Abstract
The hypoxia-inducible factor (HIF) family of transcription factors directs a coordinated cellular response to hypoxia that includes the transcriptional regulation of a number of metabolic enzymes. Chuvash polycythemia (CP) is an autosomal recessive human disorder in which the regulatory degradation of HIF is impaired, resulting in elevated levels of HIF at normal oxygen tensions. Apart from the polycythemia, CP patients have marked abnormalities of cardiopulmonary function. No studies of integrated metabolic function have been reported. Here we describe the response of these patients to a series of metabolic stresses: exercise of a large muscle mass on a cycle ergometer, exercise of a small muscle mass (calf muscle) which allowed noninvasive in vivo assessments of muscle metabolism using 31P magnetic resonance spectroscopy, and a standard meal tolerance test. During exercise, CP patients had early and marked phosphocreatine depletion and acidosis in skeletal muscle, greater accumulation of lactate in blood, and reduced maximum exercise capacities. Muscle biopsy specimens from CP patients showed elevated levels of transcript for pyruvate dehydrogenase kinase, phosphofructokinase, and muscle pyruvate kinase. In cell culture, a range of experimental manipulations have been used to study the effects of HIF on cellular metabolism. However, these approaches provide no potential to investigate integrated responses at the level of the whole organism. Although CP is relatively subtle disorder, our study now reveals a striking regulatory role for HIF on metabolism during exercise in humans. These findings have significant implications for the development of therapeutic approaches targeting the HIF pathway.
Keywords: exercise, lactate, glycolysis, Chuvash polycythemia, von Hippel-Lindau
The hypoxia-inducible factor (HIF) family of transcription factors plays a key role in orchestrating the cellular response to varying levels of oxygen. These transcription factors are heterodimeric proteins consisting of two subunits: an oxygen-regulated HIF-α subunit (HIF-1α, HIF-2α, or HIF-3α) and a constitutive HIF-β subunit (1). At physiological levels of oxygen, HIF-α subunits undergo rapid degradation, limiting the formation of the transcription complex (2). Degradation is initiated by hydroxylation of HIF-α protein by specific prolyl hydroxylases (3–5). This hydroxylation enables binding of the von Hippel–Lindau (VHL) protein (6, 7) and the subsequent destruction of HIF-α by the ubiquitin–proteasome system. At low levels of oxygen, the rate of hydroxylation of HIF-α is reduced. This reduction impairs the binding of VHL to HIF-α, enabling accumulation of transcriptionally active HIF complexes within the cell.
Many HIF target genes have been identified in metabolic and other cellular functions (for review see refs. 8–10). In metabolism, an early finding was a role for HIF in the transcriptional regulation of genes encoding enzymes of the glycolytic pathway (11). Subsequent experiments in cell culture also have identified a role for HIF in (i) downregulating mitochondrial oxygen consumption by directly or indirectly inducing pyruvate dehydrogenase kinase (PDK), which inhibits the mitochondrial pyruvate dehydrogenase complex (PDC) from converting pyruvate into acetyl-CoA (12–14); (ii) regulating the differential expression of cytochrome c oxidase subunit 4 isoforms to optimize the efficiency of respiration at different O2 tensions (15), and (iii) inducing mitochondrial autophagy as an adaptive response to hypoxia (16). However, these observations thus far have been restricted mainly to cell culture, and their significance (or lack thereof) for the intact organism remains largely unexplored.
In humans, an opportunity to understand the effects of altered HIF physiology on integrated metabolic function is afforded by the condition of Chuvash polycythemia (CP). CP is an autosomal recessive disorder that is endemic to the region of Chuvashia (17) in the central European part of Russia. Individuals affected with CP have a homozygous germline mutation in exon 3 of the VHL gene (VHL 598C→T), which impairs the binding of the gene product VHL to HIF-α subunits. This impairment reduces the rate of HIF-α degradation and results in the stabilization of the HIF complex and increased expression of HIF-target genes under normoxic conditions (18, 19). Studies of patients with CP have revealed high hematocrit and hemoglobin values, elevated pulmonary arterial blood pressures, reduced systemic arterial pressures, and marked increases in the sensitivity of the respiratory system and the pulmonary vasculature to acute exposures to hypoxia (17, 18, 20). However, no metabolic measures have been reported in any of these studies.
In this study, we set out to determine whether any abnormalities of metabolism in patients with CP could be detected by exposing patients to the metabolic stress of exercise and of a standardized meal. In particular, we measured overall exercise capacity through an incremental exercise test on a cycle ergometer; calf muscle energy metabolites during light exercise by means of 31P magnetic resonance spectroscopy (MRS); the metabolic response (arterial and venous blood metabolites) to the consumption of a standardized meal; and in vitro skeletal muscle fiber composition, enzyme activities, and mRNA expression levels from biopsy samples obtained at rest. The results demonstrate that, under conditions of enhanced metabolic activity, CP patients generate substantially more lactate than control participants. Some, but not all, of the effects predicted from studies of the HIF system in cell culture were observed in vivo in humans who had CP.
Results
The age, sex, height, weight, body mass index, amount of physical exercise per week, and hematocrit for the CP patients and control participants are given in Table 1. Control participants were well matched for levels of physical activity in their daily life, body mass index, and age.
Table 1.
Individual and group characteristics of Chuvash polycythemia patients and control participants
| Participant | Age (y) | Sex | Height (m) | Weight (kg) | Body mass index (kg m−2) | Exercise per week (h) | Maximum work rate (W/kg) | Hematocrit (%) |
| Control #1 | 22 | M | 1.80 | 79 | 24 | <3 | 3.5 | 42 |
| Chuvash #1 | 21 | M | 1.77 | 58 | 19 | <3 | 3.1 | 62 |
| Control #2 | 22 | M | 1.83 | 72 | 21 | <3 | 4.2 | 45 |
| Chuvash #2 | 22 | M | 1.71 | 65 | 22 | <3 | 2.5 | 64 |
| Control #3 | 24 | M | 1.77 | 75 | 24 | <3 | 3.7 | 45 |
| Chuvash #3 | 25 | M | 1.72 | 61 | 21 | <3 | 3.3 | 41 |
| Control #4 | 44 | F | 1.71 | 67 | 23 | <3 | 3.3 | 36 |
| Chuvash #4 | 34 | F | 1.52 | 69 | 30 | <3 | 1.4 | 48 |
| Control #5 | 46 | F | 1.61 | 65 | 25 | <3 | 2.2 | 39 |
| Chuvash #5 | 38 | F | 1.63 | 52 | 20 | <3 | 1.5 | 41 |
| Mean control | 32 | 1.74 | 72 | 23 | <3 | 3.4 | 42 | |
| SD Control | 12 | 0.09 | 6 | 2 | 0.8 | 4 | ||
| Mean Chuvash | 28 | 1.67 | 61 | 22 | <3 | 2.4* | 51 | |
| SD Chuvash | 8 | 0.10 | 6 | 4 | 0.9 | 11 |
Age, sex, height, weight, body mass index, amount of physical exercise per week, maximum work rate achieved during the incremental test on the cycle ergometer, and hematocrit.
*The results for the CP group are significantly different (P < 0.05) from those for the control group.
CP Increases Lactate Production During Exercise and Limits Overall Exercise Capacity.
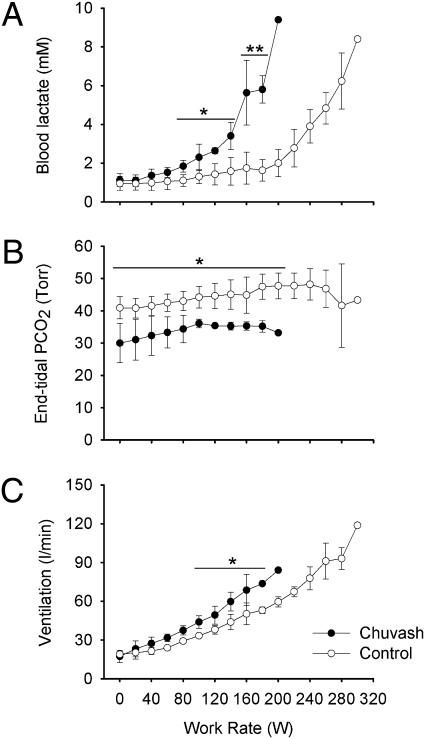
Fig. 1 illustrates mean values for venous blood lactate concentration, end-tidal partial pressure of carbon dioxide, and ventilation as a function of work rate during the incremental exercise test. Venous blood lactate concentration increased early in the CP patients, becoming significantly higher than in the control group at ∼4 min into the exercise test at a work rate of 80 W. CP patients stopped exercising at lower work rates than controls. The maximum work rates achieved per kg of body mass (reported in Table 1) were significantly lower for the CP patients, around 70% of those achieved by the control participants. There was no correlation between maximum work rate and hematocrit in the CP group. The end-tidal partial pressure of carbon dioxide was significantly lower in the CP group at rest and remained so throughout the exercise protocol. Furthermore, ventilation rose more rapidly during exercise in the CP patients than in the control participants.
Fig. 1.
Responses of CP patients and control participants to incremental exercise on a cycle ergometer. (A) Venous blood lactate concentration, (B) end-tidal partial pressure of carbon dioxide (PCO2), and (C) ventilation expressed as a function of work rate. Empty circles show results from the control group; filled circles show results from CP group. Data are mean ± SD. Number of individual values averaged per data point varies depending on number of participants who achieved the work rate; individuals’ maximum work rates are reported in Table 1. Horizontal lines indicate that each underlying average value for the CP patients differs significantly from the corresponding value for the control participants. *P < 0.05; **P < 0.01.
CP Causes Marked Phosphocreatine Depletion, Inorganic Phosphate Elevation, and Fall in pH During Calf Muscle Exercise.
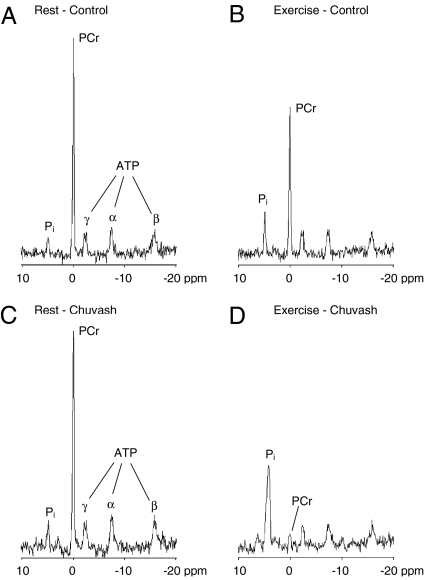
Fig. 2 illustrates 31P MR spectra for a representative control participant and a representative CP patient at rest and during calf exercise. At rest, the spectra from the two individuals appeared similar. However, during exercise, the phosphocreatine (PCr) peak almost disappeared, and the inorganic phosphate (Pi) peak was markedly higher in the CP patient than in the control participant. Similar results were obtained in all subjects within each group.
Fig. 2.
Examples of spectra obtained from calf muscle during 31P MRS. (A and B) Spectra for a representative control participant. (C and D) Spectra for a representative CP patient. (A and C) Spectra recorded at rest. (B and D) Spectra recorded in the last minute of the 5-W exercise period. The depletion of PCr and increase in Pi with exercise was much more marked in the Chuvash patient than in the control participant.
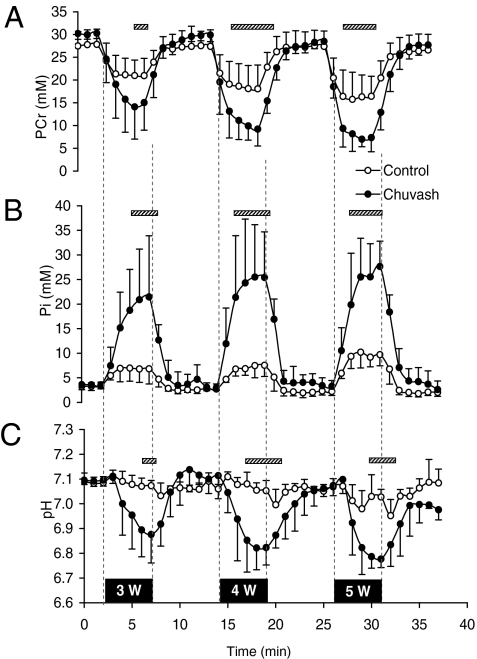
Mean values for PCr, Pi, and pH for the CP and for the control groups during the three periods of calf exercise and intervening periods of rest are shown in Fig. 3. In the CP group, marked depletion of muscle PCr and elevation of Pi was evident even at the lowest level of exercise. These effects were significant at all work rates, and even the lightest work rate (3 W) caused a greater depletion of PCr and elevation of Pi in the CP group than did the heaviest work rate (5 W) in the control group. Similarly, at all three levels of exercise, the fall in muscle pH in the CP group far exceeded the fall in muscle pH in the control participants. Again, this effect was such that the fall in pH at the lowest level of exercise in the CP group greatly exceeded the fall in pH at even the heaviest level of exercise in the control group. Muscle ATP concentration decreased in both groups in response to exercise, but its concentration did not differ significantly between the two groups at any level of exercise.
Fig. 3.
Results from CP group and control group for 31P MRS on calf muscle: (A) PCr concentration, (B) Pi concentration, and (C) pH as a function of time. The vertical broken lines indicate the onset and offset of the 5-min plantar-flexion exercise sessions, and the associated black bars indicate the power outputs (3, 4, and 5 W). Empty circles show results from the control group (n = 5); filled circles show results from the CP group (n = 5). Values are minute averages ± SD. Hatched horizontal bars indicate periods of significant difference (P < 0.05) between groups.
CP Is Associated with Elevated mRNA Levels of Muscle Phosphofructokinase, Muscle Pyruvate Kinase, and PDK in Skeletal Muscle.
Protein expression levels for the isoforms of myosin heavy chain, indicative of muscle fiber composition, are shown in Fig. S1. No significant differences were found between the CP group and the control group for the proportions of type I, IIA, IIX, and IIB fibers.
No significant differences were detected in muscle glycogen concentration, PDC total activity (PDCt), glutamate dehydrogenase (GluDH), citrate synthase (CS), 3-hydroxyacyl-CoA dehydrogenase (HAD), glyceraldehyde 3 phosphate dehydrogenase (GlyPDH), myosin heavy chains, and myosin light chains in control participants and CP patients (Table 2).
Table 2.
Results from the skeletal muscle biopsy for Chuvash polycythemia patients and control participants
| Participant | Glycogen* | PDCt† | GluDH† | CS† | HAD† | GlyPDH† | MHC | MLC |
| Control #1 | 384 | 9.1 | 9.9 | 39.0 | 20.2 | 4917 | 0.42 | 0.17 |
| Chuvash #1 | 406 | 4.8 | 7.9 | 48.5 | 17.5 | 6147 | 0.40 | 0.10 |
| Control #2 | 430 | 3.8 | 6.7 | 36.2 | 16.3 | 6421 | 2.00 | 0.90 |
| Chuvash #2 | 251 | 2.0 | 5.4 | 46.3 | 16.0 | 6246 | 0.55 | 0.18 |
| Control #3 | 346 | 7.9 | 4.0 | 37.2 | 7.4 | 3597 | 0.63 | 0.13 |
| Chuvash #3 | 323 | 4.5 | 9.4 | 65.7 | 27.8 | 6297 | 0.17 | 0.33 |
| Control #4 | 601 | 13.0 | 14.3 | 118.1 | 36.0 | 5868 | 0.15 | 0.08 |
| Chuvash #4 | 349 | 5.5 | 6.6 | 36.1 | 27.8 | 4773 | 0.36 | 0.05 |
| Control #5‡ | 500 | 4.4 | 6.9 | 32.4 | 10.4 | 6072 | ||
| Chuvash #5 | 364 | 7.8 | 5.4 | 27.9 | 9.8 | 4968 | 0.50 | 0.10 |
| Control | ||||||||
| Mean | 452 | 7.6 | 8.3 | 52.6 | 18.1 | 5375 | 0.80 | 0.32 |
| SD | 101 | 3.7 | 3.9 | 36.7 | 11.2 | 1140 | 0.82 | 0.39 |
| Chuvash | ||||||||
| Mean | 339 | 4.9 | 7.0 | 44.9 | 19.8 | 5686 | 0.40 | 0.15 |
| SD | 57 | 2.1 | 1.7 | 14.3 | 7.9 | 750 | 0.15 | 0.11 |
| P value | 0.08 | 0.07 | 0.58 | 0.73 | 0.85 | 0.69 | 0.33 | 0.50 |
CS, citrate synthase activity; GluDH, glutamate dehydrogenase activity; GlyPDH, glyceraldehyde-3-phosphate dehydrogenase activity; HAD, 3-hydroxy-acyl-coA dehydrogenase activity; MHC, myosin heavy chain; MLC, myosin light chain; PDCt, pyruvate dehydrogenase complex total activity.
*mmol glucosyl units kg−1 dry muscle.
†nmol acetyl-CoA min−1 mg−1 protein.
‡Insufficient material for MHC and MLC determination.
At the mRNA level, a significant increase in CP patients’ transcript levels was observed for muscle phosphofructokinase (PFKM), muscle pyruvate kinase (PKM) isoforms M1 (M1-PKM) and M2 (M2-PKM), and PDK isoforms 1 (PDK1), 2 (PDK2), and 4 (PDK4) (Table 3). Transcript levels did not differ significantly for hexokinase isoforms 1 (HK1) and 2 (HK2), pyruvate dehydrogenase phosphatase isoforms 1 (PDP1) and 2 (PDP2), pyruvate dehydrogenase kinase isoform 3 (PDK3), and lactate dehydrogenase A (LDHA).
Table 3.
Changes in mRNA expression levels in Chuvash polycythemia patients relative to control participants
| Gene name | Fold change | 95% CI | P value |
| HK1 | 1.4 | 0.8–2.1 | 0.06 |
| HK2 | 0.76 | 0.27–2.18 | 0.57 |
| PFKM | 1.4 | 1.1–2.0 | 0.04 |
| M1-PKM | 1.8 | 1.2–2.8 | 0.01 |
| M2-PKM | 1.8 | 1.1–3.0 | 0.04 |
| PDP1 | 0.55 | 0.27–1.11 | 0.09 |
| PDP2 | 0.67 | 0.30–1.48 | 0.28 |
| PDK1 | 2.0 | 1.1–3.5 | 0.03 |
| PDK2 | 1.9 | 1.3–2.7 | 0.004 |
| PDK3 | 1.2 | 0.6–2.1 | 0.53 |
| PDK4 | 5.5 | 1.7–18.4 | 0.01 |
| LDHA | 1.5 | 0.8–2.7 | 0.17 |
Expression data at the mRNA level for hexokinase 1 isoforms 1 (HK1) and 2 (HK2), muscle phosphofructokinase (PFKM), M1 isoform of muscle pyruvate kinase (M1-PKM), M2 isoform of muscle pyruvate kinase (M2-PKM), pyruvate dehydrogenase phosphatase isoforms 1 (PDP1) and 2 (PDP2), pyruvate dehydrogenase kinase isoforms 1 (PDK1), 2 (PDK2), 3 (PDK3), and 4 (PDK4), and lactate dehydrogenase A (LDHA). The 95% confidence interval (95% CI) is reported for each fold change.
CP Alters Pyruvate and Lactate Concentrations in Blood During the Digestion of a Standard Meal.
Fig. S2 illustrates the changes in mean arterial concentrations for plasma glucose, plasma insulin, plasma pyruvate, and blood lactate following the standard meal tolerance test. The changes in glucose and insulin did not differ significantly between the CP group and the control group. However, the increases in plasma pyruvate and blood lactate 60 min after ingestion of the standardized meal were significantly greater in the CP group than in the control group. At 60 min, the plasma pyruvate concentration was 251 ± 64 μM (mean ± SD) in the CP group compared with 122 ± 51 μM in the control group, and the blood lactate was 1.74 ± 0.27 mM in the CP group compared with 0.87 ± 0.37 mM in the control group.
Fig. S2 illustrates forearm blood flow during the standard meal tolerance test. No significant effects of the meal were detected, nor were there any significant differences between the CP group and the control group. Also illustrated in Fig. S2 are the uptakes of glucose and lactate by skeletal muscle. These uptakes increased after the meal. With the exception of one data point, no significant differences were detected between the CP group and the control group.
Discussion
This study demonstrates that a functional mutation within the HIF–VHL pathway can have a significant effect on human energy metabolism at the level of the organism as a whole. In particular, the study demonstrates that exercise, whether involving a large or a small muscle mass, is a substantial metabolic stress for patients with CP, who exhibit significantly greater lactate accumulation than normal controls. Compared with normal controls, CP patients had low maximum exercise capacities and demonstrated early and greater PCr depletion and acidosis during a light ankle plantar-flexion exercise. In patients with CP, skeletal muscle mRNA expression levels at rest were elevated for enzymes of glycolysis and PDC inhibition.
In normal healthy volunteers, maximal oxygen uptake capacity is a significant determinant of both the maximum exercise capacity and the work rate at which blood lactate begins to rise (21). In patients with CP a degree of pulmonary hypertension (20) could limit the rise in cardiac output with exercise and so cause low maximum exercise capacity and early lactate production from muscle. We did not record pulmonary arterial blood pressure in this study and so do not know how much it may have risen during the incremental exercise test. However, we studied energy metabolism in a small muscle mass during light exercise, in which CP patients demonstrated a striking depletion of PCr in muscle, accumulation of Pi, and marked muscle acidosis compared with the normal controls. Because these findings were associated with light exercise of a small muscle mass, they suggest that cardiopulmonary limitations to the delivery of oxygen are an unlikely explanation for the abnormal metabolism. The light level of exercise also makes it unlikely that the results are caused by diffusional limitations for oxygen within the muscle. Finally, three of the five patients were not noticeably polycythemic (because of clinical management via venesection), and therefore viscosity changes in blood are not likely to have limited the supply of oxygen. This last point is supported by the absence of any correlation between hematocrit and maximum work rate in the CP group.
Skeletal muscle that contains a high proportion of fast-twitch (type II) white muscle fibers is more likely to metabolize anaerobically during exercise and so produce more lactate and have greater PCr depletion at the onset of contraction. Thus a further possibility is that the skeletal muscle of CP patients may contain a high proportion of such fibers. However, this hypothesis was not supported by the biopsy results, which demonstrated almost identical proportions of fiber types in the CP patients and the normal controls.
A further possibility is that CP patients are limited in their exercise capacity not by the availability of oxygen but rather by their capacity to use oxygen. This diminished capacity could arise through limitations in substrate supply to the tricarboxylic acid cycle in a manner similar to that of the acetyl group deficit in normal humans at the start of exercise (22, 23). In particular, production of acetyl-CoA requires the activation of PDC, which is tightly regulated through an inactivation/activation cycle controlled by kinases (PDK1-4) and phosphatases (PDP1-2), respectively. In the skeletal muscle biopsies from CP patients, we detected significant elevations in transcript for PDK1, PDK2, and PDK4 (the last two being the predominant isoforms of the kinase in skeletal muscle) (24, 25). This ability of HIF to suppress oxidative metabolism is consistent with observations made in mice lacking HIF-1α in skeletal muscle; these mice had an increased level of exercise endurance associated with a lower level of lactate and a lower level of mRNA for PDK1 (26). Although there is a mouse model of CP (27, 28), no metabolic phenotype has been reported. Apart from PDK, we also observed elevated levels of transcript for two other known HIF-regulated genes (PFKM and PKM) (29, 30), but transcripts for other HIF-target genes were not significantly altered in CP patients. This result may have arisen simply as a type II error because of our limited number of patients. Overall, although PDCt activity did not differ significantly between the two groups, alterations in the expression of its regulatory kinase or phosphatase enzymes nevertheless might explain the observed abnormalities in skeletal muscle energy metabolism.
In cell culture, an increased level of HIF-1 has been associated with mitochondrial autophagy (16). Because there is a strong relationship between total mitochondrial volume and maximal oxygen uptake capacity (31), a further possible explanation of the reduced exercise capacity and enhanced lactate production of the CP patients is a reduction in mitochondrial volume. However, the lack of any difference between groups in the markers of mitochondrial volume (GluDH and CS activities) provides no evidence to support a reduced mitochondrial volume in the CP patients. In skeletal muscle of HIF-1α–null mice, the number of mitochondria also was not altered (32).
A further finding in cell culture is that HIF-1 may regulate the expression of cytochrome oxidase subunit 4 isoforms (15). It thus is possible that their expression differs in the CP patients and controls, and this difference could affect the efficiency with which they are able to consume oxygen within the muscle. Our muscle biopsy analyses were limited by the amount of tissue available, and we were unable to assess this possibility.
Given the magnitude of the effects of CP on skeletal muscle metabolism during exercise, there were remarkably few aberrations in glucose, lactate, and pyruvate metabolism after meal intake in CP patients. Glucose homeostasis after meal intake was normal. Notably, lactate uptake in the forearm in CP patients was no different from that in control participants in both the resting pre- and postprandial states. Arterial blood lactate concentration increased more in CP patients after meal intake, but the origin of this lactate is not known. Tissues with a net production of lactate are brain, intestine, blood cells, skin, and adipose tissue (33–35), whereas kidney (renal cortex) and liver remove lactate (36). Skeletal muscle will extract or produce lactate depending on metabolic state and muscular workload. There was no association between the rise in plasma lactate and hematocrit (higher in some CP patients than in controls), suggesting that generation of lactate from blood does not contribute to the difference between the groups. The production of lactate from skin also probably can be assumed to be constant. The contribution of lactate production from adipose tissue was not likely to have been very significant in a whole-body perspective, at least not in these groups of moderately lean people. Therefore the postprandial rise in lactate would seem more likely to depend on a CP-specific effect on intestinal lactate production, on a reduction in postprandial hepatic lactate clearance, or a combination of both. Changes in pyruvate concentration mirrored those of lactate, and there were no differences between groups in the lactate/pyruvate ratios in blood; these findings are consistent with there being no major disturbance of the cellular cytosolic redox state.
The meal intake gave us the opportunity to examine the postprandial rise in plasma insulin to search for an insulin-secretion defect. Such defects have been reported recently in mice with a conditional deletion of VHL within pancreatic β cells (37–39). Because the rise of meal-stimulated insulin appeared normal and also appeared to control postprandial plasma glucose concentrations within a normal range, we conclude that the subtle systemic VHL deficit present in CP patients causes no gross abnormality in insulin secretion.
With respect to their cardiopulmonary phenotype, CP patients generally appear to phenocopy sea-level natives who have been acclimatized to the hypoxia of high altitude (20). In relation to exercise, however, the predominant view has been that both acclimatized sea-level natives and high-altitude natives accumulate less lactate in the blood during exercise and have lower peak values for lactate after exhaustive exercise than do unacclimatized individuals (40, 41). This situation has been termed the “lactate paradox” (41–43). In contrast, our CP patients exhibit the opposite effect. However, not all studies have detected the reduction in lactate accumulation during exercise following acclimatization (44, 45), and the existence of the lactate paradox is a matter of debate (43, 46–50). In a similar manner, there is a debate as to whether there are any benefits associated with a “live high, train low” regimen for endurance athletes other than those that may arise from the associated erythrocytosis (51). Our results suggest that sustained activation of the HIF pathway in humans is more likely to be deleterious to such performance. Finally, the comparison between CP and reduced oxygen availability should not be drawn too closely, because oxygen not only affects the levels of HIF but is itself a key substrate in metabolism. Metabolic changes also could affect 2-oxoglutarate concentrations, and these concentrations in turn affect the level of HIF (52, 53).
In summary, CP is a subtle genetic abnormality that results in a modest increase in expression of HIF-regulated gene products in the absence of hypoxia. In this study, with our limited number of patients, we were unable to detect some of the effects that might be predicted from the much more powerful manipulations of the HIF system that can be undertaken in cell culture. Nevertheless, despite the subtle nature of the CP defect, major effects on overall metabolism could be detected as soon as metabolism was sufficiently stressed. These findings emphasize that HIF plays an important role in the overall regulation of metabolic function and that other mechanisms within the intact organism do not compensate fully for the metabolic effects of the CP modification.
Materials and Methods
Participants.
Five CP patients and five healthy control participants took part in the study. Table 1 shows the characteristics of individual participants. CP patients were identified from previous studies (20, 54). Control participants were recruited by advertisement. Each CP patient was homozygous for the classic Chuvash mutation (19) and had been treated with long-term venesection to maintain a hematocrit near normal. Nonetheless, two patients presented with high hematocrits (62% and 64%) on the first day of the experiments. No patient had undergone venesection within several weeks of the experiment. The patients had no other medical disorders, had no history of complications, and were asymptomatic except for occasional headaches and tiredness. The number of CP patients included in the study was limited by the rarity of this condition in the United Kingdom. Control participants were chosen to match CP patients for gender and, as far as possible, for a combination of age, physical fitness (based on the amount of exercise taken each week), and body build. Each participant was informed about the aims, procedure, and details of the study and signed a written informed consent form before taking part in the experiments. The study conformed to the Declaration of Helsinki and was approved by the Oxfordshire Research Ethics Committee.
Experimental Procedure.
The experiments were performed over 2 consecutive days and included an incremental bicycle exercise test to exhaustion, a small muscle mass exercise test, a standard meal tolerance test, and a muscle biopsy. These tests were ordered chronologically to minimize the effects of each test on the others as follows. On the morning of the first day, participants undertook the small muscle mass (calf) light exercise tests for biochemical investigation using 31P MRS. In the afternoon muscle biopsies were taken from the nonexercised vastus lateralis after infiltration of the biopsy area with 1% lidocaine. In the evening participants were given a low-fat meal. On the second day, the standardized meal tolerance test was administered; arteriovenous differences in metabolites concentrations were measured before and for 5 h after the consumption of the meal. The second day ended with the incremental exercise test to exhaustion on the cycle ergometer. Participants fasted overnight from 8:00 PM before each experimental day. They also were asked to avoid vigorous exercise and alcohol and caffeine consumption for 48 h before the experiments.
The methods for each test followed standard procedures. Detailed methodology for the incremental exercise test to exhaustion, small muscle mass exercise for investigation with MRS, the standard meal tolerance test, muscle biopsy analyses, and statistical analysis are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank the subjects who participated in this study. F.F. was funded by a University College Oxford Postdoctoral Research Scholarship and the Fell Fund from the Department of Physiology, Anatomy and Genetics, University of Oxford, United Kingdom. This work was supported by the British Heart Foundation (Programme Grant RG/07/004) and the Wellcome Trust, United Kingdom (Grant 075876).
Footnotes
Conflict of interest statement: P.J.R. is a scientific co-founder of and holds equity in ReOx Ltd, a company that is seeking to make HIF hydroxylase inhibitors.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1002339107/-/DCSupplemental.
References
- 1.Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 2.Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272:22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- 3.Jaakkola P, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 4.Ivan M, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: Implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 5.Epstein AC, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 6.Maxwell PH, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 7.Ohh M, et al. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 8.Semenza GL. Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem J. 2007;405:1–9. doi: 10.1042/BJ20070389. [DOI] [PubMed] [Google Scholar]
- 9.Semenza GL. Hydroxylation of HIF-1: Oxygen sensing at the molecular level. Physiology (Bethesda) 2004;19:176–182. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- 10.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 11.Firth JD, Ebert BL, Pugh CW, Ratcliffe PJ. Oxygen-regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: Similarities with the erythropoietin 3′ enhancer. Proc Natl Acad Sci USA. 1994;91:6496–6500. doi: 10.1073/pnas.91.14.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Aragonés J, et al. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet. 2008;40:170–180. doi: 10.1038/ng.2007.62. [DOI] [PubMed] [Google Scholar]
- 15.Fukuda R, et al. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, et al. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892–10903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Sergeyeva A, et al. Congenital polycythemia in Chuvashia. Blood. 1997;89:2148–2154. [PubMed] [Google Scholar]
- 18.Bushuev VI, et al. Endothelin-1, vascular endothelial growth factor and systolic pulmonary artery pressure in patients with Chuvash polycythemia. Haematologica. 2006;91:744–749. [PubMed] [Google Scholar]
- 19.Ang SO, et al. Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet. 2002;32:614–621. doi: 10.1038/ng1019. [DOI] [PubMed] [Google Scholar]
- 20.Smith TG, et al. Mutation of von Hippel-Lindau tumour suppressor and human cardiopulmonary physiology. PLoS Med. 2006;3:e290. doi: 10.1371/journal.pmed.0030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weibel ER, Hoppeler H. Exercise-induced maximal metabolic rate scales with muscle aerobic capacity. J Exp Biol. 2005;208:1635–1644. doi: 10.1242/jeb.01548. [DOI] [PubMed] [Google Scholar]
- 22.Greenhaff PL, et al. An acetyl group deficit limits mitochondrial ATP production at the onset of exercise. Biochem Soc Trans. 2002;30:275–280. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 23.Greenhaff PL, et al. Metabolic inertia in contracting skeletal muscle: A novel approach for pharmacological intervention in peripheral vascular disease. Br J Clin Pharmacol. 2004;57:237–243. doi: 10.1046/j.1365-2125.2003.01989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowker-Kinley MM, Davis WI, Wu P, Harris RA, Popov KM. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem J. 1998;329:191–196. doi: 10.1042/bj3290191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gudi R, Bowker-Kinley MM, Kedishvili NY, Zhao Y, Popov KM. Diversity of the pyruvate dehydrogenase kinase gene family in humans. J Biol Chem. 1995;270:28989–28994. doi: 10.1074/jbc.270.48.28989. [DOI] [PubMed] [Google Scholar]
- 26.Mason SD, et al. HIF-1alpha in endurance training: Suppression of oxidative metabolism. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2059–R2069. doi: 10.1152/ajpregu.00335.2007. [DOI] [PubMed] [Google Scholar]
- 27.Hickey MM, Lam JC, Bezman NA, Rathmell WK, Simon MC. von Hippel-Lindau mutation in mice recapitulates Chuvash polycythemia via hypoxia-inducible factor-2alpha signaling and splenic erythropoiesis. J Clin Invest. 2007;117:3879–3889. doi: 10.1172/JCI32614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hickey MM, et al. The von Hippel-Lindau Chuvash mutation promotes pulmonary hypertension and fibrosis in mice. J Clin Invest. 2010;120:827–839. doi: 10.1172/JCI36362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ptashne KA, Theodore J, Robin ED. Increased phosphofructokinase content during chronic hypoxia in cultured skeletal muscle (L8) cells. Biochim Biophys Acta. 1983;763:169–174. doi: 10.1016/0167-4889(83)90040-x. [DOI] [PubMed] [Google Scholar]
- 30.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- 31.Weibel ER, Bacigalupe LD, Schmitt B, Hoppeler H. Allometric scaling of maximal metabolic rate in mammals: Muscle aerobic capacity as determinant factor. Respir Physiol Neurobiol. 2004;140:115–132. doi: 10.1016/j.resp.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Mason SD, et al. Loss of skeletal muscle HIF-1alpha results in altered exercise endurance. PLoS Biol. 2004;2:e288. doi: 10.1371/journal.pbio.0020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coppack SW, Frayn KN, Humphreys SM, Whyte PL, Hockaday TD. Arteriovenous differences across human adipose and forearm tissues after overnight fast. Metabolism. 1990;39:384–390. doi: 10.1016/0026-0495(90)90253-9. [DOI] [PubMed] [Google Scholar]
- 34.Jansson PA, Smith U, Lönnroth P. Evidence for lactate production by human adipose tissue in vivo. Diabetologia. 1990;33:253–256. doi: 10.1007/BF00404805. [DOI] [PubMed] [Google Scholar]
- 35.Kreisberg RA. Glucose-lactate inter-relations in man. N Engl J Med. 1972;287:132–137. doi: 10.1056/NEJM197207202870307. [DOI] [PubMed] [Google Scholar]
- 36.Rowell LB, et al. Splanchnic removal of lactate and pyruvate during prolonged exercise in man. J Appl Physiol. 1966;21:1773–1783. doi: 10.1152/jappl.1966.21.6.1773. [DOI] [PubMed] [Google Scholar]
- 37.Cantley J, et al. Deletion of the von Hippel-Lindau gene in pancreatic beta cells impairs glucose homeostasis in mice. J Clin Invest. 2009;119:125–135. doi: 10.1172/JCI26934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puri S, Cano DA, Hebrok M. A role for von Hippel-Lindau protein in pancreatic beta-cell function. Diabetes. 2009;58:433–441. doi: 10.2337/db08-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zehetner J, et al. PVHL is a regulator of glucose metabolism and insulin secretion in pancreatic beta cells. Genes Dev. 2008;22:3135–3146. doi: 10.1101/gad.496908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hochachka PW. The lactate paradox: Analysis of underlying mechanisms. Annals of Sports Medicine. 1988;4:184–188. [Google Scholar]
- 41.West JB. Lactate during exercise at extreme altitude. Fed Proc. 1986;45:2953–2957. [PubMed] [Google Scholar]
- 42.Reeves JT, et al. Oxygen transport during exercise at altitude and the lactate paradox: Lessons from Operation Everest II and Pikes Peak. Exerc Sport Sci Rev. 1992;20:275–296. [PubMed] [Google Scholar]
- 43.West JB. Point: The lactate paradox does/does not occur during exercise at high altitude. J Appl Physiol. 2007;102:2398–2399. doi: 10.1152/japplphysiol.00039.2007. [DOI] [PubMed] [Google Scholar]
- 44.Dempsey JA, et al. Control of exercise hyperpnea under varying durations of exposure to moderate hypoxia. Respir Physiol. 1972;16:213–231. doi: 10.1016/0034-5687(72)90052-7. [DOI] [PubMed] [Google Scholar]
- 45.Klausen K, Robinson S, Micahel ED, Myhre LG. Effect of high altitude on maximal working capacity. J Appl Physiol. 1966;21:1191–1194. doi: 10.1152/jappl.1966.21.4.1191. [DOI] [PubMed] [Google Scholar]
- 46.Brooks GA. Comments on Point:Counterpoint: “The lactate paradox does/does not occur during exercise at high altitude”. J Appl Physiol. 2007;102:2408. doi: 10.1152/japplphysiol.00287.2007. author reply 2409–2410. [DOI] [PubMed] [Google Scholar]
- 47.Mazzeo RS. Comments on Point:Counterpoint: “The lactate paradox does/does not occur during exercise at high altitude”. J Appl Physiol. 2007;102:2403. doi: 10.1152/japplphysiol.00222.2007. author reply 2409–2410. [DOI] [PubMed] [Google Scholar]
- 48.van Hall G. Counterpoint: The lactate paradox does not occur during exercise at high altitude. J Appl Physiol. 2007;102:2399–2401. doi: 10.1152/japplphysiol.00039a.2007. [DOI] [PubMed] [Google Scholar]
- 49.van Hall G, et al. The lactate paradox revisited in lowlanders during acclimatization to 4100 m and in high-altitude natives. J Physiol. 2009;587:1117–1129. doi: 10.1113/jphysiol.2008.160846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hochachka PW, et al. The lactate paradox in human high-altitude physiological performance. News Physiol Sci. 2002;17:122–126. doi: 10.1152/nips.01382.2001. [DOI] [PubMed] [Google Scholar]
- 51.Gore CJ, Clark SA, Saunders PU. Nonhematological mechanisms of improved sea-level performance after hypoxic exposure. Med Sci Sports Exerc. 2007;39:1600–1609. doi: 10.1249/mss.0b013e3180de49d3. [DOI] [PubMed] [Google Scholar]
- 52.Pollard PJ, et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet. 2005;14:2231–2239. doi: 10.1093/hmg/ddi227. [DOI] [PubMed] [Google Scholar]
- 53.Zhao S, et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science. 2009;324:261–265. doi: 10.1126/science.1170944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Percy MJ, et al. Chuvash-type congenital polycythemia in 4 families of Asian and Western European ancestry. Blood. 2003;102:1097–1099. doi: 10.1182/blood-2002-10-3246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.