Abstract
A large body of research has demonstrated that variation in competitive behavior across species and individuals is linked to variation in physiology. In particular, rapid changes in testosterone and cortisol during competition differ according to an individual's or species’ psychological and behavioral responses to competition. This suggests that among pairs of species in which there are behavioral differences in competition, there should also be differences in the endocrine shifts surrounding competition. We tested this hypothesis by presenting humans’ closest living relatives, chimpanzees (Pan troglodytes) and bonobos (Pan paniscus), with a dyadic food competition and measuring their salivary testosterone and cortisol levels. Given that chimpanzees and bonobos differ markedly in their food-sharing behavior, we predicted that they would differ in their rapid endocrine shifts. We found that in both species, males showed an anticipatory decrease (relative to baseline) in steroids when placed with a partner in a situation in which the two individuals shared food, and an anticipatory increase when placed with a partner in a situation in which the dominant individual obtained more food. The species differed, however, in terms of which hormone was affected; in bonobo males the shifts occurred in cortisol, whereas in chimpanzee males the shifts occurred in testosterone. Thus, in anticipation of an identical competition, bonobo and chimpanzee males showed differential endocrine shifts, perhaps due to differences in perception of the situation, that is, viewing the event either as a stressor or a dominance contest. In turn, common selection pressures in human evolution may have acted on the psychology and the endocrinology of our competitive behavior.
Across species, including humans, males engaged in competition tend to show acute shifts in their levels of steroid hormones, such as testosterone and cortisol. These hormones change in a matter of minutes surrounding a competitive event, in anticipation of the competition and in response to its outcome (1, 2). In humans, men normally demonstrate an increase in cortisol before competition (3, 4). After the competition male winners tend to maintain their testosterone levels, whereas male losers’ testosterone decreases (5, 6). In other animals, competing males show similar rapid changes in glucocorticoids and testosterone, since these hormones are thought to mediate energy allocation toward mating effort across species (7–10). Because competition for overt markers of status and mating opportunities is more relevant to males, these effects are less consistent in females (11–14). Beyond these typical patterns, there is also high variability within and between species in the nature of the hormonal shifts surrounding competition that may be shaped by the psychology underlying competitive behavior.
Two main psychological factors have been implicated in governing the endocrine changes surrounding competition within and between species: implicit power motive and coping style. Implicit power motive, in the human literature, denotes an individual's drive to achieve high status (see ref. 15 for a review). Men with a high power motive are more likely to show increases in testosterone before competition and, depending on the outcome, stronger shifts in testosterone and glucocorticoids after competition (16, 17). Implicit power motives may drive between-species differences as well. In a comparison of a territorial and nonterritorial mouse species, only the territorial species showed an increase in testosterone after a competitive event, whereas the nonterritorial species showed no significant changes in testosterone levels (18). Coping style, on the other hand, quantifies how an individual responds physiologically across numerous stressful events, such as competition (19). Individuals with a “passive” coping style are more likely to show greater glucocorticoid increases before the competition than those with an “active” coping style, who show a less marked increase in glucocorticoids (20). Lines of mice bred for low aggression tend to exhibit passive coping styles, and the associated large glucocorticoid shift, more than lines of mice bred for high aggression (21). These results suggest that appraisal of competition and the corresponding endocrine shifts surrounding competition vary between even closely related species according to the significance of competition in that species’ behavioral ecology.
In turn, humans’ responses to competition may also have been shaped by ecological pressures. Studying humans’ closest living relatives, chimpanzees (Pan troglodytes) and bonobos (Pan paniscus), can reveal the degree to which humans’ rapid hormonal shifts surrounding competition are unique. In addition, chimpanzees and bonobos differ notably in their social behavior in the context of both competition and cooperation, providing a direct test of ecology's influence on competitive behavior and endocrinology. Male chimpanzees exhibit more severe aggression and more concern for dominance status than male bonobos (22–25). Male dominance hierarchies are more rigid and more strongly associated with basal testosterone levels among chimpanzees than among bonobos (26–30). Thus, in the terms used in human competition research, chimpanzee males may show a stronger “power motive” than bonobo males. In contrast, bonobos are better able to cooperate than chimpanzees, sharing food more readily in the wild and in captive experiments (31, 32). Previous studies have shown that bonobos exhibit a rise in cortisol before a competition over limited amounts of food, with greater increases in cortisol when that food was visibly difficult to share (implying greater social stress) (33). Because bonobo conflicts rarely escalate to severe aggression, we might classify bonobos as possessing a passive coping style—similar to the low-aggression mice.
Accordingly, we tested the hypothesis that psychological differences in the appraisal of competition in chimpanzees and bonobos are associated with species differences in rapid endocrine shifts surrounding competition. We presented chimpanzees and bonobos with an identical experimental dyadic food competition and measured testosterone and cortisol levels before and after the competitive event. We made two separate predictions about how the species difference in endocrinology might be manifested. These predictions apply principally to males, although we tested individuals of both sexes (8, 11).
Prediction 1.
Bonobo males will show an anticipatory increase in cortisol, which will be more pronounced in situations of higher stress (manifested here as social uncertainty), indicative of a passive coping style. Cortisol shifts will be less pronounced in chimpanzee males. Chimpanzees do exhibit rapid cortisol changes surrounding anesthesia (34), but we predict that in the competitive situation their cortisol will not shift as markedly as that of bonobo males.
Prediction 2.
Chimpanzee males will show an anticipatory increase in testosterone and greater sensitivity to the outcome of the competition in both testosterone and cortisol relative to bonobo males, in line with their having a greater power motive.
Alternative Hypotheses.
Chimpanzees and bonobos will show similar responses to competition, or neither species will show significant endocrine shifts surrounding competition over food.
Results
Before all food competitions (detailed below), subject pairs participated in a dominance test (SI Methods). The individual who obtained more food in this test was assigned the status of the “dominant” in the pair. Dominance in this test strongly predicted dominance in the food competitions (SI Results).
Each individual was tested as a member of only one pair. For each trial of the food competition, a controlled amount of food was placed in a specific configuration in a testing room. The subject pair viewed the placement of the food in an adjacent room and was then released into the room and allowed to eat the food. After the pair finished eating, the experimenter immediately placed new food for the subsequent trial. Three food competition trials were presented in sequence on a given day. If the dominant monopolized food on two or three of the trials on that day, this was scored as a “1” for the behavioral variable outcome, in denoting that food was obtained asymmetrically. If the dominant obtained more food on one or none of the trials (e.g., individuals shared the food relatively equally, or the dominant obtained less), a “0” was scored for outcome. Pairs participated in 3 d of testing, thus each individual was represented three times in the data set, once for each day of food competition (food configuration varied across days, as described in SI Methods).
Chimpanzees and bonobos did not differ in their relative frequencies of the outcome variable: the dominant monopolized significantly more food ≈50% of the time in both species (a χ2 test showed that the proportions of outcome were not different across the two species). Importantly, the scores for outcome were the same for both individuals in the pair (the dominant and the subordinate); thus, this variable represented asymmetry vs. sharing in the distribution of feeding rather than a win or loss. Even though the species were comparable in this measure of feeding symmetry, they did differ in more targeted coding of sharing behavior in this task (35).
In addition to the paired food competitions, each subject was presented with a solo condition that replicated the procedure of the paired conditions exactly, except that individuals were tested alone rather than in a pair. This condition served to measure individuals’ baseline hormone levels in the general test situation, without social interaction.
On each day of food competition, saliva samples were taken from both subjects immediately before the first trial, before the food was presented but after individuals were placed in their pairing. Samples were then collected again from both subjects 15 min after the third trial was finished (6, 36). Saliva samples were analyzed for testosterone and cortisol using previously validated radioimmunoassay (RIA) procedures (see Methods and ref. 37). The values of testosterone and cortisol were log-transformed to normalize the data and allow the use of parametric statistics.
Statistical Analyses.
To analyze differences between groups, we performed generalized linear model (GLM) analyses. In all of these analyses, we controlled for the within-subject factor individual, because each individual was represented in the data set three times. For all models, we examined the main effects, two-way interactions, and three-way interactions (where applicable). We controlled for multiple comparisons by using Fisher's least significant difference (LSD) procedure in post hoc tests.
Cortisol.
Pretest cortisol.
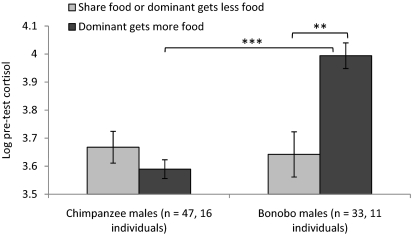
We first analyzed anticipatory effects and then looked at effects in response to the outcome of the test. For cortisol, all GLM analyses had individual as a within-subject factor and species, sex, and outcome as between-subject factors. A GLM analysis of log pretest cortisol revealed that bonobos had significantly higher log pretest cortisol than chimpanzees [Wald χ2(1) = 11.62, P = 0.001] and showed an interaction between sex and outcome [Wald χ2(1) = 6.04, P = 0.014], as well as a three-way interaction among species, sex, and outcome [Wald χ2(1) = 8.91, P = 0.003]. Post hoc tests revealed that among bonobo males, log pretest cortisol was significantly higher when the dominant was going to obtain more food than when the two individuals in a pair were going to share/the dominant was going to obtain less (Fisher's LSD, P = 0.001). In contrast, for chimpanzee males there was no difference in cortisol levels across outcomes, nor were there any significant effects among females of either species. Bonobo males also had significantly higher levels of log pretest cortisol than chimpanzee males when the dominant was going to monopolize more food (Fisher's LSD, P < 0.001) (Fig. 1). Control analyses revealed that these patterns were present equally in dominants and subordinates, independent of order (e.g., first vs. last day of food competitions), and equivalent whether a male was paired with another male or paired with a female (SI Results).
Fig. 1.
Pretest log cortisol values according to species and outcome of the food competitions, males only. Bars denote SEM. **P < 0.01; ***P < 0.001.
To ensure that these results did not reflect anticipation of food being presented or baseline cortisol differences between individuals, we examined anticipatory cortisol in comparison to solo condition (baseline) values of cortisol. Subjects’ log pretest cortisol values were highly correlated with their log pre-solo cortisol values (linear regression, r2 = 0.25, df = 162, P < 0.001). We stored the unstandardized residuals of this regression as an index of how much an individual's pretest cortisol value on a given test day departed from what would be predicted on the basis of their pre–solo day cortisol level. If the residual was positive, this represented a value higher than baseline, whereas if it was negative, this value was lower than baseline.
A GLM analysis on these residuals demonstrated that the main effects of each factor were not significant, but there was the expected significant interaction among species, sex, and outcome [Wald χ2(1) = 6.77, P = 0.009]. Post hoc tests showed that bonobo males increased in cortisol relative to their baseline when the dominant was going to obtain more food, and decreased in cortisol relative to baseline when the two individuals in a pair were going to share, with this creating a significant difference between these two outcomes (Fisher's LSD, P = 0.001). Again, there were no significant differences in chimpanzee males according to outcome and no differences among females. Bonobo males had significantly higher relative levels of cortisol than chimpanzee males when the dominant was going to monopolize significantly more food (Fisher's LSD, P = 0.01) (SI Results). These residual analyses indicate that bonobo males’ cortisol levels were sensitive to their pairing, whereas those of chimpanzee males were not.
Posttest cortisol.
We conducted a similar GLM analysis with the log posttest cortisol values. Bonobos exhibited significantly higher posttest cortisol than chimpanzees [Wald χ2(1) = 25.55, P < 0.001], and we found a significant interaction among species, sex, and outcome [Wald χ2(1) = 7.19, P = 0.007]. Because these results paralleled those obtained using the pretest cortisol values, we examined the relative contribution of the test events independent of the pretest effects. We first ran a regression of log posttest cortisol values and log pretest cortisol values (linear regression, r2 = 0.43, df = 214, P < 0.001). We then used the unstandardized residuals of this regression to assess how much an individual's posttest cortisol level departed from his or her pretest cortisol levels.
A GLM analysis of these posttest residuals revealed only a main effect of species [Wald χ2(1) = 12.54, P < 0.001]: bonobos’ cortisol tended to increase over the course of the test regardless of outcome, whereas chimpanzees’ cortisol levels did not change significantly.
Our results support a previous finding that anticipation of food competition elevates bonobo cortisol levels and that bonobos’ cortisol increases differentially according to the predicted outcome of the competition (33). The observed decrease in bonobo males’ cortisol before sharing suggests that lower levels of arousal in bonobos may in part explain their tendency to voluntarily share food with other individuals (38). The relative stability of cortisol in chimpanzee males could in theory have occurred either because they do not perform anticipatory appraisals before competition, or because such appraisals are not tied to a significant physiological response. We were able to test these alternatives, in addition to testing our main hypotheses regarding species differences in these acute endocrine shifts, with our analysis of testosterone.
Testosterone.
Pretest testosterone.
As with cortisol, we began by analyzing anticipatory effects, then moved to posttest effects. For testosterone we performed separate analyses by sex, given the known differences in testosterone levels between males and females in humans and other apes, and the prediction from the human literature and our cortisol results that the effects on this hormone would be more pronounced in males (11, 39). Thus for testosterone, all GLM analyses had individual as a within-subject factor and species and outcome as between-subject factors.
A GLM analysis of log pretest testosterone showed that bonobo females’ log pretest testosterone was significantly higher than that of chimpanzee females [Wald χ2(1) = 5.43, P = 0.02] [these baseline differences may reflect differing patterns of testosterone secretion across the menstrual cycle (40)]. In males, this analysis demonstrated a significant interaction between species and outcome [Wald χ2(1) = 5.86, P = 0.02]. Post hoc tests in males revealed that among chimpanzees, males in pairs in which the dominant was going to obtain more food had higher log pretest testosterone than males in pairs in which the two individuals were going to share (Fisher's LSD, P = 0.03). There were no distinctions in log pretest testosterone across outcomes in bonobo males. As a result, when individuals shared, male chimpanzees had significantly lower log pretest testosterone levels than bonobo males (Fisher's LSD, P = 0.02), with no species difference when the dominant monopolized the food (Fig. 2). Similar to the cortisol results, there were no effects of dominance status, test day, or pair type on these effects (SI Results).
Fig. 2.
Pretest log testosterone values according to species and outcome, males only. Bars denote SEM. *P < 0.05.
Again, we wanted to ensure that these pretest testosterone values were not simply reflections of individuals’ basal testosterone levels. We performed a regression analysis of the log pretest day testosterone values and the log pre–solo day testosterone values (linear regression, r2 = 0.13, P < 0.001, df = 132). We used the unstandardized residuals of this regression as an index of how much an individual's pretest testosterone value on a given test day departed from baseline levels.
We performed a GLM analysis on these residuals and found that in females, there was a main effect of species [Wald χ2(1) = 6.70, P = 0.01]: bonobo females exhibited higher relative testosterone on test days, whereas chimpanzee females did not. Bonobo males’ relative testosterone levels also tended to be significantly higher on test days than chimpanzee males’ relative testosterone levels [Wald χ2(1) = 4.22, P = 0.04], and we found a significant interaction between species and outcome in males as well [Wald χ2(1) = 5.24, P = 0.02] (SI Results). Post hoc tests revealed that male chimpanzees increased in testosterone relative to baseline when the dominant was going to obtain more food, and decreased relative to baseline when individuals were going to share, creating a significant difference between these two outcomes (Fisher's LSD, P = 0.02). In contrast, for bonobo males there was no difference in relative testosterone levels between the two outcomes. The decrease in chimpanzee males’ testosterone when they were going to share led to their relative testosterone levels being significantly lower than bonobo males’ relative testosterone levels in these situations (Fisher's LSD, P = 0.003).
Posttest testosterone.
A GLM analysis of log posttest testosterone values revealed a significant effect of species in females [Wald χ2(1) = 15.09, P < 0.001] and a significant interaction between species and outcome in males [Wald χ2(1) = 4.50, P = 0.03]. Because these results paralleled those found using the pretest testosterone values, we again examined subjects’ changes in response to the events of the competition as a function of their pretest testosterone levels. Log posttest testosterone and log pretest testosterone were highly correlated (linear regression, r2 = 0.44, P < 0.001, df = 178). We used the unstandardized residuals of this regression to denote how much posttest testosterone values departed from pretest testosterone values.
A GLM analysis of these posttest residuals revealed only a main effect of species in females [Wald χ2(1) = 6.54, P = 0.01]: bonobo females’ testosterone values tended to increase over the course of the test, whereas those of chimpanzee females remained relatively constant. Competition did not significantly impact posttest testosterone levels in males of either species.
In contrast to the cortisol results, whereby bonobo males showed stronger anticipatory shifts according to outcome than did chimpanzees, the patterns of anticipatory change in testosterone were stronger in chimpanzee males. This rules out the possibility that the greater cortisol response observed in bonobos might be due to their greater skill in predicting the outcome of a food competition according to pairing compared with chimpanzees.
Discussion
These results support the hypothesis that bonobos and chimpanzees differ significantly in endocrine shifts surrounding competition, and support both of our predictions regarding the nature of that species difference. Bonobo males’ cortisol increased relative to baseline before a competition in which the dominant would obtain more food, and decreased before a competition in which sharing would occur. Therefore, bonobos seemed to respond to the competition as a social stressor when food would not be shared, exhibiting a passive coping style and an associated large anticipatory shift in glucocorticoids. In the same context, chimpanzee males did not show any shifts in cortisol. Instead, their testosterone changed, showing either an anticipatory increase when the dominant was going to obtain more food or decrease when placed with a partner with whom sharing would occur. Bonobo males did not exhibit significant shifts in testosterone according to outcome. Thus chimpanzees seemed to view the competition as status-determining, similar to human men with a stronger power motive, with this driving shifts in testosterone.
These data demonstrate that between these two closely related species there are important differences in the physiological response to competition that are correlated with differences in social behavior and ecology. Our findings provide evidence for rapid endocrine changes in association with competition in chimpanzees, and corroborate previous evidence for precompetition cortisol increases in bonobos (33). These results suggest that after the divergence of chimpanzees and bonobos, selection against escalated aggression in bonobo males may have caused them to acquire a passive coping style (analogous to that observed in lines of mice bred for low aggression) (21, 41). Chimpanzees, in contrast, may have retained an ancestral state with stricter hierarchies, whereby individuals possess a high drive to achieve dominance rank, or power motive, and show corresponding large shifts in testosterone (28, 41). Future research comparing chimpanzees and bonobos can further reveal the role of hormones in the morphological, behavioral, and cognitive differences between the two species (28, 36, 37).
Interestingly, the observed endocrine shifts occurred before the competition, rather than after the test. Although it is possible that the posttest sampling interval of 15 min was too short to observe posttest effects, responses to competition in human men have been observed in that length of time (6, 36). Further, in a previous study, even 1 h after a competition over food bonobos did not exhibit any increases in cortisol beyond their anticipatory increases (33). It could be that chimpanzees and bonobos react much more slowly than humans, signifying a difference between apes and humans in the speed of endocrine response to wins or losses. Alternatively, we propose that the apes in our experiments anticipated the outcome of competition particularly easily given their mutual familiarity and ability to track each other's tolerance levels (32, 42). Individuals did not frequently vocalize or engage in aggressive behavior during the competition. This suggests that the actual process of feeding may cause relatively less arousal in apes than the anticipation of feeding competition. In turn, this indicates that the patterns of anticipatory appraisal seen in humans are not unique to our species, but that our species’ endocrine shifts in response to the outcomes of even relatively trivial competitions (such as a chess match) are derived (43).
Similar to what is seen in humans, we found the strongest effects of the competition on steroid hormones in males, whereas females did not exhibit any significant patterns. Steroid shifts surrounding competition in women are inconsistent across studies (11, 44, 45). This indicates that the pattern of minimal response by women to psychological status competitions or stressors may be an ancient hominoid trait.
Overall, the present results suggest that our closest living relatives have the capacity to anticipate and appraise the results of dyadic food competitions and that their physiology changes accordingly. Further, they support the hypothesis that species differences in the ecology of competitive behavior shape the endocrinology of competition, extending this model into nonhuman primates. These findings suggest that independent mechanisms govern the sensitivity of testosterone and cortisol to competition, and that distinct factors may affect anticipatory vs. response shifts in apes and humans. Future species comparisons can continue to illuminate how ecology has shaped species differences in behavioral endocrinology, including the selection pressures acting in human evolution.
Methods
Subjects.
The subjects for this experiment were 24 bonobos (median age 8 y, range 4–23 y) living at Lola ya Bonobo Sanctuary in the Democratic Republic of Congo and 33 chimpanzees (median age 7 y, range 5–19 y) living at Tchimpounga Chimpanzee Sanctuary in the Congo Republic (there was no species difference in subject age, Mann-Whitney U). Within bonobos, 11 males and 12 females were sampled for steroid analysis, but enough saliva volume for testosterone analysis was only obtainable for 7 of these females. One bonobo male participated in the behavioral testing but did not provide a sufficient volume of saliva to perform either testosterone or cortisol analysis. Sixteen male and 17 female chimpanzees were sampled for both cortisol and testosterone. More information about the subjects’ living circumstances and rearing histories can be found in SI Methods.
Twelve bonobo pairs and 24 chimpanzee pairs were tested. Equal numbers of adult and juvenile pairs were tested in each species. The age of the two individuals in a pair was matched as closely as possible. Equal numbers of male–male, male–female, and female–female dyads were tested in each species. Certain chimpanzees participated in repeated pairs, but for the analyses reported here, only the first pair that these subjects participated in was used. The second individual in that subject's repeated pair was still included as a subject, resulting in 24 bonobos and 33 chimpanzees in the sample.
Coding of Behavioral Variables.
All testing was videotaped. Videos of behavior in the test were coded by the first author. For reliability, a randomly chosen 20% of the trials were also coded by a second coder, who was blind to the hypotheses of the study. The reliability for the outcome measure was excellent (Cohen's κ = 0.88, P < 0.001). Outcome was usually consistent within a given pair, in that a dominant would obtain more food (or not) across each of the three food competition conditions, but could vary across condition within each pair.
In each pair, one subject was given the solo condition on a day before the three food competition days, and the other member of that pair was given the solo condition on a day after the three food competition days, thus counterbalancing any effect of test experience on the hormone values in the solo condition.
Hormonal Sampling.
During the 15-min postcompetition interval, subjects remained in the testing room with their partners. Subjects were observed so that they could not ingest any food or fecal matter during this time, making it unlikely that food debris from the test or other contaminants were present in the individuals’ mouths at the time of sample collection. In the solo condition, subjects were alone when their pretest sample was taken, and they waited alone in the testing room for the 15-min posttest interval.
To control for the effect of time of day on hormone levels, a given pair was always run within the same 2-h time window across all three test sessions and in the solo condition. The number of pairs in each age and sex category tested in the morning and the afternoon was counterbalanced as best as possible. It was not feasible to do this for all pairs owing to constraints of the testing facilities. All tests were carried out between 8:00 AM and 4:00 PM. Subjects were awake for several hours before the start of the tests, reducing the probability that the high levels of steroids observed in apes upon waking influenced results (46). These tests were not physiologically demanding for subjects, making it unlikely that exertion affected the endocrine changes seen. Further, any changes that occurred as a result of being fed would also have been present in both the solo condition and paired conditions, thus these potential effects were controlled for in the residual analyses.
Saliva samples were collected while subjects were in the test rooms, highly familiar rooms that individuals slept in each night. To collect a sample, the experimenter or caretaker first washed and disinfected his/her hands, then poured ground Sweet Tarts candy onto a cotton round. The experimenter/caretaker then stood next to the mesh of the dormitory, and if the subject approached her, she placed the cotton round inside the subject's lip so that it could suck on the cotton and ingest the Sweet Tarts while the cotton absorbed its saliva. The experimenter held on to the cotton throughout the collection procedure rather than allowing the subject to take the cotton itself, to prevent potential contamination from fecal matter on subjects’ hands. Once the cotton round had taken in enough saliva, it was placed into a syringe and squeezed to express the saliva into a test tube. Although using cotton as a collection implement may affect measurements of steroids, cotton has been shown to introduce fairly uniform rates of error across samples (47, 48). This means that although the absolute results presented here might not be comparable to those obtained without stimulation, the comparisons within this subject pool are effective because the method was consistent across subjects. The collection period for any particular sample did not span longer than 20 min.
Sweet Tarts were used to stimulate saliva because they have been shown not to alter measurements of cortisol in humans (47, 49). We performed control analyses on a small sample of human men and women to assess whether ingesting Sweet Tarts affected measurements of testosterone. Among five individuals, there was no significant change in testosterone levels in a saliva sample taken before Sweet Tarts ingestion and one taken immediately after ingestion of several Sweet Tarts (Wilcoxon signed-ranks test, P = 0.50). This suggests that Sweet Tarts have little impact on the measurement of testosterone using this RIA procedure.
Fifty microliters of 0.1% sodium azide solution was added to the ape saliva samples immediately after collection to prevent contamination and to allow samples to be kept at room temperature until they were returned to the laboratory (37). The saliva samples were analyzed in the Reproductive Ecology Laboratory at Harvard University. Salivary testosterone measurements were made using an 125I-based RIA kit (#4100, Diagnostic Systems Laboratories) with the following modifications: standards were prepared in assay buffer and run at six concentrations from 2 to 375 pg/mL. Samples were added in 100-μL amounts together with 300 μL of assay buffer. First antibody (20 μL) and labeled steroid (50 μL) were added to each tube to yield a total reaction volume of 470 μL per tube. After overnight incubation at 4 °C, 500 μL of second antibody was added to each reaction tube. Reaction tubes were subsequently centrifuged for 45 min; after aspiration of the supernatant, tubes were counted in a gamma counter for 2 min. In pilot assays, the ape testosterone values using the standard aliquot for human assays (200 μL) were too high to be readable in the assay range. Thus, we used only 100 μL of the chimpanzee and bonobo saliva for the testosterone assays, with the same standard curve as used in the human testosterone RIA protocol.
Salivary cortisol measurements were made using an 125I-based RIA kit (#2000, Diagnostic Systems Laboratories) with the following modifications: standards were prepared in assay buffer and run at six concentrations from 35 to 2,000 pg/mL. Samples were added in 25-μL amounts together with 200 μL of assay buffer. Antibody complex and labeled steroid were diluted 1:2 and added to each tube in 150-μL amounts to yield a total reaction volume of 525 μL per tube. After overnight incubation at 4 °C, 500 μL of second antibody was added to each reaction tube. Reaction tubes were subsequently centrifuged for 45 min; after aspiration of the supernatant, tubes were counted in a gamma counter for 2 min.
The average intraassay coefficient of variation (CV) was 8% for testosterone and 8% for cortisol, and average interassay CV was 16% for testosterone and 25% for cortisol. Although this interassay CV for cortisol is on the higher end of the acceptable range, all of the samples for a given individual were run in the same assay, meaning that any within-individual variation would not have been affected by interassay variation. We counterbalanced the individuals whose samples were run in each assay according to species, sex, and age.
Supplementary Material
Acknowledgments
We thank Melissa Emery Thompson, Martina Neumann, and Suzy Kwetuenda for help with data collection and analysis; Matthew McIntyre and Luke Matthews for comments on the manuscript; Stephanie Anestis and Jacinta Beehner for their immensely helpful reviews; and Rebeca Atencia, Lisa Pharoah, Debby Cox, Keith Brown, Claudine Andre, Valery Dhanani, Dominique Morel, Pierrot Mbonzo, and the caretakers of Tchimpounga Chimpanzee Sanctuary and Lola ya Bonobo for making the ape research possible. This work was performed under the authority of the Ministry of Research and the Ministry of Environment in the Democratic Republic of Congo (research permit MIN.RS/SG/004/2009), the Ministry of Scientific Research and Technical Innovation in the Congo Republic (research permit 009/MRS/DGRST/DMAST), and the US Fish and Wildlife Service (permits 09US223466/9 and 09US207589/9). This work was approved by the Institutional Care and Use Committees at Harvard and Duke Universities, and the Institutional Review Board at Harvard University for the human control samples. This work was supported in part by European Research Commission Advanced Grant Agreement 233297 and by National Science Foundation Grant NSF-BCS-08-27552-02 (to B.H.). The research of V.W. was supported in part by grants from the Leakey Foundation, National Science Foundation (DDIG 0851291), and the Wenner-Gren Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007411107/-/DCSupplemental.
References
- 1.Booth A, Shelley G, Mazur A, Tharp G, Kittok R. Testosterone, and winning and losing in human competition. Horm Behav. 1989;23:556–571. doi: 10.1016/0018-506x(89)90042-1. [DOI] [PubMed] [Google Scholar]
- 2.Mazur A, Booth A. Testosterone and dominance in men. Behav Brain Sci. 1998;21:353–363, discussion 363–397. [PubMed] [Google Scholar]
- 3.Filaire E, Maso F, Sagnol M, Ferrand C, Lac G. Anxiety, hormonal responses, and coping during a judo competition. Aggress Behav. 2001;27:55–63. [Google Scholar]
- 4.Alix-Sy D, Le Scanff C, Filaire E. Psychophysiological responses in the pre-competition period in elite soccer players. J Sport Sci Med. 2008;7:446–454. [PMC free article] [PubMed] [Google Scholar]
- 5.Schultheiss OC, Campbell KL, McClelland DC. Implicit power motivation moderates men's testosterone responses to imagined and real dominance success. Horm Behav. 1999;36:234–241. doi: 10.1006/hbeh.1999.1542. [DOI] [PubMed] [Google Scholar]
- 6.Elias M. Serum cortisol, testosterone, and testosterone-binding globulin responses to competitive fighting in human males. Aggress Behav. 1981;7:215–224. [Google Scholar]
- 7.Bernstein IS, Rose RM, Gordon TP. Behavioral and environmental events influencing primate testosterone levels. J Hum Evol. 1974;3:517–525. [Google Scholar]
- 8.Oyegbile TO, Marler CA. Winning fights elevates testosterone levels in California mice and enhances future ability to win fights. Horm Behav. 2005;48:259–267. doi: 10.1016/j.yhbeh.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Wingfield J, Hegner R, Dufty R, Ball G. The “challenge hypothesis”: Theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am Nat. 1990;136:829–846. [Google Scholar]
- 10.Wingfield JC, Sapolsky RM. Reproduction and resistance to stress: When and how. J Neuroendocrinol. 2003;15:711–724. doi: 10.1046/j.1365-2826.2003.01033.x. [DOI] [PubMed] [Google Scholar]
- 11.Kivlighan KT, Granger DA, Booth A. Gender differences in testosterone and cortisol response to competition. Psychoneuroendocrinology. 2005;30:58–71. doi: 10.1016/j.psyneuen.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Suay F, et al. Effects of competition and its outcome on serum testosterone, cortisol and prolactin. Psychoneuroendocrinology. 1999;24:551–566. doi: 10.1016/s0306-4530(99)00011-6. [DOI] [PubMed] [Google Scholar]
- 13.Filaire E, Alix D, Ferrand C, Verger M. Psychophysiological stress in tennis players during the first single match of a tournament. Psychoneuroendocrinology. 2009;34:150–157. doi: 10.1016/j.psyneuen.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 14.Booth A, Granger DA, Mazur A, Kivlighan KT. Testosterone and social behavior. Soc Forces. 2006;85:167–191. [Google Scholar]
- 15.Stanton SJ, Schultheiss OC. The hormonal correlates of implicit power motivation. J Res Pers. 2009;43:942–949. doi: 10.1016/j.jrp.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wirth MM, Welsh KM, Schultheiss OC. Salivary cortisol changes in humans after winning or losing a dominance contest depend on implicit power motivation. Horm Behav. 2006;49:346–352. doi: 10.1016/j.yhbeh.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Schultheiss OC, et al. Effects of implicit power motivation on men's and women's implicit learning and testosterone changes after social victory or defeat. J Pers Soc Psychol. 2005;88:174–188. doi: 10.1037/0022-3514.88.1.174. [DOI] [PubMed] [Google Scholar]
- 18.Fuxjager M, Marler C. How and why the winner effect forms: Influences of contest environment and species differences. Behav Ecol. 2010;21:37–45. [Google Scholar]
- 19.Salvador A, Costa R. Coping with competition: Neuroendocrine responses and cognitive variables. Neurosci Biobehav Rev. 2009;33:160–170. doi: 10.1016/j.neubiorev.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Koolhaas JM, de Boer SF, Buwalda B, van Reenen K. Individual variation in coping with stress: A multidimensional approach of ultimate and proximate mechanisms. Brain Behav Evol. 2007;70:218–226. doi: 10.1159/000105485. [DOI] [PubMed] [Google Scholar]
- 21.Veenema AH, Koolhaas JM, de Kloet ER. Basal and stress-induced differences in HPA axis, 5-HT responsiveness, and hippocampal cell proliferation in two mouse lines. Ann N Y Acad Sci. 2004;1018:255–265. doi: 10.1196/annals.1296.030. [DOI] [PubMed] [Google Scholar]
- 22.Muller M, Wrangham R. Sexual Coercion in Primates and Humans: An Evolutionary Perspective on Male Aggression Against Females. Cambridge, MA: Harvard Univ Press; 2009. [Google Scholar]
- 23.Kano T. The Last Ape: Pygmy Chimpanzee Behavior and Ecology. Stanford, CA: Stanford Univ Press; 1992. [Google Scholar]
- 24.Wrangham RW. Evolution of coalitionary killing. Am J Phys Anthropol. 1999;42(Suppl 29):1–30. doi: 10.1002/(sici)1096-8644(1999)110:29+<1::aid-ajpa2>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- 25.Hohmann G. Association and social interactions between strangers and residents in bonobos (Pan paniscus) Primates. 2001;42:91–99. [Google Scholar]
- 26.Muehlenbein MP, Watts DP, Whitten PL. Dominance rank and fecal testosterone levels in adult male chimpanzees (Pan troglodytes schweinfurthii) at Ngogo, Kibale National Park, Uganda. Am J Primatol. 2004;64:71–82. doi: 10.1002/ajp.20062. [DOI] [PubMed] [Google Scholar]
- 27.Marshall AJ, Hohmann G. Urinary testosterone levels of wild male bonobos (Pan paniscus) in the Lomako Forest, Democratic Republic of Congo. Am J Primatol. 2005;65:87–92. doi: 10.1002/ajp.20099. [DOI] [PubMed] [Google Scholar]
- 28.Vervaecke H, de Vries H, van Elsacker L. Dominance and its behavioral measures in a captive group of bonobos (Pan paniscus) Int J Primatol. 2000;21:47–68. [Google Scholar]
- 29.Muller M. Agonistic relations among Kanyawara chimpanzees. In: Boesch C, Hohmann G, Marchant L, editors. Behavioural Diversity in Chimpanzees and Bonobos. Cambridge, UK: Cambridge Univ Press; 2002. pp. 112–123. [Google Scholar]
- 30.Muller M, Wrangham R. Dominance, aggression and testosterone in wild chimpanzees: A test of the ‘challenge hypothesis’. Anim Behav. 2004;67:113–123. [Google Scholar]
- 31.Fruth B, Hohmann G. How bonobos handle hunts and harvests: Why share food? In: Boesch C, Hohmann G, Marchant L, editors. Behavioural Diversity in Chimpanzees and Bonobos. Cambridge, UK: Cambridge Univ Press; 2002. pp. 231–243. [Google Scholar]
- 32.Hare B, Melis AP, Woods V, Hastings S, Wrangham R. Tolerance allows bonobos to outperform chimpanzees on a cooperative task. Curr Biol. 2007;17:619–623. doi: 10.1016/j.cub.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 33.Hohmann G, Mundry R, Deschner T. The relationship between socio-sexual behavior and salivary cortisol in bonobos: Tests of the tension regulation hypothesis. Am J Primatol. 2008;71:223–232. doi: 10.1002/ajp.20640. [DOI] [PubMed] [Google Scholar]
- 34.Anestis SF, Bribiescas RG. Rapid changes in chimpanzee (Pan troglodytes) urinary cortisol excretion. Horm Behav. 2004;45:209–213. doi: 10.1016/j.yhbeh.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 35.Wobber V, Wrangham R, Hare B. Bonobos exhibit delayed development of social behavior and cognition relative to chimpanzees. Curr Biol. 2010;20:226–230. doi: 10.1016/j.cub.2009.11.070. [DOI] [PubMed] [Google Scholar]
- 36.Gladue M, Boechler M, McCaul K. Hormonal response to competition in human males. Aggress Behav. 1989;15:409–422. [Google Scholar]
- 37.Lipson S, Ellison P. Development of protocols for the application of salivary steroid analyses to field conditions. Am J Hum Biol. 1989;1:249–255. doi: 10.1002/ajhb.1310010304. [DOI] [PubMed] [Google Scholar]
- 38.Hare B, Kwetuenda S. Bonobos voluntarily share their own food with others. Curr Biol. 2010;20:R230–R231. doi: 10.1016/j.cub.2009.12.038. [DOI] [PubMed] [Google Scholar]
- 39.Sannen A, Heistermann M, van Elsacker L, Moehle U, Eens M. Urinary testosterone metabolite levels in bonobos: A comparison with chimpanzees in relation to social system. Behaviour. 2003;140:683–696. [Google Scholar]
- 40. Jurke M, Sommovilla R, Czekala N, Wrangham R (2000) Testosterone across the cycle of female bonobos (Pan paniscus). Am J Primatol 51:65-66.
- 41.Wrangham R, Pilbeam D. African apes as time machines. In: Galdikas B, Briggs N, Sheeran L, Shapiro G, Goodall J, editors. All Apes Great and Small. New York: Kluwer Academic/Plenum Publishers; 2001. pp. 5–18. [Google Scholar]
- 42.Melis AP, Hare B, Tomasello M. Chimpanzees recruit the best collaborators. Science. 2006;311:1297–1300. doi: 10.1126/science.1123007. [DOI] [PubMed] [Google Scholar]
- 43.Mazur A, Booth A, Dabbs J. Testosterone and chess competition. Soc Psychol Q. 1992;55:70–77. [Google Scholar]
- 44.Bateup H, Booth A, Shirtcliff E, Granger D. Testosterone, cortisol, and women's competition. Evol Hum Behav. 2002;23:181–192. [Google Scholar]
- 45.van Anders SM, Watson NV. Effects of ability- and chance-determined competition outcome on testosterone. Physiol Behav. 2007;90:634–642. doi: 10.1016/j.physbeh.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 46.Muller MN, Lipson SF. Diurnal patterns of urinary steroid excretion in wild chimpanzees. Am J Primatol. 2003;60:161–166. doi: 10.1002/ajp.10103. [DOI] [PubMed] [Google Scholar]
- 47.Smider NA, et al. Salivary cortisol as a predictor of socioemotional adjustment during kindergarten: A prospective study. Child Dev. 2002;73:75–92. doi: 10.1111/1467-8624.00393. [DOI] [PubMed] [Google Scholar]
- 48.Granger DA, Shirtcliff EA, Booth A, Kivlighan KT, Schwartz EB. The “trouble” with salivary testosterone. Psychoneuroendocrinology. 2004;29:1229–1240. doi: 10.1016/j.psyneuen.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 49.Talge NM, Donzella B, Kryzer EM, Gierens A, Gunnar MR. It's not that bad: Error introduced by oral stimulants in salivary cortisol research. Dev Psychobiol. 2005;47:369–376. doi: 10.1002/dev.20097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.