Abstract
Fibroblast growth factor 21 (FGF21) has been identified as a potent metabolic regulator. Administration of recombinant FGF21 protein to rodents and rhesus monkeys with diet-induced or genetic obesity and diabetes exerts strong antihyperglycemic and triglyceride-lowering effects and reduction of body weight. Despite the importance of FGF21 in the regulation of glucose, lipid, and energy homeostasis, the mechanisms by which FGF21 functions as a metabolic regulator remain largely unknown. Here we demonstrate that FGF21 regulates energy homeostasis in adipocytes through activation of AMP-activated protein kinase (AMPK) and sirtuin 1 (SIRT1), resulting in enhanced mitochondrial oxidative function. AMPK phosphorylation levels were increased by FGF21 treatment in adipocytes as well as in white adipose tissue from ob/ob mice. FGF21 treatment increased cellular NAD+ levels, leading to activation of SIRT1 and deacetylation of its downstream targets, peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) and histone 3. Activation of AMPK and SIRT1 by FGF21 in adipocytes enhanced mitochondrial oxidative capacity as demonstrated by increases in oxygen consumption, citrate synthase activity, and induction of key metabolic genes. The effects of FGF21 on mitochondrial function require serine/threonine kinase 11 (STK11/LKB1), which activates AMPK. Inhibition of AMPK, SIRT1, and PGC-1α activities attenuated the effects of FGF21 on oxygen consumption and gene expression, indicating that FGF21 regulates mitochondrial activity and enhances oxidative capacity through an AMPK–SIRT1–PGC1α–dependent mechanism in adipocytes.
Keywords: adipocytes, diabetes, obesity, mitochondrial function
AMP-activated protein kinase (AMPK) is a major metabolic energy sensor and master regulator of metabolic homeostasis (1). LKB1, a serine threonine kinase, is a major regulator of AMPK activation. LKB1 directly phosphorylates Thr-172 of AMPK and activates its kinase activity (2). LKB1 expression is induced upon exercise and acts as a critical mediator of gluconeogenesis in the liver (3). Recently, AMPK has been shown to play an important role in the therapeutic benefits of metformin (1, 4), thiazolidinediones (5), and exercise (6, 7), all cornerstones in the management of type 2 diabetes and metabolic syndrome. Activation of AMPK maintains energy balance by switching on catabolic pathways, enhancing oxidative metabolism and mitochondrial biogenesis (1). Recently, AMPK was found to enhance NAD+-dependent type III deacetylase sirtuin 1 (SIRT1) activity by increasing cellular NAD+ levels, resulting in modulation of the activity of downstream SIRT1 targets (8).
SIRT1 plays an important role in metabolic function and longevity in mammals (9, 10). Activation of SIRT1 also results in selective nutrient utilization and enhanced mitochondrial oxidative function to regulate energy balance. Both AMPK and SIRT1 act in concert with the master regulator of mitochondrial biogenesis, peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), to regulate energy homeostasis in response to environmental and nutritional stimuli (1, 11). PGC-1α interacts with multiple transcription factors to stimulate mitochondrial metabolic capacity (12).
Fibroblast growth factor (FGF21) has been identified as a potent metabolic regulator. Systemic administration of FGF21 to rodents and rhesus monkeys with diet-induced or genetic obesity and diabetes exerts strong antihyperglycemic and triglyceride-lowering effects and leads to reduction of body weight (13–16). Recent studies have shown that β-klotho, a single-pass transmembrane protein related to klotho, functions as a cofactor required for FGF21 signaling (17–19). Because β-klotho acts as an essential cofactor for FGF21 signaling, its expression limits the tissue-specific activities of FGF21 to the liver, pancreas, and adipose tissues, where β-klotho is predominantly expressed (17–19).
More recently, the role of FGF21 in the liver has been established; FGF21 has been shown to play an important role in regulating energy homeostasis during starvation (20–22). FGF21 is induced by fasting and is required for activation of hepatic lipid oxidation, triglyceride clearance, and ketogenesis during fasting (20–22). Despite the importance of FGF21 as a regulator of hepatic metabolism, much less is known about the role of FGF21 in adipose tissue. Additionally, the mechanisms by which FGF21 acts to regulate glucose and energy homeostasis remain unclear.
Here we demonstrate that FGF21 regulates energy expenditure by modulating AMPK and SIRT1 activities via LKB1 in adipocytes. FGF21 alters cellular NAD+ levels, resulting in activation of SIRT1 and deacetylation of its downstream targets, PGC-1α and histone 3 (H3). The activation of these key metabolic sensors results in enhancement of mitochondrial oxidative function, accounting for the beneficial metabolic effects of FGF21.
Results
FGF21 Increases AMPK Activity.
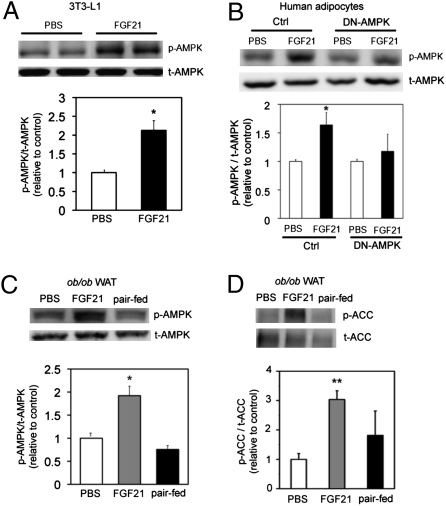
To gain insight into the mechanism by which FGF21 regulates energy expenditure, we treated 3T3-L1 adipocytes with FGF21 (4.0 μg/mL) for 3 d and analyzed the levels of activated AMPK by Western blot. FGF21 treatment robustly increased the levels of AMPK phosphorylation (p-AMPK) by 53% (P < 0.05; n = 3), but total AMPK protein (t-AMPK) levels remained unchanged (Fig. 1A). Interestingly, treatment of human differentiated adipocytes with FGF21 (4.0 μg/mL for 3 d) induced a significant (58%) increase in levels of p-AMPK (P < 0.05; n = 3), but t-AMPK protein levels were not altered (Fig. 1B). Inhibition of AMPK activity in human adipocytes with adenovirus overexpressing the dominant-negative form of the alpha2 subunit of AMPK (DN-AMPK) abolished the FGF21-stimulated increase in p-AMPK (Fig. 1B).
Fig. 1.
FGF21 increases AMPK activity. (A and B) Western blot and quantification of (A) p-AMPK in 3T3-L1 cells treated for 3 d with FGF21 (4.0 μg/mL) and (B) human adipocytes. Data are average of three independent experiments. (C and D) Western blot and quantification of (C) p-AMPK and (D) p-ACC in WAT from vehicle-treated (white bar), FGF21-treated (gray bar), and paired-fed (black bar) mice. n = 8 animals/group. *P < 0.05 (Student's t test). **P < 0.01.
To explore if FGF21 increases AMPK activity in vivo, we administered recombinant human FGF21 protein to ob/ob mice for 2 wk via continuous infusion with osmotic (Azlet) pumps. Consistent with previous reports (13–16), FGF21 administration led to a significant reduction in total body weight (Fig. S1A), which was associated with a small but significant decrease in food intake at days 10 and 14 (Fig. S1B). Body/tissue composition measurements revealed a significant reduction in total body fat mass and liver lipid content in FGF21-treated animals (Fig. S1C). We analyzed the levels of phosphorylated AMPK in white adipose tissues (WAT) from vehicle- and FGF21-treated animals and found increased (53%) levels of p-AMPK (P < 0.05; n = 8) in FGF21-treated mice, indicating enhanced activation of AMPK (Fig. 1C). These data suggest that FGF21 regulates energy expenditure through activation of AMPK.
To determine if the effects of FGF21 are independent of body weight, we performed paired-feeding studies to match the body weight of vehicle-treated animals to that of FGF21-treated mice. In agreement with previous studies (13–16), nonfasted blood glucose levels were significantly decreased in FGF21-treated animals but not in vehicle-treated or paired-fed animals (Fig. S1D). An oral glucose tolerance test revealed that FGF21 treatment significantly improved glucose tolerance (Fig. S1E) and decreased the area under the curve by 45% (Fig. S1F). Importantly, the levels of p-AMPK were increased by 53% in FGF21-treated animals but were not increased in paired-fed animals (Fig. 1C). In addition, FGF21-treated animals had increased (66%) levels of phosphorylated acetyl-CoA carboxylase (p-ACC), a downstream target of AMPK (Fig. 1D). These data suggest that FGF21 can enhance the kinase activity of AMPK in vivo. These results demonstrate that the effects of FGF21 on energy homeostasis and AMPK activation are independent of changes in body weight.
FGF21 Increases NAD+ Metabolism and SIRT1 Activity.
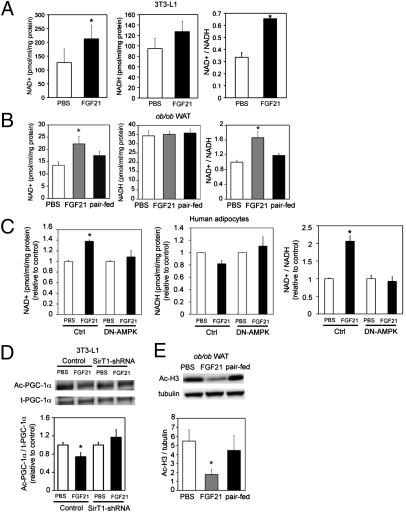
Recently, it has been shown that AMPK indirectly activates SIRT1 by modulating intracellular NAD+/NADH levels (8). Because FGF21 increases AMPK activity, we sought to determine if FGF21 could alter intracellular NAD+/NADH levels. Consistent with our hypothesis, FGF21 increased the NAD+/NADH ratio by 48% (P < 0.05; n = 3) and 52% (P < 0.05, n = 3) over controls in 3T3-L1 adipocytes and human adipocytes, respectively (Fig. 2 A and C). Importantly, the NAD+/NADH ratio was increased by 40% (P < 0.05, n = 8) in WAT from ob/ob mice treated with FGF21 but was not increased in paired-fed animals (Fig. 2B). Inhibition of AMPK activity with DN-AMPK attenuated FGF21-stimulated increases in the NAD+/NADH ratio, suggesting that AMPK activation by FGF21 is upstream of SIRT1 activation (Fig. 2C).
Fig. 2.
FGF21 increases cellular NAD+ and decreases H3 acetylation. (A) NAD+/NADH levels in 3T3-L1 adipocytes. 3T3-L1 adipocytes were treated for 3 d with FGF21 (4.0 μg/mL; black bars) or PBS (white bars). (B) NAD+/NADH levels in WAT from vehicle-treated (white bar), FGF21-treated (gray bar), and paired-fed (black bar) mice. n = 8 animals/group. (C) NAD+/NADH levels in human adipocytes transduced with adenovirus expressing control vector or DN-AMPK. Transduced adipocytes were treated for 3 d with FGF21 (4.0 μg/mL; black bars) or PBS (white bars). (D) Western blot and quantification of acetylated PGC-1α (Ac-PGC-1α) in 3T3-L1 adipocytes. 3T3-L1 adipocytes were transduced with adenovirus expressing SIRT1-shRNA and Flag–PGC-1α. Flag–PGC-1α was immunoprecipitated and blotted with pan acetylated lysine antibody. t-PGC-1α, total PGC-1α. (E) Western blot and quantification of acetylated H3 (Ac-H3) in WAT from vehicle-treated (white bar), FGF21-treated (gray bar), and paired-fed (black bar) mice. All data are averages of three independent experiments. n = 8 animals/group. *P < 0.05 (Student's t test).
To determine whether the FGF21-induced elevation of the NAD+/NADH ratio could affect SIRT1 activity, we determined the acetylation status of the known SIRT1 substrates, PGC-1α and H3. 3T3-L1 adipocytes were cotransduced with adenovirus expressing shRNA construct against SIRT1 and adenovirus expressing a Flag–PGC-1α construct. Transduced cells were treated with FGF21 (4.0 μg/mL) for 3 d. Treatment of 3T3-L1 adipocytes with FGF21 decreased PGC-1α acetylation by 28% (P < 0.05; n = 3), an effect that was attenuated with shRNA knockdown of SIRT1 (Fig. 2D). Furthermore, decreased acetylation of H3 (68%; P < 0.05; n = 8) was observed in WAT from FGF21-treated animals but not in paired-fed mice (Fig. 2E). These data indicate that FGF21 alters NAD+ metabolism and activates SIRT1 activity in adipocytes in vitro and in vivo. Together, these results suggest that FGF21 controls energy metabolism through activation of AMPK and SIRT1.
FGF21 Increases Mitochondrial Gene and Protein Expression.
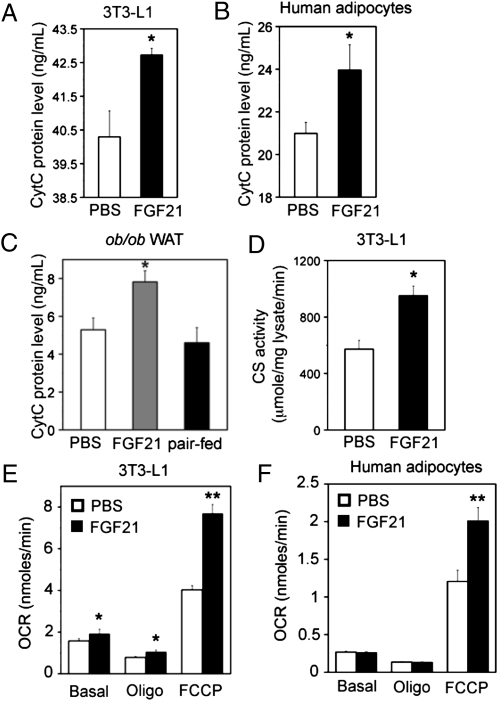
AMPK and SIRT1 are both critical regulators of mitochondrial biogenesis and function (1, 9, 10). To investigate the role of FGF21 in mitochondrial biogenesis and function, we analyzed the expression of genes involved in mitochondrial function by quantitative PCR in 3T3-L1 adipocytes. Treatment of 3T3-L1 adipocytes with FGF21 induced a significant (1.6-fold) increase in the expression of carnitine palmitoyltransferase 1A (CPT1a) (the enzyme involved in the transport of fatty acids across the mitochondrial membrane for fatty acid β-oxidation), a 1.6-fold increase in the expression of isocitrate dehydrogenase 3 alpha (Idh3a) [responsible for the rate-limiting step of the tricarboxylic acid (TCA) cycle], and a 2.1-fold increase in cytochrome c (CytC) (Fig. S2A). Furthermore, these changes in gene expression correlated with increased levels of CytC protein (6%; P < 0.05; n = 3; Fig. 3A).
Fig. 3.
FGF21 increases mitochondrial protein expression and function. CytC protein levels in (A) FGF21-treated 3T3-L1 cells and (B) human adipocytes. White bars: PBS treatment; black bars: FGF21 treatment. (C) CytC protein levels in WAT from vehicle-treated (white bar), FGF21-treated (gray bar), and paired-fed (black bar) mice. n = 8 animals/group. (D) Citrate synthase (CS) activity in 3T3-L1 adipocytes treated with FGF21. Oxygen consumption rate (OCR) in (E) 3T3-L1 cells and (F) human adipocytes treated with PBS (white bars) or FGF21 (black bars). Data are averages of three independent experiments. *P < 0.05; **P < 0.01 (Student's t test).
Additionally, treatment of human adipocytes with FGF21 significantly increased expression of genes involved in mitochondrial biogenesis and fatty acid β oxidation, including CPT1a (45%), peroxisome proliferator-activated receptor δ (PPARδ; 23%), and PGC-1α (20%) (Fig. S2C). These changes in gene expression also resulted in a significant (13%) increase in CytC protein levels (P < 0.05; n = 3) (Fig. 3B).
FGF21 has been shown previously to regulate gene expression in vivo (13, 16). Therefore, we examined mitochondrial protein expression in WAT from treated animals. CytC protein levels were elevated by 32% in FGF21-treated animals (P < 0.05; n = 8) but were not elevated in vehicle-treated or paired-fed mice (Fig. 3C).
FGF21 Enhances Mitochondrial Oxidative Capacity.
To determine further the effects of FGF21 on mitochondrial function, we measured mitochondrial enzymatic activity in 3T3-L1 cells treated with FGF21 by determining citrate synthase activity, a key component of the TCA cycle. FGF21 treatment of 3T3-L1 adipocytes increased citrate synthase activity significantly, by 1.7-fold, suggesting that FGF21 enhances TCA cycle activity (Fig. 3D).
To assess further the potential for FGF21 to increase mitochondrial oxidative capacity, we measured oxygen consumption in FGF21-treated 3T3-L1 and human adipocytes. FGF21 increased basal oxygen consumption by 1.2-fold (P < 0.05; n = 3) and increased oxygen consumption in oligomycin-treated 3T3-L1 adipocytes by 1.3-fold (P < 0.05; n = 3; Fig. 3E). Carbonylcyanide-p-trifluoromethoxyphenylhydrazone (FCCP) is a chemical uncoupler that abolishes the linkage between the respiratory chain and oxidative phosphorylation systems and maximizes the respiratory capacity of mitochondria. FCCP-stimulated oxygen consumption was significantly increased with FGF21 treatment, by 1.9- and 1.7-fold (P < 0.01; n = 3) in 3T3-L1 and human adipocytes, respectively, suggesting that FGF21 increases energy expenditure and enhances oxidative capacity (Fig. 3 E and F).
LKB1, AMPK, and SIRT1 Are Required for FGF21-Mediated Effects on Mitochondrial Function.
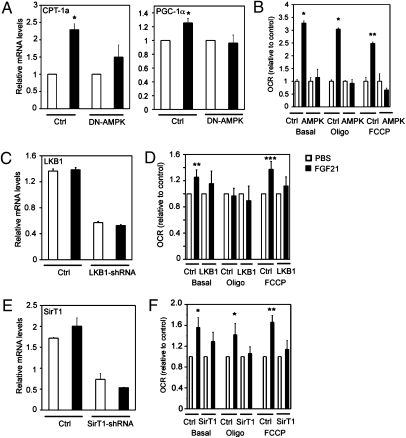
To determine if AMPK is required for FGF21-induced stimulation of mitochondrial function, we transduced human adipocytes with adenovirus overexpressing DN-AMPK. Inhibition of AMPK activity with DN-AMPK attenuated FGF21-stimulated increases in CPT-1a and PGC-1α gene expression (Fig. 4A) and oxygen consumption (Fig. 4B).
Fig. 4.
FGF21 effects in adipocytes require AMPK activity. (A) Gene expression in human adipocytes infected with adenovirus expressing DN-AMPK or GFP (Ctrl) and treated with PBS (white bars) or FGF21 (black bars). (B) Oxygen consumption in human adipocytes infected with adenovirus expressing DN-AMPK or GFP (Ctrl) and treated with PBS (white bars) or FGF21 (black bars). (C) Gene expression in human adipocytes infected with lentivirus expressing shRNA against LKB1 or control shRNA (Ctrl) and treated with PBS (white bars) or FGF21 (black bars). (D) Oxygen consumption in 3T3-L1 adipocytes infected with lentivirus expressing shRNA against LKB1 or control shRNA (Ctrl) and treated with PBS (white bars) or FGF21 (black bars). (E) Gene expression in human adipocytes infected with adenovirus expressing shRNA against SIRT1 or control shRNA (Ctrl) and treated with PBS (white bars) or FGF21 (black bars). (F) Oxygen consumption in 3T3-L1 adipocytes infected with adenovirus expressing shRNA against SIRT1 or control shRNA (Ctrl) and treated with PBS (white bars) or FGF21 (black bars). Data are averages of three independent experiments. All gene-expression data are quantitative RT-PCRs, normalized to the housekeeping gene, TATA box binding protein. *P < 0.05; **P < 0.01 (Student's t test). ***P < 0.001.
Because LKB1 regulates AMPK activity (2), we sought to determine if LKB1 is required for the effects of FGF21 on mitochondrial function by transducing human adipocytes with lentivirus expressing either shRNA against LKB1 or control shRNA. Transduction of LKB1-shRNA lentivirus resulted in decreased LKB1 mRNA levels by more than 70% in both the PBS- and FGF21-treated adipocytes (Fig. 4C). FGF21 stimulation significantly increased oxygen consumption rates in basal conditions (1.3-fold; P < 0.01; n = 3) and with FCCP treatment (1.4-fold; P < 0.001; n = 3) (Fig. 4D). The induction of cellular respiration by FGF21 was abolished when LKB1 was knocked down in these cells (Fig 4D).
Because AMPK activates SIRT1, we next evaluated whether SIRT1 mediates the effects of FGF21 on mitochondrial function downstream of AMPK. 3T3-L1 adipocytes were transduced with adenovirus expressing either SIRT1-shRNA or control shRNA. The cells were treated with FGF21 (4.0 μg/mL) for 3 d. SIRT1 mRNA levels were decreased by more than 60% by SIRT1-shRNA in both PBS- and FGF21-treated 3T3-L1 adipocytes (Fig. 4E). FGF21 treatment significantly increased oxygen consumption rates under basal conditions (1.5-fold; P < 0.05; n = 3) as well as with oligomycin (1.4-fold; P < 0.05; n = 3) and FCCP (1.7-fold; P < 0.01; n = 3) treatment (Fig. 4F). FGF21-induced increases in oxygen consumption were attenuated by shRNA knockdown of SIRT1 (Fig. 4F). Together, the data demonstrate that FGF21 enhances mitochondrial function via an LKB1-, AMPK-, and SIRT1-dependent pathway.
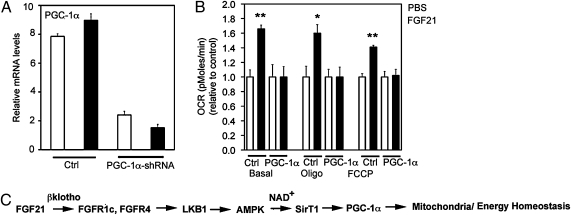
PGC-1α Is Required for FGF21-Mediated Effects on Mitochondrial Function.
Because both AMPK and SIRT1 modulate PGC-1α expression and activity (23–25), we screened FGF21-treated 3T3-L1 adipocytes for increased expression of PGC-1α by quantitative PCR. Interestingly, FGF21 did not appear to increase mRNA expression of PGC-1α in treated adipocytes (Fig. 5A). However, we found that FGF21 regulates PGC-1α activity through deacetylation via SIRT1 (Fig. 2D). To determine whether the regulation of mitochondrial function by FGF21 requires PGC-1α, we performed shRNA-knockdown studies in 3T3-L1 adipocytes. PGC-1α mRNA levels were reduced by more than 70% in 3T3-L1 adipocytes transduced with PGC-1α–shRNA adenovirus compared with controls (Fig. 5A). Knockdown of PGC-1α attenuated the effects of FGF21 on CTP-1a and CytC gene expression (Fig. S2B) and completely abolished FGF21-induced oxygen consumption at the basal condition as well as with oligomycin and FCCP treatment (Fig. 5B). Therefore, our results suggest that the coordinate effects of FGF21 on mitochondrial function require PGC-1α.
Fig. 5.
FGF21 effects in adipocytes require PGC-1α. (A) PGC-1α expression in 3T3-L1 adipocytes infected with shRNA-cytomegalovirus (CMV) (Ctrl) or shRNA–PGC-1α adenovirus and treated with PBS (white bars) or FGF21 (black bars). Data shown are from quantitative RT-PCRs, normalized to the housekeeping gene, TATA box binding protein. (B) Oxygen consumption in 3T3-L1 adipocytes infected with shRNA-CMV (Ctrl) or shRNA–PGC-1α adenovirus and treated with PBS (white bars) or FGF21 (black bars). Data are averages of three independent experiments. *P < 0.05; **P < 0.01 (Student's t test). (C) Schematic diagram showing FGF21 activation of AMPK via LKB1, which indirectly activates SIRT1 by increasing the cellular NAD+/NADH ratio. The pathway converges on regulation of PGC-1α activity and, ultimately, on mitochondrial oxidative function.
Discussion
Here we demonstrate that FGF21 regulates energy homeostasis via enhancement of mitochondrial function and efficiency through activation of AMPK and SIRT1 in vitro and in vivo. Our data support a working model for FGF21 shown in Fig. 5C. FGF21 increases the phosphorylation of AMPK through LKB1. Activation of AMPK leads to increased cellular NAD+ levels, which in turn activate SIRT1 and subsequently impact multiple metabolic pathways. Here we investigated one of the pathways which regulate mitochondrial oxidative function. The results show that the effect of FGF21 in enhancing mitochondrial function is mediated through PGC-1α.
The activation of AMPK and SIRT1 converge on enhanced mitochondrial oxidative function as demonstrated by significant increases in oxygen consumption, citrate synthase activity, and induction of key metabolic genes, including CPT-1a and CytC, in FGF21-treated adipocytes. Inhibition of LKB1, AMPK, and SIRT1 activities by dominant-negative or shRNA adenovirus and lentivirus attenuated the effects of FGF21 on oxygen consumption and gene expression, suggesting that FGF21 regulates mitochondrial activity and enhances oxidative capacity through an AMPK- and SIRT1-dependent mechanism. The effects of FGF21 on AMPK and SIRT1 are observed both in vitro and in vivo, suggesting that these effects are likely to be direct and not secondary to the changes in body weight and adiposity.
Helix 7 of the ligand-binding domain of PPARγ has been shown by Wang et al. (26) to be required for the response of PPARγ to endogenous ligands as well as for the expression of a subset of genes, including FGF21, during adipogenesis. Expression of this subset of genes is repressed by SIRT1, and inhibition of SIRT1 produces effects similar to those of PPARγ ligands. Interestingly, our results demonstrate that treatment of adipocytes with FGF21 inhibits PPARγ expression and enhances AMPK and SIRT1 activities. It is possible that repression of FGF21 by SIRT1 is necessary during adipogenesis, but FGF21 can enhance SIRT1 activity to maintain energy homeostasis in mature adipocytes.
A previous study by Coskun et al. (13) has shown that FGF21 induces PGC-1α expression in WAT. Consistent with their results, our study demonstrates that the effects of FGF21 on mitochondrial function require PGC-1α. We further show that FGF21 induces AMPK and SIRT1 activities, which act in concert with PGC-1α to regulate energy homeostasis.
Recently, PGC-1α has been shown to regulate hepatic expression of FGF21 negatively during fasting. Chronic reduction of hepatic PGC-1α expression in mice increased FGF21 expression (27). In this scenario, the metabolic status of the animal resembles that of starvation, when PGC-1α levels are low and FGF21 functions to mediate ketogenesis. Similar to previous studies showing that the actions of FGF21 in hepatic lipid metabolism require PGC-1α (30), our results show that treatment of adipocytes with FGF21 increases mitochondrial oxidative capacity and requires PGC-1α.
Consistent with our hypothesis, treatment of ob/ob mice with FGF21 elicits beneficial metabolic effects similar to those observed in transgenic mice that overexpress SIRT1. Both FGF21-treated and SIRT1-transgenic animals exhibit reductions in body weight and plasma insulin and glucose levels as well as improved glucose tolerance (28). Additionally, treatment of animals with AMPK activators such as metformin induces metabolic effects similar to those of FGF21, including glucose lowering (4). The convergent biological effects observed in FGF21-treated animals with SIRT1 and AMPK activation further support our hypothesis that FGF21 induces AMPK and SIRT1 activities.
Our results show that FGF21 activates AMPK through LKB1 and that the effects of FGF21 on mitochondrial function require LKB1. FGF21 signaling through β-klotho and the FGF receptors results in activation of ERK 1/2, which regulate LKB1 activity (29). It is possible that FGF21 regulates AMPK through interaction between ERK 1/2 and LKB1.
The actions of FGF21 are limited to tissues that express β-klotho, including the liver, pancreas, and adipose tissues. Our studies show that FGF21 stimulates AMPK and SIRT1 activities in adipose tissue. Recently, FGF21 was found to increase PGC-1α expression, fatty acid oxidation, and TCA cycle flux in the liver (30). It would be of interest to determine whether FGF21 also can activate AMPK and SIRT1 to act in concert with PGC-1α in the liver. FGF21 has been shown previously to protect pancreatic β-cells from glucolipotoxicity-induced apoptosis and dysfunction (31). SIRT1 and NAD+ metabolism have been shown to be critical in pancreatic β-cell function and health (32); it would be of interest to determine whether the effects of FGF21 on β-cell survival and function are mediated through SIRT1. It is likely that the effects of FGF21 on these pathways occur in other tissues, such as the liver and pancreas, in addition to adipose tissue, which collectively contribute to the improvement of energy homeostasis.
Taken together, our results reveal a scenario in which FGF21 enhances mitochondrial function and oxidative capacity via activation of AMPK and SIRT1 and their downstream targets, including PGC-1α, in adipocytes. Consequently, the physiologic integrative response to these changes in energy production and fatty acid oxidation improves lipid profiles and insulin sensitivity, accounting for the beneficial metabolic effects of FGF21. Furthermore, our data in human adipocytes demonstrate that FGF21 induces similar effects on mitochondrial function in rodents and humans and underscore the potential of FGF21 as a therapeutic for obesity and type 2 diabetes.
Methods
Animals and Treatments.
Male ob/ob mice were obtained from Jackson Laboratories at 8 wk of age. All procedures were performed in accordance with the standards of the US Department of Health and Human Services and were approved by the Novartis Animal Care and Use Committee. Mice were assigned randomly to treatment or vehicle groups, were stratified by body weight, and blood glucose levels at the fed state. Mice were treated with PBS (vehicle) or FGF21 via continuous s.c. infusion with osmotic pumps (Azlet). Blood samples were taken from conscious, fed animals by tail snip, and glucose levels were determined using Precision G Blood Glucose Testing System (Abbott Laboratories). A glucose tolerance test was performed after overnight fasting and challenge with a 1-g/kg glucose load.
Cell Culture, in Vitro Treatments, and Adenoviral Transduction.
3T3-L1 cells were obtained from the American Type Culture Collection. 3T3-L1 fibroblasts were grown to confluence in growth medium (DMEM supplemented with 10% FCS, 100 U/mL penicillin, and 100 μg/mL streptomycin). Differentiation was induced by incubating the cells in growth medium supplemented with 3-isobutyl-1-methylxanthine, dexamethasone, and insulin (Sigma-Aldrich) for 4 d. The cells were incubated in growth medium for an additional 4 d to complete adipocyte conversion. At day 8, cells were incubated in growth medium plus FGF21 (4.0 μg/mL) or vehicle (PBS) for 72 h. Media containing FGF21 or vehicle were replaced every 24 h. Human adipocytes were obtained from Cell Applications, Inc. Human preadipocytes were grown to confluence in preadipocytes medium. Differentiation was induced by incubating cells in adipocyte differentiation medium for 10 d. For knockdown experiments, 3T3-L1 adipocytes were infected with adenovirus expressing shRNA constructs against SIRT1 (ViraQuest), and PGC-1α (33) (Welgen) via lipofectamine 2000 (Invitrogen). For LKB1 knockdown, human adipocytes were transduced with lentivirus expressing shRNA constructs against LKB1. LKB1-shRNA lentivirus was purchased from Santa Cruz Biotechnology. DN-AMPK α2 adenovirus was purchased from Eton Bioscience and amplified by ViraQuest. For acetylated PGC-1α experiments, 3T3-L1 adipocytes were infected with adenovirus expressing SIRT1-shRNA, in addition to adenovirus expressing Flag–PGC-1α construct.
Expression and Purification of Recombinant Human FGF21.
The cDNA encoding residues 33–209 of human FGF21 (Q9NSA1; SwissProt) with an N-terminal 6× His tag followed by a tobacco etch virus (TEV) cleavage site was cloned into the NcoI and XhoI sites of pET-15b (Novagen). The His tag was removed by treatment with TEV protease. The sample then was concentrated in a 5-K molecular weight cut-off unit (Amicon) and injected onto a Superdex 200 gel filtration column (GE Healthcare) equilibrated in PBS. Protein concentration was determined using the Bradford protein assay according to the manufacturer's protocol (Bio-Rad).
RNA Isolation, Reverse Transcription, and Real-Time Quantitative PCR.
RNA was isolated from cells and tissues using TRIzol reagent (Invitrogen) and purified using the Qiagen RNeasy Mini kit (Qiagen). Isolated RNA was reverse-transcribed into cDNA by following the protocol from SuperScript III First-Strand Synthesis System (Invitrogen). Real-time quantitative PCR reactions were performed in triplicate on an ABI Prism 7900HT (Applied Biosystems) and were normalized to TATA box binding protein. Assay-on-Demand Gene Expression primers and probes were obtained from Applied Biosystems.
Oxygen Consumption and Citrate Synthase Activity Assays.
Citrate synthase activity was measured according to instructions provided with the citrate synthase kit obtained from Sigma-Aldrich. A total of 2 μg of protein was used for the assay. The XF24 Extracellular Flux Analyzer (Seahorse Biosciences) was used to measure oxygen consumption. The oxygen consumption rate was calculated using the fixed delta (50 mmHg) technique for determining the slope, following the manufacturer's instructions.
NAD+/NADH and CytC Assays.
NAD+ and NADH levels were determined according to instructions provided with the NAD/NADH assay kit purchased from Abcam. CytC protein levels were measured using a CytC ELISA obtained from R&D Systems.
Western Blotting and Immunoprecipitation.
Cells were lysed in cell lysis buffer (Cell Signaling Technology) supplemented with protease and phosphatase inhibitors (Sigma-Aldrich). Lysates were sonicated for 1 min and centrifuged at 14, 000 × g for 10 min at 4 °C. For immunoprecipitation experiments, 800 μg of total protein lysates were incubated overnight at 4 °C with anti-Flag agarose beads (Sigma). Lysates were resolved by 4–20% Tris-glycine SDS/PAGE and transferred to nitrocellulose membranes. Total and p-AMPK antibodies were obtained from Cell Signaling Technology. Pan acetylated lysine (4G12) and total PGC-1α antibodies were purchased from Millipore. Total and acetylated H3 antibodies were purchased from EMD Biosciences.
Statistical Analyses.
Data are presented as mean ± SEM. Statistical analyses were performed using Student's t test. Significant differences of P < 0.05 are identified with an asterisk.
Supplementary Material
Acknowledgments
We thank Dr. Marc Montminy (Salk Institute, La Jolla, CA) for providing the adenoviral PGC-1α–shRNA reagents, and Dr. Shari Caplan (Novartis Institute for BioMedical Research, Inc.) for providing FGF21 protein, and Kevin Godbout, YaJun Feng, Jinsheng Liang, Valerie Beaulieu, Richard Deacon, Olivia-Claire Bare, and Sandra Souza for technical assistance.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006962107/-/DCSupplemental.
References
- 1.Hardie DG. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 2.Shaw RJ, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw RJ, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou GC, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fryer LGD, Parbu-Patel A, Carling D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277:25226–25232. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 6.Barnes BR, et al. Changes in exercise-induced gene expression in 5′-AMP-activated protein kinase gamma3-null and gamma3 R225Q transgenic mice. Diabetes. 2005;54:3484–3489. doi: 10.2337/diabetes.54.12.3484. [DOI] [PubMed] [Google Scholar]
- 7.Narkar VA, et al. AMPK and PPARd agonists are exercise mimetics. Cell. 2009;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantó C, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: Understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 10.Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett. 2008;582:46–53. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reznick RM, Shulman GI. The role of AMP-activated protein kinase in mitochondrial biogenesis. J Physiol. 2006;574:33–39. doi: 10.1113/jphysiol.2006.109512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Z, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 13.Coskun T, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 14.Kharitonenkov A, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kharitonenkov A, et al. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148:774–781. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- 16.Xu J, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kharitonenkov A, et al. FGF-21/FGF-21 receptor interaction and activation is determined by betaKlotho. J Cell Physiol. 2008;215:1–7. doi: 10.1002/jcp.21357. [DOI] [PubMed] [Google Scholar]
- 18.Kurosu H, et al. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem. 2007;282:26687–26695. doi: 10.1074/jbc.M704165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogawa Y, et al. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci USA. 2007;104:7432–7437. doi: 10.1073/pnas.0701600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badman MK, et al. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Gälman C, et al. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metab. 2008;8:169–174. doi: 10.1016/j.cmet.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Inagaki T, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Atherton PJ, et al. Selective activation of AMPK-PGC-1alpha or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J. 2005;19:786–788. doi: 10.1096/fj.04-2179fje. [DOI] [PubMed] [Google Scholar]
- 24.Rodgers JT, et al. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 25.Terada S, et al. Effects of low-intensity prolonged exercise on PGC-1 mRNA expression in rat epitrochlearis muscle. Biochem Biophys Res Commun. 2002;296:350–354. doi: 10.1016/s0006-291x(02)00881-1. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Qiang L, Farmer SR. Identification of a domain within peroxisome proliferator-activated receptor gamma regulating expression of a group of genes containing fibroblast growth factor 21 that are selectively repressed by SIRT1 in adipocytes. Mol Cell Biol. 2008;28:188–200. doi: 10.1128/MCB.00992-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estall JL, et al. PGC-1alpha negatively regulates hepatic FGF21 expression by modulating the heme/Rev-Erb(alpha) axis. Proc Natl Acad Sci USA. 2009;106:22510–22515. doi: 10.1073/pnas.0912533106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bordone L, et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 29.Sapkota GP, et al. Phosphorylation of the protein kinase mutated in Peutz-Jeghers cancer syndrome, LKB1/STK11, at Ser431 by p90(RSK) and cAMP-dependent protein kinase, but not its farnesylation at Cys(433), is essential for LKB1 to suppress cell growth. J Biol Chem. 2001;276:19469–19482. doi: 10.1074/jbc.M009953200. [DOI] [PubMed] [Google Scholar]
- 30.Potthoff MJ, et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci USA. 2009;106:10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wente W, et al. Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes. 2006;55:2470–2478. doi: 10.2337/db05-1435. [DOI] [PubMed] [Google Scholar]
- 32.Ramsey KM, Mills KF, Satoh A, Imai S. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell. 2008;7:78–88. doi: 10.1111/j.1474-9726.2007.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koo SH, et al. PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat Med. 2004;10:530–534. doi: 10.1038/nm1044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.