Abstract
Cerebral cavernous malformations (CCM) are frequent vascular abnormalities caused by mutations in one of the CCM genes. CCM1 (also known as KRIT1) stabilizes endothelial junctions and is essential for vascular morphogenesis in mouse embryos. However, cellular functions of CCM1 during the early steps of the CCM pathogenesis remain unknown. We show here that CCM1 represents an antiangiogenic protein to keep the human endothelium quiescent. CCM1 inhibits endothelial proliferation, apoptosis, migration, lumen formation, and sprouting angiogenesis in primary human endothelial cells. CCM1 strongly induces DLL4-NOTCH signaling, which promotes AKT phosphorylation but reduces phosphorylation of the mitogen-activated protein kinase ERK. Consistently, blocking of NOTCH activity alleviates CCM1 effects. ERK phosphorylation is increased in human CCM lesions. Transplantation of CCM1-silenced human endothelial cells into SCID mice recapitulates hallmarks of the CCM pathology and serves as a unique CCM model system. In this setting, the multikinase inhibitor Sorafenib can ameliorate loss of CCM1-induced excessive microvascular growth, reducing the microvessel density to levels of normal wild-type endothelial cells. Collectively, our data suggest that the origin of CCM lesions is caused by perturbed Notch signaling-induced excessive capillary sprouting, which can be therapeutically targeted.
Keywords: endothelial cells, vascular malformations, CCM, KRIT1
Cerebral cavernous malformations (CCM) [OMIM: 116860] are frequent vascular abnormalities, predominately localized in the brain, affecting up to 0.5% of the human population (1). CCM lesions are characterized by grossly enlarged vascular channels, often lacking support of mural cells. The carriers can develop a symptomatic disease with headaches, seizures, focal neurological deficits, or hemorrhages. The disease is caused by mutations in one of the three known CCM genes, namely KRIT1 (CCM1), OSM (CCM2), or PDCD10 (CCM3), and can occur sporadically or familial with high penetrance (2). Recent data suggest a “two-hit mechanism” for the cause of localized lesions, where a germ-line loss of one allele is followed by a loss of the second allele of individual endothelial cells (3, 4).
CCM1 is expressed in astrocytes and endothelial cells, and can be associated to microtubules, membranes, and adherens junctions, but also the nucleus (5–8). The CCM1 protein is part of a large protein complex together with CCM2 and CCM3, components of the cytoskeleton and cell junctions, as well as components of signal transduction pathways and lipids (9). The interaction with RAP1 is essential for stabilizing endothelial cell-cell contacts (5). Additionally, the strong binding to the cytoplasmic β1-integrin binding protein-1 ICAP1 (ITGB1BP1) could affect β1-integrin conformation and endothelial-extracellular matrix interactions (10); however, detailed molecular and cellular functions remain elusive.
A Ccm1 null mutation in mouse causes embryonic lethality. Many major blood vessels of mutant embryos are dilated, and others are narrowed and the expression of arterial marker genes is strongly reduced, suggesting a defect in arterial/venous differentiation (11). In contrast, loss of ccm1 in zebrafish does not alter arterial marker gene expression, but leads to dilation of major vessels because of excessive spreading of endothelial cells (12). The clinical observation that CCM lesions grow in response to VEGF (13) suggests that their formation could be driven by angiogenesis: the growth of new blood vessels from preexisting ones. We consequently hypothesized that CCM lesions may reflect a pathological angiogenesis. The experiments revealed a role of CCM1 as an activator of vascular DELTA-NOTCH signaling. Correspondingly, perturbed DELTA-NOTCH signaling was identified as a pathogenetic cause of CCM1 mutation-mediated vascular malformations.
Results
CCM1 Is a Negative Regulator of Sprouting Angiogenesis.
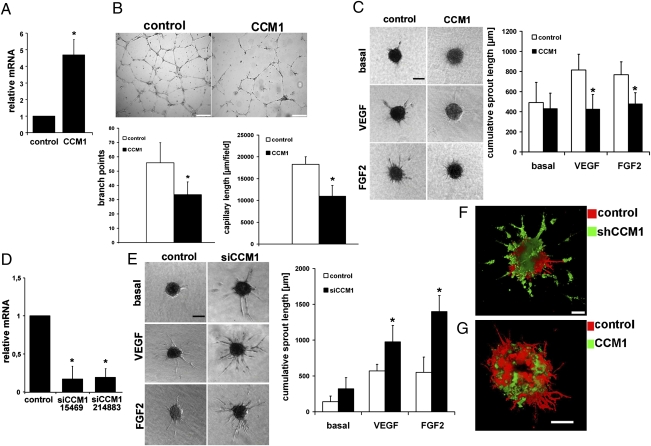
Human umbilical vein endothelial cells (HUVEC) express CCM1 mRNA as determined by quantitative real-time RT-PCR (qPCR). When cultured on Matrigel, HUVEC spontaneously organized into cords and formed a honeycomb-like network. Forced expression of CCM1 inhibited such network formation (Fig. 1 A and B). HUVEC remained isolated or in clumps of cells and formed fewer tubes and branch points compared with GFP overexpression as control.
Fig. 1.
CCM1 inhibits sprouting angiogenesis. (A–C) HUVEC were adenovirally transduced with CCM1 or GFP as control leading to an about 5-fold overexpression of CCM1 mRNA as determined by qPCR (A). (B) CCM1 inhibited tube formation and branching on a Matrigel matrix. (Scale bars, 2,000 μm.) (C) CCM1 inhibited VEGF (25 ng/mL) or FGF2 (25 ng/mL) stimulated sprouting angiogenesis in a collagen matrix. (Scale bar, 200 μm.) (D) Down-regulation of CCM1 mRNA expression by two siRNAs (Ambion) led to about 80% reduction of mRNA levels. (E) Small interfering RNA-mediated CCM1 silencing strongly enhanced endothelial sprouting in collagen gels under basal conditions and after VEGF or FGF2 stimulation. (Scale bar, 200 μm.) (F and G) Control HUVEC were labeled with the red fluorescent membrane dye PKH26 and mixed with CCM1 shRNA (F) or cDNA (G) expressing HUVEC stained with the green membrane dye PKH67. After sprouting in collagen beds (25 ng/mL VEGF), spheroids were imaged with confocal laser microscopy and 3D images were calculated. (F) CCM1 depletion caused more and larger sprouts. (Scale bar, 100 μm.) (G) CCM1-expressing HUVEC formed few regular sprouts compared with control. *P < 0.05. (Scale bar, 100 μm.) Error bars are means ± SD of n = 10 in C and E.
CCM1 expression caused a drastic reduction of VEGF- or FGF2-induced sprout formation in a 3D spheroidal system of endothelial differentiation and capillary formation (14) (Fig. 1C). The short sprouts failed to form a lumen. Because CCM lesions are typically caused by a loss of CCM1 function, we investigated whether down-regulation of this gene would lead to enhanced angiogenesis. Two independent CCM1 siRNAs, as well as a lentiviral shRNA vector, led to substantial silencing of CCM1 mRNA expression (Fig. 1D). HUVEC with silenced CCM1 expression formed significantly more long capillary sprouts compared with nonsilencing siRNA-transfected control cells (Fig. 1E). When CCM1 shRNA transduced HUVEC (green fluorescent) were mixed with control cells (red fluorescent), the CCM1-depleted HUVEC formed more and larger sprouts (Fig. 1F and Movie S1). Cells overexpressing CCM1 formed only very few and short sprouts when mixed with control HUVEC (Fig. 1G and Movie S2). Control cells exhibited normal sprouting behavior, suggesting a cell-autonomous function of CCM1. These experiments established CCM1 as a negative regulator of angiogenesis.
CCM1 Impairs Endothelial Migration, Proliferation, and Apoptosis.
Angiogenesis involves multiple cellular processes, including endothelial cell migration and proliferation. Migration of CCM1-expressing HUVEC was significantly delayed compared with control. This delay was detected in a scratch wound assay (Fig. 2A) and in the Boyden chamber using VEGF or FGF2 as chemoattractants (Fig. 2B). Conversely, CCM1 silencing enhanced endothelial migration (Fig. 2 A and B), which was associated with an increased number of polarized focal adhesions at cellular protrusions (Fig. 2C).
Fig. 2.
CCM1 inhibits endothelial migration and proliferation. (A) Confluent HUVEC were wounded and migration was assayed. Short hairpin RNA against CCM1 enhanced closure of the gap, whereas lentiviral CCM1 expression inhibited cell migration (arrows). Because shRNA vectors also expressed GFP, fluorescence pictures are shown, whereas CCM1 over expression is shown as bright field image. (Scale bars, 200 μm.) (B) Transmigration through a collagen coated filter (8-μm pore size) toward VEGF (25 ng/mL) or FGF2 (25 ng/mL) was inhibited by CCM1 and enhanced after CCM1 knockdown. (C) Antibody staining against focal adhesion kinase (FAK) revealed a higher number and more polarized focal adhesion contacts at the cell periphery after CCM1 knockdown. (Scale bar, 50 μm.) (D–G) CCM1 inhibited endothelial proliferation as shown by reduced BrdU incorporation (D), decreased fraction of cells in S-phase as determined by FACS (E), and up-regulation of the cell cycle inhibitors p21 and p27 mRNA (F). (G) Western blot analysis 48 h after CCM1 adenovirus transduction showed that CCM1 reduced phosphorylation of ERK1/2 proteins, whereas siRNA treatment elevated phospho-ERK1/2 levels. (H) Protein lysates of human CCM lesion exhibit high phospho-ERK1/2 amounts. (I) Apoptosis of HUVEC under normal culture conditions and 2 h after addition of 250 nmol/L staurosporine was measured by detection of caspase-3 and -7 activities with a luminescent substrate. CCM1 expression reduced the rate of staurosporine-induced cell death. (J and K) CCM1 expression increased the amount of phosphorylated AKT protein at serine 473. *P < 0.05. Error bars are means ± SD of n = 5.
Silencing of CCM1 caused a moderate, nonsignificant enhancement of HUVEC proliferation as determined by BrdU incorporation and cell-number counting. The apoptosis rate under basal conditions and in response to the apoptosis-inducing agent staurosporin was also not significantly altered (Fig. S1). Conversely, forced CCM1 expression led to reduced cell proliferation (Fig. 2D). This finding was associated with a reduced fraction of S-phase cells, an increased G1-phase population (Fig. 2E), and elevated mRNA levels of the cell cycle inhibitors p21 (Waf1 or CIP1) and p27 (KIP1) (Fig. 2F). CCM1 expression significantly reduced ERK phosphorylation, whereas higher phospho-ERK levels could be detected after CCM1 silencing (Fig. 2G). These findings were corroborated by analysis of protein lysates derived from surgical material of human CCM lesions. Compared with control brain tissue, lesions from six CCM patients (15) had significantly higher levels of phosphorylated ERK protein (Fig. 2H).
CCM1 expression did not induce apoptosis under normal culture conditions and even protected HUVEC from staurosporine-induced apoptosis (Fig. 2I). In line with the potent antiapoptotic and prosurvival functions of active AKT (16, 17), CCM1 expression significantly elevated the amount of active AKT protein phosphorylated at serine 473 (Fig. 2J), whereas knockdown of CCM1 decreased AKT phosphorylation (Fig. 2K). Collectively, the data establish a role of CCM1 in shifting the balance from ERK-mediated proliferation and migration to AKT-mediated cell survival and endothelial quiescence.
CCM1 Regulates Sprouting Angiogenesis in Vivo.
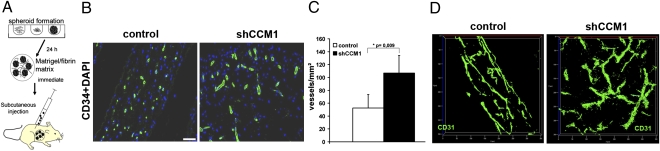
Human endothelial cells during angiogenesis can functionally be studied in vivo by grafting them in immunocompromized mice, where they will form a network of perfused capillaries that anastomoses with the mouse vasculature (18, 19). We sought to advance this assay to mimic a genetic human vascular disease in mice (Fig. 3A). Lentivirally CCM1-silenced HUVEC and control shRNA transduced cells were comparatively studied for their ability to form vascular networks following xenotransplantation. Twenty-eight days after transplantation, CCM1-silenced HUVEC had formed a significantly denser vessel network in vivo (Fig. 3C). These vessels also had more protrusions and had larger diameters than control HUVEC vasculatures (Fig. 3 B and D and Movie S3 and Movie S4), thus representing hallmarks of the CCM disease.
Fig. 3.
CCM1 inhibits angiogenesis in transplanted endothelial cells. (A) Scheme of the spheroid-based angiogenesis assay. (B) Representative sections through plugs stained against human CD34 (green) for endothelial cells. HUVEC expressing control shRNA formed a regular capillary vascular network. CCM1 silenced HUVEC formed a denser network with larger vessels. (Scale bar, 50 μm.) (C) Quantification of microvessel density determined by the number of vessels per square milimeter. n = 7 plugs of control and CCM1 shRNA. (D) Thick sections (50 μm) were stained with human specific anti-CD31 to stain the endothelial network and assessed by confocal laser microscopy (LSM710; Zeiss). shRNA against CCM1 led to formation of a denser network with larger vessels compared with control.
Global Gene Expression Analysis to Uncover Molecular CCM1 Effects.
Comparative microarray analyses of CCM1 expressing and control GFP-transduced HUVEC identified 262 transcripts with at least 2-fold changed expression (Fig. S2 and Table S1). The transcriptional changes of 12 candidates were verified by qPCR Table S2. The majority of the regulated transcripts were involved in cell-cycle progression, proliferation, and cell migration (Fig. 4B) and these genes were also highly functional connected with each other (Fig. S3 and Fig. S4).
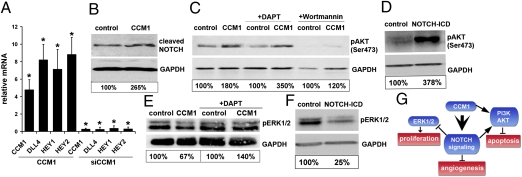
Fig. 4.
CCM1 activates DELTA-NOTCH signaling. (A) Quantitative RT-PCR showing significantly elevated mRNA levels of the NOTCH ligand DLL4 and the target genes HEY1 and HEY2 48 h after adenoviral CCM1 transduction. Small interfering RNA treatment against CCM1 down-regulated expression of DLL4 and NOTCH target genes. (B) CCM1 expression in HUVEC increased the amounts of cleaved NOTCH receptor proteins as detected by a cleavage-specific antibody. (C) Western blotting revealed strongly increased levels of phospho-AKT after 36 h of adenoviral CCM1 transduction. The NOTCH inhibitor DAPT (25 μM) could not prevent AKT phosphorylation. Inhibition of PI3K activity by Wortmannin (2 μM) prevented AKT phosphorylation independent of CCM1 expression. (D) Adenoviral expression of constitutive active NOTCH1 (intracellular domain, ICD) increased phospho-AKT levels. (E) Adenoviral CCM1 expression decreased ERK1/2 phosphorylation and this could fully be prevented with the NOTCH inhibitor DAPT (25 μM). (F) Active NOTCH1 strongly inhibited ERK1/2 phosphorylation. (G) Proposed scheme of CCM1-mediated regulation of endothelial functions. *P < 0.05.
Among the regulated genes, we identified a considerable number of transcripts associated with angiogenesis (Fig. S2C). Angiogenic factors (EGR1, ESM1, ROBO4) and genes involved in cell migration (CDC42EP2, ETV5, HMMR, IQGAP3) were down-regulated. In contrast, extracellular matrix molecules (COL1A2, FIBULIN2) and BMP/TGFβ related/regulated genes (ANGPTL4, BAMBI, COL1A2, ESM1, GDF3, TGFA) were up-regulated. Notably, CCM1 induced HEY1, a direct target of NOTCH signaling (20), which acts as a strong inhibitor of sprouting angiogenesis (21).
CCM1 Acts Upstream of the DELTA-NOTCH Pathway.
In addition to the up-regulation of HEY1, we identified a significant induction of the NOTCH ligand DLL4 and the NOTCH target gene HEY2 (Fig. 4A), which was accompanied by higher levels of cleaved, and thus activated, NOTCH protein (Fig. 4B). Active NOTCH signaling inhibits endothelial proliferation, migration, sprouting, and branching, whereas loss-of-function causes the opposite effects with excessive sprouting angiogenesis (22).
Silencing of CCM1 expression significantly diminished DLL4, HEY1, and HEY2 mRNA levels (Fig. 4A). The down-regulation of the NOTCH ligand DLL4 was between 50 and 80%, a sufficient reduction known to cause excessive angiogenesis (23–25). NOTCH activity is tightly linked to CCM1 because the down-regulation of CCM1 mRNA after 8 h was followed by rapid DLL4, HEY1, and HEY2 mRNA reduction. Conversely, CCM1 mRNA expression, observable after 18 h of transduction, was accompanied by rapid DLL4, HEY1, and HEY2 induction (Fig. S5).
DLL4-NOTCH Signaling Regulates AKT and ERK Phosphorylation.
CCM1 increased AKT phosphorylation (Figs. 2 and 4C). AKT is phosphorylated after interaction with phosphatidylinositol trisphosphates (PIP3) at the cell membrane. PIP3 is generated from PIP2 by phosphatidylinositol-3-kinase (PI3K), and CCM proteins can interact with PIP2 or PIP3 molecules (9, 26). The PI3K inhibitors Wortmannin and LY294002 almost completely abolished CCM1-induced AKT phosphorylation (Fig. 4), implicating that CCM1 functions are transmitted in a PIP3-dependent manner.
Active NOTCH1 could strongly induce phosphorylation of AKT (Fig. 4D). However, we ruled out the possibility that CCM1-induced AKT phosphorylation was only a secondary effect because of induction of NOTCH signaling. The blockage of NOTCH with the γ-secretase inhibitor DAPT did not prevent phosphorylation of AKT1 after CCM1 expression (Fig. 4C).
Furthermore, CCM1 inhibited ERK phosphorylation (Figs. 2 and 4E). However, this inhibition was abolished when HUVEC were treated with DAPT, suggesting that this CCM1 effect is fully mediated through NOTCH signaling. Our assumption was supported by the finding that constitutive active NOTCH1 strongly inhibited ERK1/2 phosphorylation (Fig. 4F). Taken together, these data imply that CCM1 promotes AKT phosphorylation in a NOTCH-dependent and independent manner, and it inhibits EKR1/2 phosphorylation indirectly through activation of the DELTA-NOTCH cascade (Fig. 4G).
Rescue of the CCM1 Phenotype.
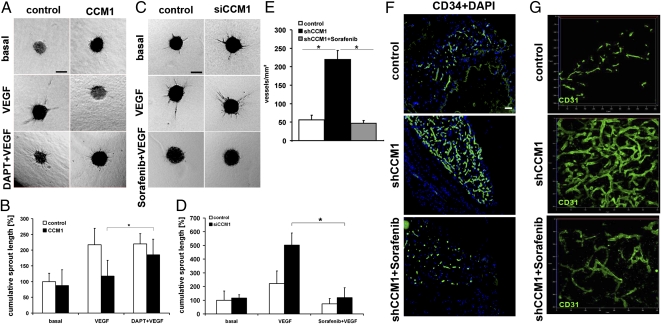
To rescue the endothelial defects caused by CCM1 gain-of-function, cells overexpressing CCM1 were treated with the γ-secretase inhibitor DAPT to prevent NOTCH activation. This treatment was sufficient to restore the responsiveness to VEGF and caused an almost normal sprouting behavior of HUVEC (Fig. 5 A and B).
Fig. 5.
Rescue of the CCM1 phenotype. (A and B) CCM1 expression inhibited sprouting angiogenesis of HUVEC after stimulation with VEGF (25 ng/mL). The NOTCH cleavage inhibitor DAPT (25 μM) could almost completely counteract this defect. (Scale bar in A, 200 μm.) (C and D) Sorafenib (10 μM) could block VEGF-induced sprouting in control and CCM1 siRNA silenced HUVEC. (Scale bar in C, 200 μm.) (E–G) The spheroid-based angiogenesis assay was employed to test Sorafenib in vivo. Twenty-eight days after implantation, mice were treated with Sorafenib or solvent for 7 d. (E) Quantification of microvascular density. (F) Representative sections show a hyperdense vascular network of CCM1-silenced endothelial cells which can be reverted by Sorafenib. (Scale bar, 50 μm.) (G) Three-dimensional reconstruction of the vascular networks by confocal microscopy (LSM710, Zeiss). Sorafenib normalizes the CCM vasculature. Error bars are means ± SD of n = 3 plugs; *P < 0.05.
Finally, we aimed at developing a unique therapeutic strategy to improve the treatment of CCM. Our data showed that loss of CCM1 leads to enhanced ERK signaling and angiogenesis. The multiple kinase inhibitor Sorafenib (Nexavar) is a clinically approved anti-angiogenic drug which inhibits the Raf/ERK pathway, as well as VEGF receptor signaling, besides some other tyrosine kinases (27). Although doses smaller than 5 μM were not effective, the addition of 10 μM Sorafenib could almost completely block VEGF-induced sprouting after CCM1-silencing in HUVEC (Fig. 5 C and D). Thus, antiangiogenic therapy might be beneficial to prevent disease progression. To test this theory, we grafted CCM1-depleted HUVEC s.c. into the flanks of SCID mice. Two groups were formed after 28 d. One group received 40 mg/kg per day Sorafenib per oral gavage, whereas the control group was mock-treated with the solvent. The plugs were analyzed after treatment for 7 d. The mock-treated mice showed again the formation of a hyper-dense and irregular vasculature with large vessel diameters. Treatment with Sorafenib was able to strongly ameliorate the excessive vasculature. Sorafenib decreased the microvessel density to rates comparable with untreated endothelial cells (Fig. 5 E–G). Taking these data together, we conclude that the elucidation of CCM1-regulated signaling pathways should open previously unexplored therapeutic avenues to prevent progression of cavernous malformations with antiangiogenic substances.
Discussion
CCMs are common vascular lesions that can dynamically change in number and size over time. Although mutations in CCM1 (KRIT1), CCM2 (OSM), and CCM3 (PDCD10) have been detected in the majority of cases, little is known about their cellular functions and thus about the pathogenesis of this disease (2). Here, we show that CCM1 is a pivotal inhibitor of angiogenesis. Thus, we suggest that uncontrolled sprouting significantly contributes to the pathogenesis of CCM and could be therapeutically targeted in the future.
We hypothesized, based on the chaotic vascular architecture and the dynamic progression of CCM lesions in response to local VEGF (13), that CCM lesions are originally caused by enhanced angiogenesis. Our data strongly supported this hypothesis and showed that CCM1 is needed to keep the endothelium quiescent. CCM1 mutations are typically loss-of-function mutations and recently a two-hit mechanism with biallelic loss of a CCM gene was proposed to explain the local development of CCM lesions (3, 4). This study supports such a scenario. The reduction or loss of CCM1 in an endothelial cell would make it very susceptible to growth factors to initiate angiogenesis. The decreased formation of cell junctions and enhanced permeability (5) would support angiogenic sprouting. Because we found that CCM1 loss-of-function also caused a loss of appropriate DELTA-NOTCH signaling, one could assume that the sprouting process must be highly disorganized with many additional capillary branches (22). Three studies have recently reported that CCM2 is essential for cardiovascular development and maintaining vascular integrity (28–30). Thus, it appears that the CCM proteins, which are not related but can form a complex (9), together keep adult endothelial cells quiescent.
Beside the existing CCM mouse model Ccm1+/−; Trp53+/− (31), there is a need to develop a suitable in vivo CCM model that allows rapid screening of a larger set of therapeutic substances. We modified a spheroid-based xenotransplantation assay (18, 19) to mimic the human CCM pathology in mice. CCM1-silenced HUVEC formed a much denser vascular network with more protrusions and drastically enlarged microvessels. Thus, this model can be used to generate a human vasculature in mice showing hallmarks of the CCM disease within only 4 w. The model is also well suited for drug testing, as demonstrated with Sorafenib treatment. The model also has the great advantage that the newly developed vessels are solely formed by human endothelial cells, so that human-specific drugs can be screened. The use of nonbrain-derived endothelial cells transplanted s.c. did not prevent the formation of CCM hallmarks, suggesting that CCM1 seems to play a pan-endothelial role. This finding is also reflected by the widespread cardiovascular defects in Ccm1−/− mice, and by the manifestation of CCM lesions outside of the central nervous system (2).
We could demonstrate that CCM1 acts genetically upstream of NOTCH signaling. CCM1 robustly induced expression of the NOTCH ligand DLL4 and target genes HEY1 and HEY2. The phenotype of our CCM1 manipulation experiments is almost identical to the effects of endothelial NOTCH signaling in HUVEC and in mice (22), and we could successfully rescue the defects caused by CCM1 expression with a NOTCH cleavage inhibitor. We showed that CCM1 reduced phospho-ERK1/2 levels but increased AKT phosphorylation in endothelial cells. The latter could not be prevented by the NOTCH inhibitor DAPT, suggesting that CCM1 can induce AKT phosphorylation in the absence of NOTCH. Because CCM proteins interact with the lipids PIP2 and PIP3 (9, 26), we suggest that the CCM complex supports the phosphorylation of AKT, which is recruited by PIP3. Blocking PI3K-AKT diminished NOTCH signaling in HUVEC and this is in line with a recent report showing that AKT1 can activate NOTCH1 in melanoma cells (32). Thus, we propose that CCM1 activates NOTCH by stimulation of AKT phosphorylation and cleaved NOTCH1 further stimulates AKT phosphorylation in a positive feedback loop. Additionally, we found that active NOTCH1 could strongly diminish ERK phoshorylation. Thus, CCM1 leads to potent reduction of ERK-mediated cell proliferation but to an increase of AKT-driven endothelial survival via NOTCH signaling.
We envisage that antiangiogenic therapy could be beneficial to prevent progression of CCM lesions. Sorafenib, an approved antiangiogenic drug, targets VEGF receptors and the Raf/ERK pathway (27). Our data suggest that ERK signaling is enhanced in the angiogenic endothelium of CCM lesions, making Sorafenib an ideal candidate for initial drug testing. Indeed, Sorafenib efficiently blocked angiogenic sprouting of HUVEC silenced for CCM1 expression. In vivo, Sorafenib led to a drastic regression of CCM1-deficient blood vessels. Thus, antiangiogenic therapy might be beneficial for the treatment of cerebral cavernous malformations in the future.
Materials and Methods
Plasmids, RNAi.
CCM1 cDNA was cloned into pENTR3c-IRES2-EGFP. Control siRNA-1 and siRNAs against CCM1 (15469, 214883) (5) were from Ambion. CCM1 shRNAs (RHS4430-98913140, -98820292, RMM4431-98978703) were from Open Biosystems. RHS4430-98820292 achieved excellent knockdown.
Endothelial Migration, Proliferation, Apoptosis, Tube Formation, and Sprouting.
Endothelial behavior was analyzed as described (33). Apoptosis was determined using the Caspase-Glo 3/7 Assay (Promega).
Spheroid-Based Transplant Assay.
HUVEC were transduced with lentivirus and selected with 0.37 μg/mL Puromycin. Spheroids were s.c. injected into 6- to 8-week-old female CB17 SCID mice (Charles River) (18, 19).
Microarray Analyses.
Total RNA was harvested, transcriped to biotin-labeled cRNA, and hybridized on Illumina Human Sentrix-8 BeadChips. Data analysis was performed with Illumina BeadStudio V3.
Western Blotting and Human Lesion Samples.
Proteins were separated with PAGE and blotted to nitrocellulose. Antibodies: cleaved NOTCH (Val-1744, Cell Signaling), ERK1 (K-23, Santa Cruz), phospho-ERK (E-4, Santa Cruz), phospho-AKT (Ser473) (D9E, Cell Signaling), AKT (9272, Cell Signaling) and GAPDH (6C5, Abcam). Human CCM specimens were described (15).
Statistical Analysis.
Results are expressed as mean ± SD. Comparisons between groups were analyzed by t test (two-sided). P values <0.05 were considered as statistically significant.
For further descriptions, please see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Anja Telzerow for excellent technical assistance and the Genomics and Proteomics Core Facility at the DKFZ Heidelberg for performing the microarray studies. This work was supported by Grants DFG FI-1568/1-1 from the Deutsche Forschungsgemeinschaft (to A.F.), SFB/TR23 (to A.F. and H.G.A.), and GRK880 (to A.F. and H.G.A.), and BayGene (U.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE18014).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1000132107/-/DCSupplemental.
References
- 1.Moriarity JL, et al. The natural history of cavernous malformations: A prospective study of 68 patients. Neurosurgery. 1999;44:1166–1171. [PubMed] [Google Scholar]
- 2.Labauge P, Denier C, Bergametti F, Tournier-Lasserve E. Genetics of cavernous angiomas. Lancet Neurol. 2007;6:237–244. doi: 10.1016/S1474-4422(07)70053-4. [DOI] [PubMed] [Google Scholar]
- 3.Akers AL, Johnson E, Steinberg GK, Zabramski JM, Marchuk DA. Biallelic somatic and germline mutations in cerebral cavernous malformations (CCMs): Evidence for a two-hit mechanism of CCM pathogenesis. Hum Mol Genet. 2009;18:919–930. doi: 10.1093/hmg/ddn430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pagenstecher A, Stahl S, Sure U, Felbor U. A two-hit mechanism causes cerebral cavernous malformations: Complete inactivation of CCM1, CCM2 or CCM3 in affected endothelial cells. Hum Mol Genet. 2009;18:911–918. doi: 10.1093/hmg/ddn420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glading A, Han J, Stockton RA, Ginsberg MH. KRIT-1/CCM1 is a Rap1 effector that regulates endothelial cell cell junctions. J Cell Biol. 2007;179:247–254. doi: 10.1083/jcb.200705175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunel M, et al. KRIT1, a gene mutated in cerebral cavernous malformation, encodes a microtubule-associated protein. Proc Natl Acad Sci USA. 2002;99:10677–10682. doi: 10.1073/pnas.122354499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guzeloglu-Kayisli O, et al. KRIT1/cerebral cavernous malformation 1 protein localizes to vascular endothelium, astrocytes, and pyramidal cells of the adult human cerebral cortex. Neurosurgery. 2004;54:943–949. doi: 10.1227/01.neu.0000114512.59624.a5. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Rigamonti D, Dietz HC, Clatterbuck RE. Interaction between krit1 and malcavernin: Implications for the pathogenesis of cerebral cavernous malformations. Neurosurgery. 2007;60:353–359. doi: 10.1227/01.NEU.0000249268.11074.83. [DOI] [PubMed] [Google Scholar]
- 9.Hilder TL, et al. Proteomic identification of the cerebral cavernous malformation signaling complex. J Proteome Res. 2007;6:4343–4355. doi: 10.1021/pr0704276. [DOI] [PubMed] [Google Scholar]
- 10.Zawistowski JS, Serebriiskii IG, Lee MF, Golemis EA, Marchuk DA. KRIT1 association with the integrin-binding protein ICAP-1: A new direction in the elucidation of cerebral cavernous malformations (CCM1) pathogenesis. Hum Mol Genet. 2002;11:389–396. doi: 10.1093/hmg/11.4.389. [DOI] [PubMed] [Google Scholar]
- 11.Whitehead KJ, Plummer NW, Adams JA, Marchuk DA, Li DY. Ccm1 is required for arterial morphogenesis: Implications for the etiology of human cavernous malformations. Development. 2004;131:1437–1448. doi: 10.1242/dev.01036. [DOI] [PubMed] [Google Scholar]
- 12.Hogan BM, Bussmann J, Wolburg H, Schulte-Merker S. Ccm1 cell autonomously regulates endothelial cellular morphogenesis and vascular tubulogenesis in zebrafish. Hum Mol Genet. 2008;17:2424–2432. doi: 10.1093/hmg/ddn142. [DOI] [PubMed] [Google Scholar]
- 13.Jung KH, et al. Cerebral cavernous malformations with dynamic and progressive course: Correlation study with vascular endothelial growth factor. Arch Neurol. 2003;60:1613–1618. doi: 10.1001/archneur.60.11.1613. [DOI] [PubMed] [Google Scholar]
- 14.Korff T, Augustin HG. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J Cell Biol. 1998;143:1341–1352. doi: 10.1083/jcb.143.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Y, et al. Involvement of PTEN promoter methylation in cerebral cavernous malformations. Stroke. 2009;40:820–826. doi: 10.1161/STROKEAHA.108.526376. [DOI] [PubMed] [Google Scholar]
- 16.Dudek H, et al. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 17.Hemmings BA. Akt signaling: Linking membrane events to life and death decisions. Science. 1997;275:628–630. doi: 10.1126/science.275.5300.628. [DOI] [PubMed] [Google Scholar]
- 18.Alajati A, et al. Spheroid-based engineering of a human vasculature in mice. Nat Methods. 2008;5:439–445. doi: 10.1038/nmeth.1198. [DOI] [PubMed] [Google Scholar]
- 19.Laib AM, et al. Spheroid-based human endothelial cell microvessel formation in vivo. Nat Protoc. 2009;4:1202–1215. doi: 10.1038/nprot.2009.96. [DOI] [PubMed] [Google Scholar]
- 20.Fischer A, Gessler M. Delta-Notch—and then? Protein interactions and proposed modes of repression by Hes and Hey bHLH factors. Nucleic Acids Res. 2007;35:4583–4596. doi: 10.1093/nar/gkm477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor KL, Henderson AM, Hughes CC. Notch activation during endothelial cell network formation in vitro targets the basic HLH transcription factor HESR-1 and downregulates VEGFR-2/KDR expression. Microvasc Res. 2002;64:372–383. doi: 10.1006/mvre.2002.2443. [DOI] [PubMed] [Google Scholar]
- 22.Roca C, Adams RH. Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 2007;21:2511–2524. doi: 10.1101/gad.1589207. [DOI] [PubMed] [Google Scholar]
- 23.Duarte A, et al. Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev. 2004;18:2474–2478. doi: 10.1101/gad.1239004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gale NW, et al. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci USA. 2004;101:15949–15954. doi: 10.1073/pnas.0407290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krebs LT, et al. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev. 2004;18:2469–2473. doi: 10.1101/gad.1239204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Béraud-Dufour S, Gautier R, Albiges-Rizo C, Chardin P, Faurobert E. Krit 1 interactions with microtubules and membranes are regulated by Rap1 and integrin cytoplasmic domain associated protein-1. FEBS J. 2007;274:5518–5532. doi: 10.1111/j.1742-4658.2007.06068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilhelm S, et al. Discovery and development of sorafenib: A multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5:835–844. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 28.Boulday G, et al. Tissue-specific conditional CCM2 knockout mice establish the essential role of endothelial CCM2 in angiogenesis: Implications for human cerebral cavernous malformations. Dis Model Mech. 2009;2:168–177. doi: 10.1242/dmm.001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kleaveland B, et al. Regulation of cardiovascular development and integrity by the heart of glass-cerebral cavernous malformation protein pathway. Nat Med. 2009;15:169–176. doi: 10.1038/nm.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitehead KJ, et al. The cerebral cavernous malformation signaling pathway promotes vascular integrity via Rho GTPases. Nat Med. 2009;15:177–184. doi: 10.1038/nm.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plummer NW, et al. Loss of p53 sensitizes mice with a mutation in Ccm1 (KRIT1) to development of cerebral vascular malformations. Am J Pathol. 2004;165:1509–1518. doi: 10.1016/S0002-9440(10)63409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bedogni B, Warneke JA, Nickoloff BJ, Giaccia AJ, Powell MB. Notch1 is an effector of Akt and hypoxia in melanoma development. J Clin Invest. 2008;118:3660–3670. doi: 10.1172/JCI36157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nacak TG, et al. The BTB-Kelch protein KLEIP controls endothelial migration and sprouting angiogenesis. Circ Res. 2007;100:1155–1163. doi: 10.1161/01.RES.0000265844.56493.ac. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.