Abstract
Background
Tuberculosis (TB) is one of the major public health problems in Zambia. However, information about lineages of M. tuberculosis complex (MTBC) isolates useful for epidemiology investigations is unknown. In this study, we investigated the diversity of MTBC isolates from Ndola, a typical Zambian urbanized city with a documented high HIV prevalence.
Methods
This was part of a prospective cohort study in subjects with sputum smear-positive pulmonary TB. Spoligotyping was used to genotype the MTBC isolates and establish the circulating lineages. The 15-locus Mycobacterial Interspersed Repetitive Units - Variable Number Tandem Repeats (MIRU-VNTR) typing was used to study recent transmission.
Results
A total of 98 different spoligotypes were identified among 273 MTBC isolates. The majority (64.8%) of the isolates belonged to 9 known families, while 96 (35.2%) of the isolates were orphans. While LAM (41.8%) was the largest spoligotype family observed, most of the isolates (87.7%) belonging to the SAF1 family, with a significant portion coming from the T (13.6%), and X (5.9%) families. A few isolates (3.6%) belonged to the CAS, EAI, H, S, X1-LAM9 or U families. MIRU-VNTR typing was highly discriminatory (h = 0.988) among the 156 isolates tested in our sample, and increased the discrimination among 82 SAF1 isolates from 6 to 46 distinct patterns. In addition, 3.2% (5/156) of cases with available MIRU-VNTR results harbored more than one MTBC strain.
Conclusions
Our findings show a limited diversity of MTBC in Ndola with a high clustering rate (37.7%), which indicates that recent transmission plays an appreciable role in the dynamics of TB disease in this setting. This conclusion emphasizes the importance of early diagnosis and timely treatment. The results also confirm that MIRU-VNTR typing is suitable for studying the molecular epidemiology of TB in Ndola.
Background
Zambia is ranked among the world's top 10 high TB incidence countries with an incidence rate of 280 smear-positive tuberculosis (TB) cases per 100,000 inhabitants [1]. The World Health Organization (WHO) estimates the prevalence of all forms of TB in Zambia at 707/100,000 [1]. According to the National Tuberculosis Leprosy Program (NTLP), in 2004, the Copperbelt Province was responsible for nearly a third (27.6%) of the nation's notified TB cases. It was also one of the provinces with the highest Human Immunodeficiency Virus (HIV) prevalence (17%) in Zambia [2]. While efforts have been made to identify drivers of the HIV pandemic in Zambia, similar efforts for the TB epidemic lag far behind. A number of surveillance activities, both biological and socio indicator - studies, which are useful in identifying the risk factors that play a role in driving the HIV epidemic in Zambia, have been implemented. However, efforts in TB have mainly relied on generic recommendations to prevent TB. The epidemiology of TB in Zambia still remains largely unknown, and consequently, epidemiological data cannot help focus TB control strategies. In high TB incidence settings, determination of distinct transmission patterns is often indefinable, but may be greatly enhanced by the use of both molecular and conventional epidemiological tools.
Use of molecular markers for strain-specific differentiation of Mycobacterium tuberculosis-complex (MTBC) isolates in epidemiological studies became available in the last decades. Some of the more popular MTBC typing methods being used include IS6110-based restriction fragment length polymorphism (RFLP) [3] and PCR-based methods like spoligotyping [4], mycobacterial interspersed repetitive units - variable number of tandem repeats (MIRU-VNTR) [5-8], single-nucleotide polymorphisms [9,10] and large-sequence polymorphism analysis [11-14]. Furthermore, apart from differentiating MTBC strains, MIRU-VNTR typing can easily identify mixed infections in patient isolates. Even so, the choice of typing methods used in studies should be considered carefully to provide meaningful analysis because their ability to discriminate MTBC in different settings varies widely. In addition, knowledge on the (over-) representation of specific genotype families in a community can be important, especially if these families have been implicated in disease complications like drug resistance, severe disease, or increased transmissibility.
In this study, we set out to investigate the circulating MTBC isolates, and use spoligotyping and 15-locus MIRU-VNTR typing to distinguish MTBC isolates from Ndola, an urban city on the Copperbelt Province of Zambia.
Methods
Study setting and population
This was part of a prospective cohort study in subjects with sputum smear-positive pulmonary TB, conducted in the Ndola urban district on the Copperbelt Province of Zambia. The Ndola District Health Management Team (DHMT) is responsible for health care service delivery in the district and has a catchment population of about 374,750 persons [15]. The district has 21 health centres, 3 military clinics, and 2 tertiary care hospitals. Most of these health centres are able to deliver TB treatment and care and are referred to as treatment centres, but only 6 are able to provide laboratory services for smear microscopy and are referred to as diagnostic centres.
For this study, sputum samples were collected from all consecutive sputum smear-positive pulmonary TB subjects at 4 of the 6 existing TB diagnostic centres between January and July 2006 as per routine. Both previously-untreated (new) and previously-treated (retreatment) cases were enrolled. The two clinics not included in the study were left out mainly because of their comparatively low population catchment areas at the time and because of logistical problems. All TB patients were treated according to the national TB guidelines [16] in line with the WHO treatment guidelines [17]. Smear-negative subjects were excluded because of logistical limitations such as budgetary and manpower constraints.
Laboratory methods
After routine microscopy, sputum smear-positive samples were stored in cetylpyridinium chloride (CPC) transport medium and kept at ambient temperature until they were taken to the Tropical Diseases Research Centre (TDRC) on a weekly basis. The samples were later transported from TDRC to the Chest Diseases Laboratory (CDL), a reference laboratory in Lusaka, for culture on Löwenstein-Jensen (LJ) medium following decontamination using the Petroff method [18]. Culture tubes were incubated at 37°C and were read weekly for growth for at least eight weeks. Successfully grown cultures were transported back to TDRC for storage and onward transportation of isolates to the Institute of Tropical Medicine (ITM, Antwerp, Belgium) for further analysis.
Data collection methods
The clinics were provided with a register dedicated for the study to record study-subject information, which included socio-demographics (name, sex, age, residence) and clinical data (case type, smear-microscopy results for three time points during treatment treatment regimen followed, and treatment outcome). HIV data could not be collected at that time, because routine counseling and testing (CT) for TB patients had not yet been implemented by 2006, and it was not logistically possible to capture this data in the study. As quality control, the study register was checked against the clinic TB registers at the end of the collection period.
DNA extraction
To obtain genomic DNA for spoligotyping and MIRU-VNTR typing, mycobacterial colonies grown on LJ medium were resuspended in 200 μl 1 × Tris EDTA buffer (10 Mm Tris-HCl, 1 Mm Ethylenediaminetetracetic acid disodium [pH8.0]) and then boiled for 10 min. The suspension was centrifuged at 15 000 g for 1 min to pellet cell debris. The supernatant containing DNA was harvested and used in PCR reactions.
DNA fingerprinting
Spoligotyping was performed using a commercial kit (Isogen Bioscience B.V., Maarssen, The Netherlands) according to Kamerbeek et al. [4]. Standardized MIRU-VNTR typing based on 15 loci was performed using the manual method [19] or by the automated method at Genoscreen in Lille, France.
Investigation of laboratory cross contamination
The investigation of possible laboratory cross contamination or error was performed by reviewing the DNA-fingerprint patterns of clustered isolates from samples that were processed on the same day from respective laboratories.
Fingerprint analysis
Spoligotyping and MIRU-VNTR patterns were compared to the international Spol DB4.0 database using MIRU-VNTRplus, a freely available web-based program [20]. This allowed assignment of shared international spoligotype numbers (ST) to known profiles. Spoligotypes that were not present in the Spol DB4.0 are referred to as 'orphan' types. MIRU-VNTR profiles with double alleles at a single locus were considered to be clonal variants of the same strain, whereas those with double alleles at 2 or more loci were considered to be mixed infections [21,22]. Identical spoligotypes and MIRU-VNTR patterns were considered to be in a cluster. Dendograms were generated using the dice coefficient and the unweighted pair group method with arithmetic averages (UPGMA). The clustering rate was defined as (nc- c)/n, where nc is the total number of clustered cases, c is the number of clusters, and n is the total number of cases in the sample. A cluster was defined as two or more patterns with identical DNA genotypes. The discriminatory power of DNA fingerprinting methods was calculated using the method described by Hunter and Gaston [23].
Ethical Consideration
Before beginning the study, approval for the study protocol was obtained from the Ethics Committee at TDRC. In addition, approval and support was also obtained from the Director of the Ndola DHMT. The protocol was implemented in such a way as to have minimum interference with routine work at the clinics. The study did not require any additional (invasive) sampling, data collected was anonymized, there was no direct contact with the patients, and the outcome of the research data would not have an influence on patient management. As a result, we did not ask informed consent from the subjects.
Statistical methods
Epidemiological and laboratory analysis data were double entered and descriptive analyses done in Epi Info™ (Version 3.2.2, Centers for Disease Control and Prevention, Atlanta, GA, USA). All the electronic records were manually counterchecked against the source records for completeness and consistency.
The two sided Pearson's asymptotic and exact chi square tests were appropriately used to assess associations of sex, age, geographic origin and drug-resistance profiles with spoligotyping families or MIRU-VNTR clusters using SAS® 9.2 (SAS Institute Inc., Cary, NC, USA.) and StatXact® 4.0.1 (Cytel Software Corp., Cambridge, MA, USA.). A P value less than 0.05 was considered statistically significant.
Results
Subjects and isolates
A total of 361 sputum smear-positive PTB subjects from the four selected diagnostic centres in Ndola were enrolled into the study from January to July 2006. Isolates were successfully obtained for 273 subjects, representing 54.7% (273/499) of all smear-positive PTB patients recorded in Ndola district during this period. Samples for the remaining 88 subjects included in the study, yielded either contaminated (n = 10) or negative (n = 78) cultures.
Of the cultures from 273 different subjects available for DNA fingerprinting, 85 (31.1%) were female and 188 (68.9%) were male with an age range between 14 and 79 years and a median age of 31 years. All the isolates were confirmed to be M. tuberculosis with an overall low drug resistance level (unpublished data).
Characterization of M. tuberculosis lineages
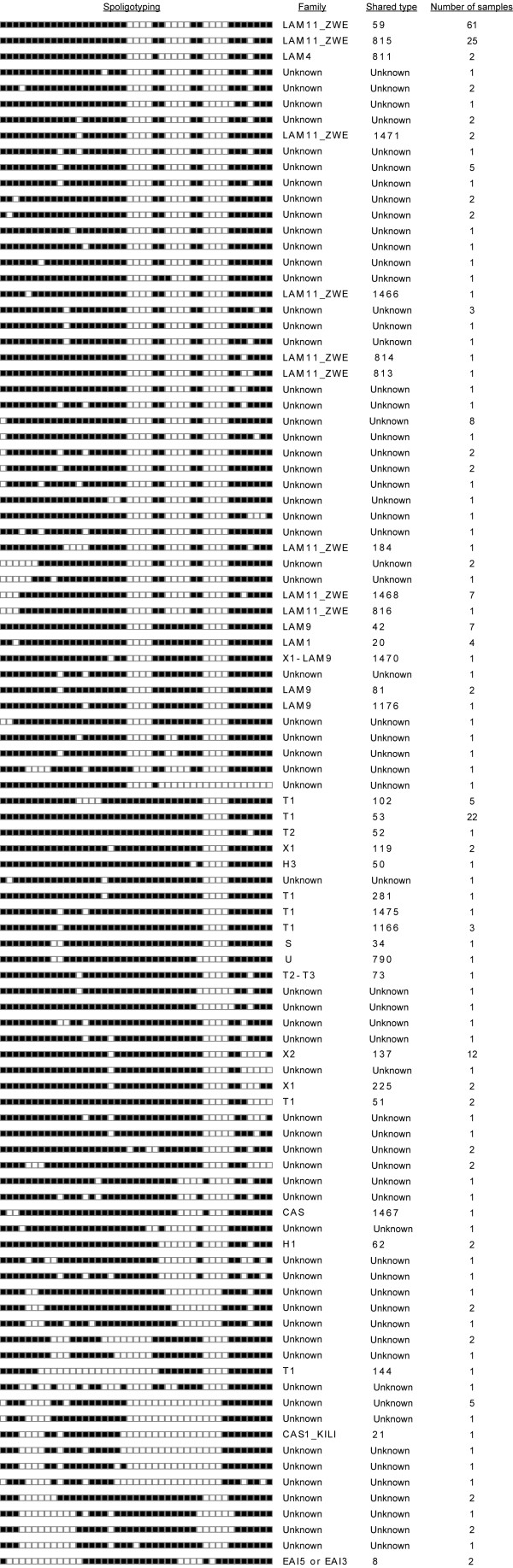
We used spoligotyping to determine lineages of circulating M. tuberculosis strains in Ndola. A total of 98 different spoligotypes were obtained among the 273 isolates analyzed. Patterns from 177 isolates belonged to nine families in the Spol DB4.0, whereas 96 (35.2%) isolates could not be matched to any lineage, and are thus referred to as 'orphan'.
The largest spoligotype family was the Latin American Mediterranean (LAM) that accounted for 41.8% (114 isolates) of the total isolates, most (100 isolates) of which belonged to the LAM11_ZWE sub-family designated Southern Africa Family 1 (SAF1) [24]. The next most common family was the T family with 37 isolates (13.6%), followed by the X family at 5.9% (16 isolates). A few isolates (3.6%) belonged to the CAS, EAI, H, S, X1-LAM9 or U families (Figure 1). Although our 'orphan' isolates were not described in the Spol DB4.0, six of them showed spoligotypes identical to previously reported orphan isolates in Zambia (n = 2), Zimbabwe (n = 2) and Cape Town (n = 1) [24]. There was a uniform distribution of spoligotypes from the various study centers (data not shown). Further, no significant statistical differences were observed in the distribution with regards to age (p = 0.5073), sex (p = 0.0896) and treatment history (p = 0.1824) between the group for whom we were able to perform spoligotyping and the group for which we could not.
Figure 1.
Distinct Spoligotypes of M. tuberculosis isolates from Ndola. The dendogram was generated using the dice coefficient and the unweighted pair group method with arithmetic averages (UPGMA) using the MIRU-VNTRplus program [20].
Transmission analysis
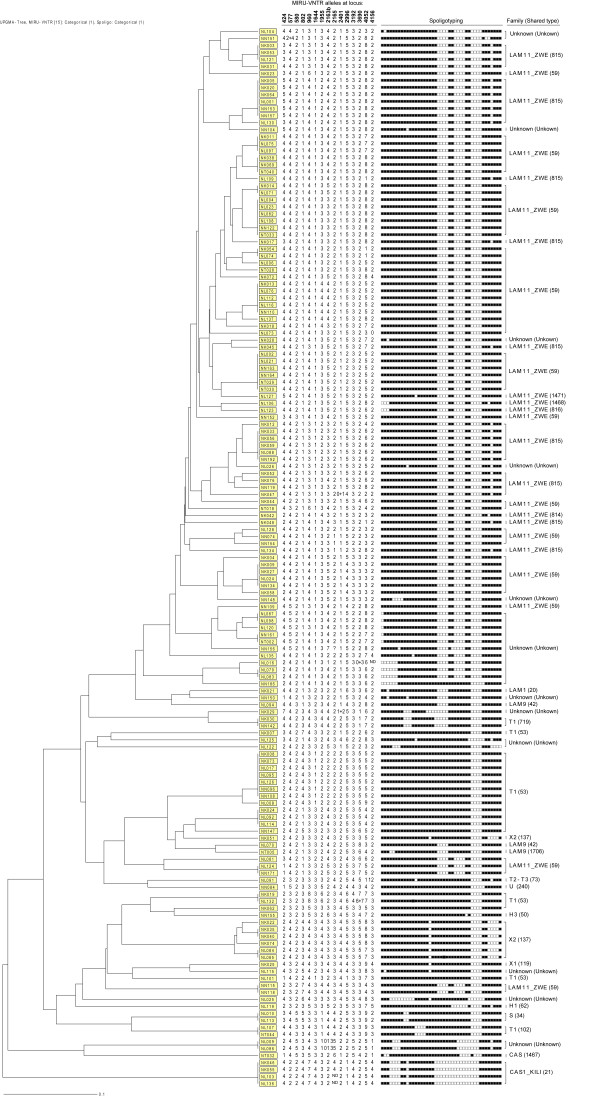
To gain insight into the transmission rate of M. tuberculosis in Ndola, 156 (57.1%) out of the 273 samples with spoligotyping results were randomly selected and typed by 15-locus MIRU-VNTR.
MIRU-VNTR analysis revealed five isolates with clonal subpopulations, i.e. the presence of double alleles at a single locus suggestive of possible ongoing evolution within a strain, and five mixed infection cases (3.2%) i.e. isolates with double alleles at 2 to 5 MIRU-VNTR loci among the 156 isolates with MIRU-VNTR results. The mixed infection cases were removed from the analysis whereas the isolates with clonal subpopulations were included in the analysis with the double alleles at a single locus treated as missing data. Thus, further analysis was performed on the 151 isolates (Figure 2).
Figure 2.
Spoligotyping and MIRU-VNTR clustering of representative M. tuberculosis isolates from Ndola. The dendogram generated using the dice coefficient and the unweighted pair group method with arithmetic averages (UPGMA) using the MIRU-VNTRplus program [20]. ND: Not determemined.
Not surprisingly, spoligotyping alone had the lowest ability to differentiate the isolates in our sample (clustering rate of 74.2%) and a discriminatory power of 0.840. MIRU-VNTR alone yielded a clustering rate of 39.1% and a discriminatory power of 0.988. The highest discrimination was achieved when spoligotyping and MIRU-VNTR were used together (h = 0.989; clustering rate of 37.7%), which was marginally better than that of MIRU-VNTR alone (Table 1 and Figure 2).
Table 1.
Discriminatory power of spoligotyping and 15-locus MIRU-VNTR among 151 M. tuberculosis isolates from Ndola, Zambia
| Genotyping method | Number of different profiles | Number of isolates with unique profile | Number of clusters | Number of isolates in clusters | Clustering rate (%) | h index |
|---|---|---|---|---|---|---|
| Spoligotyping | 39 | 27 | 12 | 124 | 74.2 | 0.840 |
| MIRU-VNTR | 92 | 65 | 27 | 86 | 39.1 | 0.988 |
| MIRU-VNTR + spoligotyping | 94 | 68 | 26 | 83 | 37.7 | 0.989 |
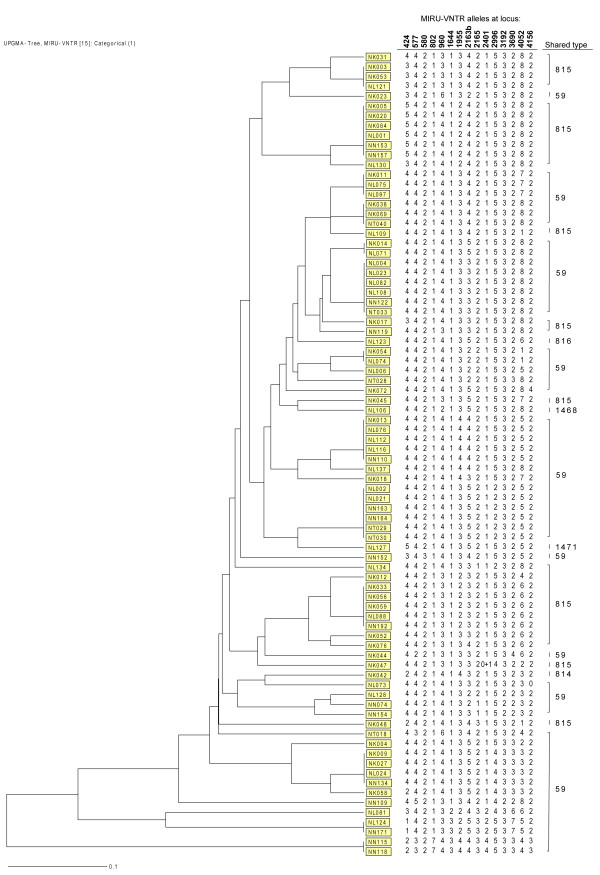
MIRU-VNTR typing of SAF1 isolates
We also assessed the genotypic similarity of isolates belonging to the major spoligotype family SAF1 among the above 151 isolates by 15-locus MIRU-VNTR. Of the 82 SAF1 isolates evaluated, MIRU-VNTR split the family into 46 different patterns i.e. 13 clusters comprising 49 isolates and 33 unique patterns (Figure 3). All isolates that were different by spoligotyping were also different by MIRU-VNTR. The differentiation of SAF1 isolates in clusters was mostly limited to one or two loci.
Figure 3.
MIRU-VNTR clustering of M. tuberculosis isolates belonging to the SAF1 family. The dendogram was generated using the dice coefficient and the unweighted pair group method with arithmetic averages (UPGMA) using the MIRU-VNTRplus program [20].
We did not observe any significant differences between the group of patients for whom we performed MIRU-VNTR and those we did not with regards to age (p = 0.8884), sex (p = 0.7350) and treatment history (p = 0.1536).
Discussion
This study reports the first utilization of spoligotyping and MIRU-VNTR typing to study the diversity of M. tuberculosis isolates in Ndola using a large number of samples. Ndola is an urbanized city with a high prevalence of HIV (17%) and is representative of many urban towns along the line of rail in Zambia. Our results demonstrate that the SAF1 family, and to a lesser extent the T family are the main circulating TB genotypes in Ndola, causing half (50.2%) of the TB cases in the city. The predominance of ST59 and ST53 of the SAF1 has been shown to be ubiquitous in the southern African region [24,25]. The small group of genotypes accounting for most TB cases in Ndola may imply their long-standing presence in the area. The clonal variations we saw for the SAF1 family appear to support this notion.
The 15-locus MIRU-VNTR in our sample performed very well. Although the comparison with IS6110-RFLP was not available, the high discriminatory value achieved by MIRU-VNTR in this study suggests that the technique is suitable for studying the molecular epidemiology of TB in Ndola. The high clustering rate (37.7%) exhibited in this study suggests likely high transmission in the community that may have occurred both before and during our study period since the study included both new and retreatment cases. We did not find any evidence of laboratory cross-contamination as a possible explanation for the high cluster rate. Also, we did not observe any significant differences between age, sex and treatment history among analyzed and non-analyzed cases by either of the typing techniques. The potential association of specific genotypes or clusters with the HIV status of the patients could not be investigated because HIV data was not routinely captured by the clinics format during the time of study. Conventional epidemiological investigations of contacts could not be performed due to budgetary constraints.
Our relatively short time window of sampling (7 months) is probably still acceptable to interpret the observed clustering as resulting from 'recent transmission' [26] and might not be surprising in a high TB and HIV incidence setting. Studies on clustering rates for other African countries are rare and diverse both in methodology and results; only a few of them have used highly discriminatory typing techniques. In Botswana, 25% of investigated isolates from four communities (18 months sampling) clustered by IS6110-RFLP [27] whereas a population-based nationwide study with a 21 months sampling period showed a 38% cluster rate [28]. Clustering was not associated with HIV or demographic characteristics in both studies except for prior imprisonment in the first study. In Ethiopia, 41.2% of 121 isolates (12 months sampling) clustered by IS6110 and a clear association with HIV infection and female sex was observed [29]. In Benin, 34% of isolates (15 months sampling) clustered by 12-loci MIRU-VNTR and spoligotyping, with no parameters linked to clustering [30]. These and our cluster rates are higher than the estimated 9-13% of TB cases due to recent transmission in Malawi, where nearly half of the cases acquired TB from an HIV-positive subject [31].
Given the relatively short time window of our study compared to other African studies, our clustering rate should be considered high, and probably reflects a high recent transmission rate emphasizing the importance of early diagnosis and timely treatment. Further investigation on the link with HIV infection is required. We acknowledge that the interpretation of transmission dynamics data in this study may be limited because we did not include smear negative subjects, who are known to contribute to TB transmission [32-34].
On the one hand, these findings lend support to the premise that M. tuberculosis in endemic areas with predominant family strains can still possess sufficient genetic diversity when the appropriate molecular method is applied, enabling more detailed epidemiologic investigations. On the other hand, since differentiation of SAF1 isolates by 15-locus MIRU-VNTR was mostly limited to one or two loci, application of the standardized 24-locus MIRU-VNTR [35] - not used in this study due to financial constraints - may increase the ability to discriminate more MTBC among the clustered SAF1 strains.
This study also detected mixed infections in five subjects. Except for 1 of these 5 subjects, who exhibited INH resistance, isolates from the other 4 subjects, were pansusceptible to the anti-TB drugs tested. The rate of mixed infections detected in this study (at least 3.1% observed among 156 isolates with MIRU-VNTR results) is comparable to previous reports from high-incidence populations [21,22,36-38]. Although this observation does not necessarily pose a serious threat for patient management owing to the relatively low level of drug resistance in this setting, it is potentially an important factor to consider particularly for treatment of compliant subjects with unexplained changes in drug resistance patterns during the course of chemotherapy.
Conclusion
This study has shown that the majority of MTBC isolates in Ndola belongs to the SAF1 family with a high clustering rate and that the 15-locus MIRU-VNTR typing is suitable for studying the molecular epidemiology of TB in Ndola. Finally, the probable high recent transmission rate underlines the importance of early diagnosis and timely treatment.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
CM was involved in the design, implementation of the study, and drafted the manuscript. ICS conceived and designed the study and critically revised the manuscript. FP critically revised the manuscript and LR was involved in the implementation and critically revised the manuscript. DK performed statistical analysis and critically revised the manuscript. NK critically revised original study design and the manuscript. All the authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Chanda Mulenga, Email: chandamulenga@yahoo.com.
Isdore C Shamputa, Email: shamputai@niaid.nih.gov.
David Mwakazanga, Email: mwakazangad@yahoo.com.
Nathan Kapata, Email: nkapata@gmail.com.
Françoise Portaels, Email: fportaels@itg.be.
Leen Rigouts, Email: lrigouts@itg.be.
Acknowledgements
This study was supported by funds from a grant of the Belgian Directorate-General for Development Cooperation (DGDC) from which Chanda Mulenga is a scholarship recipient, and the Damien Action, Brussels, Belgium. We thank the technical staff at the Chest Diseases Laboratory, Zambia for their excellent work. We also acknowledge Webster Kasongo for coordinating the study in Zambia.
References
- World Health Organization. Global Tuberculosis Control WHO Report. WHO/HTM/TB/2009.411, Geneva, Switzerland, WHO; 2009. [Google Scholar]
- Central Statistical Office (CSO), Ministry of Health (MOH), Tropical Diseases Research Centre (TDRC), University of Zambia (UNZA), and Macro International Inc. Zambia Demographic and Health Survey 2007. Calverton, Maryland, USA: CSO and Macro International Inc; 2009. [Google Scholar]
- van Embden JD, Cave MD, Crawford JT, Dale JW, Eisenach KD, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick TM, Small PM. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31(2):406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J. Simultaneous detection and strain differentiation of Mycobacterium tuberculosisfor diagnosis and epidemiology. J Clin Microbiol. 1997;35(4):907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frothingham R, Meeker-O'Connell WA. Genetic diversity in the Mycobacterium tuberculosis complex based on variable numbers of tandem DNA repeats. Microbiology. 1998;144(Pt 5):1189–1196. doi: 10.1099/00221287-144-5-1189. [DOI] [PubMed] [Google Scholar]
- Le Fleche P, Fabre M, Denoeud F, Koeck JL, Vergnaud G. High resolution, on-line identification of strains from the Mycobacterium tuberculosis complex based on tandem repeat typing. BMC Microbiol. 2002;2:37. doi: 10.1186/1471-2180-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savine E, Warren WM, van der Spuy GD, Beyers N, van Helden PD, Locht C, Supply P. Stability of variable-number tandem repeats of mycobacterial interspersed repetitive units from 12 loci in serial isolates of Mycobacterium tuberculosis. J Clin Microbiol. 2002;40(12):4561–4566. doi: 10.1128/JCM.40.12.4561-4566.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supply P, Lesjean S, Savine E, Kremer K, van Soolingen D, Locht C. Automated high-throughput genotyping for study of global epidemiology of Mycobacterium tuberculosis based on mycobacterial interspersed repetitive units. J Clin Microbiol. 2001;39(10):3563–3571. doi: 10.1128/JCM.39.10.3563-3571.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filliol I, Motiwala AS, Cavatore M, Qi W, Hazbón MH, Bobadilla del Valle M, Fyfe J, García-García L, Rastogi N, Sola C, Zozio T, Guerrero MI, León CI, Crabtree J, Angiuoli S, Eisenach KD, Durmaz R, Joloba ML, Rendón A, Sifuentes-Osornio J, Ponce de León A, Cave MD, Fleischmann R, Whittam TS, Alland D. Global phylogeny of Mycobacterium tuberculosis based on a single nucleotide polymorphism (SNP) analysis: insights into tuberculosis evolution, phylogenetic accuracy of other DNA fingerprinting systems, and recommendations for a minimal standard SNP set. J Bacteriol. 2006;188(2):759–772. doi: 10.1128/JB.188.2.759-772.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutacker MM, Mathema B, Soini H, Shashkina E, Kreiswirth BN, Graviss EA, Musser JM. Single-nucleotide polymorphism-based population genetic analysis of Mycobacterium tuberculosis strains from 4 geographic sites. J Infect Dis. 2006;193(1):121–128. doi: 10.1086/498574. [DOI] [PubMed] [Google Scholar]
- de Jong BC, Antonio M, Awine T, Ogungbemi K, de Jong YP, Gagneux S, DeRiemer K, Zozio T, Rastogi N, Borgdorff M, Hill PC, Adegbola RA. Use of spoligotyping and large sequence polymorpisms to study the populations structure of Mycobacterium tuberculosis complex in a cohort study of consecutive smear-positive cases in The Gambia. J Clin Microbiol. 2009;47(4):994–1001. doi: 10.1128/JCM.01216-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores L, Van T, Narayanan S, DeRiemer K, Kato-Maeda M, Gagneux S. Large sequence polymorphisms classify Mycobacterium tuberculosis strains with ancestral spoligotyping patterns. J Clin Microbiol. 2007;45(10):3393–3395. doi: 10.1128/JCM.00828-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagneux S, DeRiemer K, Van T, Kato-Maeda M, de Jong BC, Narayanan S, Nicol M, Niemann S, Kremer K, Gutierrez MC, Hilty M, Hopewell PC, Small PM. Variable host-pathogen compatibility in Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2006;103(8):2869–2873. doi: 10.1073/pnas.0511240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MB, Pichler VK, McIntosh F, Mattia A, Fallow A, Masala S, Domenech P, Zwerling A, Thibert L, Menzies D, Schwartzman K, Behr MA. Major Mycobacterium tuberculosis lineages associate with patient country of origin. J Clin Microbiol. 2009;47(4):1119–28. doi: 10.1128/JCM.02142-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Central Statistics Office. Summary Report, Zambia 2000 Census of Population and Housing. Central Statistics Office, Lusaka, Zambia; 2003. [Google Scholar]
- Ministry of Health. The National TB and Leprosy Control Programme. Third. Ministry of Health, Lusaka, Zambia; Tuberculosis and TB/HIV Manual. [Google Scholar]
- World Health Organization. WHO/CDS/TB/2003.313. 3. World Health Organization, Geneva, Switzerland; 2003. Treatment of tuberculosis: guidelines for national programmes. [Google Scholar]
- Petroff SA. A new and rapid method for the isolation and cultivation of tubercle bacilli directly form the sputum and feces. J Exp Med. 1915;21(1):38–42. doi: 10.1084/jem.21.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supply P, Mazars E, Lesjean S, Vincent V, Gicquel B, Locht C. Variable human minisatellite-like regions in the Mycobacterium tuberculosis genome. Mol Microlbiol. 2000;36(3):762–771. doi: 10.1046/j.1365-2958.2000.01905.x. [DOI] [PubMed] [Google Scholar]
- Allix-Béguec C, Harmsen D, Weniger T, Supply P, Niemann S. Evaluation and strategy for use of MIRU-VNTRplus, a multifunctional database for online analysis of genotyping data and phylogenetic identification Mycobacterium tuberculosis complex isolates. J Clin Microbiol. 2008;46(8):2692–2699. doi: 10.1128/JCM.00540-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamputa IC, Rigouts L, Eyongeta LA, El Aila NA, van Deun A, Salim AH, Willery E, Locht C, Supply P, Portaels F. Genotypic and phenotypic heterogeneity among Mycobacterium tuberculosis isolates from pulmonary tuberculosis patients. J Clin Microbiol. 2004;42(12):5528–5536. doi: 10.1128/JCM.42.12.5528-5536.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamputa IC, Jugheli L, Sadradze N, Willery E, Portaels F, Supply P, Rigouts L. Mixed infection and clonal representativeness of a single sputum sample in tuberculosis patients from a penitentiary hospital in Georgia. Respir Res. 2006;17(7):99. doi: 10.1186/1465-9921-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter PR, Gaston MA. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J Clin Microbiol. 1988;26(11):2465–2466. doi: 10.1128/jcm.26.11.2465-2466.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chihota V, Apers L, Mungofa S, Kasongo W, Nyoni IM, Tembwe R, Mbulo G, Tembo M, Streicher EM, van der Spuy GD, Victor TC, van Helden P, Warren RM. Predominance of a single genotype of Mycobacteium tuberculosis in regions of Southern Africa. Int J Tuberc Lung Dis. 2007;11(3):311–318. [PubMed] [Google Scholar]
- Easterbrook PJ, Gibson A, Murad S, Lamprecht D, Ives N, Ferguson A, Lowe O, Mason P, Ndudzo A, Taziwa A, Makombe R, Mbengeranwa L, Sola C, Rastogi N, Drobniewski F. High rates of clustering of strains causing tuberculosis in Harare, Zimbabwe: a molecular epidemiological study. J Clin Microbiol. 2004;42(10):4536–4544. doi: 10.1128/JCM.42.10.4536-4544.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn JR, Bauer J, de Boer AS, Borgdorff MW, Fine PEM, Godfrey-Faussett P, Vynnycky E. Interpreting DNA fingerprint clusters of Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 1999;3(12):1055–1060. [PubMed] [Google Scholar]
- Lockman S, Sheppard JD, Braden CR, Mwasekaga MJ, Woodley CL, Kenyon TA, Binkin NJ, Steinman M, Montsho F, Kesupile-Reed M, Hirschfeldt C, Notha M, Moeti T, Tappero JW. Molecular and conventional epidemiology of Mycobacterium tuberculosis in Botswana: a population-based prospective study of 301 pulmonary tuberculosis patients. J Clin Microbiol. 2001;39(3):1042–1047. doi: 10.1128/JCM.39.3.1042-1047.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockman S, Sheppard JD, Mwasekaga M, Kenyon TA, Binkin NJ, Braden CR, Woodley CL, Rumisha DW, Tappero JW. DNA fingerprinting of a national sample of Mycobacterium tuberculosis isolates, Botswana, 1995-1996. Int J Tuberc Lung Dis. 2000;4(6):584–587. [PubMed] [Google Scholar]
- Bruchfeld J, Aderaye G, Palme IB, Bjorvatn B, Ghebremichael S, Hoffner S, Lindquist L. Molecular epidemiology and drug resistance of Mycobacterium tuberculosis isolates from Ethiopian pulmonary tuberculosis patients with and without human immunodeficiency virus infection. J Clin Microbiol. 2002;40(5):1636–1643. doi: 10.1128/JCM.40.5.1636-1643.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affolabi D, Anyo G, Faïhun F, Sanoussi N, Shamputa IC, Rigouts L, Kestens L, Anagonou S, Portaels F. First molecular epidemiological study of tuberculosis in Benin. Int J Tuberc Lung Dis. 2009;13(3):317–322. [PubMed] [Google Scholar]
- Crampin AC, Glynn JR, Traore H, Yates MD, Mwaungulu L, Mwenebabu M, Chaguluka SD, Floyd S, Drobniewski F, Fine PE. Tuberculosis transmission attributable to close contacts and HIV status, Malawi. Emerg Infect Dis. 2006;12(5):729–735. doi: 10.3201/eid1205.050789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tostmann A, Kik SV, Kalisvaart NA, Sebek MM, Verver S, Boeree MJ, van Soolingen D. Tuberculosis transmission by patients with smear negative pulmonary tuberculosis in a large cohort in the Netherlands. Clin Infect Dis. 2008;47(9):1135–1142. doi: 10.1086/591974. [DOI] [PubMed] [Google Scholar]
- Hernandez-Garduno E, Cook V, Kunimoto D, Elwood RK, Black WA, FitzGerald JM. Transmission of tuberculosis from smear negative patients: a molecular epidemiology study. Thorax. 2004;59(4):286–290. doi: 10.1136/thx.2003.011759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn SD, Edwards D, Wood R. Tuberculosis transmission from patients with smear negative pulmonary tuberculosis in sub-Saharan Africa. Clin Infect Dis. 2009;48(4):496–497. doi: 10.1086/596550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supply P, Allix C, Lesjean S, Cardoso-Oelemann M, Rüsch-Gerdes S, Willery E, Savine E, de Haas P, van Deutekom H, Roring S, Bifani P, Kurepina N, Kreiswirth B, Sola C, Rastogi N, Vatin V, Gutierrez MC, Fauville M, Niemann S, Skuce R, Kremer K, Locht L, van Soolingen D. Proposal for standardization of optimized mycobacterial interspersed repetitive unit-variable-number tandem repeat typing of Mycobacterium tuberculosis. J Clin Microbiol. 2006;44(12):4498–4510. doi: 10.1128/JCM.01392-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavrum R, Mphahlele M, Ovreås K, Muthivhi T, Fourie PB, Weyer K, Grewal HM. High diversity of Mycobacterium tuberculosis genotypes in South Africa and preponderance of mixed infections among ST53 isolates. J Clin Microbiol. 2009;47(6):1848–1856. doi: 10.1128/JCM.02167-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamputa IC, Lee J, Allix-Béguec C, Cho E, Lee J, Rajan V, Lee EG, Min JH, Carroll MW, Goldfeder LC, Kim JH, Kang HS, Hwang S, Eum S, Park SK, Lee H, Supply P, Cho SN, Via LE, Barry CE. Genetic diversity of Mycobacterium tuberculosis isolates from a tertiary tuberculosis hospital in South Korea. J Clin Microbiol. 2010;48(2):387–394. doi: 10.1128/JCM.02167-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren RM, Victor TC, Streicher EM, Richardson M, Beyers N, Gey van Pittius NC, van Helden PD. Patients with active tuberculosis often have different strains in the same sputum specimen. Am J Respir Crit Care Med. 2004;169(5):610–614. doi: 10.1164/rccm.200305-714OC. [DOI] [PubMed] [Google Scholar]