Abstract
Genes and prior experience both influence the behavior of animals, but the relative contribution of each to fighting behavior in Drosophila remains unclear. To address this issue, we bred hyperaggressive flies by selecting winners of fights over 34–37 generations. Males of this strain initiate fights sooner, retaliate more often, and regularly defeat opponents from the nonselected parent Canton-S strain. After a defeat, however, these highly aggressive flies lose their second fights against socially naïve counterparts. Defeated flies also lunge and retaliate less after experiencing a loss, suggesting that the subsequent losses result from flies becoming less aggressive. Remarkably, flies that were once capable of engaging in high-intensity boxing and tussling patterns of behavior for extended periods of time often do not even engage in mid-intensity lunging after a single defeat. Furthermore, these formerly highly aggressive flies lose all competitive advantage over nonselected Canton-S after experiencing a loss. Lastly, females were more likely to copulate with males from the nonselected parent line than with the hyperaggressive strain.
Keywords: behavior, fighting, flies, learning, memory
A loser effect, an increased probability that a defeated animal will lose a subsequent fighting contest, has been demonstrated in Drosophila melanogaster. Shortly after experiencing a loss, a male Drosophila never wins a second fight against a familiar winner, unfamiliar winner, or socially naïve opponent. When two losers are paired 30 min after their first fight, hierarchical relationships are rarely formed because the majority of fights do not escalate to mid- or high intensity (1). Although defeated Drosophila lose subsequent fighting contests, it's unclear whether flies also become less aggressive after losing experience.
Behavioral changes resulting from social defeat are prevalent throughout the animal kingdom (2). Resident hamsters normally display territorial aggression toward an intruding hamster placed into their cage; however, after repeated defeats, residents behave defensively and will flee from an intruder (3). A similar behavioral phenomenon has also been documented in invertebrates. Male crickets do not reengage in aggressive encounters after experiencing defeat (4), and the aggression intensity of American lobsters is reduced for at least 1 d after repeated defeats (5). In addition to promoting submissive behavior in subsequent fighting contests, chronic social defeat often causes changes unrelated to aggression such as weight loss and decreased mating and feeding behaviors. These observations have prompted the use of social defeat to model depression- and anxiety-like states in animals (6–9).
Although it has been well documented that prior experience influences a variety of behaviors in Drosophila (10, 11), it is also true that behavior is affected by genetic background. To what extent genes and experience contribute to fighting behavior in Drosophila is unknown. Here, we bred a strain of hyperaggressive flies by selecting winners of male fights over 34–37 generations. Males of this strain were indeed more aggressive than the nonselected Canton-S strain, but the intensity of aggression displayed by females did not differ from the parent line. Although these hyperaggressive flies were endowed with superior fighting ability and routinely won fights against Canton-S, they were poorer courters compared with the nonselected parent line. After a defeat, these hyperaggressive flies became much less aggressive, lost their subsequent fighting contests against socially naïve counterparts, and lost all competitive advantage over Canton-S.
Results
Bullies Are More Aggressive than Nonselected Canton-S.
Beginning with the wild-type Canton-S strain of D. melanogaster, highly aggressive “bullies” were bred by the following selection procedure. Two to four males were placed in a fighting chamber, and the winners of fighting contests that escalated to high-intensity boxing and tussling behaviors were selected. Single male winners were then mated to virgin female siblings, and their progeny were used in the next round of selection. Experiments described here were performed with bullies selected for 34–37 generations. Similar selection schemes were used by other investigators to produce highly aggressive flies (12, 13).
Bullies were indeed substantially more aggressive than the nonselected Canton-S parent population. To illustrate, retaliatory behavior (lunging back after receiving a lunge) often leads to high-intensity boxing and tussling behaviors. Although observed in only 23% of Canton-S pairings, retaliation occurred in 69% of fighting contests between two male bullies (Fig. 1A). Bullies also lunged sooner than Canton-S. The first lunge in a fight between two Canton-S males occurred after an average of 8.1 encounters (meetings between the flies), whereas bullies lunged after an average of 3.4 encounters (Fig. 1B). Moreover, as expected for a hyperaggressive strain, when paired in fights against nonselected Canton-S flies, bullies won 94% of the fighting contests (see below). Bullies even won 81% of their fights against flies from a strain that was recently acquired from the wild (see Fig. 3B).
Fig. 1.
Male bullies are more aggressive than Canton-S; females are not. (A) Twenty-three percent of Canton-S males and 69% of bullies retaliated in dyadic fighting contests (two-tailed χ2). (B) Canton-S males lunged after an average of 8.1 encounters; bullies lunged after an average of 3.4 encounters (Mann–Whitney). (C and D) Canton-S vs. Canton-S and Bully vs. Bully female fights. (C) No difference in number of attacks (i.e., shoves and headbutts) between bully and Canton-S females (Mann–Whitney). (D) Canton-S females began fighting after an average of 21.6 s; bully females began fighting after an average of 64.9 s (Mann–Whitney). (E and F) Canton-S vs. Bully female fights. (E) No difference in number of attacks between bully and Canton-S (Mann–Whitney). (F) No difference in latency to attack between bully and Canton-S (Mann–Whitney). ─, mean;  , SEM. ***P < 0.001; *P < 0.05; NS, not significant. n values are indicated by the numbers in parentheses above the bars or data points.
, SEM. ***P < 0.001; *P < 0.05; NS, not significant. n values are indicated by the numbers in parentheses above the bars or data points.
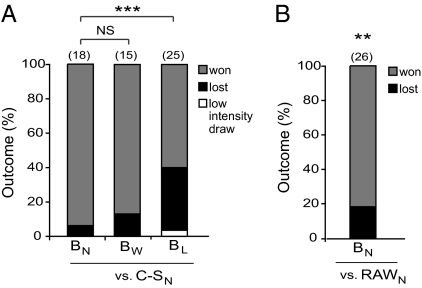
Fig. 3.
Bullies lose their advantage over Canton-S after a loss. (A) Ninety-four percent of naïve bullies won against naïve Canton-S. Outcomes of winner bullies against naïve Canton-S did not differ from naïve bullies, but only 60% of loser bullies won against naïve Canton-S (two-tailed χ2). A low-intensity draw occurred when flies displayed little to no lunging and a dominance relationship did not form within 30 min. Low intensity draw was grouped with losses for statistical analysis. (B) Naïve bullies won 81% of their fights against naïve flies recently acquired from the wild. For statistical analysis, outcomes were compared with expected values of 50/50 in two-tailed χ2 analysis. Bw, winner bullies; BL, loser bullies; BN, naïve bullies; C-SN, naïve Canton-S; RAWN, naïve flies from a strain recently acquired from the wild (Materials and Methods); ***P < 0.001; **P < 0.01; NS, not significant. n values are indicated by numbers in parentheses above the bars.
Female Bullies Are Not Hyperaggressive.
Although our selection process for bullies was male-specific, it was possible that the traits we selected for by this procedure were sex-nonspecific. In such a case, we would expect female bullies to be hyperaggressive also. Two different approaches were taken to address this issue. In the first, fights between two bully females were compared with fights between pairs of wild-type Canton-S females. Earlier studies have shown that Canton-S females do not form dominance relationships, lunge, box, or tussle (14–16). We found that female bullies are similar to Canton-S in this regard. Because females fight differently than males, it was necessary to use an alternative set of parameters to score aggression intensity. To measure female aggressiveness, we scored the number of attacks (i.e., shoves and headbutts) and the latency to attack. Fig. 1C demonstrates that there is no difference in the number of attacks between bully pairs and Canton-S pairs. Bullies attack each other an average of 14.1 times in 30 min, and Canton-S attack 13.8 times on average. There was a very small but significant difference between bullies and Canton-S females for the latency to attack (P = 0.048), but it was the Canton-S females who attacked sooner (Fig. 1D). In a parallel set of experiments, we paired female bullies in fights against Canton-S and again found no significant difference between bullies and Canton-S females for the number of attacks (Fig. 1E). In these pairings, we observed no difference in the latency to attack between the two strains (Fig. 1F). Taken together, our data indicates that female bullies are not hyperaggressive and that we have selected a male-specific trait.
Hyperaggressive Flies Suffer Consequences of Social Defeat.
To determine whether hyperaggressive flies suffer any consequences of social defeat, we analyzed the behavior of bullies in second fights. We began by pairing two socially naïve bullies in standard fighting chambers for 90 min. Thirty minutes later, winners and losers from the first fights fought a second fight against an age- and size-matched socially naïve bully. Fig. 2A demonstrates that 89% of the bullies that were defeated in their first fight (loser bullies) also lost their second fight. Winner bullies, by contrast, were equally likely to win or lose their second fights against a socially naïve bully (Fig. 2A).
Fig. 2.
Bullies lose fights and become less aggressive after social defeat. (A) Second fight outcomes of winner and loser bullies paired against an age- and size-matched socially naïve bully. Losers: 89% lost, 11% resulted in a high-intensity draw. Winners: 38% lost, 33% won, and 29% resulted in a high-intensity draw. Winner/loser outcomes were determined by flies forming a clear dominance relationship: The winner gained control of the food cup territory by lunging and chasing the loser off of the territory. A high-intensity draw occurred when flies engaged in boxing and tussling behaviors, but a dominance relationship did not form within 30 min. For statistical analyses, outcomes were compared with expected values of 50/50 in two-tailed χ2 analysis. High-intensity draw was grouped with wins for statistical analyses. (B–D) Loser flies became less aggressive after a loss. Bullies who lost both fights (BL-L) lunged and retaliated less in fight 2 compared with fight 1. The lunging and retaliatory behaviors quantified below occurred before the establishment of a dominance relationship. (B) Sixty-five percent of BL-L retaliated in fight 1, but only 29% retaliated in fight 2 (two-tailed χ2). (C) The number of lunges by BL-L decreased in fight 2 (Wilcoxon signed-rank test). Solid lines indicate flies that lunged less in fight 2 compared with fight 1; dotted lines indicate flies that lunged more. (D) Seventy-six percent of BL-L lunged in fight 1, but only 35% lunged in fight 2 (two-tailed χ2). (E) Of the flies that engaged in high-intensity boxing and tussling during fight 1, 88% of winners and 33% of losers engaged in midintensity lunging during fight 2 (Fisher's exact test). Bw, winner bullies; BL, loser bullies, BN, naïve bullies; ***P < 0.001; **P < 0.01; *P < 0.05, NS, not significant. n values are indicated by numbers in parentheses above bars or data points.
Because the results show that even inherently strong fighters lose subsequent fights after experiencing defeat, it might be that defeated flies become less aggressive. To test this idea, bullies who lost both fights (BL-L) were examined for behavioral changes between fight 1 and fight 2. Indeed, we found that BL-L retaliated and lunged less in their second fights. Whereas 65% of BL-L retaliated in fight 1, only 29% retaliated in fight 2 (Fig. 2B). Similarly, BL-L lunged less in their second fights compared with first fights (Fig. 2C). Moreover, although the majority of BL-L lunged during first fights, only 35% of BL-L lunged after a loss (Fig. 2D).
In a final experiment evaluating levels of aggressiveness, we compared winner and loser bullies directly to look for behavioral changes after fighting experience. Because bullies often escalate to boxing and tussling behaviors, we compared only the flies who displayed these high-intensity fighting patterns during their first fight. As expected, winner and loser bullies initially had similar inherent fighting abilities: 81% of winners and 79% of losers escalated to boxing and tussling during fight 1. We then asked, how many of the flies who did escalate to high intensity in fight 1, engaged in mid-intensity lunging during fight 2. Whereas 88% of the winners lunged in their second fights, only 33% of the losers displayed any mid-intensity lunging behavior (Fig. 2E). Together, these results demonstrate that social defeat causes decreased aggression in formerly hyperaggressive Drosophila males (Movie S1).
Bullies Lose Competitive Advantage over Canton-S After Experiencing a Loss.
Because bullies routinely win fights against age- and size-matched Canton-S but lose to naïve bullies after experiencing a loss, we next asked what happens when loser bullies are paired with socially naïve Canton-S in their second fights. In these experiments, two naïve bullies fought for 90 min, and 30 min later loser bullies were paired with an age- and size-matched socially naïve Canton-S male. Although the outcome of winner bullies against naïve Canton-S did not differ from that of naïve bullies, we found that loser bullies no longer had a competitive advantage against Canton-S. Forty percent of the loser bullies lost or drew their next fights against naïve Canton-S males (Fig. 3A).
Hyperaggressive Flies Court and Copulate Less than Canton-S.
Casual observations of bullies in the standard fighting chamber indicated that they seemed to spend less time courting compared to the Canton-S parent strain, suggesting an inverse relationship between aggression and courtship behavior. To test this idea, we compared the behavior of bullies to that of Canton-S in classical courtship experiments. In these experiments, a live Canton-S virgin female was paired with either a bully or Canton-S male in a standard courtship chamber. No significant difference was observed for courtship vigor index (CVI) between Canton-S and bullies (Fig. 4A). Although the percentage of males that initiated courtship was the same between Canton-S and bullies (Fig. 4B), bullies began courting significantly sooner than Canton-S (Fig. 4C). Despite having a head start however, bullies were less successful copulators than Canton-S. Whereas every Canton-S male that initiated courtship eventually copulated, 19% of the bullies failed to copulate with virgin females (Fig. 4D). Of those that succeeded in copulation, no significant difference was found for copulation latency between the two strains.
Fig. 4.
Bullies are poorer courters than Canton-S. Courtship behavior of bullies was compared with Canton-S in classical courtship assays (A–D) and in a courtship competition assay (E–H). (A) No difference was found between bullies and Canton-S for courtship vigor index (CVI) in the classical assay. Average CVI for Canton-S = 0.82; bullies = 0.78 (Mann Whitney). (B) No difference between bullies and Canton-S for courtship initiation in the classical assay. Canton S initiated courtship in 91% of the assays; bullies initiated in 88% (two-tailed χ2). (C) Bullies had a shorter courtship latency (average 191 s) compared with Canton-S (average 424 s) in the classical assay (Mann–Whitney). (D) One hundred percent of the Canton-S males that initiated courtship copulated with the female, but only 81% of the bullies copulated. (E) In the competition assay, Canton-S had a higher CCVI (average CCVI = 0.192) than bullies (average CCVI = 0.085) (Mann–Whitney). (F) Ninety-seven percent of the Canton-S males and 70% of the bullies initiated courtship in the competition assay (two-tailed χ2). (G) No difference in courtship latency between Canton-S and bullies (Mann–Whitney). (H) Females copulated with the Canton-S male rather than the bully in 83% of the mating competition assays (two-tailed χ2). ─, mean;  , SEM; ***P < 0.001; **P < 0.01; *P < 0.05, NS, not significant. n values are indicated by numbers in parentheses above bars or data points.
, SEM; ***P < 0.001; **P < 0.01; *P < 0.05, NS, not significant. n values are indicated by numbers in parentheses above bars or data points.
In a second approach, we put bullies and Canton-S in direct competition with each other for a single live virgin female and determined how much time each male spent courting and fighting. Males engaged in fighting <10% of the time in this experimental situation, displaying mostly low-intensity behavioral patterns (wing flicking and fencing) in close proximity to the female. Consistent with our initial observations, Canton-S males spent twice as much time courting as bullies in the mating competition chamber (Fig. 4E). Furthermore, 30% of the bullies never initiated courtship in the competition assay, whereas only 3% of the Canton-S males failed to initiate (Fig. 4F). Of the males that did initiate courtship, no significant difference was found for courtship latency (Fig. 4G). In addition to bullies courting less than Canton-S, females preferentially copulated with Canton-S in 83% of the mating competition assays (Fig. 4H). In a similar experiment, Dierick and Greenspan (12) reported that females were twice as likely to copulate with nonselected Canton-S than with their strain of highly aggressive flies. Again, as was the case with the classical courtship assays, of the males that copulated in the competition assays, no significant difference was found for copulation latency. Taken together, our data indicates that bullies are less successful copulators, especially when in competition with Canton-S males. This effect might be due, in part, to bullies spending less time courting females.
Discussion
Many different factors contribute to the outcome of fights including age, size, inherent fighting ability, and previous fighting experience (2, 17–20). The availability of a highly aggressive strain of Drosophila derived from wild-type Canton-S makes it possible to weigh the contributions of different factors in determining the outcome of fly fights. Experiments reported here show that genetic inbreeding can produce highly aggressive flies who will win an overwhelming majority of fights against Canton-S because of their superior fighting ability. Their exceptional capabilities are labile, however, in that these flies become much less aggressive after a single loss. Defeated bullies routinely lose fights against socially naïve bullies and have no competitive advantage over the less aggressive parent Canton-S strain. These experiments demonstrate that both inherent ability and fighting experience influence the outcome of fights. By examining both factors in these experiments, we were able to demonstrate that fight-induced losing experience has a profound influence on the outcome of subsequent fights, trumping even the important influences that genetic inbreeding has had on fighting ability. After defeat, the majority of flies that had engaged in high-intensity boxing and tussling in first fights no longer escalated to mid-intensity lunging in their second fights. This dramatic decrease in aggression demonstrates that even flies with superior fighting abilities are subjected to the consequences of social defeat. Because aggression is both time- and energy-consuming, the ability to disengage from a fight would be advantageous for an animal that learns he is an inferior fighter, which might explain why so many species throughout the animal kingdom suffer consequences of social defeat (21).
Over the course of 34–37 generations, highly aggressive flies were bred that routinely win fights against flies from both the common laboratory Canton-S strain and a strain recently acquired from the wild. If bullies are more aggressive than flies normally found in the wild, the possibility is raised that there might be an evolutionary cost associated with hyperaggression. Consistent with this hypothesis, we found that bullies are less successful at courtship compared with the parent Canton-S strain. When competing for a single virgin female, 1/3 of the bullies fail to initiate courtship. Those that do initiate spend half as much time courting as their Canton-S opponents. Decreased courtship activity by bullies explains, at least in part, why females were five times more likely to copulate with Canton-S males in the competition assay. Bullies were also less successful than Canton-S in classical courtship assays in which a single virgin female was paired with a single male. Even without the presence of a competitor, one of five bullies did not successfully copulate with the female. These courtship behavior studies of bullies and Canton-S suggest that there is an evolutionary tradeoff between hyperaggression and mating success. Alternatively, the inverse relationship between aggression and courtship could be due to genetic linkage, inbreeding, or genetic drift because only one line of hyperaggressive flies was tested in these studies. However, we argue that genetic drift and inbreeding are unlikely to be a factor given that a previous report (12) also found a negative correlation between hyperaggression and copulation success. Because losing experience decreases aggression even in hyperaggressive flies, it would now be interesting to see if loser bullies perform better than winner and naïve bullies in courtship competition assays. Further experiments will be necessary to address this issue.
Materials and Methods
Generation of Hyperaggressive Flies.
Beginning with wild type Canton-S, hyperaggressive bullies were bred by selecting winners for 34–37 generations. Two to four males were placed in a standard fighting chamber, and the winners of fighting contests that escalated to high-intensity boxing and tussling behaviors were selected. Three to five crosses were set up between single male winners and 8–15 virgin female siblings; their progeny were used in the subsequent round of selection. Nonselected Canton-S controls were passed each generation.
Animal Care and Fighting Assays.
Animal care, the standard fighting chamber, and the standard fighting assay have been described (1, 14, 22). Briefly, flies were grown in temperature- and humidity-controlled incubators (25 °C, 50% humidity) on a 12 h light/dark cycle. Because grouped housing conditions decreases aggression (23), pupae were isolated ≈24 h before eclosion and housed in individual vials for 6 d before use in fighting assays. At least 1 d before fighting, a small dot of a water-based acrylic paint was applied to the dorsal thorax of the flies so that individuals could be easily identified. On the day of the fight, two socially naïve animals were gently aspirated together into the standard fighting chamber, and behavior was videotaped for 90 min. All behavior assays were performed during the circadian “activity” period (first three hours of the light cycle). The strain of flies recently acquired from the wild was obtained from the University of California at San Diego Drosophila Species Stock Center (stock number 14021–0231.55) and was originally isolated from Mill Creek, Arkansas, in 2007 by William Etges.
Social Defeat Assays.
For fight 1, two socially naïve males were placed in a standard fighting chamber for 90 min. After fight 1, flies were returned to their isolation vials for 30 min and then paired for 90 min with an age- and size-matched socially naïve bully or Canton-S male for fight 2. Fights were videotaped, and tapes were scored blindly. Winner/loser outcomes were determined once flies formed a clear dominance relationship in which the winner gained control of the food cup territory by lunging and chasing the loser off of the territory. A draw occurred if a dominance relationship did not form within the first 30 min of the fighting contest. Approximately 13% of fights between two naïve bullies resulted in a high-intensity draw; in these cases, neither fly was used in a second fight.
Courtship Assays.
Male pupae were collected 24 h before eclosion and placed into individual vials containing cornmeal medium. Virgin female flies were selected on the same day and housed in larger vials in groups of ≈15 flies. Courtship assays were performed 4–6 d after collection.
Classical assay.
Canton-S or bully males were taken from isolation vials and aspirated into individual courtship chambers (3.5-cm2 watch glasses on top of glass plates; ref. 24) and kept alone for ≈5 min. One virgin female was then added to each chamber. Behavior was filmed for 90 min. Tapes were watched blindly and analyzed for courtship latency, copulation success, and courtship vigor index (CVI). Courtship latency was measured as the amount of time from the addition of the female into the courtship chamber until the male's first wing extension. Copulation was scored as successful if it was observed within the 90 min that the animals were paired. CVI is defined as the fraction of time the male spent courting from courtship initiation until copulation or the end of observation at 10 min (25).
Competition assay.
A mating competition chamber was designed by using a single well of a MULTIWELL 12-well plate (Becton Dickinson) containing a small food cup (cap from a microcentrifuge tube filled with standard cornmeal food and a dab of yeast paste). Up to 6 wells of a 12-well plate were used when filming the assays. One bully and one Canton-S male (age- and size-matched and socially naïve) were placed into the mating competition chamber. One virgin female was added to each chamber ≈1 min after the males, and behavior was filmed for 90 min. Tapes were watched blindly, and each male was analyzed for courtship latency, copulation success, and a competitive courtship vigor index (CCVI). The CCVI was defined as the fraction of time the males spent courting from the first social interaction (courtship or aggression) until copulation or after the 10-min observation period.
Supplementary Material
Acknowledgments
We thank Olga Alekseyenko, Sarah Certel, Yick-Bun Chan, María de la Paz Fernández, Adelaine Leung, Mireya Nadal-Vicens, Francesca Reindel, Kathy Siwicki, and Joanne Yew for many helpful discussions and comments on this manuscript. This work was supported by National Institute of General Medical Sciences Grants GM074675 and GM067645 (to E.A.K.), National Science Foundation Grant IOS-075161 (to E.A.K.), and National Institute of General Medical Sciences National Research Service Award F32GM082086 (to J.K.M.P.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007016107/-/DCSupplemental.
References
- 1.Yurkovic A, Wang O, Basu AC, Kravitz EA. Learning and memory associated with aggression in Drosophila melanogaster. Proc Natl Acad Sci USA. 2006;103:17519–17524. doi: 10.1073/pnas.0608211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu Y, Earley RL, Wolf LL. Modulation of aggressive behaviour by fighting experience: Mechanisms and contest outcomes. Biol Rev Camb Philos Soc. 2006;81:33–74. doi: 10.1017/S146479310500686X. [DOI] [PubMed] [Google Scholar]
- 3.Potegal M, Huhman K, Moore T, Meyerhoff J. Conditioned defeat in the Syrian golden hamster (Mesocricetus auratus) Behav Neural Biol. 1993;60:93–102. doi: 10.1016/0163-1047(93)90159-f. [DOI] [PubMed] [Google Scholar]
- 4.Hofmann HA, Stevenson PA. Flight restores fight in crickets. Nature. 2000;403:613. doi: 10.1038/35001137. [DOI] [PubMed] [Google Scholar]
- 5.Rutishauser RL, Basu AC, Cromarty SI, Kravitz EA. Long-term consequences of agonistic interactions between socially naive juvenile American lobsters (Homarus americanus) Biol Bull. 2004;207:183–187. doi: 10.2307/1543205. [DOI] [PubMed] [Google Scholar]
- 6.Avgustinovich DF, Gorbach OV, Kudryavtseva NN. Comparative analysis of anxiety-like behavior in partition and plus-maze tests after agonistic interactions in mice. Physiol Behav. 1997;61:37–43. doi: 10.1016/s0031-9384(96)00303-4. [DOI] [PubMed] [Google Scholar]
- 7.Berton O, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 8.Krishnan V, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Kudryavtseva NN, Bakshtanovskaya IV, Koryakina LA. Social model of depression in mice of C57BL/6J strain. Pharmacol Biochem Behav. 1991;38:315–320. doi: 10.1016/0091-3057(91)90284-9. [DOI] [PubMed] [Google Scholar]
- 10.Pitman JL, et al. There are many ways to train a fly. Fly (Austin) 2009;3:3–9. doi: 10.4161/fly.3.1.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siwicki KK, Ladewski L. Associative learning and memory in Drosophila: Beyond olfactory conditioning. Behav Processes. 2003;64:225–238. doi: 10.1016/s0376-6357(03)00137-2. [DOI] [PubMed] [Google Scholar]
- 12.Dierick HA, Greenspan RJ. Molecular analysis of flies selected for aggressive behavior. Nat Genet. 2006;38:1023–1031. doi: 10.1038/ng1864. [DOI] [PubMed] [Google Scholar]
- 13.Edwards AC, Rollmann SM, Morgan TJ, Mackay TF. Quantitative genomics of aggressive behavior in Drosophila melanogaster. PLoS Genet. 2006;2:e154. doi: 10.1371/journal.pgen.0020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nilsen SP, Chan YB, Huber R, Kravitz EA. Gender-selective patterns of aggressive behavior in Drosophila melanogaster. Proc Natl Acad Sci USA. 2004;101:12342–12347. doi: 10.1073/pnas.0404693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vrontou E, Nilsen SP, Demir E, Kravitz EA, Dickson BJ. fruitless regulates aggression and dominance in Drosophila. Nat Neurosci. 2006;9:1469–1471. doi: 10.1038/nn1809. [DOI] [PubMed] [Google Scholar]
- 16.Zhou C, Rao Y, Rao Y. A subset of octopaminergic neurons are important for Drosophila aggression. Nat Neurosci. 2008;11:1059–1067. doi: 10.1038/nn.2164. [DOI] [PubMed] [Google Scholar]
- 17.Chase ID, Tovey C, Spangler-Martin D, Manfredonia M. Individual differences versus social dynamics in the formation of animal dominance hierarchies. Proc Natl Acad Sci USA. 2002;99:5744–5749. doi: 10.1073/pnas.082104199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoefler CD. Is contest experience a trump card? The interaction of residency status, experience, and body size on fighting success in Misumenoides formosipes (Araneae: Thomisidae) J Insect Behav. 2002;15:779–790. [Google Scholar]
- 19.Hoyer SC, et al. Octopamine in male aggression of Drosophila. Curr Biol. 2008;18:159–167. doi: 10.1016/j.cub.2007.12.052. [DOI] [PubMed] [Google Scholar]
- 20.Schuett GW. Body size and agonistic experience affect dominance and mating success in male copperheads. Anim Behav. 1997;54:213–224. doi: 10.1006/anbe.1996.0417. [DOI] [PubMed] [Google Scholar]
- 21.Rutte C, Taborsky M, Brinkhof MW. What sets the odds of winning and losing? Trends Ecol Evol. 2006;21:16–21. doi: 10.1016/j.tree.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Chen S, Lee AY, Bowens NM, Huber R, Kravitz EA. Fighting fruit flies: A model system for the study of aggression. Proc Natl Acad Sci USA. 2002;99:5664–5668. doi: 10.1073/pnas.082102599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Dankert H, Perona P, Anderson DJ. A common genetic target for environmental and heritable influences on aggressiveness in Drosophila. Proc Natl Acad Sci USA. 2008;105:5657–5663. doi: 10.1073/pnas.0801327105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siwicki KK, et al. The role of cuticular pheromones in courtship conditioning of Drosophila males. Learn Mem. 2005;12:636–645. doi: 10.1101/lm.85605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krstic D, Boll W, Noll M. Sensory integration regulating male courtship behavior in Drosophila. PLoS ONE. 2009;4:e4457. doi: 10.1371/journal.pone.0004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.