Abstract
Objective:
To characterize change in depressive symptoms before and after the onset of dementia in Alzheimer disease (AD).
Method:
We used data from the Chicago Health and Aging Project, a longitudinal cohort study of risk factors for AD in a geographically defined population of old people. Two subsets were analyzed. In 357 individuals who developed incident AD during the study, self-report of depressive symptoms (Center for Epidemiologic Studies Depression Scale) was obtained at 3-year intervals for a mean of 8 to 9 years. In 340 individuals who agreed to annual data collection, informant report of depressive symptoms (Hamilton Depression Rating Scale) was obtained for a mean of 3 years after a diagnosis of AD (n = 107), mild cognitive impairment (n = 81), or no cognitive impairment (n = 152).
Results:
The incident AD group reported a barely perceptible increase in depressive symptoms during 6 to 7 years of observation before the diagnosis (0.04 symptoms per year) and no change during 2 to 3 years of observation after the diagnosis except for a slight decrease in positive affect. In those with annual follow-up, neither AD nor its precursor, mild cognitive impairment, was associated with change in informant report of depressive symptoms during a mean of 3 years of observation.
Conclusion:
Depressive symptoms show little change during the development and progression of AD to a moderate level of dementia severity.
GLOSSARY
- AD
= Alzheimer disease;
- CES-D
= Center for Epidemiologic Studies Depression Scale;
- MCI
= mild cognitive impairment.
Depressive symptoms predict the development of Alzheimer disease (AD)1-6 and its precursor, mild cognitive impairment (MCI),7-9 and are commonly reported in persons with these conditions.10,11 Yet the bases of the association between depression and AD are not known. Understanding the association is likely to require a thorough understanding of the temporal course of depressive symptoms during the evolution of dementia in AD, but few longitudinal studies have been done.12,13 Further complicating matters, the accuracy of self-report about depressive symptoms is compromised by dementia,14 making it difficult to track symptoms during the transition from no cognitive impairment to dementia.
The objective of the present study was to characterize change in depressive symptoms prior to and following the onset of dementia in AD. Data are from the Chicago Health and Aging Project, a longitudinal study of aging and AD in a geographically defined biracial community. At 3-year intervals, the entire population completed a brief self-report measure of depressive symptoms, and persons deemed dementia-free at a previous wave of data collection were sampled for clinical classification of dementia and AD. In primary analyses, we characterized change in self-reported depressive symptoms before and after dementia onset in AD. In addition, following the clinical evaluation, depressive symptoms were assessed annually by informant report in a subset of participants. In secondary analyses, we assessed change in informant report of depression following dementia onset in AD.
METHODS
Participants.
Subjects are from the Chicago Health and Aging Project, a longitudinal study of aging and AD.15 The study began in 1993 with a census of a geographically defined region of Chicago. Persons aged 65 years or older were interviewed and a stratified random sample underwent a clinical evaluation. Three years later, the population interview was repeated and a stratified random sample of those deemed free of dementia in the initial wave of data collection underwent clinical evaluation. Those 2 steps (population interview, clinical evaluation of previously dementia-free subset) have been repeated thereafter at 3-year intervals, with the fifth wave of data collection in progress at the time of these analyses. Beginning in 2001, persons who reached age 65 after the original census were also enrolled.
Analyses are based on 2 subsets of the population. Eligibility in the first group required a diagnosis of AD on clinical evaluation (see next section) after being dementia-free in an earlier wave of data collection. Of 2,592 individuals invited to participate in the second, third, fourth, or fifth clinical evaluation (in progress at time of these analyses), 1,745 (67.3%) completed the evaluation; 357 of these persons met criteria for AD, and all 357 had longitudinal data on depressive symptoms, with a mean of 3.6 valid scores per individual and only 2.1% of scores (28/1,317) missing for reasons other than death. Primary analyses are based on this group.
The second group consisted of individuals who agreed to undergo annual evaluations in a study of AD consequences. All persons who completed a clinical evaluation in the parent study (see next section) between April 1, 2000, and March 21, 2003, were recruited plus persons evaluated before April 2000 and found to have AD. Of 530 eligible, 413 (77.9%) agreed to participate and completed baseline evaluation procedures. There were 35 deaths before the first annual follow-up. Of the 378 survivors, 340 (89.9%) had longitudinal data on depressive symptoms, with a mean of 4.0 annual evaluations per individual, and 38 were lost to follow-up.
Standard protocol approvals, registrations, and patient consents.
After complete description of the study, written informed consent was obtained from all subjects. The study was approved by the Institutional Review Board of Rush University Medical Center.
Clinical evaluation.
Persons sampled for clinical classification underwent a uniform structured evaluation that included a medical history, complete neurologic examination, detailed cognitive performance testing, standard laboratory studies, and brain MRI in those with cognitive impairment and inconclusive clinical evidence of stroke. On the basis of this evaluation, an experienced physician diagnosed dementia and AD using the criteria of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association.16 They require a history of cognitive decline and impairment in at least 2 cognitive domains, 1 of which must be memory to meet AD criteria. People who did not meet dementia criteria but had impairment in 1 or more cognitive domains were classified as MCI. Individuals meeting these criteria have been shown to have intermediate levels of mortality,15,17 cognitive decline,17,18 and dementia-related pathologic burden19 compared to no cognitive impairment and dementia groups. Because distinguishing prevalent and incident MCI is difficult in the Chicago Health and Aging Project, the condition was only examined in secondary analyses.
Assessment of depressive symptoms.
Self-report of depressive symptoms was assessed in the population interview at 3-year intervals with a 10-item version20 of the Center for Epidemiologic Studies Depression Scale (CES-D).21 On each item, a brief statement was read and the participant indicated whether he or she had felt that way much of the past week. The score is the number of symptoms reported, ranging from 0 to 10. This version of the CES-D has been shown to closely correspond to the original 20-item scale20 and to predict incidence of AD,5 rate of cognitive decline,22 and risk of mortality.23 In secondary analyses, we formed subscores of depressed affect (3 items), positive affect (2 items), somatic complaint (3 items), and interpersonal problem (2 items) based on factor analyses of this20 and the original24 version of the scale.
In the subgroup with annual evaluation, data on affect were obtained by interviewing a knowledgeable informant, usually the spouse or child of the participant. Depressive symptoms were assessed with an informant report version25 of the Hamilton Depression Rating Scale.26 Three of the original 17 items were dropped: the retardation and agitation items because they require interviewer observation, and the genital symptoms item because of the advanced age of the participants. Total score on the 14-item scale could range from 0 to 42. The scale had good internal consistency as evidenced by a Cronbach coefficient α of 0.82 at baseline, and has previously been shown to have good interrater reliability.14 Based on previous factor analytic research,24 we created 4 subscores: depression (4 items), anxiety (3 items), insomnia (3 items), and somatic complaint (3 items).
Data analysis.
We analyzed change in depression scores using generalized estimating equation models27 with a logit-link function of the proportion of depressive symptoms endorsed and binomial error structure. The analyses of the incident AD group estimated annual rate of change in depression score separately before and after the diagnosis of AD. Additional models separately tested whether race, sex, age, or education modified change before or after the diagnosis and analyzed depression subscores. In analyses of the group with annual follow-up, those with no cognitive impairment formed a reference group that was contrasted with MCI and AD groups. The models included a term for time in years since baseline, indicators for MCI and AD, and the interactions of the indicators with time. The initial analysis used the Hamilton Depression Rating Scale score as the outcome; subsequent analyses tested for interactions with demographic variables and used Hamilton Depression Rating Scale subscores as outcomes. All models controlled for age, sex, race, and education.
RESULTS
Self-report of depressive symptoms.
Initial analyses focused on a group of 357 individuals who developed AD during the course of the study. At the time of diagnosis, they had a mean age of 82.5 years (SD 5.7) and a mean of 12.0 years of education (SD 3.5), 60.5% were women, and 59.6% were African American. They had a mean Mini-Mental State Examination score of 20.4 (SD 5.2), indicative of mild dementia. They had participated in the study for a mean of 8 to 9 years.
At the time of diagnosis, CES-D scores were positively skewed, ranging from 0 to 10 (median 1.0; interquartile range 3.0). To analyze change in these scores, we constructed a generalized estimating equation model that allowed rate of change to vary before and after the diagnosis of incident AD. This and all subsequent models controlled for age, sex, race, and education. In the initial analysis, scores increased during a mean of 6.2 years of observation before the diagnosis of AD (estimate = 0.04, SE = 0.01, p = 0.004) and showed no change during a mean of 2.4 years of observation after the diagnosis (estimate = −0.00, SE = 0.02, p = 0.913). Figure 1 shows the 9-year path of change in CES-D score predicted for a typical participant who was diagnosed with AD at year 6, expressed in raw score units. Depressive symptoms increased prior to dementia onset, but only at a rate of approximately 0.04 unit per year, an increase of about 1 symptom per 25 years.
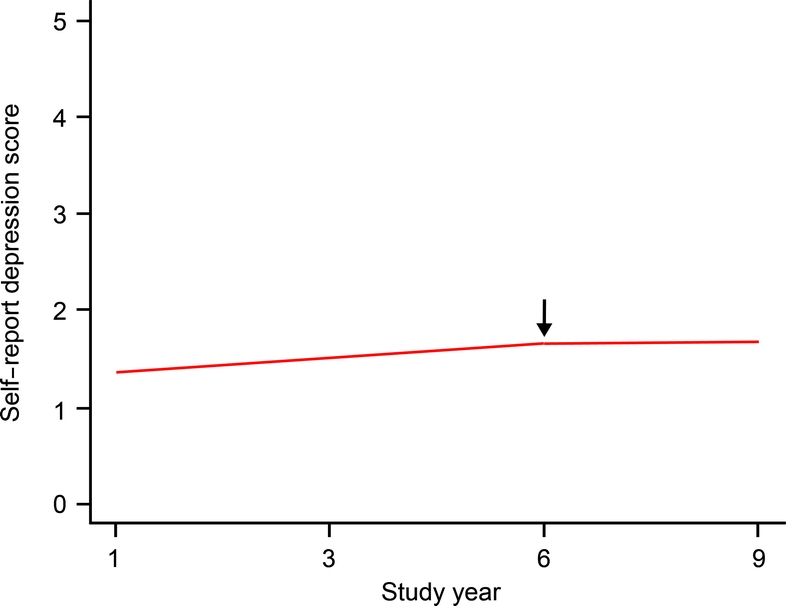
Figure 1 Change in self-report of depressive symptoms.
Predicted 9-year path of change in self-report of depressive symptoms in a typical participant diagnosed with Alzheimer disease at year 6.
Because little is known about depression and AD in different racial subgroups, we repeated the initial analysis with terms for the interactions of race with prediagnosis time and postdiagnosis time. Neither interaction was significant. In subsequent analyses, there was no evidence that sex, age, or education modified the trajectory of depressive symptoms before or after AD was diagnosed.
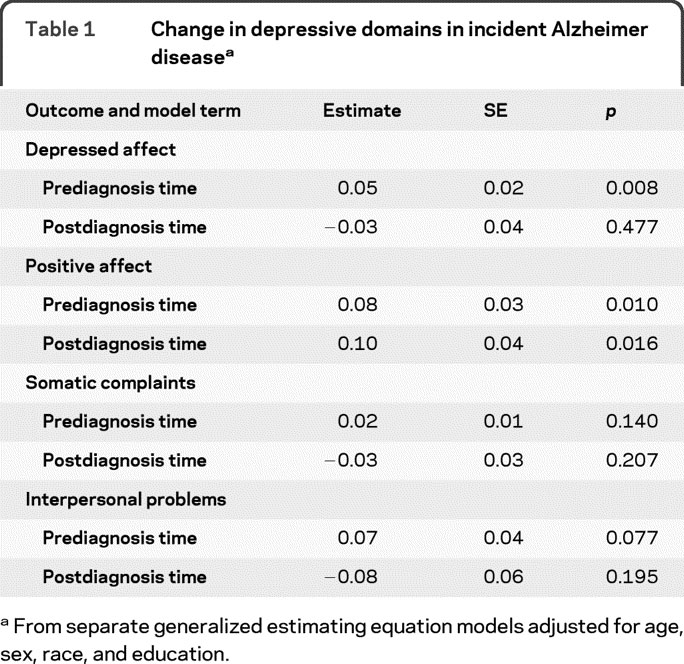
To determine whether some aspects of depression were selectively affected, we repeated the initial model using depression subscores instead of the total score (table 1). In these analyses, report of depressed affect increased slightly before dementia onset but not after it; report of positive affect decreased slightly before and after dementia onset; and no change was reported in somatic or interpersonal depressive symptoms.
Table 1 Change in depressive domains in incident Alzheimer disease
Informant report of depressive symptoms.
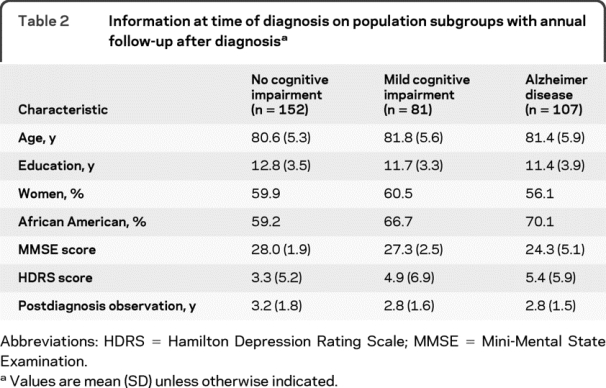
Dementia in AD appears to reduce the accuracy of self-report about depression even with scales that minimize respondent burden.14 Because of this potential bias, we conducted additional analyses of change in depressive symptoms ascertained through annual informant report in a subgroup of 340 participants followed for a mean of 3.0 years (SD 1.7, range 0.8–8.7). At the baseline of the substudy, 107 had AD, 81 had its precursor, MCI, and 152 had no cognitive impairment. As shown by the Mini-Mental State Examination scores in table 2, dementia in the AD group was predominantly mild.
Table 2 Information at time of diagnosis on population subgroups with annual follow-up after diagnosis
At baseline, scores on the Hamilton Depression Rating Scale were positively skewed, ranging from 0 to 35 (median 2; interquartile range 6), with higher values indicating more depression. In a generalized estimating equation model (table 3), persons with AD, but not those with MCI, had higher baseline Hamilton scores than the no cognitive impairment group. However, Hamilton scores did not change during follow-up in any of the groups, as shown by the terms for time, MCI × time, and AD × time in table 3 and graphically in figure 2. In separate subsequent analyses, there was no evidence that race, sex, age, or education modified these findings.
Table 3 Change in informant report of depressive symptoms in mild cognitive impairment and Alzheimer disease
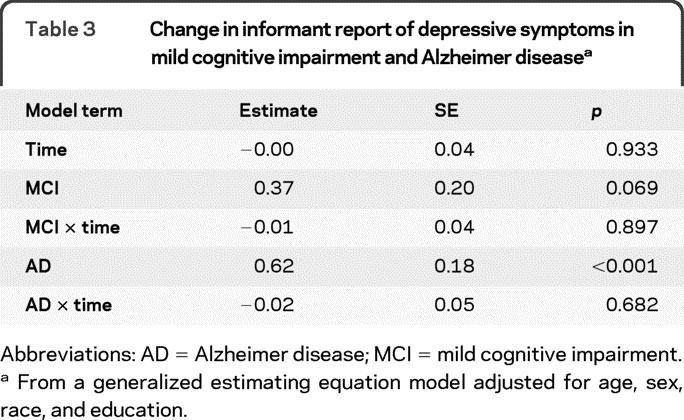
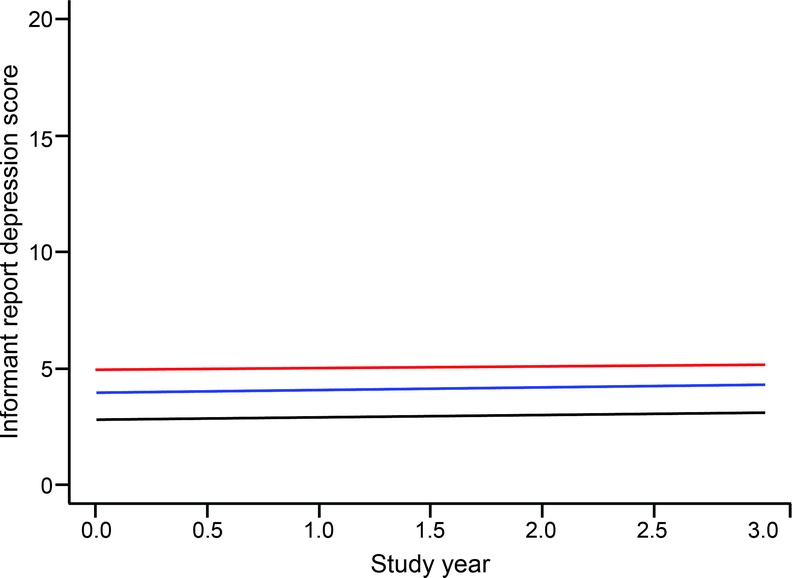
Figure 2 Change in informant report of depressive symptoms
Predicted 3-year paths of change in informant report of depressive symptoms in persons with no cognitive impairment (black line), mild cognitive impairment (blue line), or Alzheimer disease (red line), adjusted for age, sex, race, and education.
To see if some depressive symptoms were selectively affected, we repeated the original analysis with specific Hamilton Depression Rating Scale symptom domain scores as outcomes in place of the total Hamilton score. In these analyses, there was no change in any of 3 subgroups (no cognitive impairment, MCI, AD) on any of the 4 domain scores (depression, anxiety, insomnia, somatic complaint).
DISCUSSION
In a biracial urban population, we characterized change in depressive symptoms in older persons as they developed AD and the disease progressed. There was a barely perceptible increase in symptoms before the diagnosis and even less change after it, with comparable results in African American and white subjects. The findings suggest that AD has little systematic effect on depressive symptoms.
Knowledge about depressive symptoms in AD comes mainly from cross-sectional studies. Thus, in defined populations, persons with MCI, dementia, or AD have generally been found to have more depressive symptoms than persons without cognitive impairment,10,11 but these data provide limited insight into the basis of the association between depression and dementia. We are aware of only 2 previous studies that tracked depressive symptoms during the development of AD. One study of older Catholic clergy found no change in self-report of depressive symptoms (10-item, binary response CES-D) during a mean of 3.9 years before the incidence of AD and 3.2 years before the incidence of MCI.12 The other study, like the present one, took place in a defined population and found an increase in self-report of depressive symptoms prior to dementia onset.13 During up to 14 years of observation, there was a mean increase of about 3 points on the original 20-item version of the CES-D, which has 4 response options (scored 0–3) and a 0–60 score range. This represents an increase of about 0.21 unit per year, which is 0.4% of the scale range. By comparison, the average annual increase before AD was diagnosed in the present study was 0.04 unit, which is also 0.4% of the scale range. Taken together, therefore, these 3 studies are in substantial agreement in suggesting that very little change in depressive symptoms occurs prior to dementia onset in AD.
There has been little longitudinal research on depressive symptoms following dementia incidence in AD. In the Religious Orders Study, there was no increase in self-report of depressive symptoms during a mean of 2.8 years of observation after AD incidence.12 The present results replicate and extend those findings by showing virtually no change in depressive symptoms following dementia onset by informant report or self-report in either African American or white subjects.
These data beg the question of why an illness as devastating as AD has so little impact on depressive symptoms. That depressive symptoms do not appear to systematically increase in old age28,29 indicates that chronic illness does not invariably depress mood. In addition, as AD develops, poor memory and impaired executive control are likely to disrupt the continuity of mood states and their ability to regulate behavior. That is, incipient AD may disrupt depressive behavior in somewhat the same way that it disrupts more adaptive functions. Consistent with this idea, longitudinal studies of patients with AD identified in medical settings and followed longer than the present study suggest that depressive symptoms may eventually decrease in the disease.30,31
The relation of depression to the pathologic features of AD has been difficult to establish. In a small study of persons without dementia, depression was associated with elevated cortical uptake of Pittsburgh Compound B, suggesting elevated β-amyloid.32 In persons with AD, a history of depression has been associated with relatively higher levels of AD pathologic lesions.33,34 However, in 2 large clinical–pathologic studies of persons with and without dementia, level of depressive symptoms in the last years of life was not related to the pathologic hallmarks of AD or other common dementias.35,36 Thus, although AD37 and cerebrovascular disease38 may contribute to late-life depression, current data suggest that at least some of the association between depressive symptoms and subsequent cognitive dysfunction1-9 reflects factors other than the pathologic lesions traditionally linked with late-life dementia. Consistent with this idea, depressive symptoms have been associated with reduced density of dendrites and spines in the CA3 region of the hippocampus,39 a characteristic finding in animal models of chronic stress.40 Understanding the neurobiologic pathways linking depressive symptoms to cognitive impairment could suggest novel strategies for delaying symptom onset in dementia.
This study has several strengths. Participants were sampled from a defined population, making it likely that a broad spectrum of incident disease was represented and that the findings are generalizable. Diagnoses of MCI, dementia, and AD were based on a uniform clinical evaluation and widely used criteria applied by an experienced physician, reducing the likelihood of diagnostic error. The availability of psychometrically established measures of depressive symptoms by both self and informant report plus the high rate of follow-up participation enhanced our ability to identify even subtle nonlinear changes in depression. An important limitation is that dementia severity in the subjects with incident AD was mostly mild to moderate so that these results do not rule out change in depressive symptoms during the end stages of the disease.
ACKNOWLEDGMENT
The authors thank the residents of Morgan Park, Washington Heights, and Beverly who participated in the study. They also thank Ann Marie Lane for community development and oversight of project coordination; Michelle Bos, Holly Hadden, Flavio LaMorticella, and Jennifer Tarpey for coordination of the study; Todd Beck for analytic programming; and the staff of the Rush Institute for Healthy Aging.
DISCLOSURE
Dr. Wilson serves as a Consulting Editor for Aging, Neuropsychology, and Cognition and Psychology and Aging and receives research support from the NIH/NIA (R01AG024871 [Principal Investigator], P30AG10161 [Coinvestigator], R01AG11101 [Coinvestigator], R01AG15819 [Coinvestigator], R01AG021972 [Coinvestigator], U24AG026395 [Coinvestigator], R01AG017917 [Coinvestigator], R01AG009966 [Coinvestigator], and U01AG016979 [Coinvestigator]) and NIH/NIEHS (ES10902 [Coinvestigator]). G. Hoganson receives research support from the NIH/NIA (P30AG10161 [Study Coordinator]). Dr. Rajan receives research support from the NIH (R01AG032247 [Biostatistician], R01AG11101 [Biostatistician], R01AG09966 [Biostatistician], R01ES10902–01 [Biostatistician], and K23AG030944-0251 [Biostatistician]). Dr. Barnes serves on the editorial board of the Journal of Aging and Health and receives research support from the NIH (R01AG022018 [Principal Investigator], R01NR009543 [Neuropsychologist], P30AG010161 [Coinvestigator], R01ES010902 [Coinvestigator], R01AG031553 [Neuropsychologist], and R01AG032247 [Coinvestigator]) and the Alzheimer's Association. Dr. Mendes de Leon serves as an Associate Editor of the Journals of Gerontology Social Sciences and on the editorial boards of Psychosomatic Medicine, the Journal of Aging and Health, the International Journal of Behavioral Medicine, and Archives of Internal Medicine and receives research support from the NIH (R01 ES010902 [Principal Investigator], R01 AG032247 [Principal Investigator], R01 AG011101 [Coinvestigator], R01 HL084209 [Coinvestigator], AG 022018 [Coinvestigator], and NIH AG 033172 [Coinvestigator]). Dr. Evans served on a Data Monitoring Committee for Eli Lily and Company and receives research support from the NIH (AG11101 [Principal Investigator], AG09966 [Principal Investigator], AG030146 [Principal Investigator], AG10161 [Coinvestigator], AG021972 [Coinvestigator], ES10902 [Coinvestigator], NR009543 [Coinvestigator], HL084209 [Coinvestigator], AG036650 [Principal Investigator], and AG12505 [Coinvestigator]).
Address correspondence and reprint requests to Dr. Robert S. Wilson, Rush Alzheimer's Disease Center, Rush University Medical Center, 600 South Paulina St., Suite 1038, Chicago, IL 60612 rwilson@rush.edu
Editorial, page 12
See also pages 27 and 35
Study funding: Supported by NIH (NIA AG11101, NIA AG10161, and NIEHS ES 10902). The organizations funding this study had no role in the design and conduct of the study; in the collection, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript. Dr. Wilson had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure: Author disclosures are provided at the end of the article.
Received October 22, 2009. Accepted in final form February 3, 2010.
REFERENCES
- 1.Berger AK, Fratiglioni L, Forsell Y, Winbald B, Backman L. The occurrence of depressive symptoms in the preclinical phase of AD: a population-based study. Neurology 1999;53:1998–2002. [DOI] [PubMed] [Google Scholar]
- 2.Devanand DP, Sano M, Tang MX, et al. Depressed mood and the incidence of Alzheimer's disease in the elderly living in the community. Arch Gen Psychiatry 1996;53:175–182. [DOI] [PubMed] [Google Scholar]
- 3.Gatz JL, Tyas SL, St. John P, Montgomery P. Do depressive symptoms predict Alzheimer's disease and dementia? J Gerontol A Biol Sci Med Sci 2005;60:744–747. [DOI] [PubMed] [Google Scholar]
- 4.Modrego PJ, Ferrandez J. Depression in patients with mild cognitive impairment increases the risk of developing dementia of Alzheimer type: a prospective cohort study. Arch Neurol 2004;61:1290–1293. [DOI] [PubMed] [Google Scholar]
- 5.Wilson RS, Barnes LL, Mendes de Leon CF, et al. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology 2002;59:364–370. [DOI] [PubMed] [Google Scholar]
- 6.Wilson RS, Krueger KR, Arnold SE, et al. Loneliness and risk of Alzheimer's disease. Arch Gen Psychiatry 2007;64:234–240. [DOI] [PubMed] [Google Scholar]
- 7.Barnes DE, Alexopoulos GS, Lopez OL, Williamson JD, Yaffe K. Depressive symptoms, vascular disease, and mild cognitive impairment. Arch Gen Psychiatry 2006;63:273–280. [DOI] [PubMed] [Google Scholar]
- 8.Geda YE, Knopman DS, Mrazek DA, et al. Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment: a prospective cohort study. Arch Neurol 2006;63:435–440. [DOI] [PubMed] [Google Scholar]
- 9.Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA. Chronic distress and incidence of mild cognitive impairment. Neurology 2007;68:2085–2092. [DOI] [PubMed] [Google Scholar]
- 10.Lyketsos CG, Lopez O, Jones C, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA 2002;288:1475–1483. [DOI] [PubMed] [Google Scholar]
- 11.Geda YE, Roberts RO, Knopman DS, et al. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging. Arch Gen Psychiatry 2008;65:1193–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson RS, Arnold SE, Beck TL, Bienias JL, Bennett DA. Change in depressive symptoms during the prodromal phase of Alzheimer disease. Arch Gen Psychiatry 2008;65:439–446. [DOI] [PubMed] [Google Scholar]
- 13.Amieva H, Goff ML, Millet X, et al. Prodromal Alzheimer's disease: successive emergence of the clinical symptoms. Ann Neurol 2008;64:492–498. [DOI] [PubMed] [Google Scholar]
- 14.Gilley DW, Wilson RS, Fleischman DA, Harrison DW, Goetz CG, Tanner CM. Impact of Alzheimer's-type dementia and information source on the assessment of depression. Psychol Assessment 1995;7:42–48. [Google Scholar]
- 15.Wilson RS, Aggarwal NT, Barnes LL, Bienias JL, Mendes de Leon CF, Evans DA. Biracial population study of mortality in mild cognitive impairment and AD. Arch Neurol 2009;66:767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer's disease: report of the NINCDS/ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 17.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology 2002;59:198–205. [DOI] [PubMed] [Google Scholar]
- 18.Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA. Mild cognitive impairment: risk of Alzheimer's disease and rate of cognitive decline. Neurology 2006;67:441–445. [DOI] [PubMed] [Google Scholar]
- 19.Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer's disease pathology and cerebral infarctions. Neurology 2005;64:834–841. [DOI] [PubMed] [Google Scholar]
- 20.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D depression symptoms index. J Aging Health 1993;5:179–193. [DOI] [PubMed] [Google Scholar]
- 21.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- 22.Wilson RS, Mendes de Leon CF, Bennett DA, Bienias JL, Evans DA. Depressive symptoms and cognitive decline in a community population of older persons. J Neurol Neurosurg Psychiatry 2004;75:126–129. [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson RS, Bienias JL, Mendes de Leon CF, Evans DA, Bennett DA. Negative affect and mortality in older persons. Am J Epidemiol 2003;158:827–835. [DOI] [PubMed] [Google Scholar]
- 24.Shafer AB. Meta-analysis of the factor structures of 4 depression questionnaires: Beck, CES-D, Hamilton, and Zung. J Clin Psychol 2006;62:123–146. [DOI] [PubMed] [Google Scholar]
- 25.Williams JBW. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry 1989;45:742–747. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zegar SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics 1988;44:1049–1060. [PubMed] [Google Scholar]
- 28.Davey A, Halverson CF, Zonderman A, Costa PT Jr. Change in depressive symptoms in the Baltimore Longitudinal Study of Aging. J Gerontol B Psychol Sci Soc Sci 2004;59:P270–P277. [DOI] [PubMed] [Google Scholar]
- 29.Barefoot JC, Mortensen EL, Helms MJ, Avlund K, Schroll M. A longitudinal study of gender differences in depressive symptoms from age 50 to 80. Psychol Aging 2001;16:342–345. [DOI] [PubMed] [Google Scholar]
- 30.Holtzer R, Scarmeas N, Wegesin DJ, et al. Depressive symptoms in Alzheimer's disease: natural course and temporal relation to function and cognitive status. J Am Geriatr Soc 2005;53:2083–2089. [DOI] [PubMed] [Google Scholar]
- 31.Li YS, Meyer JS, Thornby J. Longitudinal follow-up of depressive symptoms among normal versus cognitively impaired elderly. Int J Geriatr Psychiatry 2001;16:718–727. [DOI] [PubMed] [Google Scholar]
- 32.Butters MA, Klunk WE, Mathis CA, et al. Imaging Alzheimer's pathology in late-life depression with PET and Pittsburgh Compound-B. Alzheimer Dis Assoc Disord 2008;22:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rapp MA, Schnaider-Beeri M, Grossman HT, et al. Increased hippocampal plaques and tangles in patients with Alzheimer's disease with a lifetime history of major depression. Arch Gen Psychiatry 2006;63:161–167. [DOI] [PubMed] [Google Scholar]
- 34.Rapp MA, Schnaider-Beeri M, Purohit DP, Perl DP, Haroutunian V, Sano M. Increased neurofibrillary tangles in patients with Alzheimer's disease with comorbid depression. Am J Geriatr Psychiatry 2008;16:168–174. [DOI] [PubMed] [Google Scholar]
- 35.Wilson RS, Schneider JA, Bienias JL, Arnold SE, Evans DA, Bennett DA. Depressive symptoms, clinical AD, and cortical plaques and tangles in older persons. Neurology 2003;61:1102–1107. [DOI] [PubMed] [Google Scholar]
- 36.Wilson RS, Arnold SE, Schneider JA, Li Y, Bennett DA. Chronic distress, age-related neuropathology, and late life dementia. Psychosom Med 2007;69:47–53. [DOI] [PubMed] [Google Scholar]
- 37.Butters MA, Young JB, Lopez O, et al. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin Neurosci 2008;10:345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alexopoulos GS, Meyers BS, Young RC, et al. Clinically defined vascular depression. Am J Psychiatry 1997;154:562–565. [DOI] [PubMed] [Google Scholar]
- 39.Soetanto A, Wilson RS, Talbot K, et al. Anxiety and depression are associated with microtubule-associated protein 2 and synaptopodin-immunolabeled dendrite and spine densities in hippocampal CA3 of older humans. Arch Gen Psychiatry 2010;67:448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magarinos AM, McEwan BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience 1995;69:83–88. [DOI] [PubMed] [Google Scholar]


