SUMMARY
CD8 T cell tolerance, once thought to be largely a result of clonal deletion, is now appreciated to be much more complex, additionally involving multiple permutations of partial loss of effector function in residual clonal populations. This is especially important in the context of tumor immunity, in which persistent tolerized cytotoxic CD8 T cells (CTL), if reactivated, could potentially mount a protective response. Previously we have shown that antigen-presenting cells (APCs) with a targeted disruption of STAT3 break tolerance in CD4 T cells. Here we evaluate the STAT3-defective APC in terms of its ability to induce a productive CTL response. Our data demonstrate that macrophages derived from conditional STAT3 knockout mice are superior to wild-type macrophages in terms of their ability to prime cognate CTL responses, and to cross-present tumor-derived antigen to CTLs in vitro. CTLs cultured with STAT3-deficient APCs demonstrated a stronger proliferative response and produced increased amounts of IFN-γ and TNF-α, all of which have been shown to be diminished in tumor-tolerized CD8 T cells. Targeting STAT3 signaling represents therefore an enticing strategy to augment CTL responses in the tumor-bearing host.
2. INTRODUCTION
CD8 T cells represent an important effector arm in the immune response to cancer. Despite clear demonstrations of their capacity to recognize tumor-associated antigens (TAA) and mount measurable responses, cytotoxic lymphocytes (CTL) rarely achieve protective anti-tumor immunity. Evidence from murine models suggested that CD8 T cell responses to self-antigens which survive thymic deletion are efficiently suppressed via a mechanism of peripheral clonal deletion which eliminates self-reactive clones that escape central tolerance1,2. However, ample evidence derived from both animal and human studies would indicate that this peripheral clonal deletion is incomplete and that residual CTL populations persist that are fully capable of antigen-driven expansion, functional activation, and extravasation into tumor sites3,4,5,6.
Defective function in any one or multiple facets of CTL activation can result in the establishment of a state of tolerance. An effective CTL response requires adequate expansion of reactive clones, sustained activation of these clones with associated IFN-γ and TNF-α production, engagement of lytic effector mechanisms including perforin production, and sufficient recruitment to the target tissue. All of these aspects of CTL activation have been shown to demonstrate impairment, independently or collectively, in various forms and models of CD8 T cell tolerance in the context of tumor immunology and autoimmunity, and with relatively consistent findings in patient populations and murine models3,4,6,7,8,9,10,11. The underlying mechanism by which these CTLs are tolerized is similarly multifaceted and is influenced by the efficacy and potency of the antigen presenting cell (APC) engaging the CTL, the avidity of the T cell for the peptide epitopes being presented, as well as the presence of inhibitory factors encountered in the tumor environment and lymphatics produced by tumor cells, stroma, and/or regulatory cell populations.
The potency of an immune response is dictated in large part by the potency of the APC and its ability to optimally prime the T cell response. This, in turn, is influenced by such factors as the particular APC cell type as well as the context – inflammatory versus non-inflammatory - in which the APC acquires the antigens for processing and presentation. Not surprisingly, APCs isolated from tumors are relatively inefficient at priming protective responses, alternatively inducing T cell anergy or tolerance12,13. Furthermore, the presence of myeloid-derived suppressor cells (MDSC) in the tumor microenvironment and draining lymph nodes further dampen potential cognate T cell responses via the production and activity of immunosuppressive cytokines, arginase, reactive oxygen species, and nitric oxide14. Targeted alteration in the function of these myeloid populations with the intent to disrupt aberrant antigen presentation and/or active suppression represents a viable strategy for breaking or reversing T cell tolerance to TAAs.
STAT3 has now been implicated as a crucial component in multiple immunosuppressive signaling pathways associated with tumorigenesis, including IL-10 and VEGF-mediated mechanisms. Initial studies demonstrated that conditional ablation of STAT3 function in macrophages and neutrophils promoted hyperactive inflammatory responses15. Studies in our lab have demonstrated the ability of macrophages defective in STAT3 signaling to act as efficient APCs and to re-engage tumor-tolerized CD4 T cells16. The impact that STAT3 ablation may have on macrophage’s APC function in terms of their potential role in CD8 T cell activation and reversal of tumor tolerance remains to be elucidated.
In this study, we assessed the cognate responses of naïve CD8 T cells to a TAA using in vitro culture conditions. STAT3-deficient macrophages were compared to congenic normal controls in terms of their ability to stimulate cytokine production and proliferation. Our results indicate that the STAT3 defect enhances the ability of macrophages to stimulate a CD8 response against its cognate peptide. Furthermore, these APCs are also superior in their ability to stimulate CD8 T cells with antigen captured and processed from a tumor cell source.
3. MATERIALS & METHODS
3.1 Mice
BALB/c mice 6–8 weeks old were purchased from NCI (Frederick, MD), while C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). CLN4 CD8 T cell transgenic mice recognizing amino acids 518–527 from the influenza hemagluttinin (HA), initially generated by Dr. Linda Sherman (Scripps Clinics) and kindly provided to us by Dr. Hyam Levitsky (Johns Hopkins University), were bred in house in specific-pathogen-free conditions. Breeder pairs of OT-I CD8 T cell transgenic mice recognizing amino acids 257–264 of ovalbumin were purchased from Jackson Labs and bred in-house with periodic monitoring of transgenic phenotype by flow cytometric staining for Vα2 and Vβ5 expression. B6.LysMCre-Stat3flox/− (B6.Stat3KO) mice were a generous gift from Dr. Shizuo Akira and were bred and maintained according to previously published protocols. BALB/c.LysMCre-Stat3flox/− (BALB/c.Stat3KO) mice were generated by crossing the LysMCre-Stat3flox/− phenotype onto the BALB/c background strain. C.129S2-Stat4tm1Gru/J (BALB/c.Stat4KO) and C.129S2-Stat6tm1Gru/J (BALB/c.Stat6KO) mice were purchased from Jackson Laboratories (Bar Harbor, ME). All experiments were performed in accordance with protocols approved by the Animal Care and Use Committees of the University of South Florida.
3.2 Peptides
Two peptides were used for cognate stimulation of CD8 T cells. The HA peptide corresponds to amino acids 518–526 of the native protein (hemagglutinin influenza) and more specifically the amino acid sequence IYSTVASSL. The OVA peptide used corresponds to amino acids 257–264 of the native protein (Ovalbumin), with the amino acid sequence SIINFEKL. Peptides were reconstituted in PBS and maintained at −80°C in stock concentrations of 5mg/mL.
3.3 Tumor Cells
The B cell lymphoma cell lines A20 and A20HA were maintained in culture in RPMI 1640 supplemented with 10% FBS, 50U/mL penicillin/streptomycin, 50mM β-ME, and incubated at 37°C in 5% CO217. EL4 and EL4mOVA thymoma cell lines, generously provided by Stephen Schoenberger (La Jolla Institute of Allergy & Immunology), were maintained in culture in IMDM supplemented with 10% FBS, 50U/mL penicillin/streptomycin, 50mM β-ME, and incubated at 37°C in 5% CO2.
3.4 Flow Cytometry
All antibodies were purchased from BD Pharmingen (San Diego, CA). CLN4 T cells were identified according to staining with CD8α PerCP (53–6.7), Vβ8 FITC (F23.1), and Thy1.1 PE (OX-7). IFN-γ production was measured by intracellular cytokine staining techniques according to the protocol by BD Pharmingen. Briefly, Lympholyte™-enriched cells were plated at 2×106 cells/well in 96 well U-bottom plates in complete media supplemented with 1µL/mL GolgiPlug and +/− 0.5 µg/mL peptide. Cells were cultured as such for 4 hours at 37°C, then stained for surface markers, permeabilized, and finally stained for IFN-γ (XMG1.2) or TNF-α (MP6-XT22) with an APC-conjugated antibody. For proliferation assays, cells were loaded with CFSE (Molecular Probes, Eugene, OR) at a concentration of 5µM for 10 minutes in HBSS, and then washed 4 times in ice-cold media. All samples were read on a BD FACSCalibur Instrument (Beckton Dickinson, San Jose, CA) and data was analyzed with FlowJo Software (Treestar, Inc).
3.5 Isolation of Peritoneal Macrophages and In Vitro Culture Assay
Peritoneal exudate macrophages (PEM) were elicited from BALB/c, BALB/c.LysMCre-Stat3flox/−, C57BL/6, B6.LysMCre-Stat3flox/−, BALB/c.Stat4KO and BALB/c.Stat6KO mice by intraperitoneal injection of thioglycollate. Cells were collected 4–5 days later by peritoneal lavage with ice-cold complete media. Extensive studies in our lab have shown that the harvested cell population is comprised of 95% macrophages as determined by staining with CD11b and F4/8016. Purified cells were plated at a density of 1×106 cells/well in 6-well culture plates overnight at which time non-adherent cells were washed away. Further analysis of an aliquot of this adherent population revealed that they are 100% macrophages (data not shown). A20 and A20HA cells, or EL4 and EL4mOVA cells, were irradiated with a dose of 3000 rad just prior to being added to the macrophage cultures. These were co-cultured for 4 hours before non-adherent cells were again washed away with 3 washes with ice-cold HBSS with vigorous pipetting. The percent of tumor cells that remained in the macrophage’s monolayer’s culture after extensive washing was less than 5%. These few remaining irradiated tumor cells undergo rapid apoptosis and are unlikely of directly present antigen to antigen-specific T-cells. CLN4 (for experiments using A20/A20HA) or OT-I (for experiments using EL4/EL4mOVA) cells were freshly harvested from spleens and lymph nodes, enriched by Lympholyte™ gradient centrifugation, and labeled with CFSE as discussed prior and then added to the macrophage cultures at 1×106 cells/well. Cultures were incubated for 72 hours at 37°C in 5% CO2 incubator, then re-stimulated and stained for cytokine production as described above.
4. RESULTS AND DISCUSSION
4.1 STAT3KO Macrophages are Superior APCs
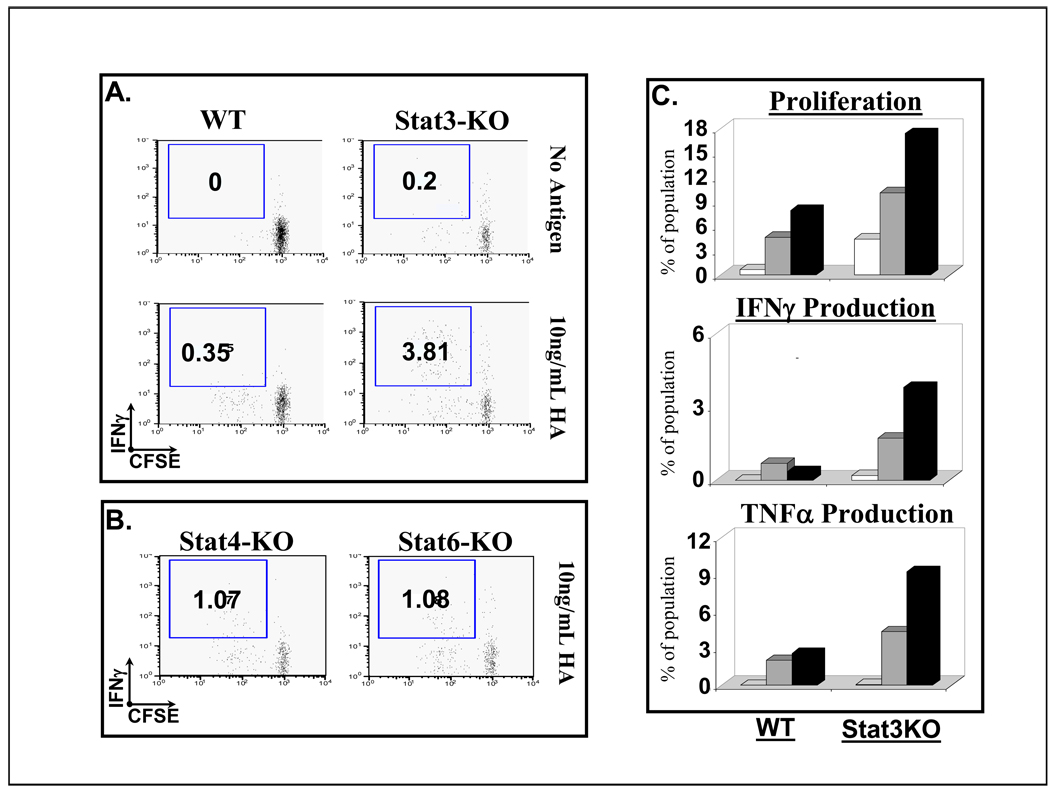
We first sought to establish that macrophage APCs with a functionally deficient STAT3 would demonstrate an enhanced capacity to activate CD8 T cells as compared to STAT3-competent macrophages. To examine this, BALB/c.Stat3KO PEM or WT control PEM were washed and pulsed with HA peptide (10 ng/mL) or left untreated (No antigen). Then, CLN4 CD8 T cells were added to the macrophage’s monolayers. Similar to results obtained in studies of CD4 T cell responses, CD8 T cells exhibited more potent cognate responses when peptide antigen was presented by STAT3-deficient APCs. Although, both BALB/c and BALB/c.Stat3KO macrophages induced a proliferative CD8 T-cell response to cognate peptide, the STAT3KO macrophages promoted a more vigorous proliferative burst and triggered IFN-γ production by CD8 T-cells (Fig 1A: IFN-γ production in 3.81% of the proliferating CTLs when antigen presented by STAT3KO macrophages versus 0.35% when peptide was presented by BALB/c APCs). Of note, genetic disruption of other members of the STAT family do not appear to produce this effect, as evaluation of macrophages isolated from BALB/c.Stat4KO or BALB/c.Stat6KO mice in identical culture parameters only triggered a minimal increase in proliferation or IFN-γ production by antigen-specific CD8 T-cells in response to cognate peptide (Figure 1B). Shown in Figure 1C is the dose-response effect when cognate antigen is presented by BALB/c.Stat3KO macrophages. At both doses of HA-peptide used, these macrophages are clearly superior to control macrophages in triggering antigen-specific CD8+ T-cell proliferation (Fig 1C-Top). Furthermore, unlike CD8+ T-cells encountering cognate antigen in wild type (WT) macrophages, only the STAT3KO macrophages induced measurable IFN-γ and TNF-α production by CLN4 CD8 T cells in response to HA peptide (Fig 1C, Middle and bottom). Cumulatively, these data indicate that STAT3KO macrophages deliver a more potent stimulatory signal to CD8 T cells in a cognate interaction.
Figure 1. Macrophages devoid of STAT3 display increased capacity to stimulate cognate responses in naïve CD8 T cells.
A & B. CLN4 CD8 T cells were cultured in vitro for 72 hours with PEM from wild type (WT), Stat3-KO, Stat4 KO or Stat6 KO mice, previously loaded with the class-I dominant epitope of HA, IYSTVASSL at 10ng/mL (or left untreated). . Plots represent CD8+/Vβ8+/Thy1.1+ gated populations depicting proliferation, measured by CFSE dilution, versus IFN-γ production. C. Dose-response effects (white bars –no peptide; gray bars - 1ng/mL peptide pulse; black bars - 10ng/mL peptide pulse) in terms of antigen-specific proliferation as measured by CFSE dilution (top figure) and cytokine production as determined by intracellular cytokine staining (middle and bottom figures).
4.2 Improved In Vitro Cross-Priming by STAT3KO APCs
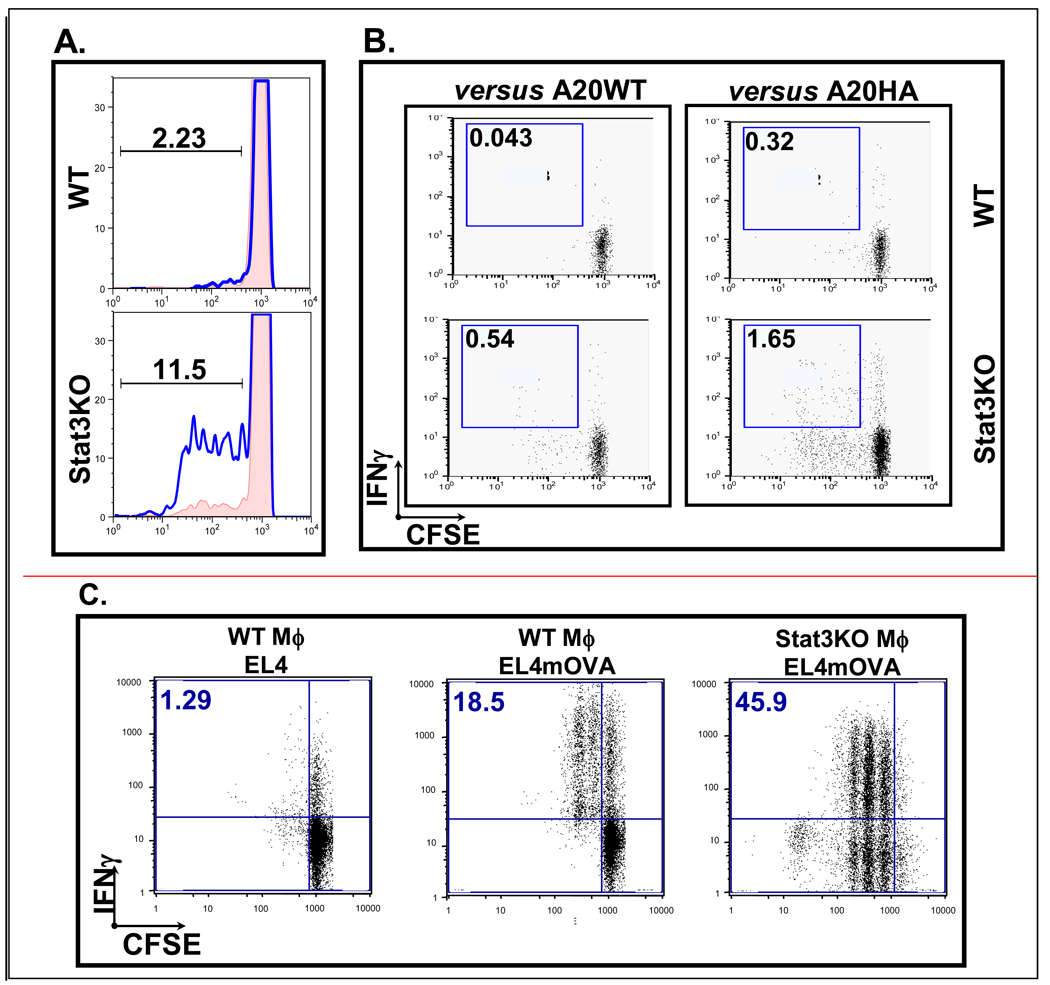
Next, we asked whether PEM devoid of STAT3 will display an improved ability as compared to wild type PEM to capture and cross-present antigen. Once again, CLN4 CD8 T cells were used to assess cognate interactions with macrophage APCs, but this time, antigen was acquired from irradiated A20HA tumor cells co-cultured with PEM for 4 hours. Figure 2A shows that CLN4 CD8 T cells proliferate vigorously when cultured with BALB/c.Stat3KO macrophage but only minimally when cultured with BALB/c macrophage (WT). Furthermore, WT macrophages are incapable of inducing IFN-γ production by CLN4 responder cells, whereas proliferating CLN4 cells show a 5-fold increase in IFN-γ production in the presence of Stat-3 deficient PEM (Figure 2B). Minimal proliferation and no IFN-γ production were seen in T cells cultured with WT macrophages, arguing against any significant contribution by irradiated A20HA B-cells (which could have potentially remained in the cultures after extensive washing) presenting HA antigen directly to CLN4 CD8 responder cells.
Figure 2. Macrophages devoid of STAT3 cross-present antigen acquired from a tumor cell more efficiently than WT macrophages.
(A) STAT3-deficient but not WT macrophages can capture and cross-present antigen from irradiated A20HA tumor cells and stimulate proliferation in CLN4 CD8 T cells responding specifically to HA antigen. (B) In the context of HA antigen presented by STAT3-deficient macrophages, intracellular cytokine staining shows that proliferating CLN4 T cells produce measurably more IFN-γ. (C) Similar results are seen when EL4mOVA tumor cells are used as an antigen source for B6.Stat3KO macrophages, with OVA-specific OT-I CD8 T cells used a measure of cognate stimulation.
To confirm the above observations, we used next a second experimental model with OT-I CD8 T cells as responders and B6.Stat3KO macrophages (or C57BL/6 macrophages) as presenters of OVA257–264 derived from irradiated EL4mOVA (Figure 2C). Whereas greater than 60% of the OT-I cells exposed to C57BL/6 APCs remained unresponsive, greater than 90% of OT-I cells exposed to B6.Stat3KO macrophage engaged in proliferation and approximately 50% of the activated population was IFN-γ+ by intracellular cytokine staining. CTL cross-presentation by STAT3KO macrophages becomes more important in light of prior published evidence by Albert et.al.18 indicating that STAT3 competent macrophages are unable to cross-present apoptotic bodies to CTLs.
Clearly, in the absence of a functional STAT3 protein, macrophage APCs exhibit increased CD8 T cell activating capacity, but even more important, our data demonstrate for the first time that STAT3KO macrophages are inherently superior than their WT counterparts at acquiring and processing antigen for effective cross-presentation to CTLs. This finding is particularly relevant in tumor-tolerized CTL responses due to the fact that tumor-induced CD8 T-cell tolerance, although multifactorial, it is becoming increasingly recognized as being established and maintained by the APC engaging the CTL19. Although we extensively tried to address the impact of the STAT3-defect on the ability of the APC to overcome CD8 T cell tolerance, in a fashion similar to our prior work with CD4 T-cells16, our in vitro culture conditions proved to be inadequate for this task. In part, this was due to the fact that the vast majority of clonal responder CTLs, in the face of tolerogenic signals such as tumor-induced tolerance or high-dose peptide challenge, undergo clonal deletion2,20,21. Residual clones may be increasingly dependent on persistent antigen presentation for continued survival and avoiding activation-induced cell death. This would likely explain prior findings that tolerant CD8 T cells in tumor models are eventually localized predominantly in the tumor beds10. Ongoing studies using an in vivo tolerance model system will address whether the STAT3-deficient APC can maintain this enhanced ability to capture antigen in the suppressive tumor microenvironment and continue to efficiently cross-prime anti-tumor CTL responses.
During the past several years, sufficient evidence has been generated supporting the conclusion that cancers are not immunologically inert entities. Yet, despite the presence of specific T cell responses against tumor-associated antigens (TAAs), the immune system rarely confers adequate and effective protection. There are many factors contributing to this observation, including (but not limited to) potent and durable central and peripheral tolerance mechanisms, suboptimal activation of professional antigen presenting cells (APCs) at the tumor site leading to ineffective T cell priming, as well as an strong immune suppressive and tolerogenic milieu within the tumor microenvironment itself. The targeted disruption of STAT3 function can potentially influence all of these factors. The hyper-inflammatory nature of STAT3-deficient APCs has been shown to disrupt peripheral tolerance mechanisms. This is evidenced by the fact that LysMCre.Statflox/− mice develop an autoimmune colitis15. Likewise, inhibition of STAT3 would, in theory, counter any suboptimal APC activation at the tumor site thus preventing suboptimal T cell priming due to APC inefficiency. Furthermore, STAT3-defective APCs might be less susceptible to the immunosuppressive environment of the tumor due to the fact that many of the primary mechanisms of immune suppression within the tumor are dependent on STAT3 signaling. For instance, IL-10 and VEGF, both considered to contribute significantly to the dampening of immune responses rely on signaling pathways incorporating STAT3 22,23,24. Taken together, targeting STAT3 signaling in the relevant tumor-associated APC populations represents a potential strategy to conceivably enhance CTL activity and overcome CD8 T cell tolerance, a major obstacle in the current development of effective cancer immunotherapies.
Acknowledgments
This work was supported by PHS grant CA87583 and CA100850
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morgan DJ, Kurts C, Kreuwel HTC, Holst KL, Heath WR, Sherman LA. Ontogeny of T cell tolerance to peripherally expressed antigens. PNAS. 1999;96:3854–3858. doi: 10.1073/pnas.96.7.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurts C, Kosaka H, Carbone FR, Miller JFAP, Heath WR. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8+ T cells. J Exp Med. 1997;186(2):239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vezys V, Olson S, Lefrancois L. Expression of intestine-specific antigen reveals novel pathways of CD8 T cell tolerance induction. Immunity. 2000;12:505–514. doi: 10.1016/s1074-7613(00)80202-2. [DOI] [PubMed] [Google Scholar]
- 4.Lee PP, Yee C, Savage PA, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 5.Janicki CN, Jenkinson SR, Williams NA, Morgan DL. Loss of high-avidity tumor-specific CD8+ T cells following tumor infiltration. Cancer Res. 2008;68:2993–3000. doi: 10.1158/0008-5472.CAN-07-5008. [DOI] [PubMed] [Google Scholar]
- 6.Zippelius A, Batard P, Rubio-Godoy V, Bioley G, Lienard D, et al. Effector function of human tumor-specific CD8 T cells in melanoma lesions: a state of local functional tolerance. Cancer Res. 2004;64:2865–2873. doi: 10.1158/0008-5472.can-03-3066. [DOI] [PubMed] [Google Scholar]
- 7.Ohlen C, Kalos M, Cheng LE, Shur AC, et al. CD8(+) T cell tolerance to a tumor-associated antigen is maintained at the level of expansion rather than effector function. J Exp Med. 2002;195(11):1407–1418. doi: 10.1084/jem.20011063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frey AB, Monu N. Effector phase tolerance: another mechanism of how cancer escapes antitumor immune response. J Leukoc Biol. 2006;79:652–662. doi: 10.1189/jlb.1105628. [DOI] [PubMed] [Google Scholar]
- 9.Dumortier H, van Mierlo GJD, Egan D, van Ewijk W, Toes R, Offringa R, Melief CJM. Antigen presentation by an immature myeloid dendritic cell line does not cause CTL deletion in vivo, but generates a CD8+ central memory-like T cells that can be rescued for full effector function. J Immunol. 2005;175:855–863. doi: 10.4049/jimmunol.175.2.855. [DOI] [PubMed] [Google Scholar]
- 10.Bai A, Higham E, Eisen HN, Wittrup KD, Chen J. Rapid tolerization of virus-activated tumor-specific CD8+ T cells in prostate tumors of TRAMP mice. PNAS. 2008;105:13003–13008. doi: 10.1073/pnas.0805599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg SA, Sherry RM, Morton KE, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169–6176. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 12.Norian LA, Rodriguez PC, O’Mara LA, et al. Tumor-infiltrating regulatory dendritic cells inhibit CD8+ T cell function via L-arginine metabolism. Cancer Res. 2009;69(7):3086–3094. doi: 10.1158/0008-5472.CAN-08-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munn DH, Sharma MD, Lee JR, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 14.Talmadge JE. Pathways mediating the expression and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin Cancer Res. 2007;13(18):5243–5248. doi: 10.1158/1078-0432.CCR-07-0182. [DOI] [PubMed] [Google Scholar]
- 15.Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Forster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of STAT3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 16.Cheng F, Wang HW, Cuenca A, Huang M, Ghansah T, Brayer J, Kerr WG, Takeda K, Akira S, Schoenberger SP, Yu H, Jove R, Sotomayor EM. Critical role for STAT3 signaling in immune tolerance. Immunity. 2003;19(3):425–436. doi: 10.1016/s1074-7613(03)00232-2. [DOI] [PubMed] [Google Scholar]
- 17.Staveley-O’Carroll K, Sotomayor E, Montgomery J, Borrello I, Hwang L, Fein S, Pardoll D, Levitsky H. Induction of antigen-specific T cell anergy: an early event in the course of tumor progression. PNAS. 1998;95(3):1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bardwaj N. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36 and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188(7):1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toes RE, Offringa R, Blom RJ, Melief CJ, Kast WM. Peptide vaccination can lead to tumor growth through specific T-cell tolerance induction. PNAS. 1996;93(13):7855–7860. doi: 10.1073/pnas.93.15.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koniaras C, Bennett SR, Carbone FR, Heath WR, Lew AM. Peptide-induced deletion of CD8 T cells in vivo via apoptosis in situ. Int Immunol. 1997;9:1601–1605. doi: 10.1093/intimm/9.10.1601. [DOI] [PubMed] [Google Scholar]
- 22.Finbloom DS, Winestock KD. IL-10 induces the tyrosine phosphorylation of tyk2 and Jak1 and the differential assembly of STAT1 alpha and STAT3 complexes in human T cells and monocytes. J Immunol. 1995;155(3):1079–1090. [PubMed] [Google Scholar]
- 23.Ohm JE, Carbone DP. VEGF as a mediator of tumor associated immunodeficiency. Immunol Res. 2001;23:263–272. doi: 10.1385/IR:23:2-3:263. [DOI] [PubMed] [Google Scholar]
- 24.Manning EA, Ullman JGM, Leatherman JM, Asquith JM, Hansen TR, Armstrong TD, Hicklin DJ, Jaffee EM, Emens LA. A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin Cancer Res. 2007;13(13):3951–3959. doi: 10.1158/1078-0432.CCR-07-0374. [DOI] [PubMed] [Google Scholar]