In our study, we found that the GFP-positive cells transduced with Ad-Fiber-BAP/EGF expressed more ABCG2 mRNA and had higher BrdU incorporation index than the parallel GFP-negative population. Furthermore, Ad-Fiber-BAP/EGF transduced cells had reduced mRNA expression of the epithelial differentiation markers, involucrin and keratin K12, compared to their untransduced counterparts. In contrast, the untargeted Ad-Fiber-BAP vector mediated showed no differences in ABCG2, EGFR, keratin, or involucrin expression, and no differences in proliferation. These data suggest that the EGF-targeted Ad5 vector increased gene delivery into EGFR-expressing epithelial PCs and provide proof of principle that gene delivery can be tuned towards particular cell populations for ocular surface gene therapy. These data also support strategies to deliver therapeutic genes into either epithelial PCs or epithelial DCs to mediate gene therapy with varied cell specificity and persistence.
Acknowledgements
The authors thank the Lions Eye Bank of Texas for their great support in providing human corneoscleral tissues. This study was supported by NIH Grants EY014553 (DQL) and EY11915 (SCP), National Eye Institute, Bethesda, MD, a grant from Lion Eye Bank Foundation, an unrestricted grant from Research to Prevent Blindness, the Oshman Foundation and the William Stams Garish Fund, and by funding from the Department of Ophthalmology at Baylor College of Medicine to M.A.B.
Footnotes
Presented in part as abstract at the annual meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, May 1–5, 2005.
References
- Benihoud K, Yeh P, Perricaudet M. Adenovirus vectors for gene delivery. Curr. Opin. Biotechnol. 1999;10:440–447. doi: 10.1016/s0958-1669(99)00007-5. [DOI] [PubMed] [Google Scholar]
- Chen YX, Krull CE, Reneker LW. Targeted gene expression in the chicken eye by in ovo electroporation. Mol. Vis. 2004a;10:874–883. [PubMed] [Google Scholar]
- Chen Z, de Paiva CS, Luo L, Kretzer FL, Pflugfelder SC, Li DQ. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004b;22:355–366. doi: 10.1634/stemcells.22-3-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- Danjo Y, Gipson IK. Specific transduction of the leading edge cells of migrating epithelia demonstrates that they are replaced during healing. Exp. Eye Res. 2002;74:199–204. doi: 10.1006/exer.2001.1115. [DOI] [PubMed] [Google Scholar]
- de Paiva CS, Chen Z, Corrales RM, Pflugfelder SC, Li DQ. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells. 2005;23:63–73. doi: 10.1634/stemcells.2004-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DA, Byrd JN, Jr., Dean BS. Nuclear targeting of plasmid DNA in human corneal cells. Curr. Eye Res. 1999;19:66–75. doi: 10.1076/ceyr.19.1.66.5344. [DOI] [PubMed] [Google Scholar]
- Dua HS, Azuara-Blanco A. Limbal stem cells of the corneal epithelium. Surv. Ophthalmol. 2000;44:415–425. doi: 10.1016/s0039-6257(00)00109-0. [DOI] [PubMed] [Google Scholar]
- Fehervari Z, Rayner SA, Oral HB, George AJ, Larkin DF. Gene transfer to ex vivo stored corneas. Cornea. 1997;16:459–464. [PubMed] [Google Scholar]
- Kim HS, Jun SX, de Paiva CS, Chen Z, Pflugfelder SC, Li DQ. Phenotypic characterization of human corneal epithelial cells expanded ex vivo from limbal explant and single cell cultures. Exp. Eye Res. 2004;79:41–49. doi: 10.1016/j.exer.2004.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin DF, Oral HB, Ring CJ, Lemoine NR, George AJ. Adenovirus-mediated gene delivery to the corneal endothelium. Transplantation. 1996;61:363–370. doi: 10.1097/00007890-199602150-00005. [DOI] [PubMed] [Google Scholar]
- Lavker RM, Sun TT. Epidermal stem cells: properties, markers, and location. Proc. Natl. Acad. Sci. USA. 2000;97:13473–13475. doi: 10.1073/pnas.250380097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Carvajal M, Carraway CA, Carraway K, Pflugfelder SC. Expression of the receptor tyrosine kinases, epidermal growth factor receptor, ErbB2, and ErbB3, in human ocular surface epithelia. Cornea. 2001;20:81–85. doi: 10.1097/00003226-200101000-00016. [DOI] [PubMed] [Google Scholar]
- Oral HB, Larkin DF, Fehervari Z, Byrnes AP, Rankin AM, Haskard DO, Wood MJ, Dallman MJ, George AJ. Ex vivo adenovirus-mediated gene transfer and immunomodulatory protein production in human cornea. Gene Ther. 1997;4:639–647. doi: 10.1038/sj.gt.3300443. [DOI] [PubMed] [Google Scholar]
- Oshima Y, Sakamoto T, Yamanaka I, Nishi T, Ishibashi T, Inomata H. Targeted gene transfer to corneal endothelium in vivo by electric pulse. Gene Ther. 1998;5:1347–1354. doi: 10.1038/sj.gt.3300725. [DOI] [PubMed] [Google Scholar]
- Parrott MB, Adams KE, Mercier GT, Mok H, Campos SK, Barry MA. Metabolically biotinylated adenovirus for cell targeting, ligand screening, and vector purification. Mol. Ther. 2003;8:688–700. doi: 10.1016/s1525-0016(03)00213-2. [DOI] [PubMed] [Google Scholar]
- Ritchie MH, Fillmore RA, Lausch RN, Oakes JE. A role for NF-kappa B binding motifs in the differential induction of chemokine gene expression in human corneal epithelial cells. Invest Ophthalmol. Vis. Sci. 2004;45:2299–2305. doi: 10.1167/iovs.03-0367. [DOI] [PubMed] [Google Scholar]
- Schermer A, Galvin S, Sun T-T. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J. Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlotzer-Schrehardt U, Kruse FE. Identification and characterization of limbal stem cells. Exp. Eye Res. 2005;81:247–264. doi: 10.1016/j.exer.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Selvamurugan N, Kwok S, Alliston T, Reiss M, Partridge NC. Transforming growth factor-beta 1 regulation of collagenase-3 expression in osteoblastic cells by cross-talk between the Smad and MAPK signaling pathways and their components, Smad2 and Runx2. J. Biol. Chem. 2004;279:19327–19334. doi: 10.1074/jbc.M314048200. [DOI] [PubMed] [Google Scholar]
- Tseng SC. Concept and application of limbal stem cells. Eye. 1989;3(Pt 2):141–157. doi: 10.1038/eye.1989.22. [DOI] [PubMed] [Google Scholar]
- Tseng SC, Kruse FE, Merritt J, Li DQ. Comparison between serum-free and fibroblast-cocultured single-cell clonal culture systems: evidence showing that epithelial anti-apoptotic activity is present in 3T3 fibroblast-conditioned media. Curr. Eye Res. 1996;15:973–984. doi: 10.3109/02713689609017643. [DOI] [PubMed] [Google Scholar]
- Tsubota K, Inoue H, Ando K, Ono M, Yoshino K, Saito I. Adenovirus-mediated gene transfer to the ocular surface epithelium. Exp. Eye Res. 1998;67:531–538. doi: 10.1006/exer.1998.0557. [DOI] [PubMed] [Google Scholar]
- Xiao J, Nataraja K, Rajala MS, Astley RA, Ramadan RT, Chodosh J. Vitronectin: a possible determinant of adenovirus type 19 tropism for human corneal epithelium. Am. J. Ophthalmol. 2005;140:363–369. doi: 10.1016/j.ajo.2005.03.077. [DOI] [PubMed] [Google Scholar]
- Zagon IS, Sassani JW, Verderame MF, McLaughlin PJ. Particle-mediated gene transfer of opioid growth factor receptor cDNA regulates cell proliferation of the corneal epithelium. Cornea. 2005;24:614–619. doi: 10.1097/01.ico.0000153561.89902.57. [DOI] [PubMed] [Google Scholar]