Abstract
To test current hypotheses on the contribution of the basal ganglia (BG) to motor control, we examined the effects of muscimol-induced inactivations in the skeletomotor region of the internal globus pallidus (sGPi) on visually-directed reaching. Injections were made in 2 monkeys trained to perform four out-and-back reaching movements in quick succession toward four randomly-selected target locations. Following sGPi inactivations: (1) Peak velocity and acceleration were decreased in nearly all sessions whereas movement duration lengthened inconsistently. (2) Reaction times were unaffected on average, although minor changes were observed in several individual sessions. (3) Outward reaches showed a substantial hypometria that correlated closely with bradykinesia, but directional accuracy was unaffected. (4) End-point accuracy was preserved for the slow visually-guided return movements. (5) No impairments were found in the rapid chaining of out-and-back movements, in the selection or initiation of four independent reaches in quick succession, or in the quick on-line correction of initially mis-directed reaches. (6) Inactivation-induced reductions in the magnitude of movement-related muscle activity (EMG) correlated with the severity of slowing and hypometria. There was no evidence for inactivation-induced alterations in the relative timing of EMG bursts, excessive co-contraction, or impaired suppression of antagonist EMG. Therefore, disconnecting the BG motor pathway consistently produced bradykinesia and hypometria, but seldom affected movement initiation time, feedback-mediated guidance, the capacity to produce iterative reaches, or the ability to abruptly reverse movement direction. These results are discussed with reference to the idea that the BG motor loop may regulate energetic expenditures during movement (i.e., movement “vigor”).
Keywords: Globus Pallidus, Effort, Error correction, Reaching, Muscimol
What does the basal ganglia (BG) contribute to the normal control of movement? Since the early work of Wilson (1914) and Vogt (1911), the BG has been thought to play important roles in the control of movement and posture. This inference was based primarily on the motor consequences of degenerative diseases affecting the BG, Parkinson's Disease (PD) being the most common. The pathology of these diseases consists, however, of networks of dysfunction, not only in the BG but also in cortical and subcortical structures (e.g. Berardelli et al. 2001; Braak and Braak 2000). Therefore, it is difficult to determine conclusively that a motor sign reflects impaired function of the BG per se, rather than dysfunction in other, possibly connected, brain areas (DeLong and Wichmann 2007). A complementary approach is to study the behavioral effects of lesioning specific territories of the BG. Of particular interest are the effects of lesions placed in the principal output nuclei of the BG (the internal segment of the globus pallidus, GPi, or substantia nigra reticulata, SNr). Such lesions can be conceived of as disconnecting the BG from the thalamocortical and brainstem structures normally influenced by BG outflow. This approach has been used extensively in non-human primates in the last three decades (Horak and Anderson 1984a; Hore and Vilis 1980; Inase et al. 1996; Kato and Kimura 1992; for review see Mink 1996; Mink and Thach 1991b; Wenger et al. 1999). Unfortunately, divergent results were often found. For instance, following inactivation of the GPi, a systematic tendency toward hypometria was reported in some experiments (Inase et al. 1996; Mink and Thach 1991b) but not in others (Horak and Anderson 1984a; Kato and Kimura 1992; Wenger et al. 1999). In the same vein, most studies found no effect of GPi inactivation on Reaction Time (RT) (Mink and Thach 1991b; Wenger et al. 1999; for reviews see Mink 1996 and Wichmann and DeLong 1996) while others did. In particular, Kato and Kimura reported a quasi-systematic increase of the RT for extension movements (Kato and Kimura 1992). Likewise, Inase et al. found that "the RT could increase, decrease or show a mixed change depending on the target direction" (Inase et al. 1996, p. 1093). Horak and Anderson also reported a significant decrease in RT 30 minutes after the injection of kainic acid within the GPi (Horak and Anderson 1984a). Although confusing, the variability observed among and within inactivation studies is not surprising given current understanding that output from the BG is organized into anatomically-segregated functionally-distinct territories that contribute to motor, associative, and limbic functions (Grabli et al. 2004; Middleton and Strick 2000). When inactivated or lesioned, these different territories are likely to induce different deficits.
In the present study, we used microinjection of muscimol, a GABA-A receptor agonist, to inactivate restricted regions of the motor territory of GPi. This territory, here designated skeletomotor (sGPi), is the main output of the BG loop that projects via thalamus to frontal motor regions (Hoover and Strick 1999). It is confined to the posterior-ventrolateral two-thirds of the nucleus where the discharge of a large proportion of the neurons is correlated with movement of one or another contralateral body segment (Anderson and Horak 1985; DeLong et al. 1985; Mink and Thach 1991a; Turner and Anderson 1997). Injections were coupled with a reaching task that required out-and-back movements to four visual targets presented in quick succession and random order. The task was designed to test three current hypotheses concerning the functional roles of the sGPi. First, converging evidence suggests the BG may contribute to the regulation of movement effort (i.e., controlling how much energy or “vigor” is devoted to individual movements; Horak and Anderson 1984b; Mazzoni et al. 2007; Niv et al. 2007; Pope et al. 2005; Turner et al. 2003b; Vaillancourt et al. 2004a). This predicts that sGPi inactivation will impair the control of movement speed and extent, while not affecting aspects of movement that are relatively energy-neutral such as RT or the direction of movement (Vindras et al. 2005; Vindras and Viviani 2002). Second, the BG may contribute to on-line feedback control of movement trajectory (Smith et al. 2000; Smith and Shadmehr 2005). Interruption of such a feedback mechanism would be expected to cause movement variability to increase progressively during movement because of the accumulation of uncorrected execution-related errors (Desmurget and Grafton 2003; Desmurget et al. 2005). Third, the BG may be important for rapidly alternating the action of agonist-antagonist muscles groups (Beuter et al. 1994, Hermsdorfer et al. 1999) and for generating discrete movements in quick succession (Houk 2001, Novak et al. 2002, Desmurget et al. 2004a). This hypothesis predicts that sGPi inactivation should differentially impair the rapid execution of out-and-back movements and the iterative production of independent motor responses.
MATERIALS AND METHODS
Animals and apparatus
Two monkeys (Macaca mulatta) were involved in this study, one male (monkey H, ∼12 kg) and one female (monkey C, ∼7.5 kg). The experimental protocol was approved by the UCSF Animal Care and Use Committee. Animals were cared for in accordance with the American Physiological Society Guiding Principles in the Care and Use of Animals (1991).
Animals were seated in a primate chair with the right hand holding a vertically oriented joystick (40 cm axis of rotation). The joystick was held at midline at the height of the mid-sternum thereby realizing bi-dimensional, quasi-horizontal movements of the right hand and arm. Voltages reflecting x and y position of the joystick were digitized at 1 kHz. A vertical LCD monitor (60 Hz refresh frequency) was placed in front of the monkeys, at eye level. A 3 mm white square cursor represented the position of the joystick with a gain of 2 (i.e. a 1 cm displacement of the joystick was represented as a 2 cm displacement of the cursor). Sagittal joystick movements moved the cursor along the monitor's vertical dimension while fronto-parallel joystick movements moved the cursor along the horizontal dimension. Five empty gray circles (Ø 40 mm), used as target zones, were displayed continuously on the LCD monitor: one central start position and four peripheral target zones positioned in the diagonal of each quadrant at a radius of 140 mm (Fig. 1A). Therefore, due to the gain of 2, a joystick displacement of 70 mm was required to move the cursor center-to-center from the central target to any of the peripheral targets. Both animals achieved these joystick displacements through combinations of shoulder and elbow rotations. Note that injection-induced hypometria was sometimes too large to allow reliable capture of the peripheral visual targets. To avoid rejecting a substantial fraction of trials and because these hypometric trials were 'representative' of the injection effect, the behavior control software was adjusted to accept movements that ended within an "end-point target zone" that was larger than the visual target zone (Ø 80 mm instead of Ø 40; which allowed an additional 20 mm hypometria). In this case, movements were accepted even if the visual cursor did not enter the visual target zone. This was done in 8 experimental sessions (4 in monkey H -a,b,e,c2-, 4 in monkey C -A,B,D,G-). The size of the target zone was always consistent pre- and post-injection.
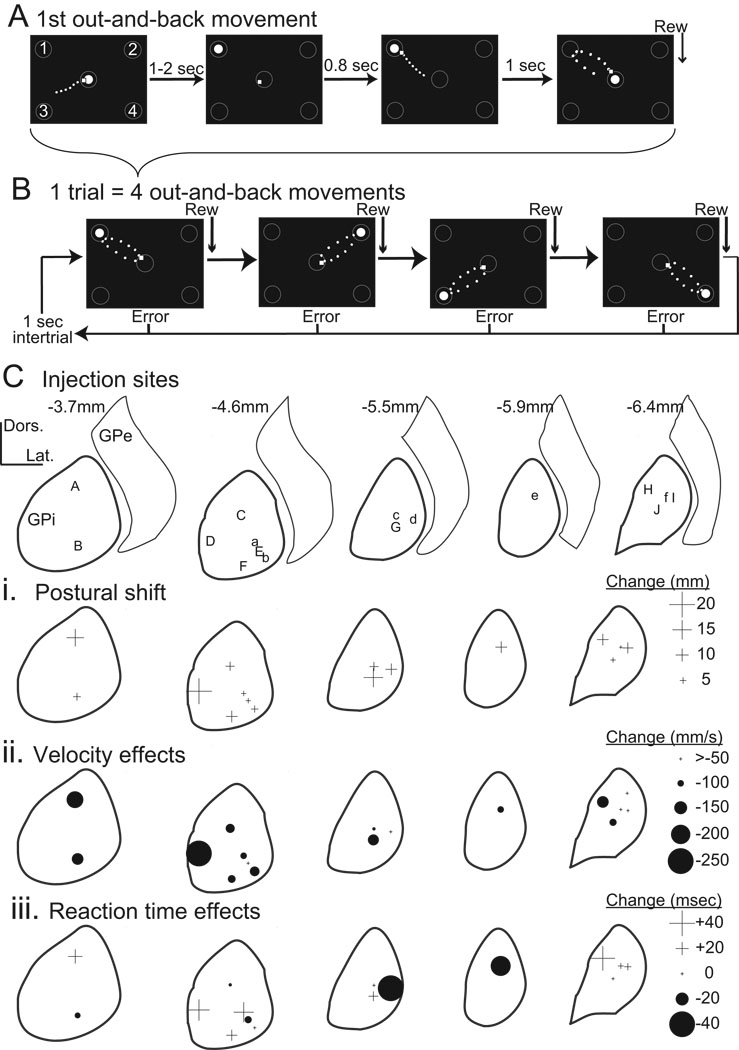
Figure 1.
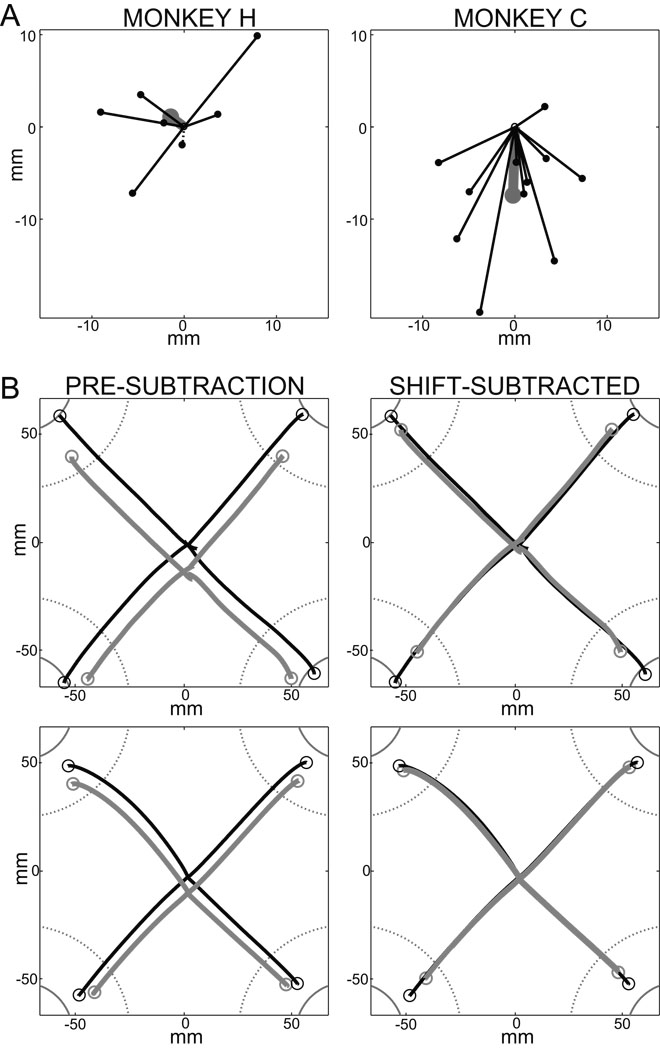
(A–B) Schematic representation of the behavioral task and apparatus. Each successful out-and-back movement was followed by a food reward (Rew). Each "trial" was composed of four-such out-and-back movements (B). Numbers 1 to 4 in the first panel of A define target numbers referred to in text. Open circles: target locations. White filled circle: instruction cue. Small white square: joystick-controlled cursor. Movements of the cursor are denoted by a dotted trail. (C) Sites of individual injections reconstructed from histology in monkeys H (lower-case letters), and C (capital letters). Letters at sites of individual injections are also listed in Table 1 and Fig. 3. Distances indicate the estimated position of the coronal section relative to the anterior commissure. The nuclear boundaries shown were derived as line drawings from a standard atlas (Szabo and Cowan, 1984) subsequently warped to align with nuclear boundaries reconstructed from histologic sections from both animals. (Ci-iii) Plots illustrate the magnitude of effects on initial posture (i), movement speed (ii), and reaction times (iii) for each injection location. Symbol sizes indicate effect magnitude (see scales at right of each row). Increases and decreases are denoted by crosses (+) and filled circles (•), respectively. Anatomical scales = 2 mm in both dorso-ventral (Dors.) and medio-lateral (Lat.) dimensions.
Behavioral task
The task required an animal to perform four out-and-back movements in quick succession between the central start position and a series of four peripheral targets (Fig. 1B). All of the results reported here address a task condition in which peripheral targets were presented in random order with replacement (i.e., on any one trial, any target could appear between zero and four times).
A single trial progressed as follows: (1) At the start of a trial, a filled white circle ("instruction cue", 23.5 mm diameter) appeared within the start position (gray circle), instructing the animal to move the cursor into this area (Fig. 1A). The animal was required to maintain the cursor in this area for a random period of 1 to 2 sec. (2) The filled white instruction cue jumped to one of the four possible target zones instructing the animal to initiate a movement to this zone. The target zone was randomly selected from the four possible locations. (3) The animal was allowed 800 ms to place the cursor in the target zone (i.e. in the empty gray target circle). (4) Immediately upon completion of the outward reach, the instruction cue jumped back to the central start position prompting the animal to return the cursor to this area. (5) A bolus of food was delivered when the cursor entered the start position. Stages 2–5 of the task were repeated four times in quick succession. For the second, third and fourth out-and-back movements the instruction cue jumped to a peripheral target 230 ms after reward delivery for the previous movement. Note that there was a delay between the instant when the cursor entered the start position and the actual termination of the return movement. On average, this delay was ∼150 ms, such that the instruction cue jumped to the next peripheral target ∼80 ms before completion of the previous out-and-back movement (see results). When the animal’s task performance did not meet the timing or spatial requirements of the task, the trial was aborted and a 1 sec inter-trial interval ensued without reward. Note that the number of movements was not strictly identical in all experimental sessions. It varied from 75 to 296 in monkey C (mean 173) and from 166 to 428 in monkey H (mean 252).
For each experiment session, pre- and post-injection data were recorded on the same day. Pre-injection data were collected first. Task performance and post-injection data collection commenced immediately after completion of the injection procedure. Both animals practiced the task a minimum of 6 months (>25,000 trials) prior to collection of the results presented here.
Surgery, electrophysiological mapping and microinjections
Animals were prepared for microinjections using aseptic technique under Isoflurane anesthesia. A cylindrical titanium recording chamber (18 mm ID) was affixed to the skull over a craniotomy in stereotaxic coordinates (Szabo and Cowan 1984) to allow trans-dural access to the left globus pallidus from a 45 deg lateral approach (for detailed description of surgical techniques, see Turner and Anderson 1997). The chamber was fixed to the skull with bone screws and dental acrylic. Bolts embedded in the acrylic allowed fixation of the head. In one animal (monkey C), pairs of fine Teflon-insulated multistranded stainless steel wires were implanted during surgery into five muscles: brachioradialis, triceps longus brachii, pectoralis, posterior deltoid, and trapezius. The wires were led subcutaneously to a connector fixed to the skull implant. Accurate placement of EMG electrodes was verified post-surgically by: (1) determining that each electrode pair provided independent EMG-like signals (thereby ruling out cross-talk); (2) observing palpable contraction of the appropriate muscle when electrical stimulation was applied to each electrode. Following surgery, animals were given prophylactic antibiotics and analgesic medication.
After recovery from surgery, animals resumed behavioral testing. During the performance of the behavioral task a single glass coated platinum/iridium electrode (FHC Bowden USA) piloted by a hydraulic microdrive (MO-95, Narishige International Japan) was used to map the boundaries of the GPi. Electrophysiological data were acquired, displayed and sorted on-line (Plexon Instruments, Dallas TX, USA). Pallidal skeletomotor territories were delineated based on anatomical boundaries and responses to active and passive movements of contralateral joints (DeLong 1971; Turner and Anderson 1997). EMG signals were differentially amplified (gain = 10k) and band pass filtered (200 Hz – 5k Hz).
Following identification of the pallidal borders, microinjection experiments were performed. Microinjections used the GABAA agonist muscimol hydrobromide (Sigma, St. Louis, MO) dissolved in artificial cerebrospinal fluid at a concentration of 1µg/µl. Injections were performed through one of two "injectrode" designs: either a silica tube fixed with cyanoacrylate glue to the side of a standard recording electrode (Kliem and Wichmann 2004) or a 30 gauge stainless steel cannula containing an in-dwelling tungsten microwire (50 micron dia., AM Systems; (Hamada and DeLong 1992). Injections were delivered by means of a 10µl Hamilton syringe attached to the silica or stainless steel tubing via Teflon tubing. Injection volumes ranged from 0.5µl to 2.0µl (see Table 1). The muscimol was injected at a rate of 0.2µl/min and the injection cannula was left in place throughout post-injection data collection. By recording single- or multi-unit neuronal activity near the site of injection we confirmed that unit activity was reduced immediately following the muscimol infusion (data not shown). At the completion of post-injection data collection, the cannula was withdrawn from the brain and the microdrive was removed from the cranial chamber. Patency of the injection system was then verified by looking for a small droplet of injectate at the cannula tip when additional injectate was expressed from the Hamilton syringe.
Table 1.
Injection sessions that met inclusion criteria for formal statistical analysis.
| MONKEY | Location (see Figure 1C) |
Injection | Volume | Distance from anterior commissure |
|---|---|---|---|---|
| H | a | muscimol | 0.5µg / 0.5µl | −4.6 |
| H | b | muscimol | 1µg / 1µl) | −4.6 |
| H | c1 | muscimol | 0.5µg / 0.5µl | −5.5 |
| H | c2 | muscimol | 0.5µg / 0.5µl | −5.5 |
| H | d | muscimol | 2µg / 2µl | −5.5 |
| H | e | muscimol | 2.5µg / 2.5µl | −5.9 |
| H | f | muscimol | 0.5µg / 0.5µl | −6.4 |
| C | A | muscimol | 0.5µg / 0.5µl | −3.7 |
| C | B | muscimol | 0.5µg / 0.5µl | −3.7 |
| C | C | muscimol | 0.5µg / 0.5µl | −4.6 |
| C | D | muscimol | 0.5µg / 0.5µl | −4.6 |
| C | E1 | muscimol | 0.5µg / 0.5µl | −4.6 |
| C | E2 | muscimol | 0.5µg / 0.5µl | −4.6 |
| C | F | muscimol | 0.5µg / 0.5µl | −4.6 |
| C | G | muscimol | 0.6µg / 0.6µl | −5.5 |
| C | H | muscimol | 0.5µg / 0.5µl | −6.4 |
| C | I | muscimol | 0.5µg / 0.5µl | −6.4 |
| C | J | muscimol | 0.5µg / 0.5µl | −6.4 |
| C | E3 | Artificial CSF | 0.5 µl | −4.6 |
Subscripts denote repeated injections at one location.
Histology
The methods used to reconstruct microinjection locations have been described in detail previously (Turner and Anderson 1997). Briefly, animals were killed by transcardial perfusion (saline followed by 10% phosphate-buffered formalin). The brains were blocked in place in the stereotaxic coronal plane, removed, fixed in formalin, cryoprotected with sucrose, frozen, cut into 50 µm sections, and stained with cresyl violet. The approximate location of each injection site was estimated by comparing the location of a penetration in the chamber, the position of injection sites along a penetration relative to electrophysiologically-identified borders, and the position of marking lesions made in the same and/or adjacent penetrations during final recording sessions.
Data analyses
All analog signals and event times were digitized online with 1 msec temporal resolution. EMG signals were full-wave rectified and low-pass filtered (500 Hz low-pass two-pole Sallen-Key filter; AD637, Analog Devices Inc.) before digitizing. Following digitization, x and y position signals from the joystick were filtered at 10 Hz with a finite impulse response dual pass filter using 33 coefficients. Movement velocity was computed from the filtered position signal using a two-points central difference derivative algorithm (Bahill and McDonald 1983). The same method was used to compute the hand's acceleration from the velocity signal. The onset and the end of the movements were computed automatically as the instant when the hand velocity reached 30 mm/sec. The results of this procedure were checked off-line and corrected, if necessary.
The characteristics of each movement (outward and return) were analyzed. Several parameters were computed including: Reaction Time (RT), Movement Duration (MD), movement Peak Acceleration (PA), Peak Velocity (PV), symmetry (SYM) and shape indexes (SHA) of the velocity profile, movement path curvature (PC), movement initial direction (DIR), hand starting location (SL), the mean movement error (SE, i.e., constant error), the spatial variability (i.e., variable error) at movement onset (IVAR) and movement end (EVAR). Measures of the shape of velocity profiles (i.e., SYM and SHA) provided information about the involvement of visual feedback in movement execution (Desmurget et al. 2005; Jeannerod 1988; Milner and Ijaz 1990). SYM was determined by computing the relative time to peak velocity (TPV/MD * 100). Perfectly symmetric profiles have a symmetry value of 50%. When the acceleration phase is shorter than the deceleration phase, the symmetry value is smaller than 50%. When the acceleration phase is longer than the deceleration phase, the symmetry value is higher than 50%. SHA was estimated by dividing the peak velocity by the average velocity of the movement. If two profiles have the same shape, this ratio is constant (Desmurget et al. 2005; Soechting 1984). Measures of hand path curvature (i.e., PC) provided additional information about the involvement of online error corrective mechanisms. PC was defined as the ratio of the largest deviation of arm trajectory from the line connecting the start and end points of the movement to the length of this line (Atkeson and Hollerbach 1985). Convex curvatures (angle between the movement line and the line joining the starting point to the point of largest deviation between 0 and π/2) were associated with positive numbers and concave curvatures (angle between 0 and −π/2) with negative numbers.
A variety of measures of movement accuracy were computed. DIR, initial movement direction, was defined as the orientation of the tangential velocity vector at the time to peak acceleration. SL, start location, was defined as the x, y coordinates of the hand, at rest, immediately prior to movement onset. SE, mean systematic error in movement, represented the mean error vector between positions of the hand and target at the time of movement termination. An hypothesis-free approach defined SE in Cartesian coordinates (SEx, SEy). Secondary analyses designed to test effects on control of movement extent defined SE in a polar frame of reference and decomposed this parameter into amplitude and direction errors (SEamp, SEdir). Amplitude errors were defined as the difference between the actual movement amplitude (norm of the vector joining the starting point to the movement end-point) and the required movement amplitude (norm of the vector joining the starting point to the target). Direction errors were defined in the same way as the angular difference between the actual movement direction (eccentricity of the movement end-point) and the required movement direction (eccentricity of the target). IVAR and EVAR, variable error at onset and end of movement, were represented by the surface of the 95% confidence ellipse of the starting and end-point distributions; the lengths of the axes of this ellipse are the square roots of the eigenvalues of the variance-covariance matrix of the end-point distribution scaled to contain 95% of the theoretical end-point population (Johnson and Wichern 1982).
Data from an experiment session were included in the formal statistical analyses described below only if the following criteria were met: (1) successful infusion of muscimol was confirmed by observing a cessation of pallidal activity near the cannula tip and the post-infusion patency test was positive; and (2) more than 6 valid movements were collected for each target both pre- and post-injection. Between-by-within subjects ANOVA and MANOVA designs were used to determine significant differences between experimental conditions for movement variables across all valid injection sessions. MANOVA designs were used only for 2-dimensional measures such as SL and SE, and for these, the F value was determined from the Wilk's lambda, using the Rao's approximation (Maxwell and Delaney 1990). A first set of analyses was conducted to identify the general features of the control movements [between factor, monkeys (2 levels: H and C); within factors: movement direction (2 levels: outward, return) and target position (4 levels: T1, T2, T3, T4)]. A second set of analyses was conducted to determine the influence of sGPi inactivation on the characteristics of the movement. These analyses were carried out independently for the outward and return movements [between factor, monkeys; within factors: injection (2 levels: pre-injection, post-injection) and target position]. A last set of analyses was conducted to determine the influence of sGPi inactivation on the ability to chain successive movements. In this case, the kinematic parameters were not averaged by target, but by ordinal position of the movement [between factor, monkeys; within factors: injection and rank (4 levels: movement 1, movement 2, movement 3, movement 4)]. For all these analyses, the different injection sessions were used as repetitions. The Tukey significant difference test was used for post-hoc comparisons of the means (Winer 1971). Since there is no unambiguous choice for an appropriate error term for post-hoc comparisons involving between by within-factor interactions (Winer et al. 1991), post-hoc evaluations involving the monkey factor were conducted using independent ANOVA (or MANOVA) for each animal (Maxwell and Delaney 1990). Note that the effects of the injection factor on the angular parameters (DIR, SEdir) was evaluated with regular statistics rather than circular statistics. This approach was valid in the context of the present study considering that these effects were well within the 90° range for which the real angular mean and the real angular standard-deviation equal the arithmetic mean and the standard-deviation (Batschelet 1965). To avoid ambiguity it should be mentioned that the effects involving the injection factor (main effect and interaction) remained strictly unchanged when the movement direction (which varies within a 360 deg range) was normalized by rotating the individual end points according to the target angle (e.g. T1: − 45 deg). For all the above analyses, the threshold for statistical significance was set at 0.05.
Additionally, within session investigations were carried out using a two-way ANOVA design for each experimental session with injection and target as the factors. Further analyses were also conducted to determine whether individual session-by-session variations were significant at the sample-level. The logic of the sample-level analysis was as follows. If the manipulation of interest had no effect (null hypothesis), then each Fisher test is supposed to be drawn from an F distribution with n1 and n2 degrees of freedom. The two-sample Kolmogorov-Smirnov (KS) goodness-of fit test uses the cumulative probabilities to assess whether two samples have been drawn from populations with the same theoretical distribution (Siegel 1956). Thus, if the null hypothesis holds, then this test should find that the measured F is drawn from the same distribution as a sample obtained by performing many (>10000) random draws of every F(df1, df2) of our observed F distribution (as already mentioned, the number of movements, and hence the degrees of freedom were not strictly identical in all sessions). The null hypothesis is rejected if the KS test finds that the 2 samples are not drawn from the same distribution (Desmurget et al. 2000; Vindras et al. 2005). Because the sample size was relatively small (monkey H, n = 7; monkey C, n=11) the statistical threshold was set at p < 0.01 to avoid false positive inferences (Vindras et al. 2005). A p < 0.01 threshold was also used for the individual within-session analyses to compensate for multiple comparisons (n = 18; Keppel 1973).
For the sake of clarity, statistical results associated with the ANOVA and MANOVA will be presented using a standard convention: (p value, variable; factors, F value). Abbreviations for the variable of interest are stated in the paragraphs above. The main factors will be designated as follows: Monkey (M; 2 levels: C, H), Target (T; 4 levels: T1, T2, T3, T4), Injection (I; 2 levels: Control and Muscimol), Rank (R; 4 levels: R1, R2, R3, R4), movement Direction (D; 2 levels: Outward, Return). A monkey by injection by target design will thus be noted: M×I×T. The factor(s) associated with the reported F values will be underlined. For instance M×I×T, F(1,16) = 2.36 will define the F value associated with the main effect of the injection factor while M×I×T, F(2,15) = 4.25 will refer to the monkey by injection interaction term.
Electromyographic (EMG) data were analyzed using methods similar to those described previously (Turner et al. 1995). For each muscle, peri-movement means were constructed of the digitized EMG signals for all valid movements to each target. The averages were aligned on the onset of outward movement and separate averages were constructed for trials performed pre- and post-injection. The EMG data were analyzed quantitatively as follows: (1) A muscle’s “preferred” and “anti-preferred” directions were identified as the target directions associated with maximal and minimal peri-movement EMG in the interval −100 to 100 msec around movement onset. (2) Epochs for measuring preferred, anti-preferred, and baseline EMG were defined manually by inspection of peri-movement means. Steps (1) and (2) were performed using grand means of each muscle’s EMG across all pre-injection recording sessions. (3) Values were obtained for a muscle’s mean preferred, anti-preferred, and baseline EMG for individual sessions pre- and post-injection (EMGpre and EMGpost, respectively). Means for baseline EMG were averaged across target directions. (4) Injection-induced changes in EMG for individual sessions were computed as 100*(EMGpost/ EMGpre)−100.
RESULTS
A total of 18 muscimol injection sessions met the criteria for inclusion in formal statistical analysis, at 16 sites in the sGPi of two monkeys (monkey H: n = 7; monkey C: n = 11; Table 1). An additional control injection of artificial CSF was made in the same structure in monkey C. All injections sites were in the skeletomotor portion of sGPi at locations where previous electrophysiological mapping demonstrated peri-movement changes in activity. The volumes and doses of each of these injections are shown in Table 1. The locations of all injections are summarized in Figure 1C.
Task performance: general features pre-injection
As shown in Figure 2 (left top), outward movements followed roughly straight hand paths with a slight bias toward a concave path curvature (PC; monkey H: −0.023, C: −0.020). They also presented nearly bell-shaped velocity profiles, even though the acceleration phase lasted slightly longer than the deceleration phase (SYM; monkey H: 0.56; C: 0.55). Movements had short durations (MD; H: 305 ms; C: 234 ms) and relatively high velocity peaks (PV; H: 426 mm/sec; C: 594 mm/sec). A substantial undershoot was observed for all targets, probably because entry of the cursor into even the proximal rim of the target circle was considered a successful target capture (undershoot; H: 29.3 mm; C: 30.8 mm).
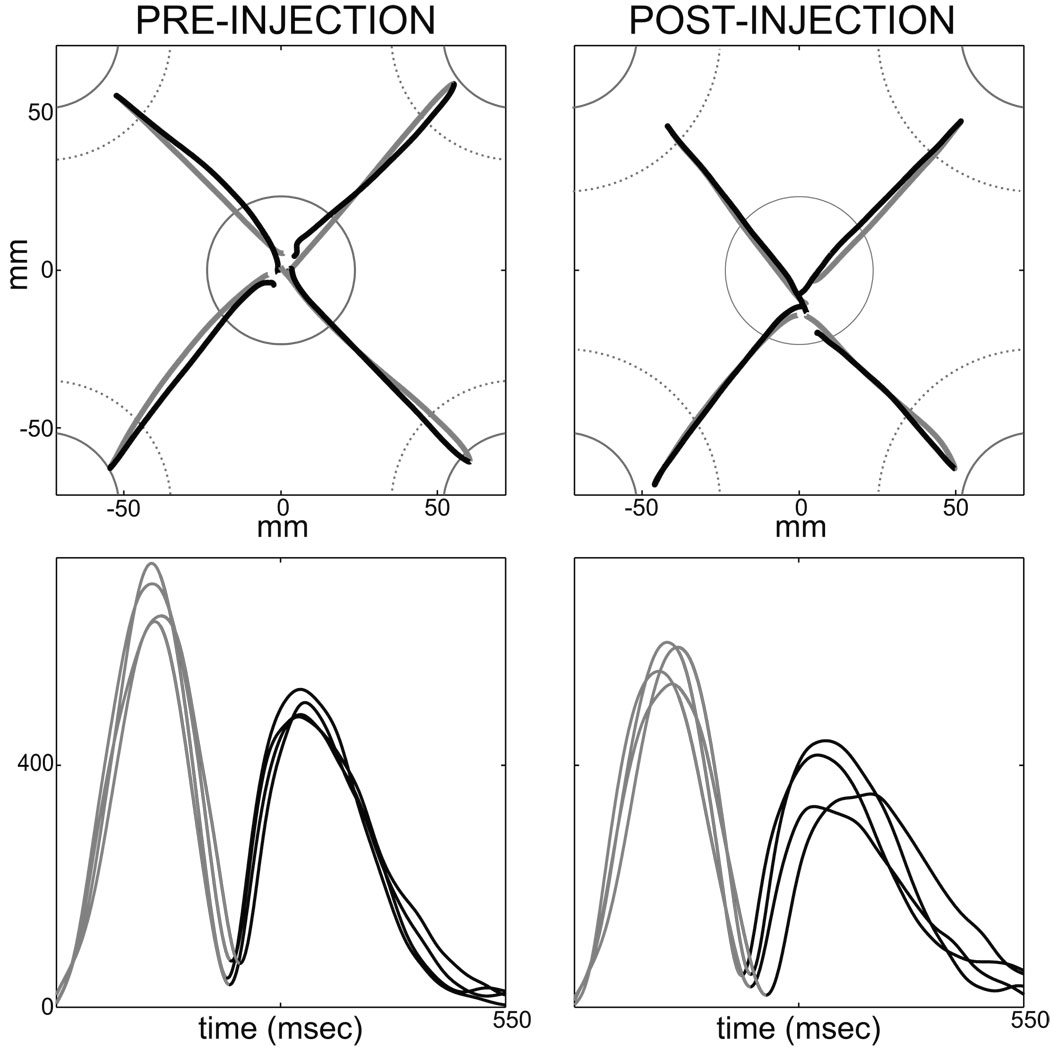
Figure 2.
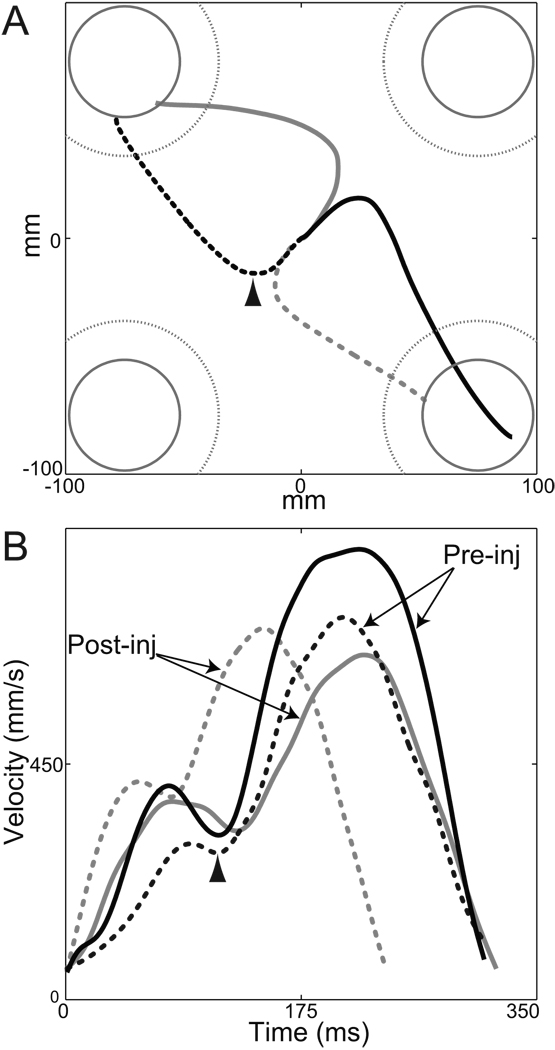
Representative pre- (left column) and post- (right column) injection movements performed by monkey C toward the 4 targets. (Movements were selected from session “G”.) The top and bottom rows show hand paths and tangential velocities, respectively. Outward movements are displayed in gray and return movements in black. For the top row, continuous gray circle (center) and arcs (peripheral targets in each corner) indicate positions of the visible targets. Dotted gray arcs indicate the peripheral target zones for cursor movements, which were larger than the visible targets to allow continued task performance following sGPi inactivation. To improve visibility of individual trajectories, the figure is scaled to show only the central region of the workspace.
As can be seen on the velocity profiles displayed in Figure 2 (left bottom), outward and return movements unfolded in quick succession. The latency was extremely short between the end of an outward reach and the beginning of the subsequent return movement (means H: 16 ms; C: 20 ms). In comparison, reaction times for outward movements were longer than 190 ms (RT; H: 191 ms; C: 194 ms). This suggests strongly that return movements were not planned after completion of the outward displacement. The short outward-to-return inter-movement delay is compatible with the conclusion that the two movements were "pre-planned" as a two-stroke out-and-back response.
A variety of observations suggested that return movements involved greater online error correction compared with the relative open-loop nature of outward movements. For both animals, return movements were distinguished by: (1) larger path curvatures that reversed toward convexity (see Table 2 and supplementary Table 3 for statistics); (2) longer durations and lower peaks velocities; (3) asymmetric velocity profiles with significantly longer deceleration phases; (4) improved end-point accuracy. (All above comparisons were significant at p < 0.001. Details for statistics are presented in supplementary Table 3.) Reinforcing the evidence for improved accuracy, only 22% of outward movements entered the visually-salient white filled instruction cue (though they did enter the larger diameter target zone). In contrast, 87% of return movements entered the central instruction cue. This suggests that, during return movements, animals actively guided the hand into the visually-salient cue in order to maximize accuracy and hence the probability of reward. The inference that return movements engaged visual feedback is consistent with the lower peak velocities, increased movement duration, and lengthened deceleration phase for these movements (Desmurget et al. 2005; Jeannerod 1988; Milner and Ijaz 1990). The greater involvement of feedback control in return movements is used below to test the hypothesized role of the BG motor circuit in feedback control mechanisms. The next three sections, however, focus on outward movements.
Table 2.
Mean measures of task performance pre- and post-injection. Separate means are shown for pre- and post-injection trials across 18 inactivations in two animals. Results are presented separately for Outward movements (to capture the peripheral target) and Return movements (to capture the central target and obtain reward). See supplemental Tables 3 and 4 for detailed statistical results.
| Mov’t | Period Re injection |
PV (mm/s) |
PA (mm/s2) |
MD (msec) |
SYM (%) |
SHA | PC |
RT/Rev (msec) |
SEamp (mm) |
SEdir (deg) |
|---|---|---|---|---|---|---|---|---|---|---|
| Outward | Pre- | 510 | 6918 | 269 | 0.55 | 1.67 | −.021 | 193 | −30.1 | 1.9 |
| Post- | 440* | 6030* | 281* | 0.54 | 1.67 | −.024 | 196 | −36.9* | 2.8 | |
| Return | Pre- | 355† | 7461† | 376† | 0.36† | 1.68 | .031† | 27.2† | −3.5† | 0.1 |
| Post- | 305* | 6458* | 388* | 0.36 | 1.64 | .018* | 35.2 | −3.2 | −0.1 |
p < 0.05 significance comparing pre- versus post-injection restricted to Outward or Return movements (3-way ANOVA Monkey×Injection×Target; F(1,16)>4.49).
p <0.05 significance comparing Outward versus Return movements (3-way ANOVA Monkey×Direction×Target; F(1,16)>4.49).
Other abbreviations: Mov’t: movement; PV: peak velocity; PA: peak acceleration; MD: movement duration; SYM: symmetry of velocity profile; SHA: shape of velocity profile; PC: path curvature of movement trajectory; RT/Rev: reaction times for outward movements and time to reverse movement direction for return movements; SEamp: systematic amplitude error; SEdir: systematic directional error.
Inactivations consistently reduced movement speed
Movement velocity
The peak velocity (PV) of outward movements was reduced consistently following sGPi inactivation (p < 0.001; Table 2; see supplemental Table 4 for detailed statistical results). The effects on velocity were often large enough to be evident in individual comparisons of movements pre- versus post-injection (Fig. 2, compare left versus right columns). The decrease was of similar magnitude in both animals (p > 0.15; M×I×T, F(1,16) = 2.20) and it was very robust across sessions (Fig. 3). In monkey H, inactivation caused PV to decrease in all 7 sessions, of which 5 were significant. In monkey C, a decrease was observed in 9 out of 11 sessions, of which all 9 were significant. Because the effect was consistent across sessions, sample-level KS statistics were highly significant for both animals (p < 0.001). sGPi inactivation also strongly reduced peak acceleration (PA; p < 0.01; Table 2) and this decrease was significant in both animals at the sample-level (p < 0.002 for both).
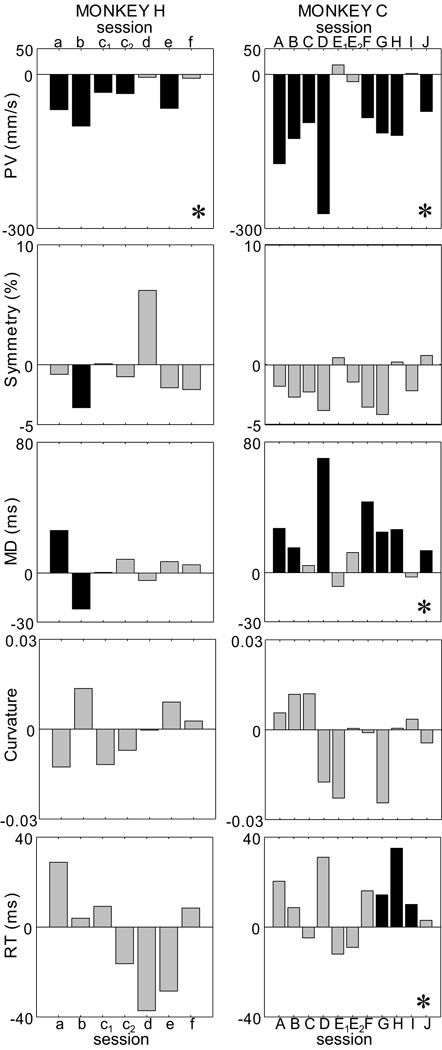
Figure 3.
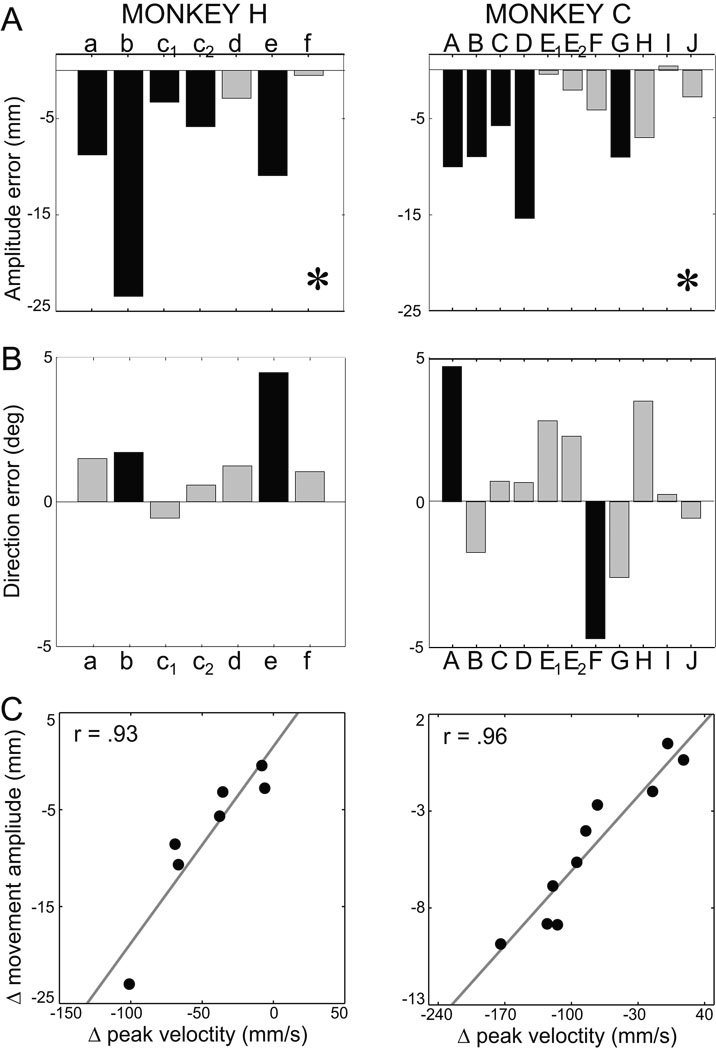
Variations of the main kinematic markers following sGPi inactivation. Each bar represents the difference between the pre- and post-injection phase for one injection session. (*) specifies significant effect at the sample-level (Kolmogorov-Smirnov test across sessions). Black bars indicate significant within-sessions variations. Grey bars indicate non-significant changes. Session labels (monkey H: a – f; monkey C: A – J) correspond to those used in Figure 1C and Table 1.
As the examples in Figure 2 (bottom) suggest, sGPi inactivations had no detectable effect on the shape of velocity profiles. Across sessions, neither measure of profile shape was affected by inactivation (p > 0.55 for SYM; p > 0.80 for SHA; Table 2 and supplemental Table 4), and there was no difference in this result between animals (p > 0.33 for both SYM and SHA; M×I×T, Fs(1,16) > 1.01). Within individual injection sessions in monkey H, only SYM changed significantly and then, only in one injection session (Fig. 3; p > 0.15; sample-level KS < 0.39). For monkey C, no injection induced a significant change in either SYM (Fig. 3) or SHA.
Movement duration
sGPi inactivations increased movement durations only slightly, but significantly (p < 0.025, MD; mean change −12 msec; Table 2 and supplemental Table 4). Although no monkey-by-injection interaction was observed (p > 0.08, MD; M×I×T, F(1,16) = 3.49), MD increases were more prominent in monkey C. Increases were observed in 9 sessions out of 11 (6 significant; Fig. 3), leading to a significant sample-level increase (p < 0.0001, mean = +21 ms; KS >0.63). In monkey H, MD increased in 5 sessions out of 7 (only one significant, Fig. 3), leading to no significance at sample-level (p > 0.20, mean = +3 ms; KS < 0.38). We show below that movement durations were less affected than might be expected because reductions in movement velocity were accompanied by substantial reductions in movement extent.
Hand paths
Movement trajectories (i.e., hand path curvatures) were not altered by sGPi inactivation (p > 0.45, PC; Table 2). This result was consistent for both monkeys (p > 0.65, PC; M×I×T, F(1,16) = 0.17; p > 0.20 for sample-level KS statistics for both animals). As illustrated in Figure 3, no within-session analyses reached significance for either animal.
Reaction Time
sGPi inactivation did not influence mean reaction times (p > 0.50, RT; Table 2). Although no monkey-by-injection interaction was observed (p > 0.10, RT; M×I×T, F(1,16) = 2.63), lengthened RTs were slightly more prevalent in monkey C (mean 10 ms increase) than in monkey H (5 ms decrease). Session-by-session analyses echoed this disparity. For monkey H, increases and decreases were equally prevalent and no change was significant (p > 0.03, sample-level KS statistic < 0.52; Fig. 3). For monkey C, 8 sessions showed a slight increase (3 significant) leading to marginal significance at the sample-level (p < 0.01, KS >0.47; 0.01 being the pre-defined alpha for sample-level tests).
Inactivations induced a modest postural drift
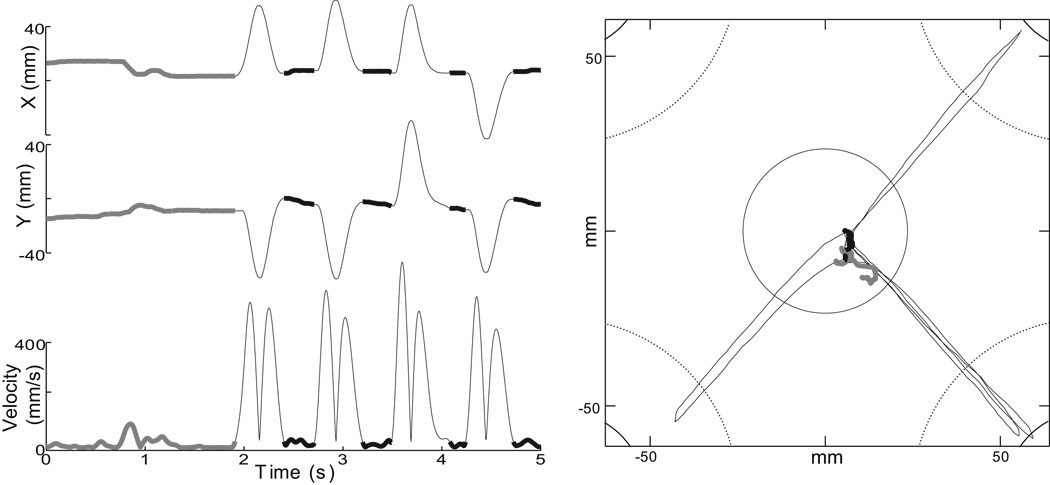
sGPi inactivation induced a significant drift in initial position of the hand in every injection session but two. Postural drift was most evident during the initial 1–2 second central hold period of trials (Fig. 4, thick gray traces). However, this drift was relatively mild and animals compensated for it such that they were able to maintain hand position within the home target (Fig. 4, right). Postural drift was less evident during the short reaction time intervals between subsequent movements (Fig. 4, thick black traces).
Figure 4.
Hand position drifted slightly during the initial hold period, prior to the first movement (thick gray traces). This drift was corrected by the animal to keep the cursor within the center home target (see plot of cursor positions at right). No drift was evident between subsequent movements (thick black traces), due to the short reaction time-length delays between the end of one movement and start of the next. Data are from one trial in monkey C session “G”. Left: X and Y positions and tangential velocity as a function of time. Right: movement of the cursor in the task plane.
For each inactivation experiment, the degree and direction of postural drift was quantified as the mean positional shift of the hand relative to the center of the home target immediately prior to first movement (i.e., SL; Fig. 5A). In monkey H, significant shifts were observed in 5 of 7 injection sessions, but the direction of the effect was not consistent across sessions (Fig. 5A, left; p < 0.0001, sample-level KS >0.86). In monkey C, all injections caused a significant shift of the hand (within-session analyses) and that shift was toward the body in all cases but one (Fig. 5A, right; p < 0.0001, sample-level KS statistic >0.99). Because the direction of the shift was not consistent in monkey H, mean injection-induced shifts differed significantly between animals (means = 1.8 mm and 7.5 mm for monkeys H and C, respectively; p < 0.02, SL; M×I×T, F(2,15) = 5.71). Post-hoc analyses confirmed that inactivation induced a systematic shift in hand position in monkey C (p < 0.02, SL; I×T, F(2,9) = 7.72) but not in monkey H (p > 0.50, SL; I×T, F(2,5) = 0.69). Session-by-session positional shifts were taken into account (below) in our analyses of the effects of sGPi inactivation on movement planning and execution.
Figure 5.
(A) Summary of the direction and magnitude of shifts in the initial position of the hand following sGPi inactivation. Shift direction was systematically toward flexion across sessions in monkey C (right), but inconsistent in monkey H (left). Each line (terminated by a dot) represents the mean difference vector between pre- and post-injection initial hand positions for one injection session. Continuous lines denote significant shifts (within-session MANOVA) whereas dashed lines represent non-significant shifts. Thick gray lines and large dots represent the mean shift across sessions. (B) Exemplar trajectories for outward movements performed pre- and post-injection (black and gray traces, respectively) by monkey C session “G”. The top row shows representative individual trials while the bottom row shows mean trajectories across all trials. The left (Pre-subtraction) column illustrates the systematic positional bias in hand position post-injection. This shift was not compensated for during movement planning or execution, resulting in roughly parallel hand trajectories for pre- and post-injection movements. The right (Shift-subtracted) column shows the same data after removal of the postural bias. Only hypometria remained following this correction. Other conventions follow those of Figure 2.
Extent planning was impaired by sGPi inactivations
End-point accuracy
An initial analysis in Cartesian coordinates (i.e., SExy) demonstrated that sGPi inactivation reduced movement accuracy (p < 0.05, SExy; M×I×T, F(2,15) = 3.69). As the two examples in Figure 5B illustrate, a large fraction of the increased error could be attributed to uncompensated inactivation-induced shifts in the initial position of the hand. Interestingly, the initial shift was not corrected during movement execution, causing pre- and post-injection trajectories to unfold along roughly parallel paths for all directions of movement.
Three complementary analyses confirmed that the initial shift was not compensated for during movement planning. First, a MANOVA tested for significant differences between the initial shift and the systematic end-point shift (i.e. the mean error vector across all target location). No effect was found, indicating that the initial hand location and the final end-point distribution were shifted by a common vector (p > 0.35, SLxy vs. SExy×M; F(2,15) = 1.05). Second, multivariate correlations were computed to investigate between-session variations of the components of the initial and end-point shift vectors (canonical correlations; Thompson 1984). A significant correlation was found, indicating that variations of the initial postural shift caused concomitant variations of the final end-point shift (p < 0.03, R = .60). Third, an ANOVA was carried out to determine whether the initial trajectory unfolded along truly parallel paths for pre- and post-injection trials. Results supported this idea by showing that the movement direction was identical in the pre- and post-injection trials at the time to peak acceleration (p > 0.15 for main effect and all interactions, DIR; M×I×T). This result also shows that the planning of movement direction was not altered following sGPi inactivation. We will return to this point later.
End-point errors associated with movement execution were identified by subtracting from the end-point distribution the systematic end-point shifts attributable to hand localization errors (Desmurget et al. 2003; Vindras et al. 1998; Vindras et al. 2005). Two examples of the result of this procedure are show in Figure 5B (right column). Following this subtraction, it became clear that hypometria was increased post-injection (p < 0.0005, SEamp; Table 2). The absence of a target-by-injection interaction indicated that the effect on extent was comparable for all target locations (p > 0.05, SEamp; M×I×T, F(3,48) = 2.75). As shown in Figure 6A, hypometria was increased in all sessions in monkey H (5 significant) and in 10 of 11 sessions in monkey C (5 significant; p < 0.001 for both animals, sample-level KS).
Figure 6.
(A–B) sGPi inactivation consistently induced hypometria while seldom affecting movement direction. Mean difference in amplitude error (SEamp, top row) and directional error (SEdir, bottom row) pre- versus post-injection for individual injection sessions. Other conventions follow those of Figure 3. (C) Injection-induced change in SEamp (Δ movement amplitude) correlated closely with injection-induced slowing of movement (Δ peak velocity). Each point represents mean results from one injection session. Correlation analyses were based on Spearman’s R (r values shown) and the continuous gray lines show the principal axis of the correlation (Sokal and Rohlf 1981 pg. 594). Results are plotted separately for monkeys H (left) and C (right).
A highly significant finding was that the severity of hypometria induced by individual injections correlated very closely with decreases in both peak velocity (p < 0.01 for both animals; Fig. 6C) and peak acceleration (p < 0.05 for both animals; Spearman’s R for monkey H = 0.74; and for monkey C = 0.94).
sGPi inactivation did not influence mean directional accuracy (p > 0.10; Table 2, supplemental Table 4) and this result did not differ between animals or targets (p > 0.10 for all interactions, SEdir; M×I×T, M×I×T and M×I×T). For monkey H, sGPi inactivation introduced a small counterclockwise rotation of movement end-point in most sessions (6 out of 7, 2 significant; Fig. 6B, right), although at the sample-level these changes were not significant (p > 0.05, KS < 0.48). For monkey C, a mixture of counterclockwise (7 sessions, 1 significant) and clockwise (4 sessions, 1 significant; Fig. 6B, left) rotations were observed, which, again, were not significant at sample-level (p > 0.10, KS < 0.35). These results agree with observations reported above showing that the initial direction of movement was not modified following sGPi inactivation. Preserved control of movement direction helps us dismiss the potential explanation that sGPi inactivation induced a general impairment in motor control (e.g., an increase in execution noise).
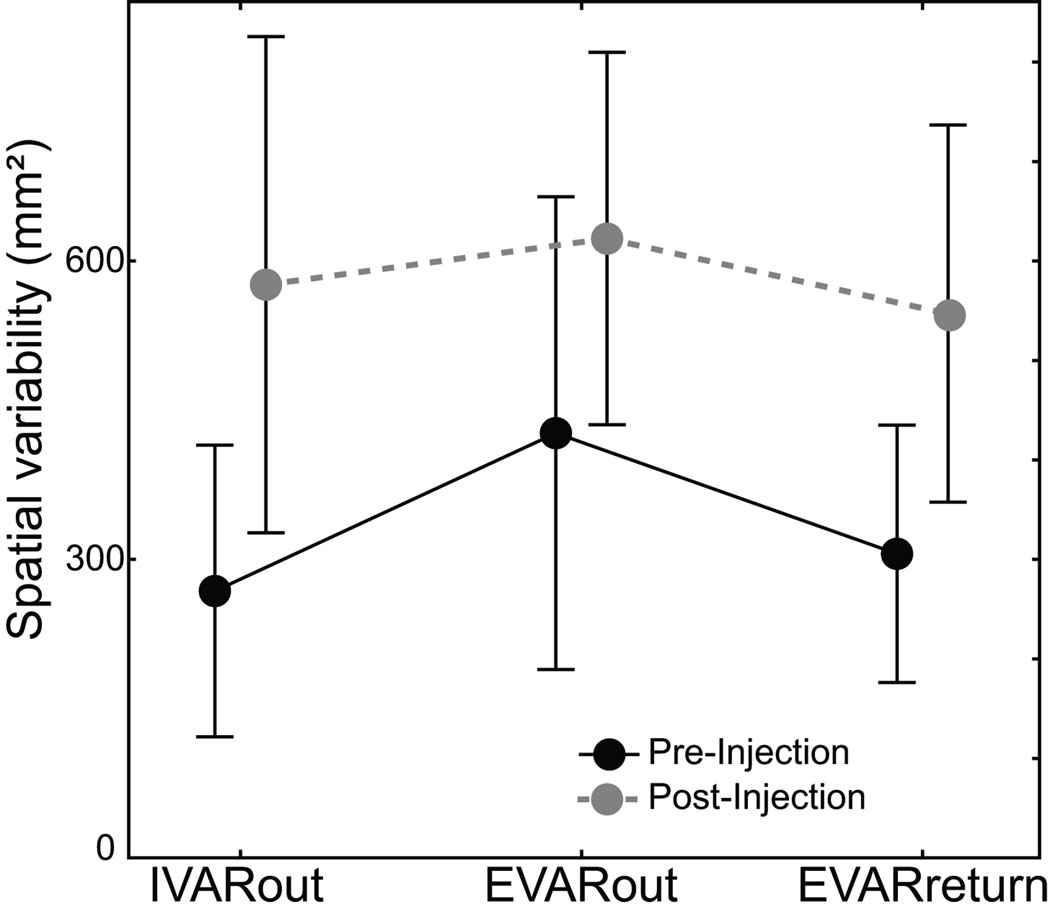
End-point variability
Injections increased the variability of hand position at the end of outward movements (p < 0.03, EVARout; pre-inj: 426 mm², post-inj: 626 mm²; M×I×T, F(1,16) = 6.01; Fig. 7). This increase was equally present in both animals (p > 0.50, M×I×T, F(1,16) = 0.36) and it was quite robust across sessions. sGPi inactivation increased variable error in 6 of the 7 sessions in monkey H, and in 9 of the 11 sessions in monkey C. Contrary to the interpretation that increased variability was due to noisier motor output or impaired feedback processing, variable error did not accumulate during movement execution. Although sGPi inactivation increased hand position variability (p < 0.0005, VAR; M×I×InitialEnd×Target, F(1,16) = 19.77), the effect did not differ between the pre- and post-movement estimates (p > 0.20, IVARout and EVARout; M×I×InitialEnd×Target, F(1,16) = 1.63). This observation was consistent in both animals (p > 0.50, IVARout and EVARout; M×I×InitialEnd×Target, F(1,16) = 0.38).
Figure 7.
sGPi inactivation did not increase execution-related variability or impair online correction of return movements despite an overall increase in positional variability post-injection. Mean variability in hand position is plotted for the beginning of outward movements (IVARout), the end of outward movements (EVARout) and the end of return movements (EVARreturn). Injections did not increase the execution-related accrual of variable errors during outward movements (EVARout– IVARout), nor did they attenuate the feedback-related reduction in error during return movements (EVARreturn– EVARout). Data are means across all sessions in both animals pre- (black) and post- (gray) injection. Error bars = SD.
Error correction remained intact during sGPi inactivations
We investigated involvement of the sGPi in online error correction by capitalizing on evidence that return movements depended differentially on online correction. Outward and return movements were affected similarly by sGPi inactivation in most respects (see Return in Table 2 and supplementary Table 4). For return movements, sGPi inactivation decreased peak velocity and peak acceleration and increased movement duration (p < 0.05 for PV, PA and MD; Table 2). The shape of the velocity profile was unchanged (p > 0.05, for SYM and SHA). In contrast to what was observed for outward movements, however, the curvature of return movements decreased following sGPi inactivations (p < 0.003, PC). A change in hand path curvature is consistent with a contribution of corrective feedback loops to movement accuracy (see below).
The most striking difference in effects of injections on return movements, relative to effects on outward movements, was for systematic end-point errors. An initial analysis of overall movement accuracy in Cartesian coordinates demonstrated that the accuracy of the return movements was not affected by injections (p > 0.07, SExy; M×I×T, F(2,15) = 3.07). A significant animal-by-injection interaction was observed however (p < 0.05, SExy; M×I×T, F(2,15) = 3.78). Post-hoc analyses indicated that injections affected accuracy of return movements in monkey C (p < 0.02, SExy; I×T, F(2,9) = 6.40) but not in monkey H (p > 0.60, SExy; I×T, F(2,5) = 0.45). This difference between animals is related to the presence of a systematic injection-induced bias in initial hand location, in monkey C only (see Fig. 5A). Return movements were directed toward the biased initial location from which outward movements were triggered, thereby inducing a systematic error in return movement endpoints in monkey C, but not in monkey H. This conclusion is supported by the close similarity between mean start locations of outward movements and end-point locations of return movements (p > 0.25, SLoutward versus SEreturn; M×I×T×OutwardReturn, F(2,15) = 1.39). This identity was true irrespective of monkey (p > 0.45; M×I×T×OutwardReturn, F(2,15) = 0.79) and injection (p > 0.30; M×I×T×OutwardReturn, F(2,15) = 1.22).
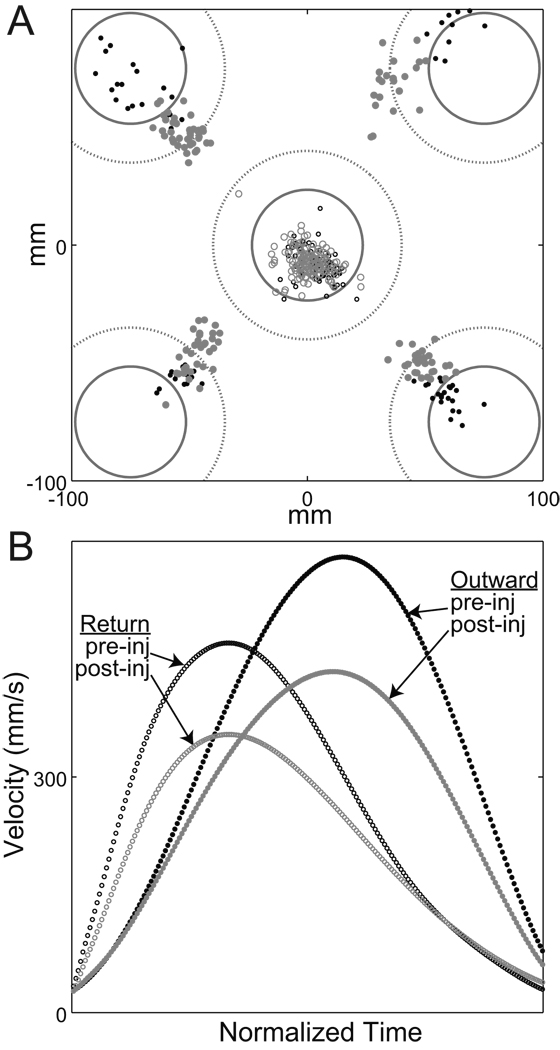
After correcting for injection-induced biases in initial posture, there was no residual effect of sGPi inactivation on the accuracy of return movements (p > 0.25 for main effect and all interactions, SExy; M×I×T, M×I×T, M×I×T, and M×I×T). In other words, although the final resting hand position was shifted post-injection, the accuracy with which this posture was reached was equivalent before and after sGPi inactivation. As suggested previously, it is likely that feedback loops contributed to this preserved accuracy. In agreement with this idea, sGPi inactivation did not degrade the animal’s ability to capture the central visually-salient instruction cue (p > 0.35; pre-inj: 87 %; post-inj 83 %; M×I×T, F(1,16) = 0.85). This point is illustrated in Figure 8A, which displays end-point locations for individual outward and return movements performed by monkey H during a single session (session “b”).
Figure 8.
(A) Return movements remained accurate following sGPi inactivation, despite frank hypometria in outward movements. End-point locations are plotted for individual outward movements (filled circles) and return movements (open circles) of pre-injection (black) and post-injection (gray) trials from one exemplar session (“b” in monkey H). Other conventions follow those of Fig. 2. (B): Pre- and post-injection return movements had lower peak velocities and longer deceleration phases compared with outward movements, consistent with the view that return movements were influenced strongly by feedback control. Mean tangential velocities are shown for the trials plotted in the top panel. To aid comparison, the durations of all velocity profiles have been normalized to one standard duration.
Another indication that return movements remained under the control of healthy feedback loops came from an analysis of variable errors. As shown in Figure 7, variability in hand position decreased during return movements relative to variability at the end of outward movements (p < 0.005, EVARoutward versus EVARreturn; M×I×InitialEnd×Target, F(1,15) = 11.27). This decrease was present both pre- and post-injection (p > 0.45; M×I×InitialEnd×Target, F(1,15) = 0.49), and it was present in both animals (p > 0.40; M×I×InitialEnd×Target, F(1,15) = 0.75).
Inactivation did not impair abrupt changes in movement direction or iterative responses
Time to reverse direction
Mean latencies between outward and return movements (i.e., the period when velocity fell below 30 mm/sec) were not affected by sGPi inactivation (p > 0.07 for main effect and all interactions; M×I×R; Return in Table 2 and supplemental Table 4). Examples of the short latencies for direction reversal are shown, pre- and post-injection, in Figure 2. For individual sGPi inactivation sessions, the time required to reverse movement direction did increase slightly in 6 of the 7 sessions in monkey H, but none of these changes were significant (p > 0.07, sample-level KS < 0.46). In monkey C, 3 sessions showed a small decrease (0 significant) and 8 an increase (3 significant), again yielding non-significant results at the sample-level (p > 0.15, KS < 0.35).
Quick successive movements
Latencies were very short between completion of one movement (first through third) and presentation of the target cue for the next movement (second through fourth) (mean = 77 ms; pre-injection: 82 ms, post-injection: 71 ms; M×I×T, F(1, 16)=1.9, p > .15). Therefore, analyses of the effects of sGPi inactivation as a function of movement order allowed us to address potential roles of the sGPi in the execution of discrete movements in quick succession. Principal kinematic measures of outward movements were averaged according to their rank [first through last (4th) movement] rather than target position (as was used in most of the preceding analyses). Several of these measures changed as a function of rank, presumably due to idiosyncratic strategies adopted by the animals. (See supplemental Fig. 11 for examples and supplemental Table 5 for statistical details.) Not one of the effects of movement rank was altered by sGPi inactivation (supplemental Fig. 11 and supplemental Table 5). Injection-by-rank interactions did not approach significance for reaction time, movement duration, peak acceleration, peak velocity, shape or symmetry of the velocity profile, movement curvature or absolute accuracy (p > 0.3 for all comparisons; details in supplemental Table 5). In other words, the idiosyncratic changes in kinematics across the four successive movements were unaffected by sGPi inactivation, even when sGPi inactivation had a significant main effect on the measure (e.g., peak velocity in supplemental Fig. 11).
Corrective reaction time
Here, we investigated individual movements in which an animal (1) initiated a movement that was directed to the wrong target and then (2) produced a discrete corrective sub-movement to reach the correct target location (Fig. 9; Meyer et al. 1988, Novak et al. 2002, Desmurget et al. 2004a, for a discussion of the discrete nature of these large corrections). We found 28 individual movements that matched this set of conditions, 16 for monkey H (7 pre-injection, 9 post-injection) and 12 for monkey C (7 pre-injection, 5 post-injection). We then measured correction times as the interval from the onset of the mis-directed movement to the inflection in the velocity profile that reflected initiation of the corrective movement (e.g., arrowhead in Fig. 9A and B). Independent t-tests for each monkey indicated that the time required to redirect movement to the correct target did not increase following sGPi inactivation (monkey H: p > 0.25; mean lag pre-inj = 137 ms, post-inj = 157 ms; monkey C: p > 0.95; mean lag pre-inj = 117 ms, post-inj = 117 ms). Thus, we found no evidence that sGPi inactivations impaired an animal's ability to detect errors in execution and correct them rapidly.
Figure 9.
sGPi inactivation did not impair the quick correction of mis-directed movements. (A): Individual hand paths are plotted in which the monkey (C) moved initially toward the wrong target before redirecting the movement toward the correct target. Two exemplar movements (distinguished by solid and dashed lines) were selected from data collected pre-injection (black traces) and post-injection (gray traces). (For the sake of clarity, all movements are displayed from a common starting location.) (B): Corresponding tangential velocity profiles are plotted. Arrowheads: The time of correction onset for one of the four traces.
Control injection, EMG data and anatomo-functional correlations
Infusion of pure artificial CSF had no effect on task performance. Performance data from the one control session performed in monkey C were subjected to within-session analysis. This injection had no effect on movement peak velocity (p > 0.10; pre-inj: 703 mm/sec, post-inj: 680 mm/sec; F(1,306) = 2.43) or movement amplitude (p > 0.90; pre-inj: −27.2 mm, post-inj: −27.1 mm; F(1,306) = 0.01). Movement duration (p > 0.10; pre-inj: 203 msec, post-inj: 210 msec; F(1,306) = 2.24) and reaction time (p > 0.90; pre-inj: 174 msec, post-inj: 173 msec; F(1,306) = 0.01) were also preserved post-injection.
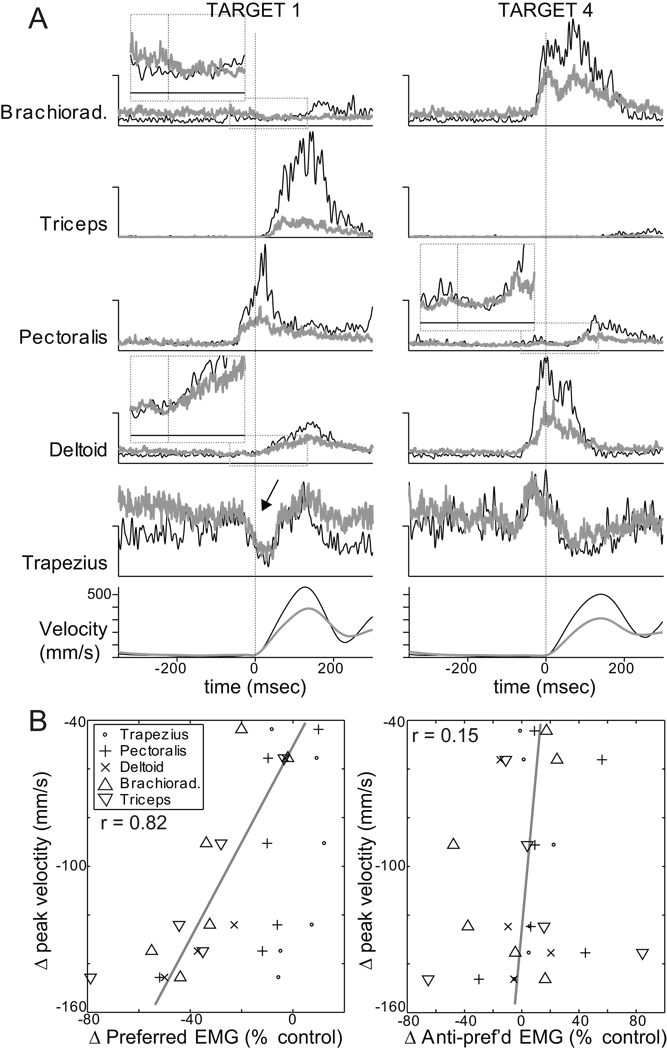
EMG recordings demonstrated reductions in movement-related EMG that correlated with the severity of inactivation-induced slowing and hypometria (Fig. 10). Successful EMG recordings were obtained during the six initial injection sessions performed in monkey C. Two of those sessions were included in the formal statistical analyses reported above (sessions “C” and “D”). One was the control injection session described in the paragraph above. The three remaining EMG recording sessions were excluded from formal statistical analysis because an inadequate number of trials were collected pre-infusion. Figure 10A illustrates the principal effects of sGPi inactivation on mean EMG during outward movements. Muscimol injections reduced the magnitude of both agonist and antagonist bursts in brachioradialis, triceps, pectoralis and deltoid muscles (Fig. 10A). Note that the relative timing of EMG bursts in the different muscles was preserved despite the major changes in burst magnitude. This was most obvious for target 1 by comparing the pre-movement onset times of pectoralis activity (pre-inj: −46 msec and post-inj: −55 msec) with the late onsets of triceps activity (pre-inj: +19 msec and post-inj: +24 msec). The control infusion had very little effect on peri-movement EMG (supplementary Fig. 12). Although baseline muscle tone (i.e., tonic EMG prior to the initial agonist burst) was modestly elevated post-injection in brachioradialis, deltoid, and trapezius, increases of similar magnitude were also observed following the control infusion (supplementary Fig. 12). Perhaps of greatest importance, sGPi inactivations did not affect peri-movement EMG for a muscle’s anti-preferred direction (i.e., for the direction associated with minimum peri-movement EMG). This was most obvious for trapezius, which was nominally involved as an agonist, but showed a marked reduction in activity before the onset of movement to target 1 (arrow in Fig. 10A). Despite an increase in baseline tone post-injection, the peri-movement reduction maintained the same magnitude and timing. Close inspection of EMGs from the prime moving muscles yielded similar qualitative observations (see insets with expanded scales for anti-preferred EMG from brachioradialis, pectoralis and deltoid; dotted boxes in Fig. 10A).
Figure 10.
sGPi inactivations attenuated peri-movement muscle activity, but did not alter relative timing or antagonist-related activity. (A): Mean rectified EMG from one injection session is plotted for two opposing directions of outward movement (left and right columns). Black and gray traces denote averages from pre- and post-injection phases, respectively. Vertical dotted lines indicate the onset of movement. Vertical scales for EMG are arbitrary but fixed for each muscle’s matching pre- and post-injection traces and left and right columns. Inset plots show averages on expanded scales around the onset of movement in anti-preferred directions for three muscles. These plots provide no evidence for injection-induced increases in co-contraction or antagonist activity. Arrow indicates a notable example of a peri-movement reduction in trapezius activity that is unaffected post-injection. Note that trial-to-trial variability in timing led to temporal smearing of later (i.e., >200 msec) aspects of the EMG and velocity averages. (B, left): Bradykinesia correlated closely with the injection-induced attenuation of agonist EMG. Mean injection-induced changes in peak velocity are plotted relative to mean changes in preferred-direction EMG for each of the six EMG recording sessions (including one control injection). (See Methods for details on EMG analysis.) A point is plotted for each muscle studied per session. Spearman correlation analysis was performed across all sessions and all muscles except trapezius (which showed nominal agonist-like activity compared with other muscles). A continuous gray line indicates the principal axis of the correlation (Sokal and Rohlf 1981 p. 594). (B, right): Bradykinesia had no consistent relationship with inactivation-induced changes in EMG during movements in muscle anti-preferred directions. Conventions follow those used in left panel.
Quantitative analysis across the 6 EMG recording sessions confirmed these observations. Injection-induced slowing and hypometria correlated closely with reductions in the size of peri-movement EMG bursts (p<0.00001; Spearman’s R = 0.82 for both PV and SEamp for movements in preferred directions for brachioradialis, triceps, pectoralis and deltoid; results for PV plotted in Fig. 10B left). Slowing and hypometria had no relationship, however, with changes in peri-movement EMG during movements in a muscle’s anti-preferred direction (p>0.5; Spearman’s R = 0.14 for both PV and SEamp; Fig. 10B right) or with post-injection changes in baseline muscle tone (p>0.5; Spearman’s R = 0.15 for both PV and SEamp). The magnitude of initial postural drift showed a weak relationship with changes in EMG only during movement in muscle anti-preferred directions, and in that case the correlation was negative (p<0.03; Spearman’s R = −0.48 for SL vector length). In other words, postural drift was greatest when peri-movement muscle tone was reduced below normal.
Figure 1C illustrates the anatomical distribution and magnitude of effects on posture, peak velocity, and reaction time. We found no clear relation between the location of injections and the patterns of behavioral deficits induced.
DISCUSSION
For the sake of clarity, we will partition the effects of sGPi inactivation into two categories: robust and variable. Effects that were consistent across sessions and animals qualify as robust. Robust effects included: slowing of movement, reduced amplitude for the rapid uncorrected outward movements, preserved accuracy for the slower visually-guided return movements and, preserved ability to chain successive motor acts. Effects that were inconsistent across sessions and animals qualify as variable. Variable effects concerned mainly movement reaction times and the existence of a flexed arm posture at rest.
Technical considerations and potential confounds
First, it is important to consider the general experimental design and the scope of our results. The inactivation technique used here shares some of the inherent limitations of any attempt to infer brain function from lesion-induced dysfunction (Aparicio et al. 2005). For instance, some impairments may not have been observed due to compensatory mechanisms operating at neuronal or behavioral levels. The use of transient pharmacologic inactivations (as opposed to permanent lesions) reduces the likelihood of compensatory mechanisms but does not eliminate it completely (Martin and Ghez 1999).
Another potential concern is that the relatively small number of infusions performed combined with the small infusion volumes may not have affected a large enough fraction of the GPi motor territory to test adequately the hypotheses posed. Additional motor impairments might have been evoked if larger injection volumes had been used. Other impairments might have been produced if injections had been placed in sub-regions of the sGPi we did not explore. Importantly, the current study did not address the possible importance of associative and limbic BG circuits, or bilateral BG circuits in the behaviors studied here. Although these are valid concerns, a strength of our results is that we observed a discrete yet consistent subset of motor impairments from focal inactivations distributed across a significant fraction of the posterior GPi. These results allow us to make inferences about functions that are common to the BG motor circuit as a whole.
A related concern is that no clear topography of effects was found within the GPi motor territory. Tract-tracing studies have identified clearly separate regions of the posterior GPi that project via thalamus to primary motor cortex (M1) and the various premotor cortices (e.g., supplementary motor cortex, SMA; Hoover and Strick 1993; Hoover and Strick 1999). One might predict different behavioral effects from inactivations of GPi regions projecting to M1, SMA, and the other motor cortices. The sGPi is also somatotopically organized (DeLong et al. 1985; Hamada et al. 1990; Romanelli et al. 2005) such that one might predict more dramatic impairments from inactivations centered on arm-related regions. In fact, some of the between-session variability we observed may be attributable to the topographic complexity of GPi subcircuits. For instance, the variable effects on reaction times and arm posture could be explained if specific subregions of the sGPi were devoted to movement initiation and maintenance of posture.
Another limitation concerns the generality of these results. It is possible the present results may not generalize to all task or motor control contexts. For example, peri-movement inhibition of antagonist EMG might be more impaired and co-contraction increased in tasks that require greater postural stabilization and suppression of antagonist muscles (Mink and Thach 1991b; Wenger et al. 1999). Similarly, significant impairments in movement initiation or execution might become evident in tasks that are more cognitively-complex, less dependent on exteroceptive sensory cues (Basso and Liu 2007; Hikosaka and Wurtz 1985; Turner and Anderson 2005), or less familiar to the subjects. It is also possible that additional task impairments might have been observed if we had also inactivated the substantia nigra pars reticulata (SNr; the other output channel of the BG). This hypothesis seem unlikely however. Indeed, few SNr neurons respond to visually-directed reaching (∼20%) and the vast majority of these neurons (∼75%) shows premotor responses (i.e. the response occurs before target presentation and stops around target appearance, before movement onset; Wichmann and Kliem 2004). In addition, when SNr is inactivated in monkeys, axial postural anomalies and rotational behavior are found, without limb dysfunction (Burbaud et al. 1998).
Slowing and hypometria after sGPi inactivation
Slowing of movement was observed in both animals and in most inactivation sessions (16 out of 18 sessions; see PV in Fig. 3). This observation is consistent with many previous reports that slowing is one of the primary motor sequelae of pallidal lesions or inactivations in neurologically-normal animals (Horak and Anderson 1984a; Hore et al. 1977; Hore and Vilis 1980; Mink and Thach 1991b). Results from earlier studies have been questioned on the grounds that the effect might be attributable to spread of damage or inactivation to surrounding structures (e.g., to GPe) (DeLong and Georgopoulos 1981; Mink 1996). Recent well-controlled experiments have confirmed, however, that infusions of small volumes of muscimol into the GPi slow planar reaching (Inase et al. 1996) and free reach-and-grasp movements (Wenger et al. 1999). A potential limitation of previous studies is that relatively stringent accuracy requirements could have led to an adaptive slowing in response to inactivation-induced increases in movement variability (Sheridan and Flowers 1990). We used relaxed accuracy constraints, however, and slowing of movement was observed nonetheless. Thus, the present results confirm and extend the previous observations from Mink's (Mink and Thach 1991b; Wenger et al. 1999) and Anderson's groups (Horak and Anderson 1984a; Inase et al. 1996).
We found large and consistent reductions in movement extent post-injection (17 of 18 sessions; see Amplitude error in Fig. 6). Several previous studies also reported reductions in movement extent after sGPi inactivation (Inase et al. 1996; Mink and Thach 1991b) although this observation was not universal (Horak and Anderson 1984a). It is quite likely that the degree of hypometria observed during sGPi inactivation depended on the timing and accuracy constraints of the task. Using relatively stringent accuracy constraints, Horak and Anderson (1984a) found no change in accuracy but consistent increases in movement duration. Using relaxed accuracy constraints, we found large reductions in movement extent but only inconsistent increases in movement duration. By controlling for injection-induced postural biases, we showed that the severity of hypometria was consistent across directions of movement and that the control of movement direction was preserved (see Direction error, Fig. 6). The dissociation here between impaired control of movement extent and preserved directional control fits well with other evidence that motor planning involves a parametric stage at which movement extent and direction are specified independently (Desmurget et al. 1998; Sainburg et al. 2003; Vindras et al. 2005).
Interestingly, extent errors correlated closely with decreases in peak acceleration and peak velocity (see Fig. 6C), thus suggesting that all three impairments arose from one underlying defect. The early timing of peak acceleration makes it unlikely that these deficits arose from impairment of some aspect of feedback control (Desmurget and Grafton 2003; Desmurget et al. 2005). (Findings regarding feedback control are discussed further below.)
Previous studies put forth two potential explanations for sGPi inactivation-induced slowing and hypometria: (1) under-activation of a normally-timed agonist/antagonist pattern of activation due to impaired feed-forward planning (Horak and Anderson 1984a); or (2) co-contraction of agonist/antagonist muscle pairs due to impaired suppression of postural reflexes (Mink and Thach 1991b; Wenger et al. 1999). The previous studies each presented EMG data consistent with the divergent interpretations (Horak and Anderson 1984a; Mink and Thach 1991b). Our EMG results, although limited in quantity, were fully consistent with the feed-forward planning hypothesis. Burst magnitudes were attenuated for muscle preferred directions, but the relative timing of activity in different muscles was maintained. The attenuation of preferred direction EMG correlated closely with the severity of injection-induced slowing and hypometria. Contrary to predictions from the impaired suppression hypothesis, we found no evidence of increased EMG during movements in a muscle’s anti-preferred direction (i.e., in a direction for which inappropriate activity could oppose, slow, and shorten the movement). Peri-movement reductions in EMG were preserved post-injection. Baseline muscle tone was increased post-injection in some muscles, thereby providing nominal support for the concept that sGPi inactivation can cause muscle co-contraction, but the increase in tone did not correlate with severity of bradykinesia or hypometria. Moreover, baseline tone was also increased following the one control injection. Therefore, whatever the explanation for the increase in resting EMG observed here, it cannot account the sGPi inactivation-induced movement slowing and hypometria.
Also bearing on the relative merit of feed-forward planning versus impaired suppression explanations, we found that the degree of slowing and hypometria was similar across four orthogonal directions of movement and that directional accuracy was unaffected by sGPi inactivation. Studies in humans have shown that maintaining constant directional accuracy despite a relatively small shift in initial posture of the arm requires substantial alterations in joint torques (Sainburg et al. 2003) and presumably in the pattern of muscle activation. Thus, it is difficult to conceive of a general disturbance in muscle agonist/antagonist balance (e.g. co-contraction) that would affect extent and speed equally for all directions of movement but have no effect on initial direction of movement, final directional accuracy, or path curvature (Inase et al. 1996; Mink and Thach 1991b; Wenger et al. 1999).
Recent models of motor control recognize the need for a general mechanism to regulate the “effort” or motor “energy” expended during a movement (Guigon et al. 2007; Todorov and Jordan 2002). This effort term, which scales with both the velocity and amplitude of movement, may link movement-related expenditure of energy to the demands of the task (Mazzoni et al. 2007) and to an animal’s previous experience of the cost/benefit contingencies of the task (Niv et al. 2007; Niv et al. 2006). (E.g., animals move more quickly to targets that predict larger rewards (Takikawa et al. 2002).) In addition to the present results, many other lines of research implicate the BG motor circuit in some aspect of motor control related to movement effort or energy expenditure: (1) single unit recording (Georgopoulos et al. 1983; Turner and Anderson 1997) and functional imaging (Turner et al. 2003b; Turner et al. 1998) have both demonstrated close correlations between activity in the BG motor circuit and the velocity or extent of movement; (2) functional imaging during an isometric task found similar correlations between motor circuit activity and the rate of change of force (Spraker et al. 2007; Vaillancourt et al. 2004a); (3) using PET imaging, we showed that pre-planning the amplitude of an upcoming movement increased BG activation relative to the pre-planning of direction or no planning (Desmurget et al. 2004b); (4) using PET imaging, Krakauer and colleagues showed that learning a new visuomotor gain selectively activates the BG network, while learning a new visuomotor rotation does not (Krakauer et al. 2004; but see Seidler et al. 2006); (5) electrical stimulation of BG output nuclei has been shown to alter reaching speed (Horak and Anderson 1984b) or saccade extent (Basso and Liu 2007); (6) numerous studies have shown that the bradykinesia associated with BG disorders (i.e., PD, Huntington’s disease, primary dystonia) reflects a sub-optimal scaling of the initial burst of agonist EMG with respect to the demands of the task (Berardelli et al. 2001; Godaux et al. 1992; Hallett and Khoshbin 1980; MacKinnon et al. 2004; Mazzoni et al. 2007; Pfann et al. 2001); (7) we found that parkinsonian hypometria is indeed a specific defect in controlling movement amplitude after subtracting systematic errors in the estimation of initial hand location (Desmurget et al. 2003); (8) focal lesions of the posterior “motor” basal ganglia in otherwise normal individuals often show parkinsonian-like bradykinesia and hypometria (Bhatia and Marsden 1994) and reductions in the rate of change of force (Aparicio et al. 2005), often with few other motor impairments. Recent reports indicate that motor slowing is also a common side-effect of therapeutic high frequency stimulation (deep brain stimulation, DBS) of the sGPi for cranial-cervical dystonia, Huntington’s disease, and Tourette’s syndrome (Diederich et al. 2005; Moro et al. 2004; Ostrem et al. 2007). Interestingly, DBS-induced slowing was observed in body segments that were unaffected by dystonia and had otherwise normal motor function (Ostrem et al. 2007). All of the findings cited above implicate the BG motor circuit in some aspect of motor control related to the “effort” or “energy” expended during a movement.
The concept that the BG motor circuit regulates motor effort is consistent with the more global hypothesis that the BG and its dopaminergic innervation regulates action motivation or “vigor” (Niv et al. 2007; Robinson et al. 2005; Salamone et al. 2007). Limbic circuits of the BG have been implicated in the appropriate scaling of response “vigor” (i.e., rate of responding or choice of effortful responses) to match the cost/benefit tradeoff of a task or context (Cagniard et al. 2006).
Preserved feedback control following sGPi inactivation
When contrasted with outward movements, return movements were characterized by decreased velocity, prolonged movement duration, elongated deceleration phase (Fig. 2), preserved final accuracy and reduced final variability relative to initial variability (Fig. 7). These characteristics suggest that return movements were performed under greater feedback guidance than the outward movements (Desmurget et al. 1995; Desmurget et al. 2005; Jeannerod 1988; Milner and Ijaz 1990). Given this assumption and the observation that these characteristics were preserved following sGPi inactivation, we conclude that sGPi inactivation did not affect on-line feedback control. This result agrees with the preserved ability of PD patients to modulate ongoing motor commands in response to small reaching errors (Desmurget et al. 2004a). It is also compatible with an absence of significant activation within the BG when requirements for feedback control are increased by subliminal target jumps (Desmurget et al. 2001). These specific results do not address, however, the hypothesis that the BG is involved in discrete path corrections in response to large target jumps triggered after movement onset (Desmurget et al. 2004a; Smith et al. 2000; Smith and Shadmehr 2005).
Preserved ability to reverse movement direction abruptly and produce iterative responses
In the present study, sGPi inactivations did not impair: (1) the short-latency transitions between outward and return-to-center movements; (2) the reaction times or kinematics of four movements performed in quick succession; (3) the production of discrete iterative corrections in the context of misdirected movements. These results are consistent with previous inactivation studies showing that GPi blockade did not impair (i) the reach-to-retrieval transition, in a reach-grasp-and-retrieve task (Wenger et al. 1999) and (ii) the generation of discrete corrective sub-movements in a single-joint reaching task (Kato and Kimura 1992). At the same time however, our observations do not echo clinical data from patients with BG-centered degenerative disorders. It is well known, for instance, that the ability to rapidly reverse movement direction is impaired in PD patients (Beuter et al. 1994, Hermsdorfer et al. 1999). It is also established that these patients are not able to generate discrete corrective sub-movements when required to correct large planning errors (Desmurget et al. 2004a). Because this type of corrective sub-movement can overlap the primary movement (Flash and Henis 1991, Milner 1992, Novak et al. 2000, 2002), it has been suggested (Desmurget et al. 2004a) that the inability of PD patients to generate discrete corrective sub-movements reflects, in part, a difficulty in stringing together successive motor acts (Agostino et al. 1992; Benecke et al. 1987) or to switching quickly from one coordinated movement to another (Cools et al. 1984, Giladi et al. 1997; Weiss et al. 1997). The present study does not allow conclusive testing of the idea that the BG is involved in switching or chaining mechanisms. However, the reasoning that normal functions of the BG can be inferred with good reliability from patient-based observations is brought into question. An alternative interpretation is that parkinsonian signs arise from a network of dysfunction, not only in the BG but also in cortical and other subcortical structures (e.g. Berardelli et al. 2001; Braak and Braak 2000, Turner et al. 2003a). Another possible alternative is that some of these functions may be mediated by other BG output circuits (i.e., anterior medial GPi or SNr; see above) that were preserved during our inactivations of sGPi. Further investigations are required to discriminate between these possibilities.
The paradox of pallidal-directed therapies
Consistent with previous studies, we found that sGPi inactivation in normal animals reproduced some of the features of PD: bradykinesia and hypometria in the presence of preserved online error correction (Desmurget et al. 2004a; Desmurget et al. 2003). These results may be seen as paradoxical from a clinical perspective, considering that ablation (pallidotomy) or DBS of sGPi reduces the same signs when they are present as components of idiopathic PD (Baron et al. 1996; Laitinen et al. 1992; Lang et al. 1997; Marsden and Obeso 1994) or experimentally-induced parkinsonism (Baron et al. 2002; Boraud et al. 1996; Lonser et al. 1999). A potential explanation arises from observations that the efficacy of pallidotomy as a treatment for bradykinesia correlates with the degree of pre-surgical impairment (Bastian et al. 2003) and that pallidotomy can attenuate the efficacy of dopamine replacement drugs (i.e., levodopa) in reducing bradykinesia (Pfann et al. 1998). Thus, the effects of pallidal-directed therapies appear to be two-edged: (1) Providing significant therapeutic benefit by eliminating the abnormal BG outflow known to be a correlate of BG disorders (Filion and Tremblay 1991; Miller and DeLong 1987; Starr et al. 2005; Wichmann and DeLong 1996). (2) Blocking any residual normal functions that the same circuit might perform. Both Pfann et al. (1998) and Bastian et al. (2003) found evidence for such a two-edged effect specifically with respect to the control of movement speed. The present results are consistent with the thesis, advanced previously by others (Berardelli et al. 1996; Pfann et al. 1998; Vaillancourt et al. 2004b), that the bradykinesia and hypometria associated with BG disorders arise, at least in part, from direct impairment of a normal function of the BG.
Summary
In summary, we analyzed the effects of sGPi inactivations on visually-directed reaching movements. Because these inactivations transiently blocked output from the primary BG motor pathway, the effects on task performance allowed us to test several current hypotheses on the contribution of this circuit to motor control. The results indicated that sGPi lesions affect the planning of movement velocity (bradykinesia) and movement gain (hypometria). These deficits could reflect a general contribution of the BG motor pathway to the allocation of motor effort. Movement initiation, movement guidance and the ability to rapidly chain successive movements were not affected.
Supplementary Material
Acknowledgments
The authors thank Kevin McCairn and Donn Simmons for their contributions to data collection, Caroline Tricot for her assistance with data analyses, and Vanessa Chan for constructive comments on the manuscript.
Grants: Supported by NIH grants NIH RO1-NS39146 R01NS44551.
Abbreviations
- RT
reaction time
- MD
movement duration
- PA
peak acceleration
- PV
peak velocity
- SYM
symmetry of the velocity profile
- SHA
shape of the velocity profile
- PC
movement path curvature
- DIR
initial direction of movement
- SL
hand starting location
- SE
systematic movement error
- IVAR and EVAR
spatial variability (i.e., variable error) at movement onset (IVAR) and movement end (EVAR)
Footnotes
Disclosures: The authors have no potential conflicts of interest.
REFERENCES
- Agostino R, Berardelli A, Formica A, Accornero N, Manfredi M. Sequential arm movements in patients with Parkinson's disease, Huntington's disease and dystonia. Brain. 1992;115:1481–1495. doi: 10.1093/brain/115.5.1481. [DOI] [PubMed] [Google Scholar]
- Anderson ME, Horak FB. Influence of the globus pallidus on arm movements in monkeys. III. Timing of movement-related information. J Neurophysiol. 1985;54:433–448. doi: 10.1152/jn.1985.54.2.433. [DOI] [PubMed] [Google Scholar]
- Aparicio P, Diedrichsen J, Ivry RB. Effects of focal basal ganglia lesions on timing and force control. Brain Cogn. 2005;58:62–74. doi: 10.1016/j.bandc.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Atkeson CG, Hollerbach JM. Kinematic features of unrestrained vertical arm movements. J Neurosci. 1985;5:2318–2330. doi: 10.1523/JNEUROSCI.05-09-02318.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahill AT, McDonald JD. Frequency limitations and optimal step size for the two-point central difference derivative algorithm with applications to human eye movement data. IEEE Trans Biomed Eng. 1983;30:191–194. doi: 10.1109/tbme.1983.325108. [DOI] [PubMed] [Google Scholar]
- Baron MS, Vitek JL, Bakay RAE, Green J, Kaneoke Y, Hashimoto T, Turner RS, Woodard JL, Cole SA, McDonald WM, DeLong MR. Treatment of advanced Parkinson's disease by posterior GPi pallidotomy: 1-year results of a pilot study. Ann Neurol. 1996;40:355–366. doi: 10.1002/ana.410400305. [DOI] [PubMed] [Google Scholar]
- Baron MS, Wichmann T, Ma D, DeLong MR. Effects of transient focal inactivation of the basal ganglia in parkinsonian primates. J Neurosci. 2002;22:592–599. doi: 10.1523/JNEUROSCI.22-02-00592.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MA, Liu P. Context-dependent effects of substantia nigra stimulation on eye movements. J Neurophysiol. 2007;97:4129–4142. doi: 10.1152/jn.00094.2007. [DOI] [PubMed] [Google Scholar]
- Bastian AJ, Kelly VE, Perlmutter JS, Mink JW. Effects of pallidotomy and levodopa on walking and reaching movements in Parkinson's disease. Mov Disord. 2003;18:1008–1017. doi: 10.1002/mds.10494. [DOI] [PubMed] [Google Scholar]
- Batschelet E. Statistical methods for the analysis of problems in animal orientation and certain biological rhythms. Washington: American Institute of Biological Sciences; 1965. [Google Scholar]
- Benecke R, Rothwell JC, Dick JPR, Day BL, Marsden CD. Disturbances of sequential movements in patients with Parkinson's disease. Brain. 1987;110:361–379. doi: 10.1093/brain/110.2.361. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Hallett M, Rothwell JC, Agostino R, Manfredi M, Thompson PD, Marsden CD. Single-joint rapid arm movements in normal subjects and in patients with motor disorders. Brain. 1996;119:661–674. doi: 10.1093/brain/119.2.661. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Rothwell JC, Thompson PD, Hallett M. Pathophysiology of bradykinesia in Parkinson's disease. Brain. 2001;124:2131–2146. doi: 10.1093/brain/124.11.2131. [DOI] [PubMed] [Google Scholar]
- Beuter A, Mergler D, de Geoffroy A, Carriere L, Belanger S, Varghese L, Sreekumar J, Gauthier S. Diadochokinesimetry: a study of patients with Parkinson's disease and manganese exposed workers. Neurotoxicology. 1994;15:655–664. [PubMed] [Google Scholar]
- Bhatia KP, Marsden CD. The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain. 1994;117:859–876. doi: 10.1093/brain/117.4.859. [DOI] [PubMed] [Google Scholar]
- Boecker H, Dagher A, Ceballos-Baumann AO, Passingham RE, Samuel M, Friston KJ, Poline J, Dettmers C, Conrad B, Brooks DJ. Role of the human rostral supplementary motor area and the basal ganglia in motor sequence control: investigations with H2 15O PET. J Neurophysiol. 1998;79:1070–1080. doi: 10.1152/jn.1998.79.2.1070. [DOI] [PubMed] [Google Scholar]
- Boraud T, Bezard E, Bioulac B, Gross C. High frequency stimulation of the internal Globus Pallidus (GPi) simultaneously improves parkinsonian symptoms and reduces the firing frequency of GPi neurons in the MPTP-treated monkey. Neurosci Lett. 1996;215:17–20. doi: 10.1016/s0304-3940(96)12943-8. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Pathoanatomy of Parkinson's disease. J Neurol. 2000;247 Suppl 2:II3–II10. doi: 10.1007/PL00007758. [DOI] [PubMed] [Google Scholar]
- Brotchie P, Iansek R, Horne MK. Motor function of the monkey globus pallidus. 2. Cognitive aspects of movement and phasic neuronal activity. Brain. 1991;114:1685–1702. doi: 10.1093/brain/114.4.1685. [DOI] [PubMed] [Google Scholar]
- Burbaud P, Bonnet B, Guehl D, Lagueny A, Bioulac B. Movement disorders induced by gamma-aminobutyric agonist and antagonist injections into the internal globus pallidus and substantia nigra pars reticulata of the monkey. Brain Res. 1998;780:102–107. doi: 10.1016/s0006-8993(97)01158-x. [DOI] [PubMed] [Google Scholar]
- Cagniard B, Beeler JA, Britt JP, McGehee DS, Marinelli M, Zhuang X. Dopamine scales performance in the absence of new learning. Neuron. 2006;51:541–547. doi: 10.1016/j.neuron.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Cools AR, Jaspers R, Schwarz M, Sontag KH, Vrijmoed-deVries M, van den Bercken J. Basal ganglia and switching motor programs. In: McKenzie JS, Kemm RE, Wilcock LN, editors. The Basal Ganglia Structure and Function. New York: Plenum Press; 1984. pp. 513–544. [Google Scholar]
- DeLong MR. Activity of pallidal neurons during movement. J Neurophysiol. 1971;34:414–427. doi: 10.1152/jn.1971.34.3.414. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Crutcher MD, Georgopoulos AP. Primate globus pallidus and subthalamic nucleus: functional organization. J Neurophysiol. 1985;53:530–543. doi: 10.1152/jn.1985.53.2.530. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Georgopoulos AP. Motor functions of the basal ganglia. In: Brookhart JM, Mountcastle VB, Brooks VB, Geiger SR, editors. Handbook of Physiology The Nervous System Motor Control Sect 1. Pt 2. Vol II. Bethesda: American Physiological Society; 1981. pp. 1017–1061. [Google Scholar]
- DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Gaveau V, Vindras P, Turner RS, Broussolle E, Thobois S. On-line motor control in patients with Parkinson's disease. Brain. 2004a;127:1755–1773. doi: 10.1093/brain/awh206. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Grafton ST. Feedback or forward control: end of a dichotomy. In: Johnson S, editor. Cognitive Neuroscience Perspectives on the Problem of Intentional Action. Boston: MIT Press; 2003. pp. 289–338. [Google Scholar]
- Desmurget M, Grafton ST, Vindras P, Grea H, Turner RS. Basal ganglia network mediates the control of movement amplitude. Exp Brain Res. 2003;153:197–209. doi: 10.1007/s00221-003-1593-3. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Grafton ST, Vindras P, Grea H, Turner RS. The basal ganglia network mediates the planning of movement amplitude. Eur J Neurosci. 2004b;19:2871–2880. doi: 10.1111/j.0953-816X.2004.03395.x. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Grea H, Grethe JS, Prablanc C, Alexander GE, Grafton ST. Functional anatomy of nonvisual feedback loops during reaching: a positron emission tomography study. J Neurosci. 2001;21:2919–2928. doi: 10.1523/JNEUROSCI.21-08-02919.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmurget M, Grea H, Prablanc C. Final posture of the upper limb depends on the initial position of the hand during prehension movements. Exp Brain Res. 1998;119:511–516. doi: 10.1007/s002210050367. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Rossetti Y, Prablanc C, Stelmach GE, Jeannerod M. Representation of hand position prior to movement and motor variability. Can J Physiol Pharmacol. 1995;73:262–272. doi: 10.1139/y95-037. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Turner RS, Prablanc C, Russo GS, Alexander GE, Grafton ST. Updating target location at the end of an orienting saccade affects the characteristics of simple point-to-point movements. J Exp Psychol Hum Percept Perform. 2005;31:1510–1536. doi: 10.1037/0096-1523.31.6.1510. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Vindras P, Grea H, Viviani P, Grafton ST. Proprioception does not quickly drift during visual occlusion. Exp Brain Res. 2000;134:363–377. doi: 10.1007/s002210000473. [DOI] [PubMed] [Google Scholar]
- Diederich NJ, Kalteis K, Stamenkovic M, Pieri V, Alesch F. Efficient internal pallidal stimulation in Gilles de la Tourette syndrome: a case report. Mov Disord. 2005;20:1496–1499. doi: 10.1002/mds.20551. [DOI] [PubMed] [Google Scholar]
- Filion M, Tremblay L. Abnormal spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res. 1991;547:142–151. [PubMed] [Google Scholar]
- Flash T, Henis EA. Arm trajectory modifications during reaching toward visual targets. J Cogn Neurosci. 1991;3:220–230. doi: 10.1162/jocn.1991.3.3.220. [DOI] [PubMed] [Google Scholar]
- Georgopoulos A, DeLong M, Crutcher M. Relations between parameters of step-tracking movements and single cell discharge in the globus pallidus and subthalamic nucleus of the behaving monkey. J Neurosci. 1983;3:1586–1598. doi: 10.1523/JNEUROSCI.03-08-01586.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giladi N, Kao R, Fahn S. Freezing phenomenon in patients with parkinsonian syndromes. Mov Disord. 1997;12:302–305. doi: 10.1002/mds.870120307. [DOI] [PubMed] [Google Scholar]
- Giladi N, McMahon D, Przedborski S, Flaster E, Guillory S, Kostic V, Fahn S. Motor blocks in Parkinson's disease. Neurology. 1992;42:333–339. doi: 10.1212/wnl.42.2.333. [DOI] [PubMed] [Google Scholar]
- Godaux E, Koulischer D, Jacquy J. Parkinsonian bradykinesia is due to depression in the rate of rise of muscle activity. Ann Neurol. 1992;31:93–100. doi: 10.1002/ana.410310117. [DOI] [PubMed] [Google Scholar]
- Goerendt IK, Messa C, Lawrence AD, Grasby PM, Piccini P, Brooks DJ. Dopamine release during sequential finger movements in health and Parkinson's disease: a PET study. Brain. 2003;126:312–325. doi: 10.1093/brain/awg035. [DOI] [PubMed] [Google Scholar]
- Grabli D, McCairn K, Hirsch EC, Agid Y, Feger J, Francois C, Tremblay L. Behavioural disorders induced by external globus pallidus dysfunction in primates: I. Behavioural study. Brain. 2004;127:2039–2054. doi: 10.1093/brain/awh220. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia and chunking of action repertoires. Neurobiol Learn Mem. 1998;70:119–136. doi: 10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- Guigon E, Baraduc P, Desmurget M. Computational motor control: redundancy and invariance. J Neurophysiol. 2007;97:331–347. doi: 10.1152/jn.00290.2006. [DOI] [PubMed] [Google Scholar]
- Hallett M, Khoshbin S. A physiological mechanism of bradykinesia. Brain. 1980;103:301–314. doi: 10.1093/brain/103.2.301. [DOI] [PubMed] [Google Scholar]
- Hamada I, DeLong MR. Excitotoxic acid lesions of the primate subthalamic nucleus result in reduced pallidal neuronal activity during active holding. J Neurophysiol. 1992;68:1859–1866. doi: 10.1152/jn.1992.68.5.1859. [DOI] [PubMed] [Google Scholar]
- Hamada I, DeLong MR, Mano N. Activity of identified wrist-related pallidal neurons during step and ramp wrist movements in the monkey. J Neurophysiol. 1990;64:1892–1906. doi: 10.1152/jn.1990.64.6.1892. [DOI] [PubMed] [Google Scholar]
- Hermsdorfer J, Marquardt C, Wack S, Mai N. Comparative analysis of diadochokinetic movements. J Electromyogr Kinesiol. 1999;9:283–295. doi: 10.1016/s1050-6411(98)00050-9. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. II. Effects of muscimol in monkey substantia nigra pars reticulata. J Neurophysiol. 1985;53:292–308. doi: 10.1152/jn.1985.53.1.292. [DOI] [PubMed] [Google Scholar]
- Hoover JE, Strick PL. Multiple output channels in the basal ganglia. Science. 1993;259:819–821. doi: 10.1126/science.7679223. [DOI] [PubMed] [Google Scholar]
- Hoover JE, Strick PL. The organization of cerebellar and basal ganglia outputs to primary motor cortex as revealed by retrograde transneuronal transport of herpes simplex virus type 1. J Neurosci. 1999;19:1446–1463. doi: 10.1523/JNEUROSCI.19-04-01446.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak FB, Anderson ME. Influence of globus pallidus on arm movements in monkeys. I. Effects of kainic acid-induced lesions. J Neurophysiol. 1984a;52:290–304. doi: 10.1152/jn.1984.52.2.290. [DOI] [PubMed] [Google Scholar]
- Horak FB, Anderson ME. Influence of globus pallidus on arm movements in monkeys. II. Effects of stimulation. J Neurophysiol. 1984b;52:305–322. doi: 10.1152/jn.1984.52.2.305. [DOI] [PubMed] [Google Scholar]
- Hore J, Meyer-Lohmann J, Brooks VB. Basal ganglia cooling disables learned arm movements of monkeys in the absence of visual guidance. Science. 1977;195:584–586. doi: 10.1126/science.402029. [DOI] [PubMed] [Google Scholar]
- Hore J, Vilis T. Arm movement performance during reversible basal ganglia lesions in the monkey. Exp Brain Res. 1980;39:217–228. doi: 10.1007/BF00237552. [DOI] [PubMed] [Google Scholar]
- Houk JC. Neurophysiology of frontal-subcortical loops. In: Lichter DG, Cummings JL, Gilford, editors. Frontal-subcortical circuits in psychiatry and neurology. New York: 2001. pp. 92–113. [Google Scholar]
- Inase M, Buford JA, Anderson ME. Changes in the control of arm position, movement, and thalamic discharge during local inactivation in the globus pallidus of the monkey. J Neurophysiol. 1996;75:1087–1104. doi: 10.1152/jn.1996.75.3.1087. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. The neural and behavioural organization of goal-directed movement. Oxford: Oxford University Press; 1988. [Google Scholar]
- Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. Building neural representations of habits. Science. 1999;286:1745–1749. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Wichern DW. Applied multivariate statistical analysis. Englewood Cliffs, New Jersey: Prentice Hall; 1982. [Google Scholar]
- Kato M, Kimura M. Effects of reversible blockade of basal ganglia on a voluntary arm movement. J Neurophysiol. 1992;68:1516–1534. doi: 10.1152/jn.1992.68.5.1516. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and Analysis: A Researcher's Handbook. Engelwood Cliffs, NJ: Prentice-Hall; 1973. [Google Scholar]
- Kimura M, Matsumoto N, Okahashi K, Ueda Y, Satoh T, Minamimoto T, Sakamoto M, Yamada H. Goal-directed, serial and synchronous activation of neurons in the primate striatum. Neuroreport. 2003;14:799–802. doi: 10.1097/00001756-200305060-00004. [DOI] [PubMed] [Google Scholar]
- Kliem MA, Wichmann T. A method to record changes in local neuronal discharge in response to infusion of small drug quantities in awake monkeys. J Neurosci Methods. 2004;138:45–49. doi: 10.1016/j.jneumeth.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Ghilardi MF, Mentis M, Barnes A, Veytsman M, Eidelberg D, Ghez C. Differential Cortical and Subcortical Activations in Learning Rotations and Gains for Reaching: A PET Study. J Neurophysiol. 2004;91:924–933. doi: 10.1152/jn.00675.2003. [DOI] [PubMed] [Google Scholar]
- Laitinen LV. Pallidotomy for Parkinson's diesease. Func Neurol. 1995;6:105–112. [PubMed] [Google Scholar]
- Laitinen LV, Bergenheim AT, Hariz MI. Leksell's posteroventral pallidotomy in the treatment of Parkinson's disease. J Neurosurg. 1992;76:53. doi: 10.3171/jns.1992.76.1.0053. [DOI] [PubMed] [Google Scholar]
- Lang AE, Lozano AM, Montgomery E, Duff J, Tasker R, Hutchinson W. Posteroventral medial pallidotomy in advanced Parkinson's disease. N Engl J Med. 1997;337:1036–1042. doi: 10.1056/NEJM199710093371503. [see comments] [DOI] [PubMed] [Google Scholar]
- Lonser RR, Corthesy ME, Morrison PF, Gogate N, Oldfield EH. Convection-enhanced selective excitotoxic ablation of the neurons of the globus pallidus internus for treatment of parkinsonism in nonhuman primates. J Neurosurg. 1999;91:294–302. doi: 10.3171/jns.1999.91.2.0294. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Velickovic M, Drafta C, Hesquijarosa A, Brin MF. Corticospinal excitability accompanying ballistic wrist movements in primary dystonia. Mov Disord. 2004;19:273–284. doi: 10.1002/mds.20017. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Obeso JA. The functions of the basal ganglia and the paradox of stereotaxic surgery in Parkinson's disease. Brain. 1994;117:877–897. doi: 10.1093/brain/117.4.877. [DOI] [PubMed] [Google Scholar]
- Martin JH, Ghez C. Pharmacological inactivation in the analysis of the central control of movement. J Neurosci Methods. 1999;86:145–159. doi: 10.1016/s0165-0270(98)00163-0. [DOI] [PubMed] [Google Scholar]
- Maxwell SE, Delaney HD. Designing experiments and analyzing data. A model comparison perspective. Belmont: Wadsworth; 1990. [Google Scholar]
- Mazzoni P, Hristova A, Krakauer JW. Why don't we move faster? Parkinson's disease, movement vigor, and implicit motivation. J Neurosci. 2007;27:7105–7116. doi: 10.1523/JNEUROSCI.0264-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer DE, Abrams DA, Kornblum S, Wright CE, Smith JEK. Optimality in human motor performance: Ideal control of rapid aimed movements. Psychol Review. 1988;95:340–370. doi: 10.1037/0033-295x.95.3.340. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Miller WC, DeLong MR. Altered tonic activity of neurons in the globus pallidus and subthalamic nucleus in the primate MPTP model of parkinsonism. In: Carpenter MB, Jayaraman A, editors. The Basal Ganglia II. New York: Plenum Press; 1987. pp. 415–427. [Google Scholar]
- Milner TE, Ijaz MM. The effect of accuracy constraints on three-dimensional movement kinematics. Neuroscience. 1990;35:365–374. doi: 10.1016/0306-4522(90)90090-q. [DOI] [PubMed] [Google Scholar]
- Milner TE. A model for the generation of movements requiring endpoint precision. Neuroscience. 1992;49:487–496. doi: 10.1016/0306-4522(92)90113-g. [DOI] [PubMed] [Google Scholar]
- Mink J. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Mink J, Thach W. Basal ganglia motor control. I. nonexclusive relation of pallidal discharge to five movement modes. J Neurophysiol. 1991a;65:273–300. doi: 10.1152/jn.1991.65.2.273. [DOI] [PubMed] [Google Scholar]
- Mink J, Thach W. Basal ganglia motor control. III. pallidal ablation: normal reaction time, muscle cocontraction, and slow movement. J Neurophysiol. 1991b;65:330–351. doi: 10.1152/jn.1991.65.2.330. [DOI] [PubMed] [Google Scholar]
- Miyachi S, Hikosaka O, Lu X. Differential activation of monkey striatal neurons in the early and late stages of procedural learning. Exp Brain Res. 2002;146:122–126. doi: 10.1007/s00221-002-1213-7. [DOI] [PubMed] [Google Scholar]
- Moro E, Lang AE, Strafella AP, Poon YY, Arango PM, Dagher A, Hutchison WD, Lozano AM. Bilateral globus pallidus stimulation for Huntington's disease. Ann Neurol. 2004;56:290–294. doi: 10.1002/ana.20183. [DOI] [PubMed] [Google Scholar]
- Mushiake H, Strick PL. Pallidal neuron activity during sequential arm movements. J Neurophysiol. 1995;74:2754–2758. doi: 10.1152/jn.1995.74.6.2754. [DOI] [PubMed] [Google Scholar]
- Niv Y, Daw ND, Joel D, Dayan P. Tonic dopamine: opportunity costs and the control of response vigor. Psychopharmacology (Berl) 2007;191:507–520. doi: 10.1007/s00213-006-0502-4. [DOI] [PubMed] [Google Scholar]
- Niv Y, Joel D, Dayan P. A normative perspective on motivation. Trends Cogn Sci. 2006;10:375–381. doi: 10.1016/j.tics.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Novak KE, Miller LE, Houk JC. Kinematic properties of rapid hand movements in a knob turning task. Exp Brain Res. 2000;132:419–433. doi: 10.1007/s002210000366. [DOI] [PubMed] [Google Scholar]
- Novak KE, Miller LE, Houk JC. The use of overlapping submovements in the control of rapid hand movements. Exp Brain Res. 2002;144:351–364. doi: 10.1007/s00221-002-1060-6. [DOI] [PubMed] [Google Scholar]
- Ostrem JL, Marks WJ, Jr, Volz MM, Heath SL, Starr PA. Pallidal deep brain stimulation in patients with cranial-cervical dystonia (Meige syndrome) Mov Disord. 2007 doi: 10.1002/mds.21580. [DOI] [PubMed] [Google Scholar]
- Peigneux P, Maquet P, Meulemans T, Destrebecqz A, Laureys S, Degueldre C, Delfiore G, Aerts J, Luxen A, Franck G, Van der Linden M, Cleeremans A. Striatum forever, despite sequence learning variability: a random effect analysis of PET data. Hum Brain Mapp. 2000;10:179–194. doi: 10.1002/1097-0193(200008)10:4<179::AID-HBM30>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penhune VB, Doyon J. Dynamic cortical and subcortical networks in learning and delayed recall of timed motor sequences. J Neurosci. 2002;22:1397–1406. doi: 10.1523/JNEUROSCI.22-04-01397.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfann KD, Buchman AS, Comella CL, Corcos DM. Control of movement distance in Parkinson's disease. Mov Disord. 2001;16:1048–1065. doi: 10.1002/mds.1220. [DOI] [PubMed] [Google Scholar]
- Pfann KD, Penn RD, Shannon KM, Corcos DM. Pallidotomy and bradykinesia: implications for basal ganglia function. Neurology. 1998;51:796–803. doi: 10.1212/wnl.51.3.796. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Clark J, Pare-Blagoev EJ, Shohamy D, Creso Moyano J, Myers C, Gluck MA. Interactive memory systems in the human brain. Nature. 2001;414:546–550. doi: 10.1038/35107080. [DOI] [PubMed] [Google Scholar]
- Pope P, Wing AM, Praamstra P, Miall RC. Force related activations in rhythmic sequence production. Neuroimage. 2005;27:909–918. doi: 10.1016/j.neuroimage.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Robinson S, Sandstrom SM, Denenberg VH, Palmiter RD. Distinguishing whether dopamine regulates liking, wanting, and/or learning about rewards. Behav Neurosci. 2005;119:5–15. doi: 10.1037/0735-7044.119.1.5. [DOI] [PubMed] [Google Scholar]
- Romanelli P, Esposito V, Schaal DW, Heit G. Somatotopy in the basal ganglia: experimental and clinical evidence for segregated sensorimotor channels. Brain Res Brain Res Rev. 2005;48:112–128. doi: 10.1016/j.brainresrev.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Rossetti Y, Stelmach G, Desmurget M, Prablanc C, Jeannerod M. The effect of viewing the static hand prior to movement onset on pointing kinematics and variability. Exp Brain Res. 1994;101:323–330. doi: 10.1007/BF00228753. [DOI] [PubMed] [Google Scholar]
- Sainburg RL, Lateiner JE, Latash ML, Bagesteiro LB. Effects of altering initial position on movement direction and extent. J Neurophysiol. 2003;89:401–415. doi: 10.1152/jn.00243.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Noll DC, Chintalapati P. Bilateral basal ganglia activation associated with sensorimotor adaptation. Exp Brain Res. 2006;175:544–555. doi: 10.1007/s00221-006-0571-y. [DOI] [PubMed] [Google Scholar]
- Sheridan MR, Flowers KA. Movement variability and bradykinesia in Parkinson's disease. Brain. 1990;113:1149–1161. doi: 10.1093/brain/113.4.1149. [DOI] [PubMed] [Google Scholar]
- Shin JC, Aparicio P, Ivry RB. Multidimensional sequence learning in patients with focal basal ganglia lesions. Brain Cogn. 2005;58:75–83. doi: 10.1016/j.bandc.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Siegel S. Nonparametric statistics for the behavioral sciences. New York: McGraw-Hill; 1956. [Google Scholar]
- Smith MA, Brandt J, Shadmehr R. Motor disorder in Huntington's disease begins as a dysfunction in error feedback control. Nature. 2000;403:544–549. doi: 10.1038/35000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington's disease but not cerebellar degeneration. J Neurophysiol. 2005;93:2809–2821. doi: 10.1152/jn.00943.2004. [DOI] [PubMed] [Google Scholar]
- Soechting JF. Effect of target size on spatial and temporal characteristics of a pointing movement in man. Exp Brain Res. 1984;54:121–132. doi: 10.1007/BF00235824. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. New York: W.H. Freeman & Co; 1981. [Google Scholar]
- Spraker MB, Yu H, Corcos DM, Vaillancourt DE. Role of individual basal ganglia nuclei in force amplitude generation. J Neurophysiol. 2007 doi: 10.1152/jn.00239.2007. 00239.02007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr PA, Rau GM, Davis V, Marks WJ, Jr, Ostrem JL, Simmons D, Lindsey N, Turner RS. Spontaneous pallidal neuronal activity in human dystonia: comparison with Parkinson's disease and normal macaque. J Neurophysiol. 2005;93:3165–3176. doi: 10.1152/jn.00971.2004. [DOI] [PubMed] [Google Scholar]
- Szabo J, Cowan WM. A stereotaxic atlas of the brain of the cynomologus monkey. J Comp Neurol. 1984;222:265–309. doi: 10.1002/cne.902220208. [DOI] [PubMed] [Google Scholar]
- Takikawa Y, Kawagoe R, Itoh H, Nakahara H, Hikosaka O. Modulation of saccadic eye movements by predicted reward outcome. Exp Brain Res. 2002;142:284–291. doi: 10.1007/s00221-001-0928-1. [DOI] [PubMed] [Google Scholar]
- Thompson B. Canonical correlation analysis: Uses and Interpretation. Beverly Hills, CA: SAGE University Paper; 1984. [Google Scholar]
- Todorov E, Jordan MI. Optimal feedback control as a theory of motor coordination. Nat Neurosci. 2002;5:1226–1235. doi: 10.1038/nn963. [DOI] [PubMed] [Google Scholar]
- Turner R, Grafton S, McIntosh A, DeLong M, Hoffman J. The functional anatomy of parkinsonian bradykinesia. Neuroimage. 2003a;19:163–179. doi: 10.1016/s1053-8119(03)00059-4. [DOI] [PubMed] [Google Scholar]
- Turner RS, Anderson ME. Pallidal discharge related to the kinematics of reaching movements in two dimensions. J Neurophysiol. 1997;77:1051–1074. doi: 10.1152/jn.1997.77.3.1051. [DOI] [PubMed] [Google Scholar]
- Turner RS, Anderson ME. Context-dependent modulation of movement-related discharge in the primate globus pallidus. J Neurosci. 2005;25:2965–2976. doi: 10.1523/JNEUROSCI.4036-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RS, Desmurget M, Grethe J, Crutcher MD, Grafton ST. Motor subcircuits mediating the control of movement extent and speed. J Neurophysiol. 2003b;90:3958–3966. doi: 10.1152/jn.00323.2003. [DOI] [PubMed] [Google Scholar]
- Turner RS, Grafton ST, Hoffman JM, Votaw JR, DeLong MR. Motor subcircuits mediating the control of movement velocity: a PET study. J Neurophysiol. 1998;80:2162–2176. doi: 10.1152/jn.1998.80.4.2162. [DOI] [PubMed] [Google Scholar]
- Turner RS, Owens JWM, Anderson ME. Directional variation of spatial and temporal characteristics of limb movements made by monkeys in a two-dimensional work space. J Neurophysiol. 1995;74:684–697. doi: 10.1152/jn.1995.74.2.684. [DOI] [PubMed] [Google Scholar]
- Uno M, Yoshida M. Monosynaptic inhibition of thalamic neurons produced by stimulation of the pallidal nucleus in cats. Brain Res. 1975;99:377–380. doi: 10.1016/0006-8993(75)90040-2. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Mayka MA, Thulborn KR, Corcos DM. Subthalamic nucleus and internal globus pallidus scale with the rate of change of force production in humans. Neuroimage. 2004a;23:175–186. doi: 10.1016/j.neuroimage.2004.04.040. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Prodoehl J, Verhagen Metman L, Bakay RA, Corcos DM. Effects of deep brain stimulation and medication on bradykinesia and muscle activation in Parkinson's disease. Brain. 2004b;127:491–504. doi: 10.1093/brain/awh057. [DOI] [PubMed] [Google Scholar]
- Van Der Graaf FH, De Jong BM, Maguire RP, Meiners LC, Leenders KL. Cerebral activation related to skills practice in a double serial reaction time task: striatal involvement in random-order sequence learning. Brain Res Cogn Brain Res. 2004;20:120–131. doi: 10.1016/j.cogbrainres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Vindras P, Desmurget M, Prablanc C, Viviani P. Pointing errors reflect biases in the perception of the initial hand position. J Neurophysiol. 1998;79:3290–3294. doi: 10.1152/jn.1998.79.6.3290. [DOI] [PubMed] [Google Scholar]
- Vindras P, Desmurget M, Viviani P. Error parsing in visuomotor pointing reveals independent processing of amplitude and direction. J Neurophysiol. 2005;94:1212–1224. doi: 10.1152/jn.01295.2004. [DOI] [PubMed] [Google Scholar]
- Vindras P, Viviani P. Altering the visuomotor gain: Evidence that motor plans deal with vector quantities. Exp Brain Res. 2002;147:280–295. doi: 10.1007/s00221-002-1211-9. [DOI] [PubMed] [Google Scholar]
- Vogt C. Quelques considérations générales sur le syndrôme du corps strié. J Psychol Neurol (Leipzig) 1911;19:479–488. [Google Scholar]
- Weiss P, Stelmach GE, Hefter H. Programming of a movement sequence in Parkinson's disease. Brain. 1997;120:91–102. doi: 10.1093/brain/120.1.91. [DOI] [PubMed] [Google Scholar]
- Wenger KK, Musch KL, Mink JW. Impaired reaching and grasping after focal inactivation of globus pallidus pars interna in the monkey. J Neurophysiol. 1999;82:2049–2060. doi: 10.1152/jn.1999.82.5.2049. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Functional and pathophysiological models of the basal ganglia. Curr Opin Neurobiol. 1996;6:751–758. doi: 10.1016/s0959-4388(96)80024-9. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Kliem MA. Neuronal activity in the primate substantia nigra pars reticulata during the performance of simple and memory-guided elbow movements. J.Neurophysiol. 2004;91:815–827. doi: 10.1152/jn.01180.2002. [DOI] [PubMed] [Google Scholar]
- Wilson SAK. An experimental research into the anatomy and physiology of the corpus striatum. Brain. 1914;36:427–492. [Google Scholar]
- Winer BJ. Statistical principles in experimental design. New York: McGraw-Hill; 1971. [Google Scholar]
- Winer BJ, Brown DR, Michels KM. Statistical principals in experimental design. New York: McGraw-Hill; 1991. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.