The basic outline of the events controlling entry into mitosis has been clear for more than 20 y: the essential mitotic driver M-phase promoting factor [MPF; a cyclin-dependent kinase (CDK) composed of CDK1 and cyclin B] becomes rapidly activated and phosphorylates a wide variety of targets, creating mitotic phosphoproteins that contribute to nuclear membrane breakdown, chromosome condensation, and spindle assembly. But is this the entire story? The article in PNAS by Burgess et al. (1) adds to evidence accumulating over the last 2 y arguing that half the picture has been missing. The levels of mitotic phosphoproteins are governed not only by MPF but also by the specific phosphatases that remove MPF-catalyzed phosphorylations. Importantly, both entry into M phase and the maintenance of M phase require the operation of a pathway, mediated by a kinase called Greatwall (Gwl), that can inactivate these phosphatases.
On the discovery of MPF, it was widely appreciated that the phosphorylations that this kinase adds to mitotic phosphoproteins (MPF phosphosites) must be removed for cells to exit from M phase after anaphase onset. This fact implied that the phosphatase(s) responsible for this reversal might, in some fashion, be turned off during M phase itself to preserve the MPF phosphosites necessary for M-phase entry and maintenance. This theory was supported by a few tantalizing early observations (2–4), and it could also in theory explain the phenomenon, widely known at the time, that the phosphatase inhibitor okadaic acid promotes M-phase entry in vertebrate systems (5, 6). However, the concept languished for many years, in large part because the identity of the phosphatases directed against MPF phosphosites remained unknown. Most of the evidence pointed to one or more forms of PP2A (2, 4, 7–9), a prominent group of heterotrimeric phosphatases, but this assignment has remained contentious even until fairly recently (10).
Within the last 2 y, Mochida and Hunt (11, 12) have placed on a much firmer footing the hypothesis that the phosphatase targeting MPF-driven mitotic phosphorylations must be down-regulated during M phase. First, by direct measurements using model substrates, they showed that Xenopus egg extracts have abundant phosphatase activity directed against MPF phosphosites during interphase, but the same extracts are almost completely devoid of this activity during M phase (11). Second, biochemical experiments established that the phosphatase measured in these assays was a form of PP2A containing a particular B55-type regulatory subunit called B55δ (12). Because the Drosophila genome has only a single B55-family gene, it is assumed hereafter that PP2A enzymes associated with any B55-type subunit similarly display activity against MPF phosphosites and are turned off during M phase.
Additional strong evidence in favor of this idea has emerged from a separate and originally unrelated series of investigations on the kinase Greatwall (sometimes called MAST-L in humans), whose role in the cell cycle was first intimated in Drosophila (13, 14). The most easily interpreted phenotype associated with loss-of-function mutations of Drosophila Gwl is a general slowing of the cell cycle, particularly during the G2-to-M transition (14). Work in Xenopus extracts then revealed that Gwl was required both for M-phase entry and [in so-called cytostatic factor-containing (CSF) extracts made from eggs arrested in metaphase of meiosis II] M-phase maintenance (15). Initial models assumed that Gwl operated exclusively in the autocatalytic loop that ensures a rapid increase in MPF activity on M-phase entry by removing inhibitory phosphorylations from the CDK1 component of MPF. However, subsequent observations suggested that Gwl could also affect the cell cycle in some mysterious fashion independent of the MPF autoregulatory loop (16). Indeed, under certain experimental conditions, the cell-cycle status of extracts seems to be dictated more intimately by Gwl than by MPF itself. For example, CSF extracts in which MPF has been almost completely inactivated by drugs can remain in M phase if they retain active Gwl kinase, but the extracts immediately lose M-phase characteristics when Gwl is removed, even if MPF is still present (16).
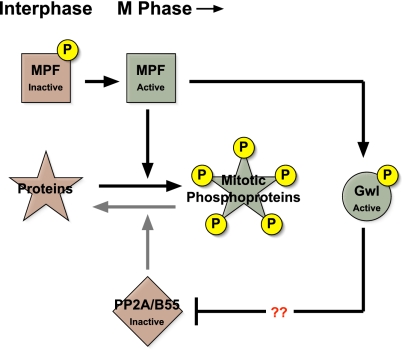
Publication of the Mochida and Hunt (11) paper described above suggested how this mystery might be resolved. Perhaps Gwl could be a critical component of the mechanism that inactivates, specifically during M phase, the phosphatases directed against MPF phosphosites. Recent publications from two separate laboratories have verified this hypothesis (17, 18), leading to the simple yet powerful general model shown in Fig. 1. MPF can activate Gwl (15), and active Gwl then promotes the inactivation of PP2A/B55 phosphatases through an unknown mechanism that may be either direct (with Gwl phosphorylating a component of the phosphatase) or indirect (with Gwl targeting a different molecule that subsequently acts on PP2A/B55).
Fig. 1.
Gwl kinase mediates the inactivation of PP2A/B55 phosphatase during M-phase entry. Green boxes indicate active enzymes; red boxes indicate inactive enzymes. MPF becomes activated through an autoregulatory process involving the removal of inhibitory phosphorylations from its CDK1 component. Active MPF then phosphorylates many targets, creating phosphoproteins involved in many downstream events in mitosis. Among the targets of MPF are enzymes (such as Cdc25 phosphatase, not shown) that participate in the autoregulatory loop and thus keep MPF activity high. MPF also phosphorylates and activates Gwl kinase. Gwl, in turn, inactivates (through a currently unknown mechanism) PP2A phosphatases with B55 regulatory subunits. If not inactivated by this Gwl-mediated process, PP2A/B55 phosphatases would prematurely reverse MPF-driven phosphorylations, preventing M-phase entry.
The power of this model lies in its explanation of how Gwl can be involved in the autoregulatory loop that activates MPF but, at the same time, can govern the cell cycle in a manner that is relatively independent of MPF. By controlling the activity of PP2A/B55, Gwl helps determine the phosphorylation of a wide variety of MPF phosphosites, including those on known regulators of MPF in the autoregulatory loop (for example, Myt1/Wee1 kinases and Cdc25 phosphatases) as well as other sites on downstream targets of MPF that effect M-phase phenomena such as nuclear envelope breakdown, chromosome condensation, and spindle assembly. The discovery of this pathway now makes it clear that M-phase entry and maintenance are not solely the result of MPF activity; instead, the turnoff of PP2A/B55 phosphatases that antagonize MPF is of near equal consequence.
In PNAS, the paper of Burgess et al. (1) is based on the use of RNAi technology to investigate the phenotypes caused by Gwl depletion in human-tissue culture cells. The authors find, as expected from the model in Fig. 1, that the loss of Gwl arrests cells in G2 of the cell cycle. Furthermore, they exhaustively analyze several phenotypes associated with partial depletion of Gwl, finding, for example, that many cells that escape the G2 arrest and enter M phase are subsequently blocked in metaphase because of a failure to inactivate the spindle-assembly checkpoint.
For the general reader, the article of Burgess et al. (1) will primarily be of significance through its authoritative demonstration that the pathway illustrated in Fig. 1 is operative in human cells. This conclusion is far from trivial, because many evolutionary lineages such as nematodes and plants do not contain obvious Gwl orthologs. How such organisms can reverse MPF-driven mitotic phosphorylations is not known but is an extremely
Gwl (MAST-L) may be a highly relevant target for anticancer therapy.
interesting question. Perhaps another kinase might replace Gwl in suppressing PP2A/B55, or perhaps these species use a completely unrelated pathway, such as one involving the phosphatase Cdc14 that targets MPF phosphosites and is essential for mitotic exit in yeasts (reviewed in ref. 19). The work of Burgess et al. (1) further implies that Gwl (MAST-L) may be a highly relevant target for anticancer therapy. Agents that inactivate Gwl would be predicted to ensure the continued action of PP2A/B55 but only in dividing cells and only during a short interval of the cell cycle (i.e., prometaphase/metaphase). Furthermore, the combination of an anti-Gwl drug with another drug directed against MPF (or some aspect of MPF activation) might be of particular efficacy in preventing the division of cancer cells. Cells treated in this manner would not only have lowered MPF levels but also heightened action of the PP2A/B55 phosphatases that could remove any residual MPF phosphosites.
The body of recent work discussed here substantially broadens our view of the mechanisms cells use to establish and maintain M phase during mitosis and meiosis. In the near future, it will be exciting to determine the extent to which this concept of phosphatase inhibition during the cell cycle might apply. In particular, several kinases other than MPF are also operative during M phase (such as Aurora and Polo-like kinases as well as Gwl), and these enzymes phosphorylate sites that look very different from the canonical (Ser/Thr)Pro sites targeted by CDKs like MPF. The phosphorylations added by these other mitotic kinases must also be removed when cells exit mitosis or meiosis. What are the identities of the phosphatases that catalyze the reversal of these other mitotic phosphosites, and are these phosphatases down-regulated during M phase, as is now established in the case of PP2A/B55?
Footnotes
The author declares no conflict of interest.
See companion article on page 12564.
References
- 1.Burgess A, et al. Loss of human Greatwall results in G2 arrest and in multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc Natl Acad Sci USA. 2010;107:12564–12569. doi: 10.1073/pnas.0914191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarke PR, Hoffmann I, Draetta G, Karsenti E. Dephosphorylation of cdc25-C by a type-2A protein phosphatase: Specific regulation during the cell cycle in Xenopus egg extracts. Mol Biol Cell. 1993;4:397–411. doi: 10.1091/mbc.4.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumagai A, Dunphy WG. Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell. 1992;70:139–151. doi: 10.1016/0092-8674(92)90540-s. [DOI] [PubMed] [Google Scholar]
- 4.Lee TH, Turck C, Kirschner MW. Inhibition of cdc2 activation by INH/PP2A. Mol Biol Cell. 1994;5:323–338. doi: 10.1091/mbc.5.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goris J, Hermann J, Hendrix P, Ozon R, Merlevede W. Okadaic acid, a specific protein phosphatase inhibitor, induces maturation and MPF formation in Xenopus laevis oocytes. FEBS Lett. 1989;245:91–94. doi: 10.1016/0014-5793(89)80198-x. [DOI] [PubMed] [Google Scholar]
- 6.Lorca T, et al. An okadaic acid-sensitive phosphatase negatively controls the cyclin degradation pathway in amphibian eggs. Mol Cell Biol. 1991;11:1171–1175. doi: 10.1128/mcb.11.2.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Che S, et al. A phosphatase activity in Xenopus oocyte extracts preferentially dephosphorylates the MPM-2 epitope. FEBS Lett. 1998;424:225–233. doi: 10.1016/s0014-5793(98)00158-6. [DOI] [PubMed] [Google Scholar]
- 8.Ferrigno P, Langan TA, Cohen P. Protein phosphatase 2A1 is the major enzyme in vertebrate cell extracts that dephosphorylates several physiological substrates for cyclin-dependent protein kinases. Mol Biol Cell. 1993;4:669–677. doi: 10.1091/mbc.4.7.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maton G, Lorca T, Girault JA, Ozon R, Jessus C. Differential regulation of Cdc2 and Aurora-A in Xenopus oocytes: A crucial role of phosphatase 2A. J Cell Sci. 2005;118:2485–2494. doi: 10.1242/jcs.02370. [DOI] [PubMed] [Google Scholar]
- 10.Wu JQ, et al. PP1-mediated dephosphorylation of phosphoproteins at mitotic exit is controlled by inhibitor-1 and PP1 phosphorylation. Nat Cell Biol. 2009;11:644–651. doi: 10.1038/ncb1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mochida S, Hunt T. Calcineurin is required to release Xenopus egg extracts from meiotic M phase. Nature. 2007;449:336–340. doi: 10.1038/nature06121. [DOI] [PubMed] [Google Scholar]
- 12.Mochida S, Ikeo S, Gannon J, Hunt T. Regulated activity of PP2A-B55 delta is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. EMBO J. 2009;28:2777–2785. doi: 10.1038/emboj.2009.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Archambault V, Zhao X, White-Cooper H, Carpenter AT, Glover DM. Mutations in Drosophila Greatwall/Scant reveal its roles in mitosis and meiosis and interdependence with Polo kinase. PLoS Genet. 2007;3:e200. doi: 10.1371/journal.pgen.0030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu J, et al. Greatwall kinase: A nuclear protein required for proper chromosome condensation and mitotic progression in Drosophila. J Cell Biol. 2004;164:487–492. doi: 10.1083/jcb.200310059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu J, Zhao Y, Li Z, Galas S, Goldberg ML. Greatwall kinase participates in the Cdc2 autoregulatory loop in Xenopus egg extracts. Mol Cell. 2006;22:83–91. doi: 10.1016/j.molcel.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, et al. Roles of Greatwall kinase in the regulation of cdc25 phosphatase. Mol Biol Cell. 2008;19:1317–1327. doi: 10.1091/mbc.E07-11-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castilho PV, Williams BC, Mochida S, Zhao Y, Goldberg ML. The M phase kinase Greatwall (Gwl) promotes inactivation of PP2A/B55delta, a phosphatase directed against CDK phosphosites. Mol Biol Cell. 2009;20:4777–4789. doi: 10.1091/mbc.E09-07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vigneron S, et al. Greatwall maintains mitosis through regulation of PP2A. EMBO J. 2009;28:2786–2793. doi: 10.1038/emboj.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Queralt E, Uhlmann F. Cdk-counteracting phosphatases unlock mitotic exit. Curr Opin Cell Biol. 2008;20:661–668. doi: 10.1016/j.ceb.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]