Abstract
Thalassemia major is characterized by chronic ineffective erythropoiesis and anemia as its primary problems. These, in turn, produce physiologic adaptations in the cardiovascular system as well as pathologic/iatrogenic processes such as iron overload, splenectomy, nutritional deficiencies, chronic oxidative stress, and lung disease. This article discusses the pathophysiology of thalassemia as it relates to the cardiovascular system, the mechanisms and monitoring of iron cardiomyopathy, pulmonary hypertension, and vascular aging in thalassemia patients.
Keywords: Thalassemia, Heart complications, Iron cardiomyopathy, Pulmonary hypertension
THALASSEMIA AND THE CARDIOVASCULAR SYSTEM
Chronic Anemia
Patients with chronic anemia increase their cardiac output to maintain oxygen delivery, resulting in increased cardiac dimensions and heart rate. Anemic patients have larger hearts on CXR, echo, and MRI measurements than patients with normal hemoglobin levels, even without any other pathology. Thus, normative data generated from non-anemic patients is inappropriate for patients with hemoglobinopathies (1). The larger cardiac dimensions, stroke volumes, and heart rates carry metabolic cost; chronically anemic patients have higher resting oxygen consumption and decreased reserves. Increased resting metabolism is also a source of increased oxidative stress, independent of the free-radical effects of iron.
Patients with thalassemia have low or normal blood pressures, despite their increased cardiac output, because they have lower vascular resistance. Lower tonic vascular tone partially compensates for the increased chamber dimensions, but it leaves patients vulnerable to the endothelial toxicity of iron overload as well as making them less tolerant and responsive to the effects of afterload-reducing agents.
Splenectomy
Hypersplenism is relatively common in the thalassemias and may necessitate spleen removal. Splenectomy may also be performed to lower blood transfusion requirements. However, the spleen plays a critically important role in removing hematologic debris from the cardiovascular system. Phosphatidylserine positive platelets, platelet fragments, and red cell fragments are powerful pro-coagulants. They also inhibit nitric oxide, stimulate vasoconstricting substances such as endothelin and vasoconstricting prostaglandins, and produce endothelial proliferation (2). The spleen also removes brittle senescent red cells from the circulation, suppressing intravascular hemolysis. Cell-free hemoglobin is a powerful oxidant and scavenger of nitric oxide. As a result, splenectomy is a strong risk factor for intravascular thrombosis and pulmonary hypertension.
Iron Overload
Patients with thalassemia develop iron overload through increased iron absorption and transfusional therapy. Iron is toxic to all the endocrine glands that support the heart. Insulin-resistance and frank diabetes are relatively common. Hyperglycemia and insulin resistance are powerful oxidative stressors to the heart, worsening the effects of iron overload. Proper insulin sensitivity is also vital for efficient cardiac energy utilization. Iron may also poison the thyroid and parathyroid gland, impairing metabolism and calcium regulation respectively. Iron-mediated adrenal insufficiency may also manifest itself during metabolic stress. Deficiencies of growth hormone and the sex steroids impair cardiac function. Iron-mediated endocrine toxicity must be excluded in TM patients with cardiac failure.
Nutritional Deficiencies
The hemoglobinopathies are a hypermetabolic state and inherently produce chronic oxidative stress. Broad-spectrum nutritional deficiencies are prevalent and may reinforce disease toxicity (3). Fat-soluble vitamin depletion is common, including vitamin A, D, E, and K, suggesting fat mal-absorption. The mechanisms and consequences are unknown. Vitamin D deficiency is associated with increased cardiac iron and decreased function, but causation has not been proven (4). Many trace metals are decreased, including selenium, zinc, and copper. B-vitamin levels are also low, particularly thiamine, riboflavin, and folate, most likely from consumption during ineffective erythropoiesis. Severe thiamine deficiency can have neurological and cardiac toxicity, whereas deficient riboflavin and folate may result in elevated homocysteine and endothelial toxicity. Carnitine deficiency is also relatively common in thalassemia and can impair cardiac function (5).
Lung Disease
The heart and lungs are intimately coupled. Patients with thalassemia major develop mild, nonprogressive restrictive lung disease. Inhomogeneous ventilation and small airways disease is common but diffusion capacity (when corrected for hemoglobin) is normal suggesting that vascular networks are normal at the alveolar level. Both the etiology and the consequences of these changes are unknown.
IRON CARDIOMYOPATHY
Mechanisms
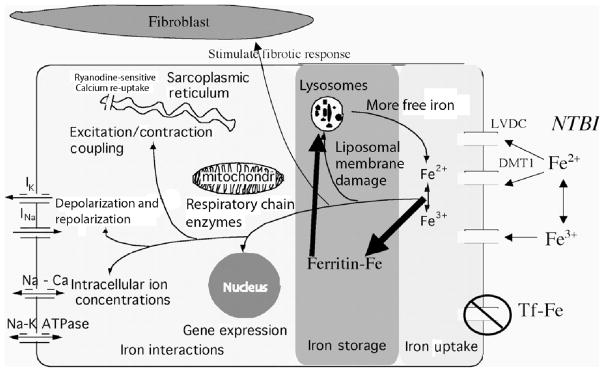
Figure 1 represents the pathophysiology of iron cardiomyopathy, artificially divided into iron uptake, iron storage, and iron toxicity (6). The heart takes up physiologic amounts of iron through transferrin receptors, but this process is tightly regulated and does not lead to iron overload. When transferrin-binding capacity is exceeded, circulating low molecular weight non-transferrin-bound iron (NTBI) species appear. NTBI is oxidatively active and can enter through nonspecific, poor-regulated cation channels in the heart, leading to cardiac iron overload. Several channel mechanisms have been proposed, including L-type voltage-dependent calcium channels, but much more work is necessary to characterize cardiac iron-uptake processes.
FIGURE 1.
Once in the heart, NTBI is rapidly bound by cytosolic ferritin to protect the cell against oxidative damage. Within hours, excess iron is shuttled off to long-term storage in lysosomes. This creates a state of abnormal MRI signal (T2*) in the heart, but most cardiac tests will be completely normal.
Eventually, heart cells lose their ability to safely buffer and stored cardiac iron. Stresses such as infection may also play a role is disturbing safe iron stores. Once labile iron is increased in the myocyte, it wreaks havoc with lysosomal, mitochondrial, and sarcoplasmic membranes, further increasing myocyte oxidative stress. Calcium, sodium, and potassium ion channels are disrupted causing conduction/repolarization disturbances, arrhythmias, and diastolic and systolic dysfunction. Iron can even modify gene expression, stimulate fibrosis, and trigger apoptosis.
Monitoring
Standard of care for cardiac monitoring varies regionally. Cardiac MRI assessment of T2* and ejection fraction on an annual or biannual basis are the gold standard when resources permit. Ejection fraction estimates by MRI are much more robust than by echo, allowing recognition of preclinical cardiac dysfunction. Cardiac T2* values less than 10 ms are at high risk of subsequent cardiac decompensation and warrant aggressive iron chelation therapy. Less severe cardiac iron overload can be managed less urgently.
Electrocardiogram and echocardiogram should be performed annually. Iron overload causes conduction delay and subtle repolarization abnormalities. Echocardiographic metrics of systolic and diastolic dysfunction are insensitive but relatively specific for cardiac iron overload; tricuspid regurgitation jet is helpful for recognition of pulmonary hypertension.
PULMONARY HYPERTENSION
Mechanisms
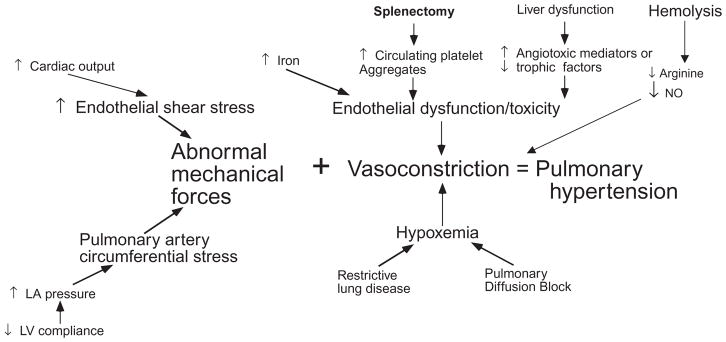
Figure 2 demonstrates the complex pathophysiology of pulmonary hypertension in thalassemia. Increased cardiac output and diastolic dysfunction cause abnormal loading of the pulmonary artery. Lung disease can exacerbate night-time hypoxia, a powerful stimulus for vasoconstriction. Iron, phosphatidylserine-expressing hematologic debris, free hemoglobin, and other circulating angiotrophic factors cause vasoconstriction and intimal proliferation.
FIGURE 2.
Diagnosis and Treatment
Patients with thalassemia intermedia are particularly vulnerable to pulmonary hypertension because of higher cardiac output, splenectomy, and greater intravascular hemolysis; a majority of patients will develop pulmonary hypertension in middle age. Thalassemia major (TM) patients have a greater contribution from iron toxicity, including more ventricular diastolic dysfunction and direct endothelial toxicity, but often have less hemolysis, lower cardiac outputs, and retain their spleens. Pulmonary hypertension is comparably rare in well-transfused TM patients. Diagnosis can usually be made by echocardiogram, and cardiac catheterization is reserved for relatively severe disease. Pulmonary hypertension in hemoglobinopathy patients responds relatively well to transfusion therapy, early in disease progression, and to sildenafil therapy in more advanced disease.
PREMATURE VASCULAR AGING
As thalassemia patients are living longer, the chronic effects of unbuffered oxidative stress on the vascular system are becoming more apparent (7). Thalassemia patients have impaired endothelial relaxation, intimal thickening, abnormal vascular stiffening, and degeneration of elastic arteries. Although some of these abnormalities improve with iron chelation, premature vascular aging is not solely iron-mediated. Further work is necessary to clarify the mechanisms and optimal therapies for this condition.
Footnotes
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- 1.Westwood MA, Anderson LJ, Maceira AM, et al. Normalized left ventricular volumes and function in thalassemia major patients with normal myocardial iron. J Magn Reson Imaging. 2007;25(6):1147–1151. doi: 10.1002/jmri.20915. [DOI] [PubMed] [Google Scholar]
- 2.Singer ST, Kuypers FA, Styles L, Vichinsky EP, Foote D, Rosenfeld H. Pulmonary hypertension in thalassemia: association with platelet activation and hypercoagulable state. Am J Hematol. 2006;81(9):670–675. doi: 10.1002/ajh.20640. [DOI] [PubMed] [Google Scholar]
- 3.Claster S, Wood JC, Noetzli L, et al. Nutritional deficiencies in iron overloaded patients with hemoglobinopathies. Am J Hematol. 2009 doi: 10.1002/ajh.21416. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wood JC, Claster S, Carson S, et al. Vitamin D deficiency, cardiac iron and cardiac function in thalassaemia major. Br J Haematol. 2008;141(6):891–894. doi: 10.1111/j.1365-2141.2008.07135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Beshlawy A, Ragab L, Fattah AA, et al. Improvement of cardiac function in thalassemia major treated with L-carnitine. Acta Haematol. 2004;111(3):143–148. doi: 10.1159/000076522. [DOI] [PubMed] [Google Scholar]
- 6.Wood JC, Enriquez C, Ghugre N, et al. Physiology and pathophysiology of iron cardiomyopathy in thalassemia. Ann N Y Acad Sci. 2005;1054:386–395. doi: 10.1196/annals.1345.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hahalis G, Kremastinos DT, Terzis G, et al. Global vasomotor dysfunction and accelerated vascular aging in beta-thalassemia major. Atherosclerosis. 2008;198(2):448–457. doi: 10.1016/j.atherosclerosis.2007.09.030. [DOI] [PubMed] [Google Scholar]