Abstract
The control of food intake has been studied using reductionism; by separately investigating environmental, physiological, and genetic variables. The general model of intake regulation attempts to reassemble the pieces into an organized whole. It postulates that intake is influenced by sets of both physiological factors which have negative feedback loops to intake and environmental factors which do not. Data and behavioral genetic analysis on a number of environmental, psychological, dietary, and social variables demonstrates that they have large impacts on the intake of free-living humans in their everyday environments and their magnitude and impact on intake are influenced by heredity. Recent evidence of built-environment influences on activity and intake further indicate the profound influence of environmental circumstances on both intake and expenditure. A computer simulation of the general model of intake regulation demonstrates that the model predicts different maintained levels of intake and body weight depending upon the external environment and that change in the environment can produce new sustained levels. It is suggested that eating is influenced by a myriad of physiological and non-physiological factors and that total intake results from the integral of their influences. It is concluded that recombining the components broken down in the reductionistic process results in a functional whole that can well describe human behavior in natural environments.
Keywords: Food Intake, Eating, Meal Size
Reductionism postulates “divide each difficulty into as many parts as is feasible and necessary to resolve it.” [1]. It states that complex phenomena can be understood by decomposing them into their component parts and studying the individual parts until all components and their interactions are understood. This has been and continues to be the primary tactic applied to the understanding of ingestive behavior. But, there is another side to reductionism. It also states that to understand the overall phenomenon the parts need to be reassembled back into the whole. It is this later component of reductionism that has received very little attention in the study of ingestive behavior.
There has been tremendous progress in describing and understanding the component parts of ingestive behavior. A plethora of remarkable and inspired research has produced numerous breakthrough findings regarding the stimuli, mechanisms, and processes underlying ingestive behavior [2,3]. But, this has not resulted in a clear understanding of its control or an ability to effectively alter intake in the natural world. In fact, the field has been unable to propose or mount an effective strategy to counteract the rampant epidemic of obesity [4-6]. This suggests that the large number of factors and processes that affect intake make it problematic to define solutions based upon single factors or processes. If this is the case, then only by developing a conceptual system that integrates all of the relevant factors, can an understanding of ingestive behavior be identified and thereby produce effective intervention strategies.
Physiological vs. Environmental Factors
The history of the study of ingestive behavior has been dominated by the search for the physiological process or processes that are responsible for the control of intake. Currently, the large majority of research conducted in the area can be classified as primarily physiological in orientation involving the search for control systems that are based upon negative feedback loops where intake affects a particular physiological mechanism that in turn negatively affects intake. These kinds of systems imply control that would result in either a set point or settling point that the system tends to defend. The operation of such systems should be apparent in compensatory responses such that deviations from the settling point should be followed after a reasonably brief period of time by a calibrated adjustment.
The compensatory processes should result in a negative correlation between sequential intakes such that large meals should be followed by small meals and visa-versa. In fact, there is no statistically significant correlation between sequential meals [7,8]. Alternatively, compensation could occur is by delaying the initiation of a meal based upon previous intake. This would produce a positive correlation between the amount eaten in a meal and the time till the next meal. But, in general no statistically significant correlations between meal size and the following interval have been found [9,10]. One final way that compensation might be seen is by adjusting the amount eaten in a meal based upon the amount of time that has elapsed since the last meal. In fact, statistically significant correlations between the duration of the interval prior to the meal and the amount eaten in the meal have been reported [9,10]. But the correlations are generally very small and account for less than 4% of the variance in meal size. These findings are generally stronger when meal size us expressed in units of food energy, but similar, albeit weaker findings occur with other measures such as the weight of solids, liquids, or combined, or individual macronutrients.
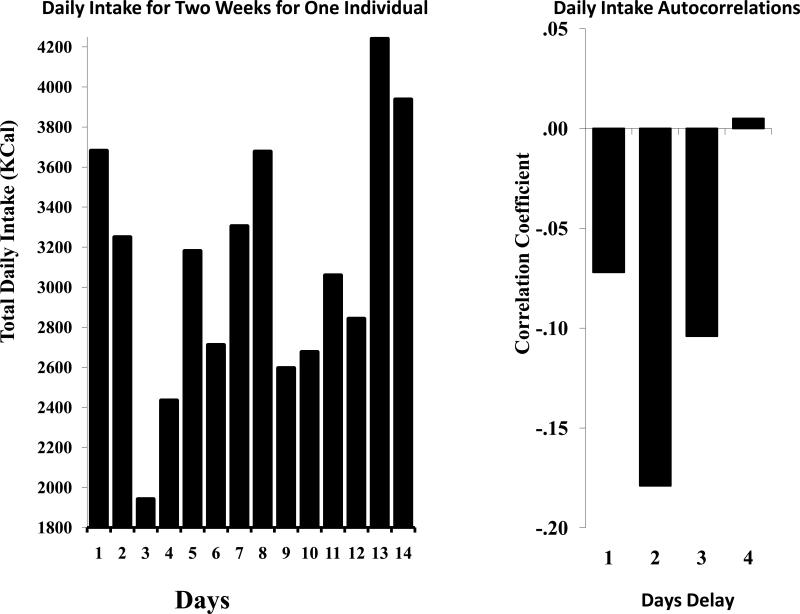
There is generally considerable variation in the amounts eaten each day (Figure 1 left). If physiological compensation was occurring then it should be observed that days where intake exceeded the average would be followed by days of reduced intake and visa-versa. In fact, there are small negative autocorrelations between total daily intakes and intakes on subsequent days (Figure 1, right), particularly with delays of two to three days [11]. But, again the correlations are very small and in combination account for less than 5% of the variance in daily intake. Hence it appears that some degree of compensation occurs suggesting a modicum of control by physiological regulation. But, the size of the effects suggest that this form of control plays a very minor role in the regulation of intake. Thus it would appear by default that non-physiological factors have much greater importance in intake regulation. In fact, estimates derived from behavioral genetic data suggests that over 86% of the variance in intake is due to non-physiological (environmental) factors [12-14].
Figure 1.
Total daily food energy intake (Kcal) over a 14-d period for a single individual (left). Autocorrelation coefficients between daily food energy intake and intake on subsequent days (right). The first bar represents the correlations calculated between the amounts ingested on a day and on the next day (1 day delay), and 2 (2 day delay), 3 (3 day delay), and 4 (4 day delay) days later.
The General Intake Regulation Model
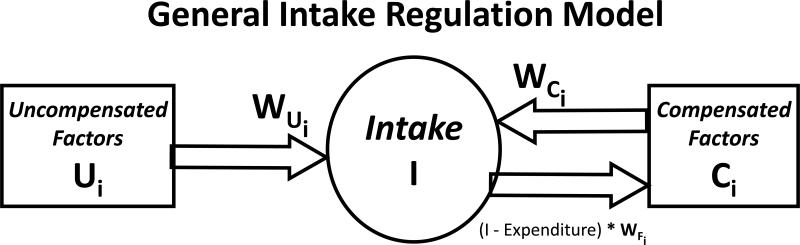
The data and reasoning reviewed above as well as other information indicated that there was a need to expand the view of intake regulation to include both physiological and environmental factors. This led us to develop the General Intake Regulation Model [15]. It was recognized that the influences of environmental factors on intake are different from physiological factors because, in general, they lack negative feedback loops with intake. As a result compensation occurs for physiological factor while environmental factors are largely uncompensated. It was also assumed that a large number of factors were responsible for intake regulation rather than single factors or small set of factors and that the occurrence of meals and the amount eaten in meals results from the sum of the operation of large sets of factors. The model is presented in Figure 2.
Figure 2.
General intake regulation model. The general intake regulation model, wherein intake (I) is controlled by two sets of factors; compensated factors (Ci ) that both affect and are affected by intake via negative-feedback loops, and uncompensated factors (Ui) that affect but are not affected by intake. Inheritance affects the system by determining the preferred level for intake, and compensated and uncompensated factors also by determining the level of impact of the factors on intake (Wi).
In the model it is postulated that there is a large set of compensated factors (Ci) each of which has a modest impact on intake that is specified by different weighting factors for each compensated factor (WCi). In turn, feedback from intake is postulated to have a modest effect upon each compensated factor and this is specified by different weighting factors for each compensated factor (WIi). In the model it is also postulated that there is a large set of uncompensated factors (Ui) each of which has a modest impact on intake that is specified by different weighting factors for each compensated factor (WUi). But, unlike the compensated factors, the uncompensated factors are postulated to lack negative feedback from intake. For all compensated and uncompensated factors, both the magnitude of the optimal levels of each factor and the magnitude of each of the weighting factors are postulated to be determined, at least in part, by heredity.
Diet Diary Methodology
In order to investigate the vast array of factors that influence intake in the model, very detailed information about intake and the level of each factor is necessary. In addition, the model suggests that the magnitude of the influence of each factor can only be understood when looked at in combination with many, if not most or all, of the other influential factors. Hence, data are required that are obtained within a multivariate context. The real world context in which most of the intake by humans occurs provides just such a context. But, there are limited options available to measure intake and associated factors in the complex real environments of free-living humans. One, flawed but adequate method is to obtain self-reports from individuals of their intake in diaries.
The vast majority of the studies that will be reviewed here obtained data on the intakes of free-living humans employing a 7-day self-report diet diary technique [16-17]. This technique allows the recording of the details of intake including the time that intake occurs along with the environmental circumstances and psychological states of the participants while they are engaged in their everyday activities. There is currently no other non-invasive technique available to do this. The data obtained, however, must be viewed cautiously as there is considerable evidence that there is significant underreporting of intake in diaries [18-26]. It is estimated that the amount of food energy reported in diaries is on average about 20% less than actual intake [18] and that the degree of underreporting increases with increasing levels of obesity [19,27-29].
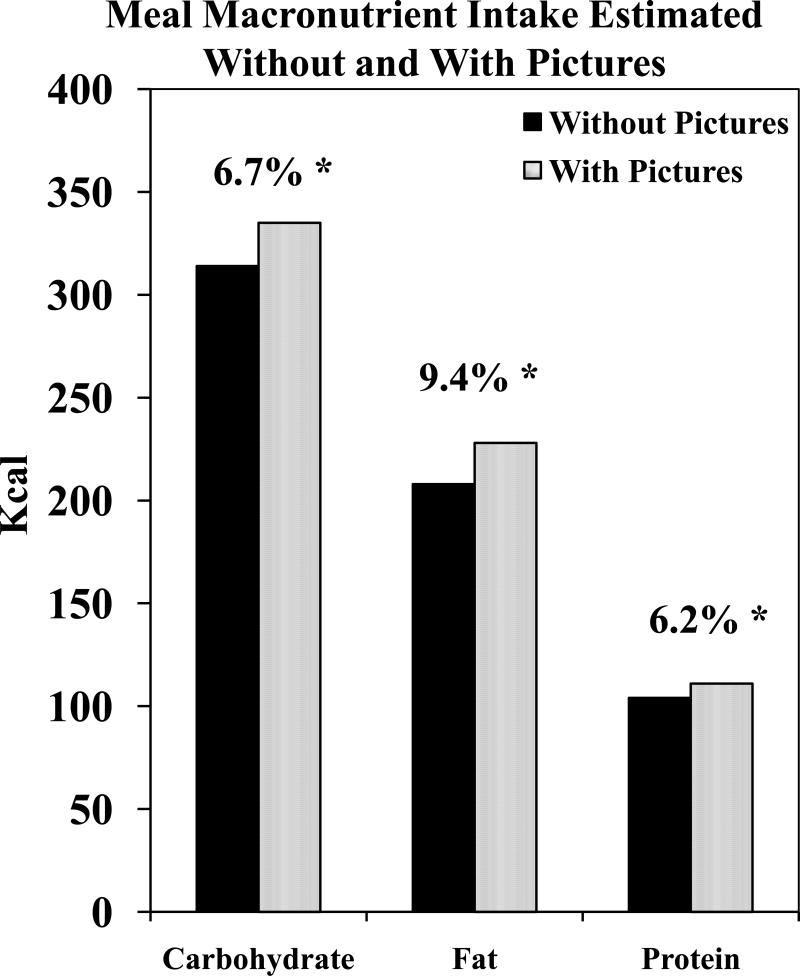
In our studies we have modified the diet-diary technique by having participants not only record their intake in the diary but to also take a picture of their food at the beginning and end of the meal. We believe that this technique helps to limit underreporting in two ways. First, because the participants know that the pictures will be viewed by the researcher, they are less likely to leave out items actually consumed. Second, viewing the pictures allows for a more accurate encoding of the intake by the experimenter. To investigate this latter claim we had a sample of diaries encoded separately by two independent coders; the first only had access to the diaries while the second had the diaries plus the benefit of the additional pictures. With the pictures included the estimates of the amount ingested increased significantly (Figure 3). We believe that this technique helps to reduce the impact of underreporting on the estimates of intake.
Figure 3.
Mean amounts of macronutrients ingested in meals (Kcal) estimated either without or with the aid of pictures of the meals. * indicates that the means are significantly (p<.05) different (t test).
All measurement techniques contain errors of measurement and/or alter intake as a result of reactivity to the measurement technique. The issue is not whether there is error, but rather how does that error affect the interpretation of the data obtained. If the errors are constant they should not affect the correlations between the variables, as correlations are unaffected by the operation of constants. If the errors are random they should only affect the correlations magnitude, reducing it by the amount of variation added by the random error. This would make it more difficult to detect a significant correlation, but would not produce a spuriously significant correlation. Only if the errors are specific to certain contexts or participant characteristics could contamination occur.
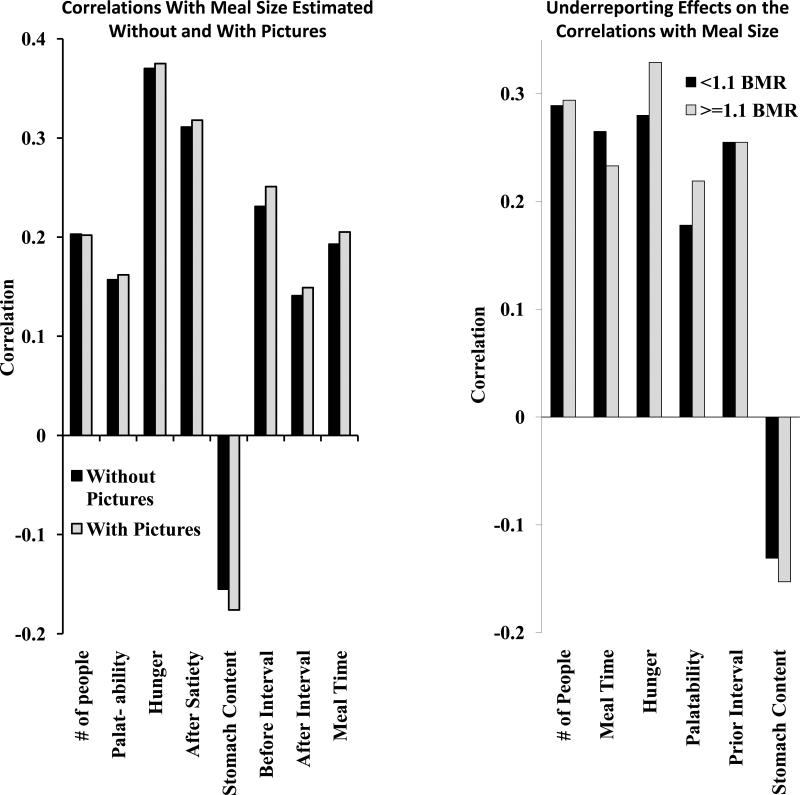
In order to determine whether the correlations between meal size and a variety of variables are affected by underreporting, correlations were calculated separately for the data from diaries that were coded without viewing the meal pictures and compared to the data that were coded with the benefit of the additional pictures. There were no significant differences between the correlations (Figure 4, left).
Figure 4.
Mean correlations between the size of the meals and a number of factors that are associated with meal size for meals whose size was estimated either without or with the aid of pictures of the meals (left) and between individuals who were either below or above the cutoff for underreporting of intake (right).
Additionally, correlations were calculated separately for the data obtained from participants who met an objective criterion for underreporting (reported intake less than 10% above their estimated basal metabolic rate) and compared to the data from participants who did not. The reported intakes were compared to the estimated basal metabolic rate for each participant; BMRest. Basal metabolic rate was estimated from the participant's weight considering age and gender according [30]. The ratio of the reported daily food energy intake (EI) to the BMRest was calculated for each participant; EI:BMRest. A reasonable cut-off for identifying unrepresentative intake is EI:BMRest < 1.1 [30-32]. Again, there were no significant differences between the correlations (Figure 4, right). These data suggest that underreporting does not affect the conclusions from within-participant correlation analysis of meal pattern data.
Behavioral Genetic Studies of Factors Affecting Meal Patterns
In order to investigate the impact on intake of a number of compensated and uncompensated factors from the General Intake Regulation Model and to investigate the postulated influence of inheritance on both the magnitude of these factors and also the magnitude of their impact on intake we performed analyses on 7-day diet diary data obtained from identical and fraternal twin pairs [12-14]. We investigated the direct relationship between these factors and intake and additionally performed a heritability analysis to determine the influence of inheritance on both the factor levels and also their impact on intake.
1. Compensated Factors
Two compensated factors could be identified in the meal pattern data, stomach content and hunger. Both qualify as compensated factors as they affect intake and in turn are affected by intake. Although it is not feasible to directly measure the contents of the stomach in free-living humans it can be estimated with a relatively simple computer model of stomach emptying. Since the stomach empties in a regular predictable fashion and we know from the diary the time of intake and the amount, it is fairly simple to estimate how much is left in the stomach at the time of the next meal [10,33]. The reported intake is estimated to empty from the stomach at a rate proportional to the square root of the caloric content of the stomach, Sn+1 = Sn - 5√Sn where S equals the stomach content in 1000 Kcal units and n equals a particular minute of the day. This procedure reflects actual measured emptying rates from the human stomach [34-36] and has been used in prior studies [10,33,37].
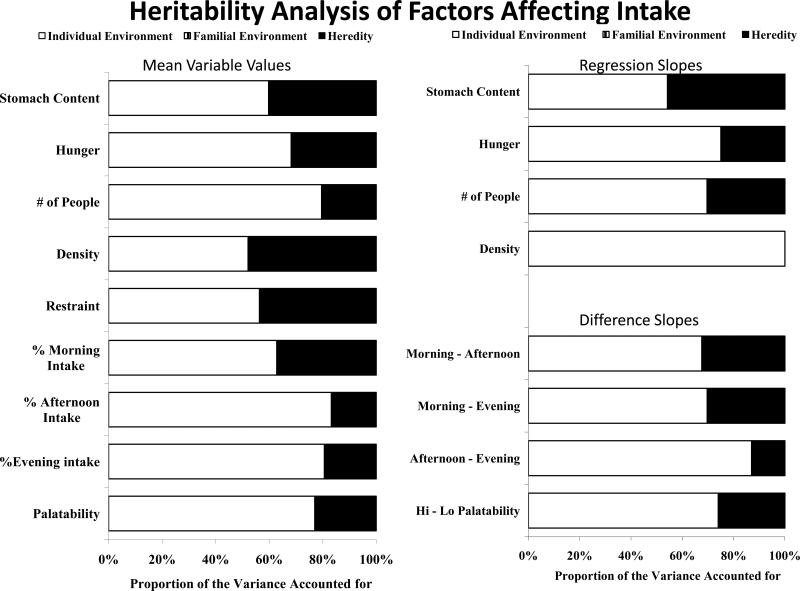
The estimated content of the stomach at the beginning of the meal correlates on average -0.24 with the size of the subsequent meal and indicates that the more that is estimated to be in the stomach at the time of the meal the smaller the meal that will be ingested [10,33,38]. The magnitude of the correlation, however, is small accounting for less than 6% of the variance in meal size. Although this is a small effect, that is exactly what the General Intake Regulation Model predicts. Since, the model postulates that there are a large number of factors affecting intake, it would be expected that each individual factor would have only a small impact on intake. The heritability analysis of the twins’ data produced significant heritability estimates for both the estimated amount in the stomach at the start of the meal and the slope of the regression between the estimated stomach content and the meal size (Figure 5) [38]. The results for the stomach content estimates suggest that heredity affects the preferred amount in the stomach at the time of the meal. In addition, the results for the slopes of the regressions suggestions that heredity also affects the impact of that stomach content on the amount ingested in the meal [38].
Figure 5.
Heritability analysis of factors affecting intake. The proportion of the variance in the factor means (left) and the slopes of the relationships between the factors and the meal size (right) that could be accounted for by the individual environment (white), family environment (striped) and heredity (black) in the linear structural modeling heritability analysis of the twin data.
Hunger levels are self-reported in the diaries by the participants both at the beginning of each meal on 7-point scales. Hunger at the beginning of the meal correlates on average 0.30 with the size of the subsequent meal and indicates that the hungrier the participant was at the beginning of the meal the more that is eaten in the meal [33]. The magnitude of the correlation is again small accounting for 9% of the variance in meal size. Again this modest effect size is what the General Intake Regulation Model predicts. The heritability analysis of the twins’ hunger data produced significant heritability estimates for both the level of hunger at the start of the meal and the slope of the regression between hunger and the meal size (Figure 5) [39]. These results suggest that heredity affects the preferred level of hunger at the time of the meal and also the impact of hunger on the amount ingested in the meal [39].
The results for the estimated content of the stomach and for self-reported hunger are supportive of the General Intake Regulation Model. Both of these factors that have negative feedback loops with intake were significantly related to intake but only accounted for small proportions of the variance. In addition, both the level and impact of these factors on intake were influenced by heredity as predicted in the model. These compensated factors are the types that have been classically investigated as regulatory factors in the control of intake. Although, other uncompensated factors have been investigated as to their effect on intake they have in general not been incorporated into a comprehensive model of the control of intake.
2. Uncompensated Factors
In the 7-d diet diaries several environmental (uncompensated) factors are measured and their impact on intake can be assessed. Participants are simply asked to list the number of people who are eating with them at the meal. The impact of the presence of other people can be assessed by correlating the number of people present with the size of the meal ingested. The number of people present correlates on average 0.30 with the size of the subsequent meal [40-43]. This indicates that the more other people present at the meal the more that the individual eats. In fact, meals eaten with other people present are on average 44% larger than meals eaten alone [40,41]. The magnitude of the correlation is modest, accounting for 9% of the variance in meal size. Significant correlations between meal size and the number of other people present were found separately for meals eaten during the breakfast period, the lunch period and the dinner period, eaten in restaurants, at home and elsewhere, eaten accompanied by alcohol intake or without alcohol, and for only snacks or only meals [43].
In alignment with the predictions of the General Intake Regulation Model. The heritability analysis of the twins’ data on the number of other people present produced significant heritability estimates for the number of other people present, and the correlation and slope of the regression between the number of other people present and the meal size (Figure 5) [44]. These results suggest the interesting and counterintuitive conclusion that heredity affects the number of other people present at the time of the meal. They also suggest that the impact of the number of other people present on the amount ingested in the meal [44]. These results support the prediction of the model that the factor magnitude and its impact would vary by individual due in part to heredity.
Another environmental factor that's known to affect intake is the density of the diet; the amount of energy in a gram of food [45-50]. Dietary density has a significant influence on intake as indicated by a correlation of 0.33 between dietary density and meal size, accounting for over 10% of the variance in meal size. This indicates that the higher the energy density of the meal the more food energy that the individual will ingest. In fact, high energy dense meals are on average eight times larger than low energy dense meals [46] Corresponding to the models predictions, the heritability analysis of the twins’ dietary density data produced strong and significant heritability estimates for dietary density accounting for 47% of the variance in dietary density (Figure 5) [51]. The correlation and slope of the relationship, however, were not significantly affected by heredity. These results suggest that heredity affects the level of dietary density that an individual chooses to ingest supporting the prediction of the model [51].
Another psychological factor that's known to affect an individuals overall intake is the tendency to actively restrain intake [52-54]. Individuals who have a moderate amount of cognitive restraint ingest 15% less per day than individuals who are low in restraint [52]. The heritability analysis of the twins’ cognitive restraint scores produced strong and significant heritability estimates for restraint accounting for 44% of the variance in restraint (Figure 5) [55]. These results suggest that heredity affects the individual's level of restraint and this in turn affects the amount of food energy ingested over the day supporting another prediction of the model.
The time of day that eating occurs is another environmental (uncompensated) factor that is known to affect intake [56-60]. Participants are simply asked to record in their diaries the exact times that the eat their meals. Time of day has a large effect on intake with meals eaten in the evening 69% larger than meals eaten in the morning and 15% larger than meals eaten in the afternoon [56]. The time of day correlates 0.20 with meal size, accounting for 4% of the variance in meal size. The heritability analysis of the twins’ time of day of intake data produced significant heritability estimates for the proportion of overall intake ingested in the morning (37%), afternoon (17%), and evening (19%) [61]. In addition, the impact of time of day on intake also appears to have significant heritability as the difference between intakes in the morning and afternoon (33%), morning and evening (30%) and afternoon and evening (13%) have significant heritability (Figure 5). These results suggest that heredity affects the distribution of eating over the day and also the impact of that distribution [61].
The palatability of the food is assessed in the diaries with a 7-point bad-good scale. The magnitude of the correlations between the palatability ratings and the size of the meal ingested is modest, 0.22, accounting for less than 5% of the variance in the meal size [62,63]. Also, the heritability analysis of the twins’ palatability data produced significant heritability estimates for both the level of palatability at the start of the meal and the difference between meal sizes eaten at low and high palatability ratings (Figure 5) [64]. These results suggest that heredity affects the individual's preferred level of palatability of the food and also the impact of that selected palatability on the amount ingested in the meal [64].
The Built Environment Influences on Intake and Activity Levels
Over the last few years, the built environment has received increasing attention as an environmental influence on intake and activity levels that may be contributing to the societal increase in obesity [65,66]. It is thought that the physical environment that humans have constructed may be promoting higher levels of intake, particularly of high density foods and also may be restricting activity levels and overall energy expenditure. Recently, we employed GPS to record the exact location of free-living participants when they were eating or active in their normal everyday environments. Activity was continuously monitored with triaxial accelerometers and eating was recorded by the participants in 7-day diet diaries. Geographical Information System (GIS) databases were then queried to determine the environmental characteristics of the participants’ locations. These characteristics were then related to the behavior of the individual. To look at its associations with overweight, normal weight individuals were compared to overweight individuals in their intake and activity levels in various environments.
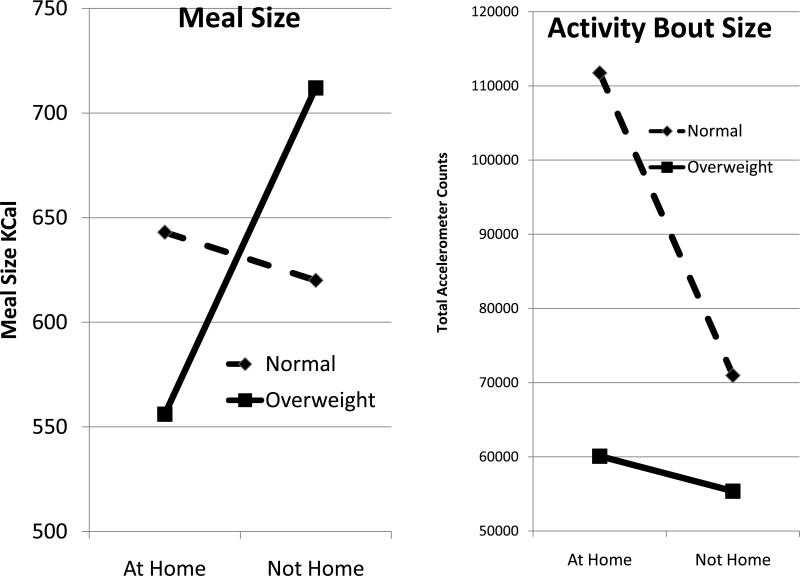
It was observed that being at home or away from their home has differential effects on normal weight vs. overweight/obese individuals (Figure 6). Overweight/obese individuals eat more when they are away from home and are less active while normal weight individuals eat about the same at home as away but are significantly more active at home. This suggests that the overweight and obese are most vulnerable to overeating and under-activity outside of the home and thus tend toward positive energy balance when outside the home environment. This suggests that the built environment impacts susceptible individuals and contributes to overweight. More data are needed to identify exactly what characteristics of the environment are particularly problematic for this susceptible group of individuals.
Figure 6.
Mean meal sizes of normal weight (BMI <25) or overweight (BMI >= 25) individuals for meals eaten at home or outside of the home (left). Mean activity bout sizes of normal weight (BMI <25) or overweight (BMI >= 25) individuals for activity bouts occurring at home or outside of the home (right).
Simulations of the General Model of Intake Regulation
In order to ascertain if the General Model of Intake Regulation can produce outcomes that parallel intake and body weight changes seen in the natural environment a computer simulation was implemented. A number of instantiations of the model were implemented to predict the influences of changes in the environment and its impact on body weight. It was found that a rather simple instantiation of the model was sufficient to assess the model's behavior. It included only four hypothetical uncompensated factors and four hypothetical compensated factors in addition to body weight. The parameterization of the model was arbitrary except that it was specified that the sum of all of the positive and negative weights would be equal to zero [15].
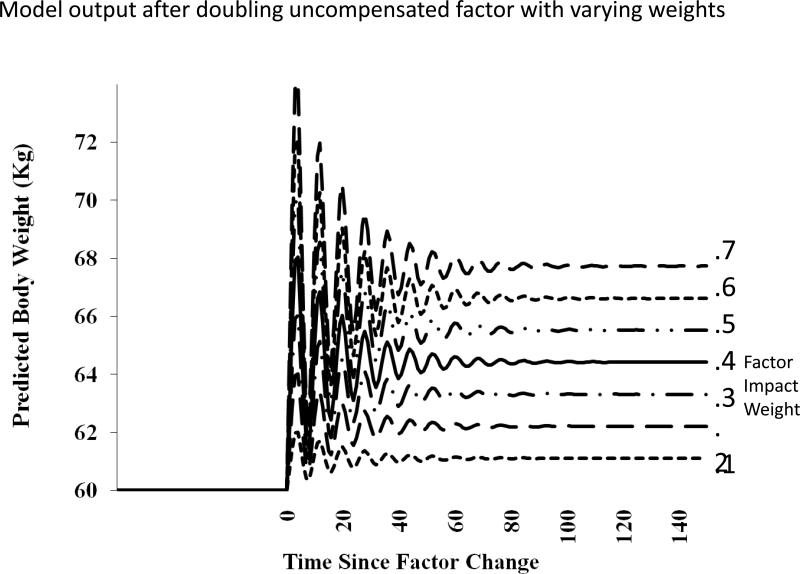
The model's response to a simulated change in the environment was investigated by doubling the level of one environmental (uncompensated) factor. The output of the model responded to the change, initially by predicting an unstable body weight that oscillated, but at a markedly higher level before stabilizing and settling at a 7% higher body weight (Figure 7). The output of the model then indicated that the new body weight would be maintained as long as no further changes occurred [15]. A simulation was then implemented to investigate how the model responded to differences in individual responsiveness that is represented in the model as weights (impact factors). In conjunction with the doubling of the environmental (uncompensated) factor as above, the weighting factor was manipulated by employing seven different levels. The output of the model indicated that when the weighting factor was low, the doubling of the uncompensated factor predicted only a small increase in body weight. But when the weighting factor was large, the model's output predicted a large increase in body weight (Figure 7). The output body weight was found to depend upon both the amount of increase in the level of the environmental (uncompensated) factor and the magnitude of the weighting factor. Hence, the model predicted that if there was a sustained change in the environment there would be a sustained change in body weight, the magnitude of which would be dependent upon the individual's inherited responsiveness to the factor.
Figure 7.
Model output after doubling uncompensated factor with varying weights. Results of a computer simulation of the general intake regulation model in response to a doubling of one uncompensated factor with seven different levels of impact weights. Four hypothetical compensated factors and four hypothetical uncompensated factors with varying weights were set to produce a stable output from the model of 60-kg body weight. One uncompensated factor's level was doubled. Seven simulations were performed with differing weights for the doubled uncompensated factor.
Discussion
The control of food intake is a very complex phenomenon that defies simple description. Large numbers of factors and processes are involved that not only act individually, but also interact. The General Model of Intake Regulation was designed to allow for the integration of these multiple components into an organized totality. It was designed to reflect a large number of factors acting simultaneously. These factors fall into two categories, compensated, including negative feedback loops with intake, that are designed to represent mainly physiological factors, and uncompensated factors, that are designed to represent mainly environmental factors, without feedback loops. It would appear that this is an reasonable way to view food intake regulation, that both compensated and uncompensated factors are simultaneously operative, and that overall intake results from the integral of the simultaneous operation of all of the components.
It also appears that inheritance plays a major role in determining the importance of and responsiveness to both compensated and uncompensated factors. It not only affects what level of the factor that tends to be maintained, but also influences how responsive the individual is to the factor. Thus, the model includes mechanisms than can account for individual differences in responsiveness. When individuals are immersed in new environments, some react and gain weight, while others appear unaffected. The simulations of the model suggests an explanation as heritable differences in responsiveness can be seen to markedly alter the effect of a change in the environment.
The recent epidemic of obesity [4-6] has occurred amid a large number of changes in the environment. These include changes in the built environment and increases over the last few decades in dietary energy densities, portion sizes, palatability, variety and availability of copious quantities of attractive foods, restaurant eating, breakfast skipping and shifting of intake to the evening, television watching including incessant advertisements for food, home delivery and attractive pre prepared foods [67]. The simulation of the model indicates that any lasting change in the environment would produce a lasting change in body mass. Hence, the model would predict that these changes would be more than sufficient to prompt an obesity epidemic [68].
The reviewed findings suggest that inheritance influences not only physiology but also influences the environment and the responsiveness of the individual to the environment. This is a surprising conclusion as the genes have classically been perceived as influencing anatomical structure and the genes were not seen as affecting the environment. The findings of genetic influences on the environment may occur through the operation of inherited psychological characteristics. For example, the heritable level of the number of other people present at meals may well result from an inherited extraversion or sociability factor [69,70]. These factors would then tend to prompt the individual to seek out preferred levels of companionship. Inborn differences in circadian oscillators [71] or in the gustatory system [72] might explain how the genes affect the time of day that people choose to eat and their preferred palatability levels respectively. Nevertheless, whether direct or indirect, the genes have the capacity to affect the selection of environments that an individual chooses to occupy and also the impact those environments might have on the individual's behavior.
The present review and model suggests that the importance of any single component that is singled out and isolated through reductionism, can only be adequately assessed when viewed as functioning within the total system. Looking at the factor in isolation with all or most other sources of variation eliminated or held constant can produce an overinflated view of its importance. For example, the palatability of food when tested in the laboratory has large and profound effects on the amount of food energy ingested. But, relative to actual intakes by humans in their natural environments, it appears to have only a very modest influence accounting for less than 5% of the variance. This appears to occur due to self-selection of foods producing a ceiling effect in palatability. In the lab, removing this selection component that is present in the natural environment, and presenting a range of palatability that would normally seldom be selected provides a misimpression of the factors actual real world importance in affecting energy intake.
In contrast to reductionism, the gestalt view suggests that qualities emerge from the organization of parts that transcend the sum of the original parts. This view would imply that attempts to simply put the pieces back together after reductionistic study would not produce an understanding of the organized totality. The present results for ingestive behavior from the application of the General Model of Intake Regulation suggests that in this instance, for this process, simple recombination of the parts can indeed produce results that reflect the actual operation of the organized totality. This is gratifying to see that when the pieces are put back together into an integrated whole, reasonable outcomes are generated. This suggests that the reductionistic process is a reasonable tactic in addressing complex biobehavioral phenomenon such as food intake regulation. But, the full implementation of reductionism must occur, including not only breaking the process into its parts, but also recombining them into a meaningful whole. Only then can reductionism produce a meaningful understanding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Descartes Rene. Rules For The Direction Of The Mind. In: Anscombe Elizabeth, Geach Peter Thomas., editors. Descartes Philosophical Writings. Thomas Nelson and Sons Ltd.; London: 1954. pp. 153–180. [Google Scholar]
- 2.Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu. Rev. Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- 3.Woods SC, D'Alessio DA. Central control of body weight and appetite. J. Clin. Endocrinol. Metab. 2008;93(11 Suppl 1):S37–50. doi: 10.1210/jc.2008-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flegal KM. The obesity epidemic in children and adults: current evidence and research issues. Med Sci Sp Exer. 1999;31:S509–S514. doi: 10.1097/00005768-199911001-00004. [DOI] [PubMed] [Google Scholar]
- 5.Mokdad AH, Serdula MK, Dietz WH, et al. The spread of the obesity epidemic in the United States, 1991–1998. JAMA. 1999;282:1519–1522. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- 6.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The Continuing Epidemics of Obesity and Diabetes in the United States. JAMA. 2001;286:1195–1200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 7.LeMagnen J, Tallon S. L'effect du jeune preable sur les caracteristiques temporelles de la prise d'aliments chez le rat. J. Physiol. (Paris) 1968;60:143–154. [PubMed] [Google Scholar]
- 8.Bernstein IL, Zimmerman JC, Czeisler AC, Weitzmanm ED. Meal patterning in free-running humans. Physiol. Behav. 1981;27:621–623. doi: 10.1016/0031-9384(81)90232-8. [DOI] [PubMed] [Google Scholar]
- 9.de Castro JM, Kreitzman SN. A microregulatory analysis of spontaneous human feeding patterns. Physiol. Behav. 1985;35:329–335. doi: 10.1016/0031-9384(85)90304-x. [DOI] [PubMed] [Google Scholar]
- 10.de Castro JM, McCormick J, Pedersen M, Kreitzman SN. Spontaneous human meal patterns are related to preprandial factors regardless of natural environmental constraints. Physiol. Behav. 1986;38:25–29. doi: 10.1016/0031-9384(86)90128-9. [DOI] [PubMed] [Google Scholar]
- 11.de Castro JM. Prior days intake has macronutrients specific delayed negative feedback effects on the spontaneous food intake of free-living humans. J. Nutr. 1998;128:61–67. doi: 10.1093/jn/128.1.61. [DOI] [PubMed] [Google Scholar]
- 12.de Castro JM. Genetic influences on daily intake and meal patterns of humans. Physiol. Behav. 1993;53(4):777–782. doi: 10.1016/0031-9384(93)90188-l. [DOI] [PubMed] [Google Scholar]
- 13.de Castro JM. Independence of genetic influences on body size, daily intake, and meal patterns of humans. Physiol. Behav. 1993;54(4):633–639. doi: 10.1016/0031-9384(93)90070-v. [DOI] [PubMed] [Google Scholar]
- 14.de Castro JM. A twin study of genetic and environmental influences on the intake of fluids and beverages. Physiol. Behav. 1993;54(4):677–687. doi: 10.1016/0031-9384(93)90076-r. [DOI] [PubMed] [Google Scholar]
- 15.de Castro JM, Plunkett S. A general model of intake regulation. Neurosc. Biobehav. Rev. 2002;26:581–595. doi: 10.1016/s0149-7634(02)00018-0. [DOI] [PubMed] [Google Scholar]
- 16.de Castro JM. Methodology, correlational analysis, and interpretation of diet diary records of the food and fluid intakes of free-living humans. Appetite. 1994;23:179–192. doi: 10.1006/appe.1994.1045. [DOI] [PubMed] [Google Scholar]
- 17.de Castro JM. Measuring real-world eating behavior. Prog. Obes. Res. 1999;8:215–221. [Google Scholar]
- 18.Livingstone MB, Prentice AM, Strain JJ, Coward WA, Black AE, Barker AE, Mckenna PG, Whitehead RG. Accuracy of weighed dietary records in studies of diet and health. Br. Med. J. 1990;300:708–712. doi: 10.1136/bmj.300.6726.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bandini LG, Schoeller DA, Cyr HN, Dietz WH. Validity of reported energy intake in obese and nonobese adolescents. Am. J. Clin. Nutr. 1990;52:421–425. doi: 10.1093/ajcn/52.3.421. [DOI] [PubMed] [Google Scholar]
- 20.Goran MI, Poehlman ET. Total energy expenditure and energy requirements in healthy elderly persons. Metab. 1992;41:744–753. doi: 10.1016/0026-0495(92)90315-2. [DOI] [PubMed] [Google Scholar]
- 21.Lissner L, Habicht J-P, Strupp BJ, Levitsky DA, Haas JD, Roe DA. Body composition and energy intake: do overweight women overeat and underreport? Am. J. Clin. Nutr. 1989;49:320–325. doi: 10.1093/ajcn/49.2.320. [DOI] [PubMed] [Google Scholar]
- 22.Livingstone MB, Prentice AM, Coward WA, Strain JJ, Black AE, Davies PSW, Steward CM, Mckenna PG, Whitehead RG. Validation of estimates of energy intake be weighted-dietary record and diet history in children and adolescents. Am. J. Clin. Nutr. 1992;56:29–35. doi: 10.1093/ajcn/56.1.29. [DOI] [PubMed] [Google Scholar]
- 23.Livingstone MB, Robson PJ, Black AE, Coward WA, Wallace JM, McKinley MC, Strain JJ, McKenna PG. An evaluation of the sensitivity and specificity of energy expenditure measured by heart rate and the Goldberg cut-off for energy intake: basal metabolic rate for identifying mis-reporting of energy intake by adults and children: a retrospective analysis. Eur. J. Clin. Nutr. 2003;57(3):455–463. doi: 10.1038/sj.ejcn.1601563. [DOI] [PubMed] [Google Scholar]
- 24.Mertz W, Tsui JC, Judd JT, Reiser S, Hallfrisch J, Morris ER, Steele PD, Lashley E. What are people really eating? The relation between energy intake derived from estimated diet records and intake determined to maintain body weight. Am. J. Clin. Nutr. 1991;54:291–296. doi: 10.1093/ajcn/54.2.291. [DOI] [PubMed] [Google Scholar]
- 25.Seale JL, Rumpler WV. Comparison of energy expenditure measurements by diet records, energy intake balance, doubly labeled water and room calorimetry. Eur. J. Clin. Nutr. 1997;51(12):856–863. doi: 10.1038/sj.ejcn.1600498. [DOI] [PubMed] [Google Scholar]
- 26.Livingstone MB, Black AE. Markers of the validity of reported energy intake. J. Nutr. 2003;133(Suppl 3):895S–920S. doi: 10.1093/jn/133.3.895S. [DOI] [PubMed] [Google Scholar]
- 27.Braam LA, Ocke MC, Bueno-de-Mesquita HB, Seidell JC. Determinants of obesity-related underreporting of energy intake. Am. J. Epidemiol. 1998;147:1081–1086. doi: 10.1093/oxfordjournals.aje.a009402. [DOI] [PubMed] [Google Scholar]
- 28.Heitmann BL, Lissner L. Dietary underreporting by obese individuals--is it specific or non-specific? Brit. Med. J. 1995;311:986–989. doi: 10.1136/bmj.311.7011.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price GM, Paul AA, Cole TJ, Wadsworth ME. Characteristics of the low-energy reporters in a longitudinal national dietary survey. Br. J. Nutr. 1997;77:833–851. doi: 10.1079/bjn19970083. [DOI] [PubMed] [Google Scholar]
- 30.Schofield WN, Schofield C, James WPT. Basal metabolic rate - review and prediction, together with an annotated bibliography of source material. Hum. Nutr.: Clin. Nutr. 1985;39c(Suppl.1):1–96. [PubMed] [Google Scholar]
- 31.Black AE, Goldberg GR, Jebb SA, Livingstone MB, Cole TJ, Prentice AM. Critical evaluation of energy intake data using fundamental principles of energy physiology: 2. Evaluating the results of published surveys. Br. J. Clin. Nutr. 1991;45:583–799. [PubMed] [Google Scholar]
- 32.Goldberg GR, Black AE, Jebb SA, Cole TJ, Murgatroyd PR, Coward WA, Prentice AM. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of Cut-off limits to indentifying under-recording. Br. J. Clin. Nutr. 1991;45:569–651. [PubMed] [Google Scholar]
- 33.de Castro JM, Elmore DK. Subjective hunger relationships with meal patterns in the spontaneous feeding behavior of humans: Evidence for a causal connection. Physiol. Behav. 1988;43:159–165. doi: 10.1016/0031-9384(88)90232-6. [DOI] [PubMed] [Google Scholar]
- 34.Hopkins A. The pattern of gastric emptying: a new view of old results. J. Physiol. (Lond.) 1966;182:144–150. doi: 10.1113/jphysiol.1966.sp007814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunt JN, Knox MT. Regulation of gastric emptying. In: Code CF, Heidel W, editors. Handbook of- Physiology: Alimentary Canal, Vol. 4: Motility. American Physiological Society; Washington D.C.: 1968. pp. 1917–1935. [Google Scholar]
- 36.Hunt JN, Stubbs DF. The volume and content of meals as determinants of gastric emptying. J. Physiol. (Lond.) 1975;245:209–225. doi: 10.1113/jphysiol.1975.sp010841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Booth DA. A simulation model of psychobiosocial theory of human food-intake control. Int. J. Vit. Nutr. Res. 1988;58:119–134. [PubMed] [Google Scholar]
- 38.de Castro JM. Inheritance of premeal stomach content influences on eating and drinking in free living humans. Physiol. Behav. 1999;66:223–232. doi: 10.1016/s0031-9384(98)00291-1. [DOI] [PubMed] [Google Scholar]
- 39.de Castro JM. Inheritance of hunger relationships with food intake in free living-humans. Physiol. Behav. 1999;67(2):249–258. doi: 10.1016/s0031-9384(99)00065-7. [DOI] [PubMed] [Google Scholar]
- 40.de Castro JM, de Castro ES. Spontaneous meal patterns in humans: influence of the presence of other people. Am. J. Clin. Nutr. 1989;50:237–247. doi: 10.1093/ajcn/50.2.237. [DOI] [PubMed] [Google Scholar]
- 41.de Castro JM, Brewer EM. The amount eaten in meals by humans is a power function of the number of people present. Physiol. Behav. 1992;51(1):121–125. doi: 10.1016/0031-9384(92)90212-k. [DOI] [PubMed] [Google Scholar]
- 42.Feunekes GIJ, De Graaf C, Van Staveren WA. Social facilitation of food intake is mediated by meal duration. Physiol. Behav. 1995;58(3):551–558. doi: 10.1016/0031-9384(95)00087-y. [DOI] [PubMed] [Google Scholar]
- 43.de Castro JM, Brewer M, Elmore DK, Orozco S. Social facilitation of the spontaneous meal patterns of humans is independent of time, place, alcohol, or snacks. Appetite. 1990;15:89–101. doi: 10.1016/0195-6663(90)90042-7. [DOI] [PubMed] [Google Scholar]
- 44.de Castro JM. Inheritance of social influences on eating and drinking in humans. Nutr. Res. 1997;17:631–648. [Google Scholar]
- 45.Bell EA, Rolls BJ. Energy density of foods affects energy intake across multiple levels of fat content in lean and obese women. Am. J. Clin. Nutr. 2001;73(6):1010–1018. doi: 10.1093/ajcn/73.6.1010. [DOI] [PubMed] [Google Scholar]
- 46.de Castro JM. Density and intake relationships in the eating behavior of free-living humans. J Nutr. 2004;134:335–41. doi: 10.1093/jn/134.2.335. [DOI] [PubMed] [Google Scholar]
- 47.de Castro JM. Stomach filling may mediate the influence of dietary energy density on the food intake of free-living humans. Physiol. Behav. 2005;86(1-2):32–45. doi: 10.1016/j.physbeh.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 48.Prentice AM. Manipulation of dietary fat and energy density and subsequent effects on substrate flux and food intake. Am. J. Clin. Nutr. 1998;67(Suppl. 3):535S–541S. doi: 10.1093/ajcn/67.3.535S. [DOI] [PubMed] [Google Scholar]
- 49.Westerterp-Plantenga MS. Effects of energy density of daily food intake on long-term energy intake. Physiol. Behav. 2004;81(5):765–771. doi: 10.1016/j.physbeh.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 50.Yao M, Roberts SB. Dietary energy density and weight regulation. Nutr. Rev. 2001;59(8 Pt 1):247–258. doi: 10.1111/j.1753-4887.2001.tb05509.x. [DOI] [PubMed] [Google Scholar]
- 51.de Castro JM. Heredity influences the dietary energy density of free-living humans. Physiol. Behav. 2006;87:192–198. doi: 10.1016/j.physbeh.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 52.de Castro JM. The relationship of cognitive restraint to the spontaneous food and fluid intake of free-living humans. Physiol. Behav. 1995;57(2):287–295. doi: 10.1016/0031-9384(94)00229-x. [DOI] [PubMed] [Google Scholar]
- 53.Ruderman AJ. Dietary restraint: a theoretical and empirical review. Psychol. Bull. 1986;99(2):247–262. [PubMed] [Google Scholar]
- 54.Laessle RG, Tuschl RJ, Kotthaus BC, Prike KM. Behavioral and biological correlates of dietary restraint in normal life. Appetite. 1989;12:3–94. doi: 10.1016/0195-6663(89)90098-6. [DOI] [PubMed] [Google Scholar]
- 55.de Castro JM, Lilenfeld L. The influence of heredity on dietary restraint, disinhibition, and perceived hunger in humans. Nutr. 2005;21(4):446–455. doi: 10.1016/j.nut.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 56.de Castro JM. Circadian rhythms of the spontaneous meal pattern, macronutrient intake and mood of humans. Physiol. Behav. 1987;40:437–466. doi: 10.1016/0031-9384(87)90028-x. [DOI] [PubMed] [Google Scholar]
- 57.de Castro JM, Bellisle F, Feunekes GIJ, Dalix AM, De Graaf C. Culture and meal patterns: a comparison of the food intake of free-living American, Dutch, and French Students. Nutr. Res. 1997;17:807–829. [Google Scholar]
- 58.de Castro JM. The time of day of food intake influences overall intake in humans. J. Nutr. 2004;134:104–111. doi: 10.1093/jn/134.1.104. [DOI] [PubMed] [Google Scholar]
- 59.de Castro JM. The time of day and the proportions of macronutrients eaten are related to total daily food intake. Br. J. Nutr. 2007;98:1077–1083. doi: 10.1017/S0007114507754296. [DOI] [PubMed] [Google Scholar]
- 60.de Castro JM. When, how much and what foods are eaten are related to total daily food intake. Br. J. Nutr. 2009;102:1228–1237. doi: 10.1017/S0007114509371640. [DOI] [PubMed] [Google Scholar]
- 61.de Castro JM. Heritability of diurnal changes in food intake in free-living humans. Nutr. 2001;17:713–720. doi: 10.1016/s0899-9007(01)00611-6. [DOI] [PubMed] [Google Scholar]
- 62.de Castro JM, Bellisle F, Dalix AM. Palatability and intake relationships in free-living humans: Measurement and characterization in the French. Physiol. Behav. 2000;68:271–277. doi: 10.1016/s0031-9384(99)00166-3. [DOI] [PubMed] [Google Scholar]
- 63.de Castro JM, Bellisle F, Dalix AM, Pearcey S. Palatability and intake relationships in free-living humans: Characterization and independence of influence in North Americans. Physiol. Behav. 2000;70:343–350. doi: 10.1016/s0031-9384(00)00264-x. [DOI] [PubMed] [Google Scholar]
- 64.de Castro JM. Palatability and intake relationships in free-living humans: Influence of heredity. Nutr. Res. 2001;21(7):935–945. doi: 10.1016/s0271-5317(01)00323-2. [DOI] [PubMed] [Google Scholar]
- 65.Papas MA, Alberg AJ, Ewing R, Helzlsouer KJ, Gary TL, Klassen AC. The built environment and obesity. Epidemiol. Rev. 2007;29:129–143. doi: 10.1093/epirev/mxm009. [DOI] [PubMed] [Google Scholar]
- 66.Sallis JF, Glanz K. Physical activity and food environments: solutions to the obesity epidemic. Milbank Q. 2009;87(1):123–154. doi: 10.1111/j.1468-0009.2009.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stroebele N, de Castro JM. The influence of ambience on food intake in humans. Nutr. 2004;20:821–838. doi: 10.1016/j.nut.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 68.Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280:1371–1374. doi: 10.1126/science.280.5368.1371. [DOI] [PubMed] [Google Scholar]
- 69.Jang KL, Livesley WJ, Vernon PA. Heritability of the big five personality dimensions and their facets: A twin study. J. Pers. 1996;64(3):577–591. doi: 10.1111/j.1467-6494.1996.tb00522.x. [DOI] [PubMed] [Google Scholar]
- 70.Saudino KJ, Pedersen NL, Lichtenstein P, McClearn GE, Plomin R. Can personality explain genetic influences on life events? J. Pers. Soc. Psych. 1997;72(1):196–206. doi: 10.1037//0022-3514.72.1.196. [DOI] [PubMed] [Google Scholar]
- 71.Kolker DE, Turek FW. The search for circadian clock and sleep genes. J. Psychopharm. 1999;13(4,Suppl. 1):S5. doi: 10.1177/026988119901304S02. [DOI] [PubMed] [Google Scholar]
- 72.Matsunami H, Montmayeur JP, Buck LB. A family of candidate taste receptors in human and mouse. Nature. 2000;6, 404(6778):601–604. doi: 10.1038/35007072. [DOI] [PubMed] [Google Scholar]