Abstract
In plants, the major route for dissipating excess light is the nonphotochemical quenching of absorbed light (NPQ), which is associated with thylakoid lumen acidification. Our data offer an interpretation for the complex relationship between changes in luminal pH and the NPQ response. Upon steady-state illumination, fast NPQ relaxation in the dark reflects the equilibration between the electrochemical proton gradient established in the light and the cellular ATP/ADP+Pi ratio. This is followed by a slower phase, which reflects the decay of the proton motive force at equilibrium, due to gradual cellular ATP consumption. In transient conditions, a sustained lag appears in both quenching onset and relaxation, which is modulated by the size of the antenna complexes of photosystem II and by cyclic electron flow around photosystem I. We propose that this phenomenon reflects the signature of protonation of specific domains in the antenna and of slow H+ diffusion in the different domains of the chloroplast.
Keywords: electrochemical proton gradient, fluorescence, antenna
Light conversion is a most critical step in photosynthesis. In low light, photon capture must be optimized to aliment CO2 assimilation by the Calvin-Benson-Bassham cycle (hereafter referred as to the Calvin cycle). Conversely, when excitation pressure exceeds the evacuation capacity of the photosynthetic apparatus, light absorption must be down-regulated to avoid overreduction of the soluble electron carriers, generation of reactive oxygen species, and possible photodamage. Plants are capable of withstanding exposure to excess light through a variety of mechanisms associated with photosystem (PS) II, which are collectively referred to as nonphotochemical quenching (NPQ; reviewed in refs. 1 and 2). According to previous definition (1), this term encompasses at least three processes: (i) qE, a quenching associated with the acidification of the luminal pH (3). qE is the dominant form of NPQ in vascular plants (1, 2). It reflects an increased thermal dissipation in the light-harvesting apparatus of PSII (LHCII) requiring the ΔpH, carotenoid deepoxidation [by the xanthophyll cycle (XC); ref. 4] and the presence of the PSII subunit PsbS (2). (ii) qT (state transitions; ref. 5) reflects changes in the relative size of PSII and PSI antenna, due to reversible phosphorylation. (iii) qI is a slowly reversible process (>1 h), related to photoinhibition (6) and to long-living quenching phenomena (7). According to Horton et al. (1), the complex kinetics of NPQ decay observed upon illumination should be indicative of the different relaxation times of the three processes. On the other hand, both qE effectors (XC and PsbS) are affected by changes in the luminal pH. The turnover of the violaxanthin (Vx) deepoxidase, the enzyme producing the “NPQ-active” carotenoids antheraxanthin and zeaxanthin (Zx), is increased at low pH (1). In parallel, PsbS senses lumen acidification via the protonation of two conserved luminal glutamic residues (2). Thus, the existence of multiple pH-sensitive steps in fluorescence quenching could provide another explanation for the complex patterns of NPQ relaxation in the dark (8).
Our analysis demonstrates that multiphasic quenching relaxation mainly reflects the existence of at least two phases in the decline of the ΔpH: a fast one, due to equilibration between the ΔμH+ and the phosphorylating potential (ΔGP = ΔGpo′ + RT ln[ATP]/([ADP]+[Pi])), and a slower one, in which the ΔGp slowly declines due to cellular ATP consumption. We also report the existence of a sustained lag in NPQ both at the onset and at the end of illumination, in prestationary conditions. We propose that this phenomenon reflects the protonation of specific domains in the antenna, and the slow H+ diffusion between the grana and the stroma compartments of the chloroplast.
Results
Induction of NPQ by Light and Dark Cycles in Arabidopsis.
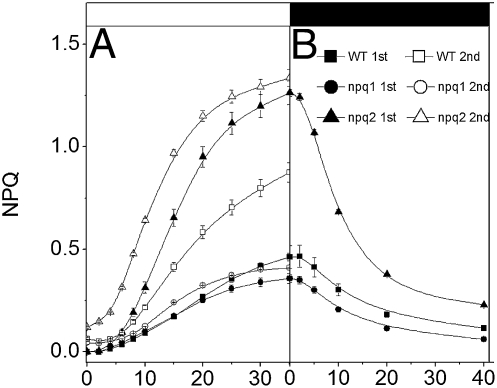
In Fig.1, dark-adapted Arabidopsis leaves (from WT and NPQ mutants; ref. 2) were exposed to two consecutive short periods of illumination (35 s) with moderate light (kiPSII ≈250 s−1), separated by 90 s of dark. During the first exposure, identical NPQ kinetics was observed for ≈20 s in the WT and in npq1, a mutant unable to accumulate Zx in the light (2). Longer illumination led to a larger NPQ generation in WT, likely reflecting the onset of Zx synthesis via the activation of XC (7, 9). Consistent with this hypothesis, a second illumination induced a much larger NPQ in WT but had lesser consequences in npq1. In npq2 plants, which constitutively accumulate Zx (2), a nearly maximum NPQ was already seen during the first illumination. A second light exposure did not considerably change the NPQ kinetics, except for a shortening of the lag phase. This indicates that NPQ onset in npq2 essentially reflects the building of a ΔpH during the first illumination, and that the reduced lag seen during the second illumination is caused by incomplete ΔpH relaxation between the two illuminations (10). A similar effect was seen in npq1 leaves, although to a much smaller extent. This suggests that either the proton gradient established in the light relaxes differently in the two lines, or that their different carotenoid composition results in a different pH sensitivity of NPQ. As expected, no significant NPQ was seen in the npq4 strain, which lacks PsbS (2), during both illuminations.
Fig. 1.
Dynamics of NPQ during consecutive illuminations. Dark-adapted Arabidopsis leaves were exposed to two consecutive 35-s green illuminations (400 μmol photons m−2 s−1 or kiPSII ≈250 s−1). (A) Solid symbols, first light exposure; open symbols, second light exposure after 90 s of dark. (B) NPQ decay kinetics measured after the 35-s illumination. Each curve represents the average of six experiments performed on different leaves of the same plant. Black bar, light off; white bar, light on.
NPQ Decay Kinetics.
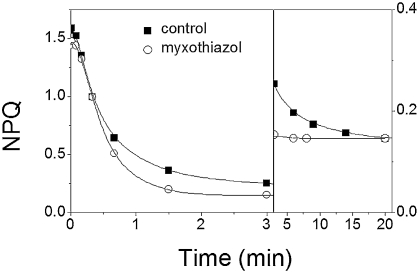
To further investigate the relationship between the proton gradient and NPQ, we measured the kinetics of quenching relaxation upon light exposure. After the first illumination, npq1 leaves display a rather monotonous decay (t½ ≈13 s; Fig. 1). In WT a first decaying phase was observed, which was identical to that observed on npq1 leaves. However, the existence of a second phase of small amplitude and longer duration could be inferred, as suggested by the presence of a residual NPQ after 40 s of darkness. This phase fully relaxed in ≈3 min. In npq2, the biphasic character of NPQ decay was more pronounced, with a first phase characterized by a t½ of ≈26 s and a second one completed in several minutes. After longer illumination (≥15 min), as required to load WT leaves with Zx and to achieve steady-state NPQ (4), similar relaxation kinetics were observed in WT and npq2 strains. This suggests that the slowing down of NPQ decay in WT is linked to Zx accumulation. Upon preloading leaves with Zx (SI Appendix, Fig. S1), three phases of NPQ decay were observed, completed in ≈3 min (Fig. 2 Left), 20 min (Fig. 2 Right), and several hours, respectively, in agreement with previous reports (1). Previous data indicate that a slowly relaxing ΔμH+ component (t½ ≈5–10 min) exists in steady-state light-exposed spinach leaves (10). This ΔμH+ (≈150 mV) is in equilibrium with the ΔGp, which has reached a maximum value in the light. The ΔμH+ comprises both an electric (ΔΨ) and a pH component (10). Its slow relaxation in the dark reflects the consumption of ATP accumulated in the light phase, until a permanent ΔμH+ is attained, the amplitude of which is set by the [ATP]/[ADP] × [Pi] ratio in the dark. Thus, we reasoned that the two phases of NPQ decay observed in the 0–3-min and 3–20-min time range (Fig. 2) could reflect the biphasic relaxation of the proton motive force (11), rather than the existence of different quenching phenomena (1). To test this possibility, we examined the kinetics of NPQ decay upon inhibition of the respiratory chain (Fig. 2). Blocking respiration completely abolishes the ΔμH+ in the dark (10), indicating ATP equilibration between the mitochondria, the cytoplasm, and the chloroplasts. In the presence of the respiratory inhibitor myxothiazol, the cytoplasm is depleted in ATP and acts as a sink of chloroplast-generated ATP. Hence, this inhibitor considerably accelerates the decay of the long-lived ΔμH+ in equilibrium with ATP (10). We first checked that the effect of myxothiazol on the ΔμH+ could be reproduced in Arabidopsis leaves, and then we compared the NPQ relaxation in leaves that were either infiltrated with a solution containing 150 mM sorbitol (control) to avoid changes in the internal osmotic force or with the same solution containing a saturating concentration of myxothiazol (20 μM). In the control, the first two phases of NPQ relaxation were completed in ≈3 min (t½ ≈25 s) and in ≈20 min (Fig. 2), in agreement with findings in noninfiltrated leaves. Conversely, myxothiazol addition led to a monotonous NPQ relaxation, by selectively suppressing the 3–20-min relaxation phase. This indicates that both the fast and the intermediate phases of NPQ decay observed are linked to changes in ΔpH: the fast one would be due to equilibration of the ΔμH+ with ΔGp, whereas the slower one should reflect ΔμH+ decay at equilibrium with the ΔGp. Both phases, therefore, represent a qE type of quenching, instead of being indicative of qE and qT, as previously suggested. Consistent with this conclusion, no differences in NPQ relaxation were seen between a mutant (stn7) that is totally impaired in state transitions because of the absence of the LHCII-kinase (12) and WT (SI Appendix, Table S1). We note that myxothiazol addition did not modify the amplitude of the slowest phase (20 min to several hours). Because this inhibitor completely suppressed the dark ΔμH+ (10), we conclude that the so-called “qI” phase of quenching is pH independent and therefore reflects either recovery from photoinhibition (6) or a Zx-related, pH-independent quenching (7).
Fig. 2.
NPQ decay rates in Arabidopsis WT leaves illuminated for 15 min (kiPSII ≈250 s−1) in the absence (squares) and presence (circles) of 12 μM myxothiazol. (A) The 0- to 3-min phase of the NPQ decay. (B) The 3- to 20-min phase with an expanded vertical scale (×4).
pH Equilibration and NPQ Dynamics in the Chloroplast.
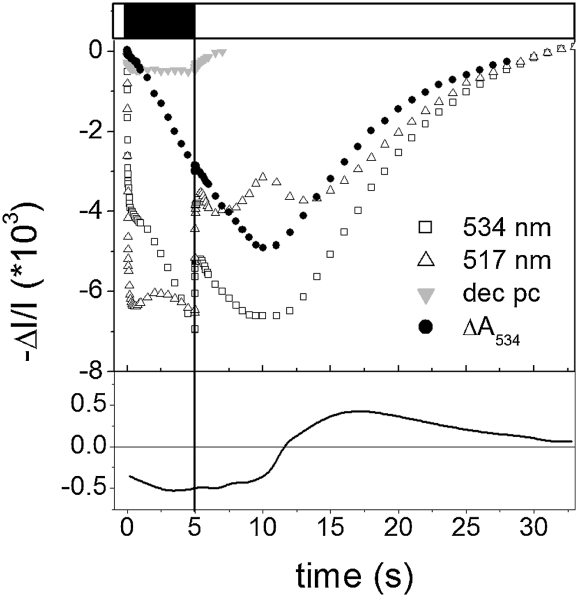
To further test the relationship between pH changes and qE formation, we studied the kinetics of generation and relaxation of NPQ and of the qE-associated spectral shift (ΔA535; SI Appendix, Fig. S2; refs. 13 and 14) in Zx preloaded leaves. In the presence of this carotenoid, qE and ΔA535 generation reflect the protonation of luminal exposed protein residues (8). We first checked that the spectrum of the ΔA535 signal measured upon switching the light off in our spinach and Arabidopsis leaves was similar to the one previously described in WT and NPQ mutants of Arabidopsis (9). Young spinach leaves were then used for these experiments, because they show larger NPQ and ΔA535 signals than Arabidopsis (SI Appendix, Fig. S2A). Leaves were infiltrated with 150 mM sorbitol, to make illumination within the leaf more homogenous, thanks to reduced light scattering. Zx-preloaded leaves were then submitted to light and dark cycles, and the kinetics of the ΔA535 signals was estimated (Fig. 3) after subtraction of the membrane potential and of plastocyanin redox signals (15). Surprisingly, the deconvoluted signal continued to relax for ≈15 s after the light was switched on, indicating the existence of a lag in NPQ onset. Interestingly, a lag phase (≈5 s) is also observed in the first derivative of the ΔA535 signal (Fig. 3, Lower), suggesting that the involvement of at least two processes limits NPQ generation. The lag phases was further investigated in experiments in which the kinetics of NPQ and ΔA535 were measured in a Zx-loaded leaf submitted to different light and dark cycles (2 min light and 5 s, 25 s, and 50 s of dark; Fig. 4). Similar kinetics and lag phases were seen in NPQ and in ΔA535 changes, extending the notion of a tight correlation between the two parameters (9, 13, 14) and the transient NPQ phase. In both cases, the duration of the lag decreased along with the rate of NPQ decay measured just before the light was switched on.
Fig. 3.
Kinetics of the NPQ-associated absorption change (ΔA535). Black bar, light off; white bar, light on. The leaf is submitted to light–dark cycles (2 min light, 5 s dark). After ≈10 cycles, reproducible kinetics was obtained. Extinction coefficients needed to deconvolute the ΔA535 from membrane potential were evaluated from (i) the ratio R of absorption changes measured at 517 nm and 534 nm. For this purpose a dark-adapted leaf was exposed to a single turnover flash, which generates a transmembrane potential without promoting any accumulation of carotenoids (R = 1 0.85). (ii) Plastocyanin (PC) measured at 870 nm. According to the red-ox spectrum of PC (15), we estimate that redox changes due to PC should be identical at 870 nm and in the 534 nm – 517 nm/1.85 kinetics. Lower: First derivative of the ΔA534 signal, estimated from the solid circles shown (Upper).
Fig. 4.
Changes in (A) ΔA535 and (B) NPQ in spinach leaves exposed to dark and light cycles of different duration (5 s, 25 s, and 50 s dark, 2 min light). Same deconvolution of ΔA535 as in Fig. 3.
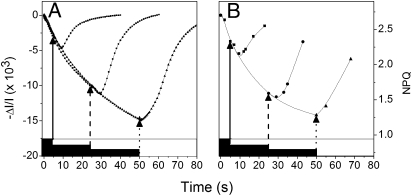
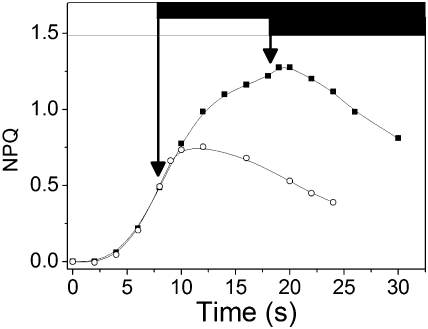
In Fig. 5, a spinach leaf was illuminated for 15 min and then dark adapted for 15 min, a time sufficient to inactivate the Calvin cycle (16), while maintaining a significant concentration of Zx (SI Appendix, Fig. S1) (4). The leaf was then submitted alternatively to 8 s or 18 s of light, separated by 3 min of dark. A lag phase (≈8 s) was seen upon switching the light off after the 8-s illumination. The duration of the lag was reduced (≈3 s) after 18 s illumination (i.e., when NPQ approached the steady-state level). Thus, similar lag phases but of opposite direction are observed when the light is switched on (Fig. 4) and off (Fig. 5).
Fig. 5.
Changes in NPQ in WT leaf illuminated for 15 min and then dark adapted for 15 min, to inactivate the Calvin cycle. The leaf is then alternatively submitted to 8 s (circles) or 18 s of illumination (squares) separated by 3 min of dark. Several cycles of illumination were performed, to ensure reproducibility of the results.
Role of Antenna Size in NPQ Dynamics.
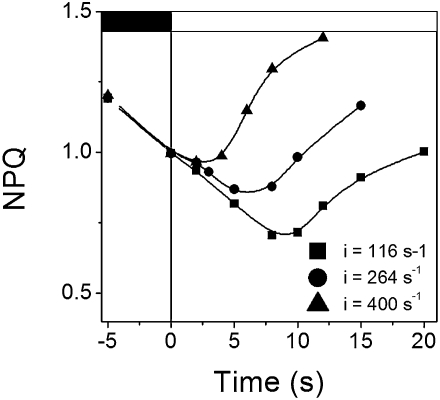
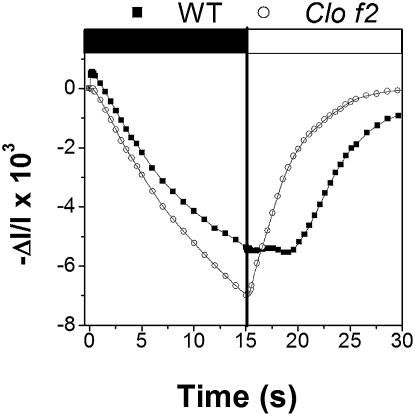
The lag phase observed for both ΔA535 and NPQ suggests the existence of limiting steps in both NPQ formation and relaxation. What is the nature of these steps? To answer this question, we tested the light intensity dependence of the lag in Zx-loaded Arabidopsis leaves (WT) exposed to light and dark cycles. After 2 min of illumination (kiPSII = 264 s−1), the leaf was dark adapted for 5 s before illumination for 20 s with three different intensities (kiPSII = 116 s−1, kiPSII = 264 s−1, and kiPSII = 400 s−1). Clearly, the duration of the lag was a decreasing function of the light intensity used during the second illumination (Fig. 6), suggesting that the rate of the limiting step could be modified by light. Previous work has suggested that the major site of NPQ is the PSII antenna (1, 2). To test whether the lag was related to a process taking place in these complexes, we measured NPQ transients in the chlorina f2 (clo-f2) mutant of barley, which lacks most of the PSII antennae due to impaired chlorophyll b synthesis (17). In the mutant, we could identify an NPQ-related spectral shit, having the same spectral features as the ones observed in WT (SI Appendix, Fig. S3). Moreover, a significant NPQ was found in clo-f2 leaves (1.2 vs. 2 in WT) when leaves were exposed to the same amount of PSII absorbed light. This corresponds to a light intensity of 800 μmol m−2 s−1 for clo-f2 and of 340 μmol m−2 s−1 for WT, to compensate for the reduced absorption capacity of the latter (18). The first phase of NPQ relaxation was faster in clo-f2 (t½ ≈23 s) than in WT (t½ ≈65 s), and the lag phase was largely reduced in the mutant (≈1 s) when compared with WT (≈6 s) under a dark–light cycle regime (1 min light, 15 s dark). This was true in the case of the ΔA535 signal (Fig. 7) and of NPQ (SI Appendix, Fig. S4). Therefore, we conclude that the absence of LHCII in clo-f2 reduces the overall thylakoid buffering capacity and eliminates the limiting step in NPQ onset, while preserving a similar mechanism of energy quenching (as suggested by the identical relationship between NPQ and the ΔA535) and a significant fraction (≈50%) of the quenchers.
Fig. 6.
NPQ lag as a function of the light intensity. Zx, preloaded Arabidopsis leaves (WT). The leaves were then submitted to light and dark cycles. After 2 min of illumination (kiPSII = 264 s−1), the leaf was dark adapted for 5 s before illumination for a short period (<20 s) with three different intensities.
Fig. 7.
Changes in ΔA535 in barley leaves that were exposed to dark–light cycles (15 s light, 15 s dark). Squares, WT; circles, clo-f2. Note that all of the spectroscopic signals (including the ΔA535) are enlarged in clo-f2 (by a factor of ≈2), owing to the lower pigment content of this mutant.
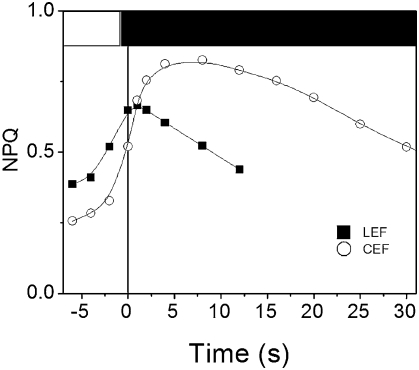
Protonation of acid pK's in the lumen is responsible for NPQ development in plants (2). Thus, we tried to modify the duration of the lag in NPQ by altering the light-generated proton gradient via changes in linear flow (LEF), which involved PSII and PSI turnover, and in cyclic flow (CEF). The latter only requires PSI activity and induces a proton gradient that is essentially not consumed for carbon assimilation. Conversely, the ΔpH induced by LEF promotes synthesis of ATP that is rapidly consumed at the level of the Calvin cycle. This is likely the reason why CEF promotes NPQ more efficiently than LEF (19). In Fig. 8, Zx-preloaded leaves were submitted to a light–dark regime (1 min light, 50 s dark, 6 s light, 12 s dark) that maintains the Calvin cycle in its active state and thus promotes essentially LEF (16). In these conditions, a short lag (2 s) was observed at the end of the 6-s light period. The same leaf was then submitted to light dark cycles with longer dark periods (2 min dark, 6 s light). This condition mainly inactivates the Calvin cycle, as evidenced by the ≈4.5-fold decrease of rate of the LEF (ref. 20; Fig. 8). Inactivation of LEF resulted in an increased NPQ onset rate, consistent with the notion that quenching is more efficiently generated by CEF. In this condition, the lag in NPQ decay (10 s) was ≈5 times longer than in LEF conditions. It seems, therefore, that although CEF enhances the ΔpH-mediated NPQ response, it slows down the limiting step of fluorescence quenching.
Fig. 8.
NPQ lag under linear and CEF conditions. Zx-loaded leaves were submitted to a light–dark cycles. Squares, 50 s dark, 6 s light, 12 s dark, 1 min light, PSII photochemical rate = kiPSII times the ΦPSII (computed from ref. 20) = 63 s−1. Circles, the same leaf was submitted to light–dark cycles (2 min dark, 6 s light), PSII photochemical rate = 14 s−1.
Discussion
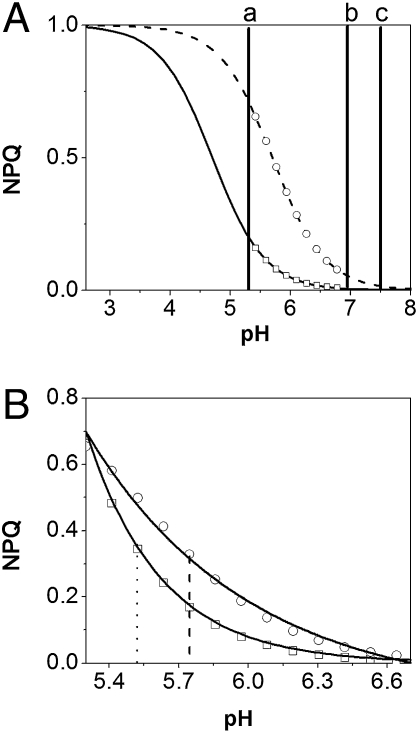
The similarity between the NPQ kinetics observed during the first seconds of illumination in WT and npq1 leaves, as well as the large difference between npq1 and npq2 kinetics, is consistent with the suggestion by Horton et al. (21) that quenchers of similar efficiency decrease fluorescence emission in Vx-loaded or Zx-loaded antenna complexes. This hypothesis assumes that the conversion of Vx into Zx shifts the apparent pK of protonable residues associated with NPQ toward less acid values (from 4.7 to 5.7, respectively; ref. 21). We assume here (Fig. 9), for simplicity, a single pK of 5.7 for groups involved in NPQ in Zx-loaded membranes, and a single pK of 4.7 for Vx-loaded membranes. By combining this hypothesis with our estimates of the ΔpH dynamics in the dark (10), it is possible to interpret most of the experimental data presented in this work. (i) Considering that the ratio between the NPQ levels reached after a 35-s illumination in the presence of Zx (npq2) and Vx (npq1) is ≈4 (Fig. 1), we can estimate the luminal pH in the range of ≈5.5 (Fig. 9A, vertical line a), corresponding to a possible ΔpH of ≈2 in the light (ref. 22; see, however, ref. 23 for a different estimate). (ii) Assuming identical ΔpH decay in npq1 and npq2 leaves after illumination, we can predict a faster NPQ relaxation in npq1 than in npq2 leaves (Fig. 9B), as reported in Fig. 1. (iii) Taking into account that the ratio between the amplitude of the 0–3-min and the 3–20-min phase is ≈12, we estimate that the ΔpH at the end of the 0–3-min phase should be ≈0.5 pH units (see difference between vertical lines a and b in Fig. 9A). This ΔpH would decrease during the 3–20-min phase following the ATP concentration. Eventually, (iv) our model explains previous observations concerning the NPQ dynamic at limiting light. In these conditions, a transient NPQ (maximum quenching level ≈0.8) is observed, which decreases along with the activation of the Calvin cycle (to a steady-state level of ≈0.15; ref. 24). Although biphasic NPQ decay is observed when light is switched off at maximum quenching, only the slow phase, completed in ≈15 min, is detected upon steady-state illumination. This suggests that weak light sustains ATP synthesis and the generation of a ΔμH+ during the first phase of illumination. Conversely, identical rates of ATP formation and consumption are achieved in steady state, and the ΔμH+ approaches the equilibrium with the ΔGp, owing to activation of the Calvin cycle. In these conditions, no fast NPQ decay is expected, because the ΔμH+ is relaxing at equilibrium.
Fig. 9.
Possible NPQ vs. pH relationship in the chlorplast. Solid line, titration curve of Vx-loaded membranes. Dashed line, titration curve of Zx-loaded membranes. (A) Luminal pH values in the light (vertical line a) and after illumination (vertical line b) are shown. Vertical line c: Stromal pH value in the dark. (B) Zoom on the acid pH range (normalized traces). NPQ is halved at lower pH in Vx- (dots) than in Zx-loaded leaves (dashed lines), suggesting a faster NPQ relaxation when the pH increases after dark adaptation of light-exposed leaves.
Overall, Two main conclusions can be derived from this model concerning plant light acclimation. First, heterogeneous NPQ relaxation upon illumination reflects a multiphasic pH- and Zx-dependant process (qE), completed in ≈20 min. This is followed by a slowest phase (20 min to several hours) that is pH independent. This slow phase likely reflects photoinhibition (6) but also a possible Zx-driven constitutive quenching (7). Second, our data suggest that by adjusting the pK of groups responsible for NPQ to a value close to the pH value in the light (i.e., far from the value established at equilibrium), plants have achieved a maximum flexibility with respect to both carbon fixation efficiency and photoprotection. This should be relevant in case of exposure to a sudden changes in light intensity (25), whereby the ΔpH can rapidly vary between the light and the dark equilibrium value (“a” and “b”) without significant changes in the chloroplast pigment composition. In this condition, extremely fast NPQ responses can be predicted even for small pH changes, owing to the very steep NPQ/pH relationship.
Although the model discussed above accounts for most of the data observed at steady state, it cannot explain the significant delay in the NPQ response, which we observe during quenching onset and relaxation. In principle, this lag could simply reflect the time required to induce a conformational change in the antenna, as required for NPQ generation (14, 21). However, our data suggest that the lag could also stem from delayed protons diffusion from their sources (PSII and the cyt b6f) toward the residues responsible for fluorescence quenching and A535 onset. Experimental evidence supports this hypothesis: (i) the finding that the duration of the lag is inversely proportional to the light intensity (Fig. 6) is compatible with the known modulation of the rate of proton release in the lumen by light (26). (ii) The abolishment of the lag in chlo-f2 (Fig. 7) correlates with a decrease in the buffering capacity of the thylakoids in this mutant, due to removal of PSII antenna complexes. (iii) The enhancement of the lag under conditions whereby the photosynthetic apparatus mainly operated according to CEF is also in line with this possibility. Although CEF augments the quenching level, as expected because of the enhanced ΔpH generation, this process increases the NPQ lag instead of reducing it. According to the model of Albertsson (27), H+ produced by CEF are mostly generated at the level of cyt b6f complexes localized in the nonappressed regions. Thus, they are released far from the quenching sites. Conversely, H+ produced by LEF are generated by cyt b6f complexes in the appressed thylakoid regions (27, 28) (i.e., in proximity of the site of quenching). Moreover, H+ are also produced by PSII in LEF, at variance with CEF. These H+ could be liberated directly within the LHCII complex (26), which according to our analysis is a major actor of the modulation of the lag. The resulting proximity between the source and the sink of protons is expected to reduce the duration of the lag.
Overall, we propose that that limited H+ diffusion between the nonappressed thylakoid regions and target groups in the grana regions (including the sites of qE quenching) is one parameter delaying NPQ changes under transient conditions. This kinetic effect would probably superimpose to the time required for conformational changes, which provide the structural frame for quenching onset (1, 21). Our kinetic analysis of the ΔA535 showing a lag not only in the absorption kinetics but also in its first derivative is fully consistent with the involvement of at the least two steps in the observed lag. Recent data (29) have shown that dissociation of a five-subunit membrane complex, composed of two monomeric Lhcb proteins (CP29 and CP24) and of trimeric LHCII, is a prerequisite for NPQ development in high light. This phenomenon could make quenching sites more accessible to protons, thus affecting the lag in NPQ.
Our concept of slow H+ equilibration within different membrane compartments is somehow reminiscent of the notion that “localized” and “delocalized” proton domains modulate NPQ (30), as revealed by the differential effect of hydrophilic and lipophilic uncoupling agents on this parameter (31). On the other hand, our model does not imply any kinetic barrier for the membrane potential (ΔΨ), which is an essential component of the ΔμH+. Thus, the rapid delocalization of the ΔΨ (in the microsecond time range; ref. 32) between the appressed and the nonappressed region of the membrane would allow maintaining a “delocalized” ATP generation synthesis mechanism as required by the Mitchell's theory.
Methods
Plant Growth and Experimental Conditions.
Arabidopsis and barley plants were grown for 5 to 6 wk in a growth chamber with a 16-h photoperiod at a light intensity of 50 μmol photons m−2 s−1 and a day/night temperature of 22/18 °C. Spinach leaves were bought at the local market. Plants were dark adapted for at least 5 h before measurement, to ensure complete carotenoid epoxidation (4). Experiments 2–8 were performed on leaves that were preilluminated for more than 15 min before measurements (Zx preloaded leaves), to induce sustained conversion of Vx to Zx.
Spectroscopic Measurements.
Absorption changes were measured using a JTS spectrophotometer (Bio-Logic), equipped with a homemade device to measure absorption spectra. Photochemical rate constants under continuous illumination were estimated by measuring the initial rate of the membrane potential increase (Ri) and the membrane potential increase (A) induced by a saturating flash (8 ns duration) on a dark-adapted leaf (16). Assuming that green light equally excites PSI and PSII centers, we have kiPSII = ≈kiPSI = ≈Ri/A.
Fluorescence Measurements.
Fluorescence changes were measured using the same setup. NPQ measurements were performed with moderate light intensity (<300 s−1), to limit development of photoinhibition. NPQ was computed according to the formula (Fmax − Fq)/Fq (33), where Fmax is the maximum fluorescence level on a fully dark-adapted leaf and Fq the maximum fluorescence level measured 100 μs after a 200-ms pulse of saturating light on illuminated leaf. Kinetics of NPQ relaxation in the short time range (<60 s) was measured using a single 200-ms pulse to avoid perturbations induced by the saturating pulses. Therefore, analysis of the NPQ relaxation required several experiments in which a single 200-ms pulse was fired at different times after switching the light off. We checked that a maximum fluorescence level was reached at the end of the 200-ms pulse and that its duration was short enough not to induce any significant decrease in photochemical activity (<5%) in light-exposed leaves. Therefore, several pulses were used to measure NPQ during the first 10 s of illumination, as reported in Fig. 1.
Supplementary Material
Acknowledgments
We thank Anne Joliot for valuable comments; Ingvar Bordman for help in fluorescence measurements; and Pierre Cardol and Marcel Kuntz for help in HPLC measurements. This work was supported by grants from the Centre National de la Recherche Scientifique.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006399107/-/DCSupplemental.
References
- 1.Horton P, Ruban AV, Walters RG. Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- 2.Li Z, Wakao S, Fischer BB, Niyogi KK. Sensing and responding to excess light. Annu Rev Plant Biol. 2009;60:239–260. doi: 10.1146/annurev.arplant.58.032806.103844. [DOI] [PubMed] [Google Scholar]
- 3.Wraight CA, Crofts AR. Energy-dependent quenching of chlorophyll alpha fluorescence in isolated chloroplasts. Eur J Biochem. 1970;17:319–327. doi: 10.1111/j.1432-1033.1970.tb01169.x. [DOI] [PubMed] [Google Scholar]
- 4.Siefermann D, Yamamoto HY. Properties of NADPH and oxygen-dependent zeaxanthin epoxidation in isolated chloroplasts. A transmembrane model for the violaxanthin cycle. Arch Biochem Biophys. 1975;171:70–77. doi: 10.1016/0003-9861(75)90008-9. [DOI] [PubMed] [Google Scholar]
- 5.Allen JF. Protein phosphorylation in regulation of photosynthesis. Biochim Biophys Acta. 1992;1098:275–335. doi: 10.1016/s0005-2728(09)91014-3. [DOI] [PubMed] [Google Scholar]
- 6.Aro EM, Virgin I, Andersson B. Photoinhibition of Photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta. 1993;1143:113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- 7.Dall'Osto L, Caffarri S, Bassi R. A mechanism of nonphotochemical energy dissipation, independent from PsbS, revealed by a conformational change in the antenna protein CP26. Plant Cell. 2005;17:1217–1232. doi: 10.1105/tpc.104.030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takizawa K, Cruz JA, Kanazawa A, Kramer DM. The thylakoid proton motive force in vivo. Quantitative, non-invasive probes, energetics, and regulatory consequences of light-induced pmf. Biochim Biophys Acta. 2007;1767:1233–1244. doi: 10.1016/j.bbabio.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Johnson MP, Pérez-Bueno ML, Zia A, Horton P, Ruban AV. The zeaxanthin-independent and zeaxanthin-dependent qE components of nonphotochemical quenching involve common conformational changes within the photosystem II antenna in Arabidopsis. Plant Physiol. 2009;149:1061–1075. doi: 10.1104/pp.108.129957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joliot P, Joliot A. Quantification of the electrochemical proton gradient and activation of ATP synthase in leaves. Biochim Biophys Acta. 2008;1777:676–683. doi: 10.1016/j.bbabio.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Gilmore AM, Björkman O. Adenine nucleotides and the xanthophyll cycle in leaves II. Comparison of the effects of CO2- and temperature-limited photosynthesis on photosystem II fluorescence quenching, the adenylate energy charge and violaxanthin de-epoxidation in cotton. Planta. 1994;192:537–544. [Google Scholar]
- 12.Bellafiore S, Barneche F, Peltier G, Rochaix JD. State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature. 2005;433:892–895. doi: 10.1038/nature03286. [DOI] [PubMed] [Google Scholar]
- 13.Heber U. Conformational changes of chloroplasts induced by illumination of leaves in vivo. Biochim Biophys Acta. 1969;180:302–319. doi: 10.1016/0005-2728(69)90116-9. [DOI] [PubMed] [Google Scholar]
- 14.Ruban AV, Pascal AA, Robert B, Horton P. Activation of zeaxanthin is an obligatory event in the regulation of photosynthetic light harvesting. J Biol Chem. 2002;277:7785–7789. doi: 10.1074/jbc.M110693200. [DOI] [PubMed] [Google Scholar]
- 15.Katoh S, Shiratori I, Takamiya A. Purification and some properties of spinach plastocyanin. J Biochem. 1962;51:32–40. doi: 10.1093/oxfordjournals.jbchem.a127497. [DOI] [PubMed] [Google Scholar]
- 16.Joliot P, Joliot A. Cyclic electron flow in C3 plants. Biochim Biophys Acta. 2006;1757:362–368. doi: 10.1016/j.bbabio.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Thornber JP, Highkin HR. Composition of the photosynthetic apparatus of normal barley leaves and a mutant lacking chlorophyll b. Eur J Biochem. 1974;41:109–116. doi: 10.1111/j.1432-1033.1974.tb03250.x. [DOI] [PubMed] [Google Scholar]
- 18.Havaux M, Dall'osto L, Bassi R. Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in Arabidopsis leaves and functions independent of binding to PSII antennae. Plant Physiol. 2007;145:1506–1520. doi: 10.1104/pp.107.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heber U, Walker D. Concerning a dual function of coupled cyclic electron transport in leaves. Plant Physiol. 1992;100:1621–1626. doi: 10.1104/pp.100.4.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Genty B, Harbinson J, Briantais JM, Baker NR. The relationship between non-photochemical quenching of chlorophyll fluorescence and the rate of photosystem 2 photochemistry in leaves. Photosynth Res. 1990;25:249–257. doi: 10.1007/BF00033166. [DOI] [PubMed] [Google Scholar]
- 21.Horton P, Ruban AV, Wentworth M. Allosteric regulation of the light-harvesting system of photosystem II. Philos Trans R Soc Lond B Biol Sci. 2000;355:1361–1370. doi: 10.1098/rstb.2000.0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heldt WH, Werdan K, Milovancev M, Geller G. Alkalization of the chloroplast stroma caused by light-dependent proton flux into the thylakoid space. Biochim Biophys Acta. 1973;314:224–241. doi: 10.1016/0005-2728(73)90137-0. [DOI] [PubMed] [Google Scholar]
- 23.Kramer DM, Sacksteder CA, Cruz JA. How acid is the lumen? Photosynth Res. 1999;60:151–163. [Google Scholar]
- 24.Finazzi G, et al. A zeaxanthin-independent nonphotochemical quenching mechanism localized in the photosystem II core complex. Proc Natl Acad Sci USA. 2004;101:12375–12380. doi: 10.1073/pnas.0404798101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Külheim C, Agren J, Jansson S. Rapid regulation of light harvesting and plant fitness in the field. Science. 2002;297:91–93. doi: 10.1126/science.1072359. [DOI] [PubMed] [Google Scholar]
- 26.Jahns P, Polle A, Junge W. The photosynthetic water oxidase: Its proton pumping activity is short-circuited within the protein by DCCD. EMBO J. 1988;7:589–594. doi: 10.1002/j.1460-2075.1988.tb02851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albertsson PA. A quantitative model of the domain structure of the photosynthetic membrane. Trends Plant Sci. 2001;6:349–358. doi: 10.1016/s1360-1385(01)02021-0. [DOI] [PubMed] [Google Scholar]
- 28.Joliot P, Lavergne J, Béal D. Platoquinione compartimentaion in chloroplasts. Evidence for domains with different rates of photo-reduction. Biochim Biophys Acta. 1992;1101:1–12. [Google Scholar]
- 29.Betterle N, et al. Light-induced dissociation of an antenna hetero-oligomer is needed for non-photochemical quenching induction. J Biol Chem. 2009;284:15255–15266. doi: 10.1074/jbc.M808625200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagle JF, Dilley RA. Models of localized energy coupling. J Bioenerg Biomembr. 1986;18:55–64. doi: 10.1007/BF00743612. [DOI] [PubMed] [Google Scholar]
- 31.Laasch H, Weis E. Photosynthetic control, “energy-dependent” quenching of chlorophyll fluorescence and photophosphorylation under influence of tertiary amines. Photosynth Res. 1989;22:137–146. doi: 10.1007/BF00035444. [DOI] [PubMed] [Google Scholar]
- 32.Witt HT. Energy conversion in the functional membrane of photosynthesis. Analysis by light pulse and electric pulse methods. The central role of the electric field. Biochim Biophys Acta. 1979;505:355–427. doi: 10.1016/0304-4173(79)90008-9. [DOI] [PubMed] [Google Scholar]
- 33.Bilger W, Björkman O. Role of the xanthophyll cycle in photoprotection elucidated by measurments of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res. 1990;25:173–186. doi: 10.1007/BF00033159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.