Abstract
Background
To investigate the extent to which three putative “environmental” risk factors, maternal and paternal punitive discipline and negative life events, share genetic influences with, and moderate the heritability of, externalizing behavior.
Methods
The sample consisted of 2647 participants, aged 12–19 years, from the G1219 and G1219Twins longitudinal studies. Externalizing behavior was measured using the Youth Self-Report, maternal punitive discipline (MPD), paternal punitive discipline (PPD) and exposure to negative life events (NLE) were assessed using the Negative Sanctions Scale and the Life Event Scale for Adolescents respectively.
Results
Genetic influences overlapped for externalizing behavior and each “environmental” risk, indicating gene-environment correlation. When controlling for the gene-environment correlation genetic variance decreased, and both shared and non-shared environmental influences increased, as a function of MPD. Genetic variance increased as a function of PPD, and for NLE the only interaction effect was on the level of non-shared environment influence unique to externalizing behavior.
Conclusions
The magnitude of the influence of genetic risk on externalizing behavior is contextually dependent, even after controlling for gene-environment correlation.
Keywords: gene-environment interaction, gene-environment correlation, externalizing behavior, negative life events, punitive discipline
Introduction
Adoption studies (Cadoret et al., 1995) and molecular genetic studies (Caspi et al., 2002; Foley et al., 2004) have suggested that gene-environment interactions (GxE) are likely to be important in understanding risk for antisocial behavior. These studies suggest that genetic factors increase susceptibility to environmental risk. For instance, Cadoret et al. (1995) demonstrated that negative family environment only increased children’s problem behavior in the context of genetic risk (having antisocial biological parents). Similarly, molecular studies have demonstrated that exposure to maltreatment was only a substantial risk for antisocial outcomes when it occurred in the context of a particular allele of the monoamine oxidase A gene (Caspi et al., 2002). These findings are consistent with the stress-diathesis model (Shanahan and Hofer, 2005), which proposes that environmental context may trigger an underlying genetic risk for a phenotype.
There is emerging evidence from twin studies for other types of gene-environment interplay in relation to antisocial outcomes, whereby environmental processes moderate genetic risk, and ultimately estimates of heritability (Button et al., 2005; Tuvblad et al., 2005). Thus in one study heritability of antisocial behavior was higher in those with low versus high levels of family dysfunction (Button et al., 2005). These findings are consistent with the Bioecological model (Bronfenbrenner and Ceci, 1994), which states that the mechanisms by which genotypes actualize into phenotypes, vary as a function of environmental context. When proximal processes are weak, i.e. when the environment is not conducive to expression of that genotype, heritability is low, as genetic potential is not realized. Results of this kind are also consistent with the “Social Push Perspective” (Raine, 2002), which suggests that a highly negative environment predisposes to negative outcomes to such an extent as to suppress genetic influence. If replicated, such findings hold important implications for our understanding of the risk processes for antisocial behavior, and also for intervention strategies.
In addition, there is the confounding issue of gene-environment correlations (rGE) -genetic influences on aspects of the environment. rGE is likely to be important in many aspects of psychopathology (Plomin et al., 1977; Scarr and McCartney, 1983), and perhaps especially so for antisocial behavior. First, antisocial parents provide sub-optimal rearing environments for their children, as well as transmitting a genetic predisposition for antisocial behavior (passive rGE). Second, antisocial behavior in children can evoke negative reactions from others (evocative rGE). Third, the outcomes of antisocial behavior can function to ‘select’ individuals into adverse environments (active rGE).
It is likely that in practice both rGE and GxE operate in the development of antisocial behavior (Rutter and Silberg, 2002). In such instances, it is difficult to determine whether increased associations between genes and environment are the result of the environment modifying the effects of genes (GxE), or the genetic risk being more prevalent within certain environments (rGE). Consequently, rGE may mask detection of GxE and vice versa. In instances where we wish to test for interactions, but suspect gene-environment correlation, we need to use statistical approaches that can disentangle these effects.
This paper explores these issues of co-occurring gene-environment interaction and correlation in relation to three well-established correlates for externalizing behavior. Maternal and paternal punitive discipline may reflect passive and/or evocative gene-environment correlations, whereas dependent negative life events could represent active gene-environment correlation.
Parental discipline
Aspects of parental discipline (Kerr et al., 2004), particularly harsh (Nix et al., 1999) and inconsistent (Rutter et al., 1998) discipline are well-documented risk factors for externalizing behavior. There is evidence of a genetic contribution to maternal discipline (Wade and Kendler, 2000), with genes contributing between 10% and 40% depending on rater. Thus, discipline may index both a risk for behavioral problems as well as being a consequence of them. Harsh discipline may impact upon children’s development, thereby influencing the child’s behavior, but equally importantly children’s behavior can evoke negative reactions from parents (Ge et al., 1994; O'Connor et al., 1998; Riggins-Caspers et al., 2003). Moreover, genetic liability appears to be moderated by parental discipline. For example, Riggins-Caspers et al (2003) found that the association between biological risk for behavioral problems and harsh physical discipline by the adoptive parent was mediated by the child’s own negative behavior (rGE), but only in those exposed to environmental risks for the negative behavior (GxE). To date, evidence of gene-environment interaction in the presence of gene-environment correlation has been limited to adoption designs.
Negative Life Events
Although an association between negative life events and externalizing behavior was proposed almost 30 years ago (Robins, 1978) and studies have demonstrated a correlation (Champion et al., 1995; Wiesner and Windle, 2004), there has been limited research into the association. There are, however, several plausible reasons to expect such links. First, negative events may carry risk for externalizing phenotypes. Alternatively, individuals who are predisposed to externalizing behavior may elicit, or seek out, such events, for example by placing themselves in high-risk situations. This interpretation is supported by evidence of a genetic contribution to negative life events (Thapar and McGuffin, 1996), particularly those dependent on individuals’ own behavior (Billig et al., 1996).
The aims of the study were to identify whether there is a shared genetic liability between externalizing behavior and three “environmental” risk factors, and to investigate whether heritability is moderated by levels of exposure to these “environmental” risks. We expect the covariation between externalizing behavior and MPD, PPD, and NLE to be partially due to common genetic risk, and that the heritability of externalizing behavior will be contextually dependent.
Method
Participants
The analyses use the G1219 and G1219Twins longitudinal studies. G1219 consists of all adolescent offspring of adults from a large-scale population-based study (GENESiS: (Sham et al., 2000). Of the 9,000 families contacted through GENESiS, a total of 3,600 (40%) participated either in this study (of adolescents) or another study on childhood hyperactivity (participants were only eligible for one or other study on the basis of their age; full details are given elsewhere: Eley et al., 2004). Of the 3,600 responses received, a total of 1,818 (20%) adolescents from 1,294 families took part in G1219, the remainder participated in the hyperactivity study. The G1219Twins are a random selection of twins, born between 1985 and 1988, identified by the UK Office of National Statistics. Health Authorities and General Practitioners contacted 2,947 families of whom 1,381 (47%) participated. The siblings were combined with the twins in order to increase power to detect shared environmental effects. Both samples were sent two postal reminders, and only respondents aged 12- to 19-years were included. The siblings were slightly older than the twins (mean recruitment age: siblings=15.20; twins=14.09; difference=1.11; t=18.06; df=3166; p< 0.001) and significantly more likely to have mothers with at least one A-level (internationally recognized pre-university qualification, typically taken at age 18; Percentage of mothers with A-level or higher: siblings=46.4%; twins=39.7%; χ2=11.33; df=1; p=0.001). Although significant, these differences were too small to represent meaningful differences. The samples do not differ significantly from one another for maternal or paternal punitive discipline or exposure to negative life events. Informed consent was obtained from parents of all adolescents under 16, and from adolescents themselves when over 16. Ethical approval for this study was given by the Research Ethics Committee of the Institute of Psychiatry and South London and Maudsley NHS Trust.
These analyses focus on the second wave of data collection (N=1,820), which took place approximately 8 months (S.D. = 5 months; Range= 0.8 to 25 months) after initial contact. Zygosity was assessed by maternal report of physical similarity (Cohen et al., 1975). Total data were available for 2648 individuals. Eighty-four twin pairs were of unknown zygosity and consequently were excluded from all genetic analyses, resulting in a sample of 2562 individuals. Of these, 589 were from sibling pairs (120 male, 200 female, 268 opposite sex), 695 were MZ twins (309 male, 386 female), 1279 were DZ twins (246 male, 376 female, 657 opposite-sex). A complete description of the number of complete and incomplete pairs (7%) is provided in Table 1. The mean age of the sample at wave 2 was 15.0 years (range 12–21); 48% were boys.
Table 1.
Mean, standard deviations and twin pair correlations for externalizing behavior, maternal punitive discipline and negative life events split by sibling type (MZ, DZ, and full siblings) and sex.
| MZ | DZ | Full Sibling | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Opposite sex | Opposite sex | |||||||||
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | |
| N individuals | 309 | 386 | 246 | 376 | 323 | 334 | 120 | 200 | 124 | 144 |
| N complete pairs | 153 | 192 | 122 | 187 | 323 | 52 | 90 | 118 | ||
| N incomplete pairs | 3 | 2 | 2 | 2 | 11 | 0 | 16 | 20 | 26 | 6 |
| Externalizing Behavior | ||||||||||
| Mean | 11.89 | 10.73 | 12.83 | 12.51 | 12.96 | 12.13 | 13.45 | 13.04 | 13.93 | 12.75 |
| SD | 7.91 | 6.19 | 8.08 | 7.27 | 7.77 | 6.61 | 7.42 | 7.44 | 7.82 | 6.55 |
| Correlation | 0.47 | 0.61 | 0.24 | 0.44 | 0.24 | 0.37 | 0.20 | 0.20 | ||
| Maternal Punitive Discipline | ||||||||||
| Mean | 7.10 | 6.73 | 6.51 | 7.39 | 6.67 | 6.84 | 5.84 | 6.09 | 5.94 | 6.69 |
| SD | 4.38 | 4.04 | 3.94 | 4.18 | 3.99 | 3.81 | 3.90 | 3.85 | 3.66 | 3.94 |
| Correlation | 0.50 | 0.38 | 0.15 | 0.35 | 0.22 | 0.32 | 0.31 | 0.26 | ||
| Paternal Punitive Discipline | ||||||||||
| Mean | 6.28 | 5.97 | 6.13 | 6.63 | 6.26 | 6.62 | 5.71 | 5.19 | 5.52 | 6.18 |
| SD | 4.96 | 4.41 | 4.94 | 4.92 | 4.67 | 4.43 | 4.49 | 3.85 | 4.24 | 4.12 |
| Correlation | 0.60 | 0.49 | 0.39 | 0.41 | 0.33 | 0.51 | 0.49 | 0.46 | ||
| Negative Life Events | ||||||||||
| Mean | −0.12 | −0.13 | 0.06 | 0.05 | 0.02 | −0.04 | 0.04 | 0.07 | 0.07 | 0.07 |
| SD | 0.98 | 0.92 | 1.02 | 1.05 | 1.00 | 0.96 | 0.94 | 1.05 | 0.98 | 0.97 |
| Correlation | 0.48 | 0.43 | 0.31 | 0.36 | 0.21 | 0.06 | 0.37 | 0.23 | ||
Levels of parental education were higher than observed in a nationally representative sample (39% vs. 32% educated to A-level or above), and parents were more likely to own their own houses (82% vs. 68%; Meltzer et al., 2000). To reduce the impact of possible response bias associated with educational level, the sample was re-weighted to match the distribution of educational qualifications in the representative sample (Meltzer et al., 2000). Only 74% of the wave 1 sample (2651 individuals) participated in wave 2; females, families with higher parental qualifications, and owner-occupiers were more likely to continue to participate. A second weight was created and multiplied with the wave 1 weight to account for both initial response and later attrition biases (roughly 26%). Weights were family-general and thus did not incur any additional individual-specific effects between members of the same family.
Measures
Externalizing behavior was measured using the Youth Self-Report (Achenbach, 1991). Items are rated as not true to very true (scored 0 to 2) and summed to provide an externalizing behavior score. The scores were positively skewed (skew=1.124; kurtosis=1.664), but did not represent a J-shaped distribution.
Ratings of maternal and paternal punitive discipline were assessed using the Negative Sanctions sub-scale adapted from a previously well-validated parent-child relationship measure (Dunn et al., 1998; Hetherington & Clingempeel, 1992). This consists of 5 items such as ‘does your mother/father yell at you for what you did?’ Internal consistency was acceptable (mother: α=0.66; father: α=0.72). The distribution of scores was not significantly skewed for maternal (skew=0.556, kurtosis=0.121) or paternal punitive discipline (skew=0.529, kurtosis=0.017).
Negative life events were assessed with the Life Event Scale for Adolescents, a checklist of 50 events for which adequate reliability has been demonstrated (Coddington, 1984). As behavioral genetic analyses cannot test the heritability of items that are necessarily shared by siblings (Purcell and Koenen, 2005), only 12 items that were individual-specific, negative, and dependent upon the individual’s own actions such as ‘failing to achieve things that you want’ were used in these analyses (Rowe et al., 2006). The Cronbach’s alpha was 0.59; the scale was positively skewed (skew=1.086; kurtosis=0.864), although it did not present a J-shaped distribution.
Analyses
Means and correlations were calculated separately for males and females, by sibling type, using SPSS (SPSS Inc., 2004). Model-fitting was performed using the structural equation modeling package, Mx (Neale, 1997). The maximum likelihood function of Mx was applied to appropriately transformed raw data (i.e. regressed on age and sex) to avoid loss of information due to missing data, and the “Weight” function of Mx was used to incorporate the sampling weights. This function controls for attrition by adjusting the log-likelihoods for each family by the sample weights described above.
We fit univariate models to our data to estimate the relative contribution of genetic (A), shared environment (environment which makes family members similar to one another; C) and non-shared environment (environment which acts to make family members different from one another; E) to the phenotypic variances. Behavioral genetic analyses utilize differences in the genetic relatedness of different sibling-types to partition the phenotypic variance. For example, MZ twins share 100% of their genes and shared environment, but none of their non-shared environment. Therefore, similarities between them result from shared genes and shared environmental factors. DZ twins and full siblings share on average only half their genes, and any shared environmental factors. Consequently, if MZ twins are more similar to one another than DZ or full siblings, genes must play a role. We also tested for sex-effects on the magnitude of A, C and E, by first fitting a full sex-limitation model, allowing for A, C, and E, estimates to differ for males and females and the genetic correlation between opposite sex twins to differ from 0.5 (the genetic correlation between same sex full sibling and DZ twin pairs as described above). Following this, we fit a scalar sex-limitation model in which the A, C, and E parameters were constrained to be the same across sex, and the opposite sex genetic correlation was fixed to 0.5, but the male and female total variances were allowed to differ, to establish if this resulted in a significant deterioration in fit.
The fit of the full models was compared with that obtained from saturated models in which there are no constraints and all possible parameters (means, variances, and covariances of/between variables) are estimated. These are descriptive models that fit the data perfectly, and thus serve as a comparative model for nested models. Comparing the -2ll fit of the ACE models with the saturated model produces a chi-square statistic, which, along with the degrees of freedom (df) of the full model, calculated as the difference in the number of parameters estimated in the two models, makes it possible to determine how well the full model explains the observed data. A low, non-significant χ2 statistic indicates that the model explains the observed data well. Detection of a significant genetic influence on the “environmental” variable would provide evidence for rGE.
Second, we estimated the extent to which genes and environment contribute to covariation between externalizing behavior and each of the “environmental” risk measures using a bivariate Cholesky Decomposition. This model partitions the variances and covariances into A, C and E. All variables that contribute to the covariance are shared in common by both phenotypes are referred to as “common” effects, and all those that contribute to the variance of externalizing behavior, but are not shared with the “environmental” variable, are referred to as “unique”, since they are unique to externalizing behavior. To avoid confusion, the term “shared” will be used to refer to environmental variance that is shared by family members and makes them similar to one another, although this is frequently referred to as “common environment” in the literature. A significant common genetic pathway indicates that the genetic factor contributing to exposure to the “environment” also contributes to the predisposition for the behavioral outcome, and thus controls for gene-environment correlation.
The final set of models examined whether the genetic and environmental influences on externalizing behavior are moderated by levels of exposure to each of the putative environmental risks, whilst simultaneously accounting for genetic and environmental correlations between each with externalizing behavior (Purcell, 2002, see Figure 1) as described above. The potential moderator is modeled both as an outcome and as a moderating variable on all the path coefficients to the externalizing behavior, which are expressed as linear functions of the moderator. As a result, the variance of externalizing behavior is the consequence of the main effects from the common genetic, shared environmental and non-shared environmental influence that are shared with the moderating variables (e.g. aC), and those variables unique to externalizing behavior (e.g. aU), and the interaction between each of these terms with the moderator variable (e.g. βXC). The significance of each interaction term was tested by dropping moderation effect and comparing the fit of these nested models with the full model. Interaction terms that could be dropped without a significant change in χ2 were excluded from the full model to determine the most parsimonious model. A significant interaction term provides evidence for environmental moderation of the levels of the variance components for externalizing behavior, whilst accounting for the presence of any gene-environment correlation identified from the univariate and bivariate models.
Figure 1.
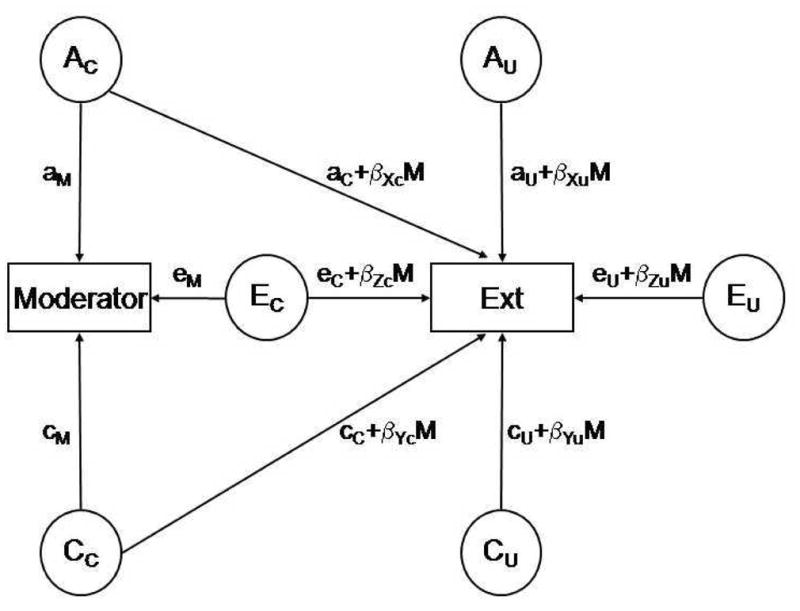
Gene-environment correlation and interaction model. AC: genetic influence common to the moderator and externalizing behavior (Ext), AU: genetic influence unique to externalizing behavior (Ext), C: shared environment influence, E: non-shared environment influence, aM: the influence of AC on the moderator variable, aC: the influence of AC on externalizing behavior, aU: the influence of AU on externalizing behavior, M: mean, β:moderationterm.
Results
Summary results
The means, standard deviations, sample size and twin pair correlations for externalizing behavior, maternal and paternal punitive discipline, and negative life events are presented in Table 1. Males had significantly higher mean levels of externalizing behavior than females (t=2.040, p=0.042), but there were no significant sex differences in the mean levels of maternal punitive discipline (t=1.765, p=0.078), paternal punitive discipline (t=1.066, p=0.287), or negative life events (t=0.501, p=0.617). There were small correlations with age for all three variables (externalizing behavior: r=0.041, p<0.05; MPD: r=−0.148, p<0.01; PPD: r=−0.111, p<0.01 and NLE: r=0.022, p=ns). All scores were regressed for age and sex, and standardized residuals were used for the genetic analyses.
Genetic model-fitting results
Results of univariate analyses are in Table 2. The full univariate externalizing behavior model fit the data significantly worse than the saturated model. Inspection of the model showed that this resulted from a lower variance for externalizing behavior in the female MZ twins (0.72) compared with the other female sibling types (0.92). However, comparison of both the AIC and the BIC of the two models demonstrate lower AIC and BIC fit statistics for the full model, indicating that the models do fit better than the saturated model. All other univariate models fit the data well, according to the Δχ2 between the full and saturated models, There were no significant sex effects on A, C, and E for any of the variables. There were significant genetic and non-shared environmental influences on all variables. Shared environmental influences were significant for the PPD measure.
Table 2.
Univariate model fit statistics for saturated and full models, and parameter estimates for genetic (A), shared environment (C) and non-shared environment (E) influences on externalizing behavior, maternal punitive discipline and negative life events.
| −2LL (df) | χ2 (df) | P | AIC | BIC | A | C | E | |
|---|---|---|---|---|---|---|---|---|
| Externalizing behavior | ||||||||
| Saturated Model | 6056.552(2420) | 1216.552 | −5618.365 | |||||
| Full Sex-limitation | 6107.701(2452) | 51.149(32)* | 0.017 | 1203.701 | −5703.126 | M= 44 (07–57) F=66 (46–72) |
03 (00–32) 00 (00–16) |
54 (43–67) 34 (28–42) |
| Scalar sex-limitation | 6117.038(2455) | 60.552(35)* 9.337(3)# |
0.005 0.025 |
1207.038 | −5713.177 | 57 (42–62) | 00 (00–11) | 43 (38–50) |
| Drop A | 6152.258(2456) | 35.220(1)‡ | 0 | 1240.258 | −5699.140 | 0 | 35 (30–40) | 65 (60–70) |
| Drop C | 6117.038(2456) | 0 (1) ‡ | 0 | 1205.038 | −5716.750 | 57(50–62) | 0 | 43 (38–50) |
| MPD | ||||||||
| Saturated Model | 6166.894(2420) | 1326.894 | −5563.194 | |||||
| Full Sex-limitation | 6198.535(2452) | 31.641(32)* | 0.484 | 1294.535 | −5661.709 | 46(20–56) 06(00–35) |
00(00–22) 31(08–43) |
54(44–65) 63(52–72) |
| Scalar sex-limitation | 6203.173(2455) | 36.279(35)* 4.638(3) # |
0.409 0.200 |
1293.173 | −5670.109 | 29(09–48) | 13(00–27) | 58(51–66) |
| Drop A | 6211.053(2456) | 7.880(1)‡ | 0.005 | 1299.053 | −5669.742 | 0 | 32(27–37) | 68(63–73) |
| Drop C | 6206.005(2456) | 2.832(1) ‡ | 0.092 | 1294.005 | −5672.266 | 45(38–51) | 0 | 55(49–62) |
| PPD | ||||||||
| Saturated Model | 6056.151(2420) | 1216.151 | −5618.566 | |||||
| Full Sex-limitation | 6093.994(2452) | 37.843(32)* | 0.220 | 1189.994 | −5713.980 | 37 (08–65) 22 (00–47) |
21 (00–45) 29 (08–48) |
42 (33–52) 49 (40–60) |
| Scalar sex-limitation | 6095.278(2455) |
39.127(35)* 1.284(3)# |
0.290 0.733 |
1185.278 | −5724.057 | 30 (12–47) | 24 (11–37) | 46 (39–53) |
| Drop A | 6105.938(2456) | 10.661(1)‡ | 0.001 | 1193.938 | −5722.300 | 0 | 44 (39–48) | 56 (52–61) |
| Drop C | 6108.543(2456) | 13.266(1) ‡ | 0.000 | 1196.543 | −5720.997 | 59 (53–64) | 0 | 41 (36–47) |
| NLE | ||||||||
| Saturated Model | 6127.723(2420) | 1287.723 | −5582.780 | |||||
| Full Sex-limitation | 6166.684(2452) | 38.952(32)* | 0.185 | 1264.684 | −5677.635 | 47(12–58) 10(00–41) |
01(00–29) 26(02–40) |
52(42–64) 64(52–75) |
| Scalar sex-limitation | 6170.170(2455) | 42.447(35)* 3.486(3) # |
0.181 0.323 |
1260.170 | −5868.611 | 32(10–50) | 10 (00–25) | 58(50–67) |
| Drop A‡ | 6178.304(2456) | 8.134(1) ‡ | 0.004 | 1266.304 | −5686.117 | 0 | 30(25–35) | 70(65–75) |
| Drop C‡ | 6171.886(2456) | 1.717(1) ‡ | 0.190 | 1259.886 | −5689.326 | 45(38–52) | 0 | 55(48–62) |
−2ll: minus twice the log likelihood fit statistic, df: degrees of freedom; χ2: chi-square fit statistic for the comparative fit of the full with the saturated model, P: probability;
compared with the saturated model;
compared with the full model;
compared with the scalar model
Externalizing behavior correlated significantly with maternal punitive discipline (r=0.33, p<0.001; male=0.30; female=0.36), paternal punitive discipline (r=0.27, p<0.001; male=0.19; female=0.27), and negative life events (r=0.47, p<0.001; male=0.46; female=0.48). Parameters could be constrained across sex for all bivariate models without significant reduction in the fit of the model (externalizing behavior and MPD: Δχ2=11.067; d.f.=9; p=0.271; externalizing behavior and MPD: Δχ2=16.241; d.f.=9; p=0.062; externalizing behavior and NLE: Δχ2=10.164; d.f.=9; p=0.337), therefore, all results are reported on data collapsed across sex. Standardized path coefficient estimates for each of the bivariate genetic models are presented in Figure 2 The path coefficients were used to estimate the proportion of total covariance between MPD, PPD, or NLE and externalizing behavior due to genes, shared environment and non-shared environment by estimating each as a proportion of the total covariance. Thus, the covariance with MPD due to genes results from: genes (0.54*0.45) / total covariance (genes: 0.54*0.45) + (shared environment: 0.12*0.00) + (non-shared environment: 0.15*0.63), or 61% of the covariance. The shared environment contributes 1% of the phenotypic covariance and the remaining 28% is due to non-shared environmental factors. Finally, we calculated the genetic correlation (rA: the extent to which the two phenotypes share genetic variation), shared environmental correlation (rC), and non-shared environmental correlation (rE) to be 0.60 (95% CI=0.34–1.0), −1.0 (1.0–1.0) and 0.23 (0.14–0.32) respectively.
Figure 2.
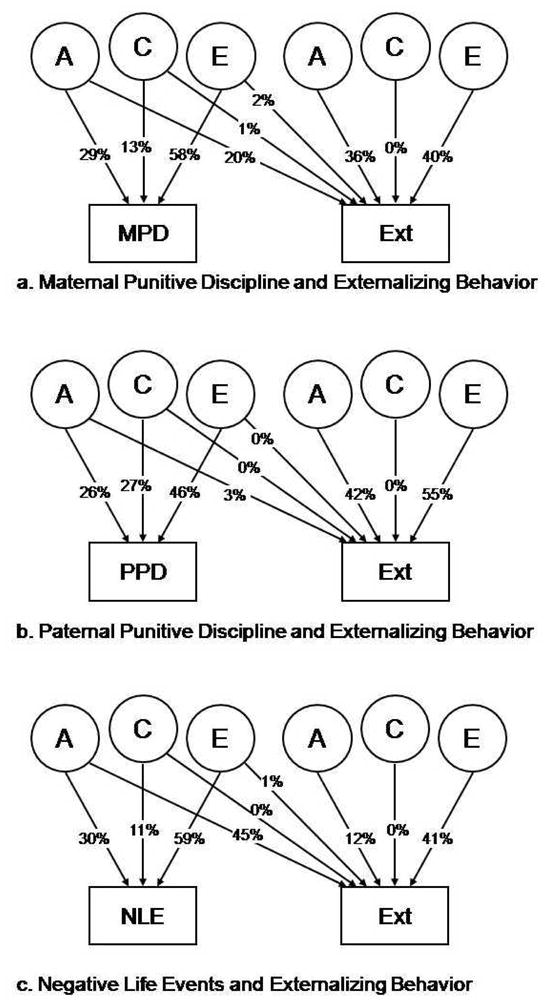
Fit for model a: −2LL=13878.130, d.f.=5112, χ2=43.954, d.f.=58, p=0.914. Fit for model b: −2LL=13543.363, d.f.=5112, χ2=52.719, d.f.=58, p=0.671. Parameter estimates for the Cholesky models. MPD: Maternal punitive discipline, Ext: externalizing behavior, NLE: negative life events
The covariance between PPD and externalizing behavior was almost entirely due to genetic covariation (98%), with a very small, non-significant, contribution (2%) from the non-shared environment. The genetic, shared and non-shared environmental correlations between externalizing behavior and PPD were 0.24 (−0.05–0.65), −1.0 (−1.0–1.0), and 0.00 (−0.11–0.10) respectively.
Finally, the covariance between NLE and externalizing behavior was also largely due to genetic covariation (77%), with negligible shared (7%) and moderate non-shared environmental (21%) influence. The genetic, shared and non-shared environmental correlations between externalizing behavior and negative life events were 0.88 (0.63–1.0), −1.0 (−1.0–1.0), and 0.19 (0.09–0.29) respectively.
Interaction effects
Figure 3 illustrates the changes in parameter estimates increased exposure to maternal punitive discipline (3a: full model, 3b: best fitting model), paternal punitive discipline (3c: full model, 3d:best fitting model), and negative life events (3e: full model, 3f: best fitting model). The graphs in Figure 3 show absolute levels of variance for A, C, and E, as suggested by Purcell (2000), not the proportion of relative phenotypic variance due to A, C, and E, and as such estimates for each do not depend on the size of the estimate for the other variance components. There was an interaction between MPD and genetic influences unique to externalizing behavior (βXU; χ2=4.64, d.f.=1, p=0.031; contact first author for fit statistics for dropping each pathway), and between MPD and both common (βZC; χ2=8.32, d.f.=1, p=0.004) and unique (βZU; χ2=37.13, d.f.=1, p=0.000) non-shared environment influences. Furthermore, there was an interaction between MPD and the shared environmental influences on externalizing behavior. It was not possible to ascertain whether this applied to the common shared environment (βYC) or the unique shared environment (βYU), as although each could be dropped individually it was not possible to drop both simultaneously (χ2=7.110, d.f.=2, p=0.029). For paternal punitive discipline moderation of both shared and unique genetic effects was significant; whilst, all other interactions were non-significant (χ2=3.390, d.f.=4, p=0.495).
Figure 3.
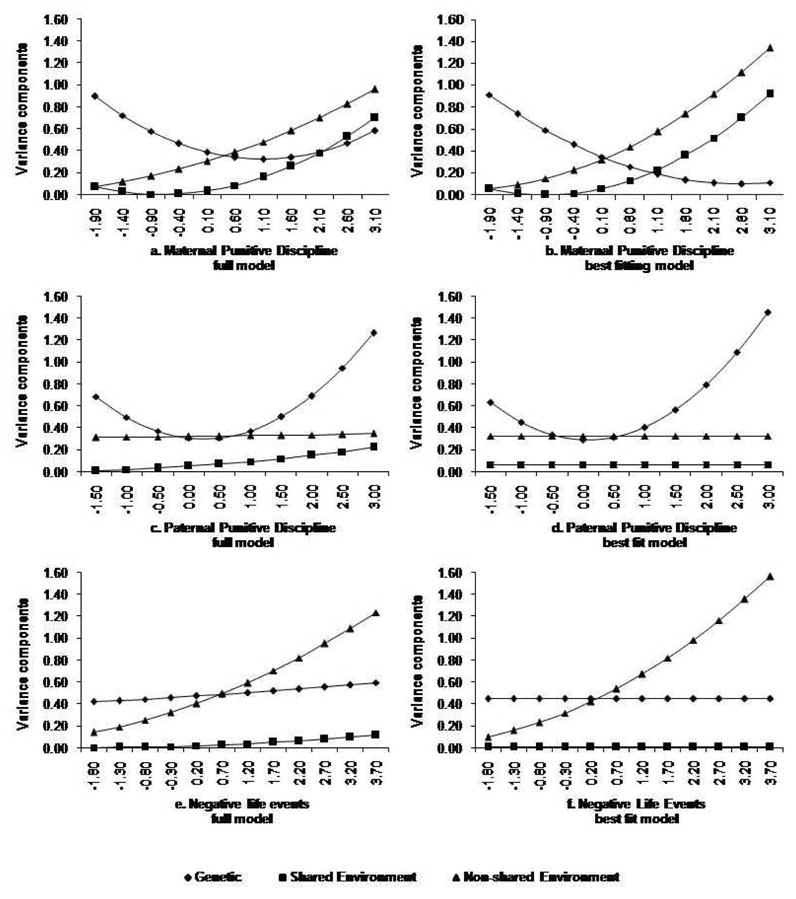
Estimates of genetic (A), shared environment (C) and non-shared environment (E) variance at different levels of maternal punitive discipline (a and b) and negative life events (c).
There was an interaction between NLE and the non-shared environment path unique to externalizing behavior (βZU: χ2=29.286, d.f.=1, p<0.001); all other interactions were non- significant (χ2=6.866, d.f.=5, p=0.231).
Given that the full siblings appeared to differ significantly from the DZ twin pairs significant on some socio-demographic and personal characteristics, inclusion of them in the current analyses may have biased the results in some way. Therefore, we conducted all of the described analyses with the twin sample only and found that results were comparable to those reported here for the complete dataset.
Discussion
Consistent with previous research we found that externalizing behavior was substantially heritable with a small, non-significant shared environment effect. Although a substantial heritability and non-significant shared environment effect for externalizing behavior is consistent with some studies that use CBCL measures (e.g. Eaves et al.1997), it is not consistent with results from all twin studies (e.g. Edelbrock et al.,1995). This may, in part, result from the use of self-report rather than parental report of externalizing behavior. Another contributing factor may have been the use of siblings of different ages rather than the sole use of twins, in which, if not controlled for, common age might act as shared environment effect. Finally, we used the total externalizing scale in these analyses, whereas many other genetic studies use aggressive and delinquent subtypes. Maternal and paternal punitive discipline, and negative life events were also heritable, with modest (though, in the case of maternal punitive discipline and negative life events, non-significant) shared environment contributions, and the remainder due to non-shared environmental variance.
Parental punitive discipline
MPD and PPD were significantly heritable indicating gene-environment correlation which may be passive (resulting from the parents providing both genes and the environment), or evocative, in which the externalizing child elicits harsh discipline from the parent (O'Connor et al., 1998). This is not to dismiss the possibility of direct environmental causality: 38% of the covariance of MPD with externalizing behavior was due to non-shared environmental influences, which could reflect direct causation of MPD on externalizing behavior. Although the genetic correlation between externalizing behavior and maternal punitive discipline was high there was genetic variance unique to each phenotype. This may in part explain why many adolescents demonstrate externalizing behavior irrespective of levels of MPD. However, genes appeared to account entirely for the correlation between PPD and externalizing across the entire population, indicating that the association was due to common genetic risk.
The common genetic influences were not moderated by exposure to MPD, indicating that externalizing behavior was genetically influenced regardless of MPD levels. However, the unique genetic effects were moderated, implying that some of the genetic risk is contextually dependent, even after controlling for rGE. Genes were more salient in the development of externalizing behavior in those who were exposed to low maternal punitive discipline. These results are consistent with the “social push perspective” (Raine, 2002), as genes were more salient in the low risk (low levels of MPD) environment. Furthermore, these results are in contrast to the bioecological model (Bronfenbrenner and Ceci, 1994) as the genetic risk in the current study appears to be greater in the environment least conducive to such behaviors (low levels of MPD), rather than in the environment most conducive to the behavior (high levels of MPD).
For PPD, the common and unique genetic risks for externalizing behavior were moderated by levels of exposure. In this example, genetic risk appears particularly salient in levels of higher exposure to paternal punitive discipline. This finding is consistent with the bioecological model as the genetic risk appears to be greater in the environment most conducive to such behaviors (high levels of PPD). The difference in moderating patterns of maternal and paternal punitive discipline on the genetic risk for externalizing behavior may be due to differences in the way adolescents react to mothers and fathers.
Negative Life Events
We also found evidence of gene-environment correlation for negative life events, and common genetic variance accounted for most of the covariation between NLE and externalizing behavior. This may be an example of active rGE; that is, people with an antisocial predisposition are more likely to seek out or elicit environments that increase their exposure to negative events. This account is consistent with results from previous analyses, demonstrating that aspects of adults’ personality explained genetic influence on controllable life events (Saudino et al., 1997). These findings are also consistent with studies that show how “risk taking” behavior contributes to the genetic variation of externalizing behavior (Krueger et al, 1994), since such behavior may increase the risk of exposure to negative life events.
These results provide evidence in support of our hypothesis that children’s externalizing characteristics increase their risk of exposure to negative life events. Although exposure did not moderate the extent to which genes play a role in externalizing behavior, it did moderate the contribution made by non-shared environmental influences, which increased as exposure to negative life events increased. As we had selected only those negative life events that were not necessarily shared by twins, NLE may potentially be acting in these twins as non-shared environmental effects, accounting for some of the covariation above that explained by common genetic effects. Consequently, as the NLEs become more prevalent they may be responsible for the apparent increased contribution of the non-shared environmental influences to the variance of externalizing behavior.
Although the absolute level of genetic risk remained unchanged in the rGE-GxE model, the proportion of the phenotypic variance attributable to the genetic risk did vary. Therefore, the increase in the variance of the non-shared environment, resulting from moderation of the NLE levels, will necessarily reduce the proportion of variance accounted for by genetic risk. This might be explained by the social push perspective (Raine, 2002) since the relative genetic risk decreases as the “environment” becomes more severe, despite the absolute level of genetic risk remaining constant.
Limitations
These findings should be considered in the context of a number of limitations. The age range of the participants is rather large (12 to 21), and encompasses a broad developmental range. Consequently, we might expect different etiological influences or mechanisms for the association between phenotypic outcomes and their correlates at different ages. At age 12, for example, adolescents with externalizing problems may have fewer opportunities to expose themselves to situations where negative events occur than older adolescents, and are also more likely to be reprimanded within the family. Although we did our best to account for age effects on the means of our measured variables, future research might benefit from looking at differences over age. Another limitation of this study was the exclusive use of self-report data, which may have led to an inflation of associations between measures. Furthermore, no paternity analyses have been performed in the G1219 study, and thus it is possible that some half-sibling pairs have been included as full pairs, which may have resulted in inflated estimates of genetic variances for each outcome. Furthermore, non-paternity might be associated with parenting style which could result in greater parenting problems in the full siblings than the identical or even DZ twins, for which shared paternity is highly likely. However, it is unclear what such an association might have on the reported results, and there is no evidence in the current study to suggest that full siblings receive more punitive discipline than DZ pairs.
Another concern of this study is that the variance of externalizing behavior for female MZ twin pairs was slightly, but significantly, lower than the variance for the other females in the sample, resulting in a bad fit of the full univariate externalizing behavior model. However, in large samples, there is often power to detect small differences that may not be meaningful. As the AIC and BIC were lower in the full model compared with the saturated model, it appeared that full model could not be rejected. Furthermore, the difference in variance for MZ female twin compared with the other female pairs may indicate that there are sibling interaction or competition effects for female externalizing behavior. However, it was not possible to test this explicitly in the current model.
Finally, the externalizing behavior scale and the negative life events were somewhat skewed; skewed data may mimic GxE effects (Purcell, 2002), although the effects are reduced when the moderator is also included in the model. Transforming data can remove true interaction effects (Falconer & Mackay, 1996). Given the sensitivity of GxE analyses to scale we opted, a priori, to use the scales in their regressed form, rather than transforming them. However, non-normal distribution of data is a possible limitation of the current study.
Although the results of this study show that variations in the levels of MPD and PPD are associated with changes in the genetic variance for externalizing behavior, we cannot directly compare these result with the adoption and molecular studies reported previously (Cadoret et al., 1995; Caspi et al., 2002). The previous GxE analyses assessed the interactive contribution of genetic and environmental risk to changes in mean levels of behavior, whereas the current analyses assess changes in the variance components of externalizing behavior as a function of MPD and PPD.
Despite these caveats, these results highlight the complex relationship between people and their surroundings in the development of behavior. We can no longer just assume that the environment influences the way people act ‘above and beyond’ genetic influences. Instead, exposure to certain environment may result from a genetic risk, and the magnitude of the genetic risk may in turn be dependent on the level of environment exposure.
Acknowledgments
Dr. Button is supported by a Medical Research Council studentship, and grant DA-011015 from the Institute for Behavioral Genetics, University of Colorado, Boulder, USA. Dr. Lau is supported by a Medical Research Council studentship, and the Mood and Anxiety Program, National Institute of Mental Health, National Institutes of Health, Bethesda, USA. Dr. Maughan is supported by the Medical Research Council, and the Medical Research Council grant G9901475. The G1219 study was supported by the W T Grant Foundation, the University of London Central Research fund and a Medical Research Council Training Fellowship and Career Development Award, which also supports Dr. Eley. We would like to thank the families for their participation, and Sally Cartwright, Georgina Hosang, Alessandra Iervolino, Holan Liang, Maria Napolitano, Robert Plomin, Richard Rowe, Pak Sham, Abram Sterne, Eileen Walsh and Richard Williamson for input to various stages of the project.
References
- Achenbach T. Manual for the Child Behavior Checklist: 4–18 and 1991 Profile. University of Vermont, Department of Psychiatry; Burlington, VT: 1991. [Google Scholar]
- Billig JP, Hershberger SL, Iacono WG, McGue M. Life events and personality in late adolescence: genetic and environmental relations. Behavior Genetics. 1996;26:543–554. doi: 10.1007/BF02361227. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U, Ceci SJ. Nature-nurture reconceptualized in developmental perspective: a bioecological model. Psychological Review. 1994;101:568–586. doi: 10.1037/0033-295x.101.4.568. [DOI] [PubMed] [Google Scholar]
- Button TMM, Scourfield J, Martin N, Purcell S, McGuffin P. Family Dysfunction Interacts with Genes in the Causation of Antisocial Symptoms. Behavior Genetics. 2005;35:115–120. doi: 10.1007/s10519-004-0826-y. [DOI] [PubMed] [Google Scholar]
- Cadoret RJ, Yates WR, Troughton E, Woodworth G, Stewart MA. Genetic-environmental interaction in the genesis of aggressivity and conduct disorders. Archives of General Psychiatry. 1995;52:916–924. doi: 10.1001/archpsyc.1995.03950230030006. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Champion LA, Goodall G, Rutter M. Behaviour problems in childhood and stressors in early adult life. I. A 20 year follow-up of London school children. Psychological Medicine. 1995;25:231–246. doi: 10.1017/s003329170003614x. [DOI] [PubMed] [Google Scholar]
- Cohen DJ, Dibble E, Grawe JM, Pollin W. Reliably separating identical from fraternal twins. Archives of General Psychiatry. 1975;32:1371–1375. doi: 10.1001/archpsyc.1975.01760290039004. [DOI] [PubMed] [Google Scholar]
- Eaves LJ, Silberg JL, Meyer JM, Maes HH, Simonoff E, Pickles A, et al. Genetics and developmental psychopathology: 2. The main effects of genes and environment on behavioral problems in the Virginia Twin Study of Adolescent Behavioral Development. Journal of Child Psychology and Psychiatry. 1997;38:965–980. doi: 10.1111/j.1469-7610.1997.tb01614.x. [DOI] [PubMed] [Google Scholar]
- Edelbrock C, Rende R, Plomin R, Thompson LA. A twin study of competence and problem behavior in childhood and early adolescence. Journal of Child Psychology and Psychiatry. 1995;36:775–785. doi: 10.1111/j.1469-7610.1995.tb01328.x. [DOI] [PubMed] [Google Scholar]
- Eley TC, Liang H, Plomin R, Sham P, Sterne A, Williamson R, Purcell S. Parental familial vulnerability, family environment, and their interactions as predictors of depressive symptoms in adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:298–306. doi: 10.1097/00004583-200403000-00011. [DOI] [PubMed] [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to Quantitative Genetics. 4. Longman; New York: 1996. [Google Scholar]
- Foley DL, Eaves LJ, Wormley B, Silberg JL, Maes HH, Kuhn J, Riley B. Childhood adversity, monoamine oxidase a genotype, and risk for conduct disorder. Archives of General Psychiatry. 2004;61:738–744. doi: 10.1001/archpsyc.61.7.738. [DOI] [PubMed] [Google Scholar]
- Ge X, Conger RD, Cadoret RJ, Neiderhiser JM, Yates WR, Troughton E, Stewart MA. The developmental interface between nature and nurture: A mutual influence model of child antisocial behavior and parent behaviors. Developmental Psychology. 1994;31:574–589. [Google Scholar]
- Hetherington EM, Clingempeel WG. Coping with marital transitions: A family systems perspective. Monographs of the Society for Research in Child Development. 1992;2–3(Serial No. 227) [Google Scholar]
- Kerr DC, Lopez NL, Olson SL, Sameroff AJ. Parental discipline and externalizing behavior problems in early childhood: the roles of moral regulation and child gender. Journal of Abnormal Child Psychology. 2004;32:369–383. doi: 10.1023/b:jacp.0000030291.72775.96. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Schmutte PS, Caspi A, Moffitt TE, Campbell K, Silva PA. Personality traits are linked to crime among men and women: evidence from a birth cohort. Journal of Abnormal Psychology. 1994;103:328–338. doi: 10.1037//0021-843x.103.2.328. [DOI] [PubMed] [Google Scholar]
- Meltzer H, Gatward R, Goodman R, Ford T. Mental health of children and adolescents in Great Britain. The Stationary Office; London: 2000. [DOI] [PubMed] [Google Scholar]
- Neale MC. Mx: Statistical modeling. 3. Box 980126 MCV; Richmond VA: 1997. [Google Scholar]
- Nix RL, Pinderhughes EE, Dodge KA, Bates JE, Pettit GS, Fadyen-Ketchum SA. The relation between mothers' hostile attribution tendencies and children's externalizing behavior problems: the mediating role of mothers' harsh discipline practices. Child Development. 1999;70:896–909. doi: 10.1111/1467-8624.00065. [DOI] [PubMed] [Google Scholar]
- O'Connor TG, Deater-Deckard K, Fulker D, Rutter M, Plomin R. Genotype-environment correlations in late childhood and early adolescence: antisocial behavioral problems and coercive parenting. Developmental Psychology. 1998;34:970–981. doi: 10.1037//0012-1649.34.5.970. [DOI] [PubMed] [Google Scholar]
- O'Connor TG, Dunn J, Jenkins JM, Pickering K, Rasbash J. Family settings and children's adjustment: differential adjustment within and across families. British Journal of Psychiatry. 2001;179:110–115. doi: 10.1192/bjp.179.2.110. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, Loehlin JC. Genotype-environment interaction and correlation in the analysis of human behavior. Psychological Medicine. 1977;84:309–322. [PubMed] [Google Scholar]
- Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Research. 2002;5:554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Purcell S, Koenen KC. Environmental mediation and the twin design. Behavior Genetics. 2005;35:491–498. doi: 10.1007/s10519-004-1484-9. [DOI] [PubMed] [Google Scholar]
- Raine A. Biosocial studies of antisocial and violent behavior in children and adults: A review. Journal of Abnormal Child Psychology. 2002;30:311–326. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Riggins-Caspers KM, Cadoret RJ, Knutson JF, Langbehn D. Biology-environment interaction and evocative biology-environment correlation: contributions of harsh discipline and parental psychopathology to problem adolescent behaviors. Behavior Genetics. 2003;33:205–220. doi: 10.1023/a:1023434206261. [DOI] [PubMed] [Google Scholar]
- Robins LN. Sturdy childhood predictors of adult antisocial behaviour: replications from longitudinal studies. Psychological Medicine. 1978;8:611–622. doi: 10.1017/s0033291700018821. [DOI] [PubMed] [Google Scholar]
- Rowe R, Maughan B, Eley TC. Links between antisocial behavior and depressed mood: The role of life events and attributional style. Journal of Abnormal Child Psychology. 2006;34:293–302. doi: 10.1007/s10802-006-9032-0. [DOI] [PubMed] [Google Scholar]
- Rutter M, Giller H, Hagell A. Antisocial behavior by young people. Cambridge University Press; New York: 1998. [Google Scholar]
- Rutter M, Silberg J. Gene-environment interplay in relation to emotional and behavioral disturbance. Annual Review of Psychology. 2002;53:463–490. doi: 10.1146/annurev.psych.53.100901.135223. [DOI] [PubMed] [Google Scholar]
- Saudino KJ, Pedersen NL, Lichtenstein P, McClearn GE, Plomin R. Can personality explain genetic influences on life events? Journal of Personality and Social Psychology. 1997;72:196–206. doi: 10.1037//0022-3514.72.1.196. [DOI] [PubMed] [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: a theory of genotype greater than environment effects. Child Development. 1983;54:424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Schmitz S, Fulker DW, Mrazek DA. Problem behavior in early and middle childhood: An initial behavior genetic analysis. Journal of Child Psychology and Psychiatry. 1995;36:1443–1458. doi: 10.1111/j.1469-7610.1995.tb01674.x. [DOI] [PubMed] [Google Scholar]
- Sham PC, Sterne A, Purcell S, Cherny SS, Webster M, Rijsdijk F, Asherson P, Ball D, Craig IW, Eley TC, Goldberg D, Gray J, Mann A, Owen M, Plomin R. GENESiS: creating a composite index of the vulnerability to anxiety and depression in a community-based sample of siblings. Twin Research. 2000;3:316–322. doi: 10.1375/136905200320565292. [DOI] [PubMed] [Google Scholar]
- Shanahan MJ, Hofer SM. Social context in gene-environment interactions: retrospect and prospect. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2005;60(Spec No 1):65–76. doi: 10.1093/geronb/60.special_issue_1.65. [DOI] [PubMed] [Google Scholar]
- Silberg JL, Erickson MT, Meyer JM, Eaves LJ, Rutter ML, Hewitt JK. The application of structural equation modeling to maternal ratings of twins' behavioral and emotional problems. Journal of Consulting and Clinical Psychology. 1994;62:510–521. [PubMed] [Google Scholar]
- SPSS Inc. SPSS for Windows, Release 12.0.2. Chicago, IL: 2004. [Google Scholar]
- Thapar A, McGuffin P. Genetic influences on life events in childhood. Psychological Medicine. 1996;26:813–820. doi: 10.1017/s0033291700037831. [DOI] [PubMed] [Google Scholar]
- Tuvblad C, Grann M, Lichtenstein P. Heritability for adolescent antisocial behavior differs with socioeconomic status: gene-environment interaction. Journal of Child Psychology and Psychiatry. 2005;47:734–743. doi: 10.1111/j.1469-7610.2005.01552.x. [DOI] [PubMed] [Google Scholar]
- Wade TD, Kendler KS. The genetic epidemiology of parental discipline. Psychological Medicine. 2000;30:1303–1313. doi: 10.1017/s0033291799003013. [DOI] [PubMed] [Google Scholar]
- Wiesner M, Windle M. Assessing covariates of adolescent delinquency trajectories: A latent growth mixture modeling approach. Journal of Youth and Adolescence. 2004;33:431–442. [Google Scholar]


