Abstract
Growth factors are implicated in several processes essential for cancer progression. Specifically, growth factors that bind to ErbB family receptors have been implicated in cell proliferation and in resistance of solid tumors to chemotherapy. We quantified ligand secretion by several human cancer cell lines, and generated mAbs against two ligands, namely TGF-α and heparin-binding EGF-like growth factor. These growth factors are frequently secreted by pancreatic tumor cell lines, including BxPC3 cells. The monoclonal antibodies were tested for their antigen specificity and ability to inhibit growth of BxPC3 cells in vitro. Combining the two antibodies resulted in enhanced inhibition of BxPC3 cell growth, both in vitro and in tumor-bearing animals. Hence, we combined the two antibodies with gemcitabine, an effective chemotherapeutic drug commonly used to treat pancreatic cancer patients. Because treatment with a combination of two monoclonal antibodies enhanced the ability of chemotherapy to inhibit BxPC3 tumors in mice, we propose a general cancer therapeutic strategy that entails profiling the repertoire of growth factors secreted by a tumor, and combining with chemotherapy several antibodies capable of blocking autocrine ligands.
Keywords: cancer therapy, growth factor, monoclonal antibody, signal transduction
The ErbB family of receptors and their ligands play important roles in development, as well as in tissue remodeling, throughout adulthood. Ligand-binding is followed by receptor dimerization and phosphorylation, and results in various cellular processes, including proliferation (1). There are four ErbB receptors. ErbB-1 (EGFR) binds seven growth factors, including the EGF, TGF-α, heparin-binding EGF-like growth factor (HB-EGF), and amphiregulin (AR) (2). ErbB-3 and ErbB-4 bind a distinct group of growth factors called neuregulins (NRGs) (3), but ErbB-2/HER2 has no known ligand (4, 5). All ErbB-family ligands share a 50 to 60 amino acid-long sequence containing six cysteines (6). EGF-like ligands bind and activate receptors on distant cells, neighboring cells, or on the cells of their origin, mechanisms termed endocrine, paracrine, or autocrine, respectively.
ErbB proteins are involved in several types of human cancer. Clinical studies indicate that overexpression of one or more ligands correlates with decreased patient survival; for example, expression of TGF-α in colorectal tumors is associated with a greater than 50-fold increased risk of liver metastases (7). In bladder cancer, the elevated expression of a number of ligands is linked to decreased patient survival (8). Likewise, increased expression of TGF-α in head and neck tumors is correlated with decreased survival (9). Moreover, tumor cell expression of some ligands is associated with resistance to chemotherapeutic drugs (10, 11). Despite these observations, the currently approved drugs for the treatment of cancers driven by the ErbB family target the receptors, rather than the ligands, and they include either monoclonal antireceptor antibodies, or tyrosine kinase inhibitors (TKIs) (12, 13).
Acquired resistance, often associated with up-regulation of ligands (14–16) or other receptors (17–20), limits efficacy of anti-ErbB drugs. Human breast-cancer cells selected in vivo for resistance to trastuzumab overexpress EGFR and ErbB ligands (18). Because of resistance and moderate clinical efficacies of antireceptor antibodies and TKIs, it is worthwhile considering alternative strategies. For example, fractions of colorectal and other tumors respond to bevacizumab, an antibody that binds the vascular endothelial growth factor (21, 22). The multiplicity of EGF-like ligands, along with extensive receptor-receptor interactions, are potential impediments to similar applications of antiligand antibodies. Nevertheless, because the repertoires of autocrine growth factors secreted by individual tumors is often limited to two to three ligands, we assumed that it would be feasible to profile the spectrum of ligands secreted by specific carcinomas, combine the respective antigrowth factor antibodies, and test their combination with chemotherapy. Here, we focused on TGF-α and HB-EGF. In parallel to the generation of antagonistic antibodies, we screened human tumor cell lines and identified a pancreatic line that secretes both growth factors. We describe in vitro assays and tests in animals, collectively supporting the feasibility of a “tailored” immunotherapeutic strategy able to enhance the effect of chemotherapy. This study opens the way for combinations of antibody pairs with receptor antagonists, such as kinase inhibitors.
Results
As a prelude to testing our working hypothesis, we examined secretion of different EGF-like ligands by tumor cell lines. For this purpose, we used an immunological kit able to detect EGF, TGF-α, HB-EGF, and AR. The assay was performed on media conditioned by 13 carcinoma cell lines of a wide variety of tumors, such as ovary, breast, lung, and pancreas (Table 1). As expected, the assay detected distinct combinations of growth factors and wide ranges of expression levels. Notably, unlike HB-EGF, AR, and TGF-α, which were abundantly secreted by several tumor cell lines, EGF was either absent or very low. Because an alternative assay that used real-time PCR and mRNA isolated from BxPC3 cells confirmed high expression of TGF-α and HB-EGF, but did not detect comparable levels of AR, NRG, betacellulin, and epiregulin, we next aimed at the generation of mAbs capable of blocking the action of HB-EGF and TGF-α.
Table 1.
In vitro secretion of EGF-like ligands by human cancer cell lines
| Cell line | Tumor type | TGF-α | EGF | HB-EGF | AR |
| MDA-MB-231 | Breast | 111 ± 1 | — | 617 ± 28 | 1607 ± 171 |
| MDA-MB-468 | Breast | — | — | 64 ± 7 | — |
| SKBR3 | Breast | — | 4.8 ± 1.0 | — | 34 ± 6 |
| A-431 | Epidermoid | 13 ± 2 | — | 223 ± 10 | 82 ± 18 |
| H1437 | Lung | 27 ± 3 | — | 61 ± 27 | 2,121 ± 85 |
| OVCAR3 | Ovary | 13 ± 10 | — | 105 ± 34 | 276 ± 21 |
| SKOV3 | Ovary | — | — | — | 28 ± 12 |
| TOV112 | Ovary | — | — | 83 ± 4 | — |
| BxPC3 | Pancreas | 21 ± 0 | — | 213 ± 62 | 3,306 ± 50 |
| MiaPaCa | Pancreas | — | — | 134 ± 42 | 3,794 ± 15 |
| PANC-1 | Pancreas | — | — | 148 ± 32 | 201 ± 13 |
| DU145 | Prostate | 26 ± 3 | — | 127 ± 26 | 2,575 ± 199 |
| PC3 | Prostate | 14 ± 10 | 2.9 ± 2.1 | 325 ± 54 | 3,822 ± 33 |
The indicated cell lines (1 × 106) were seeded in 10-cm plates, covered with 8 mL medium, and incubated for 4 d. Media were collected and ligand quantified by using the Duoset kit from R&D Systems. Ligand concentrations are indicated in pg/mL± SDs.
Cloning, Expression, and Biological Activity of EGF-Like Ligands.
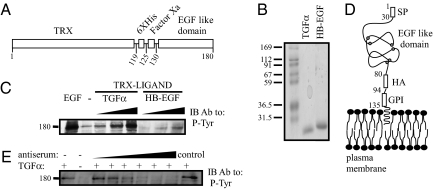
It is known that highly conserved three-disulfide bonds are responsible for the correct folding and activity of the EGF-like domain of all ErbB ligands (23). Therefore, we chose to express the EGF-like domain of EGFR-specific ligands as fusion proteins, linked to the thioredoxin protein (TRX) (Fig. 1A). The fused protein also contained a histidine repeat, as well as a factor Xa cleavage site, to enable specific release of the EGF-like domain. Following expression in bacteria, the two ligands were purified using a metal column (Fig. 1B), and their biological activity was verified by confirming their ability to induce EGFR phosphorylation (Fig. 1C). The results we obtained ensured that the recombinant ligands represented the respective functionally active conformation. Hence, mice were subsequently immunized with the active TRX-fused ligands.
Fig. 1.
Construction, expression and biological activity of recombinant EGF-like ligands. (A) A scheme of an EGF-like chimeric protein, including a thioredoxin (TRX) domain, a histidine box (6XHis), a flanking Factor Xa cleavage site, and a carboxyl-terminal EGF-like domain. Residue numbers are indicated. (B) Coomassie blue staining of an acrylamide gel showing purified TGF-α and HB-EGF prepared using bacterial expression and purified on a NiNTA column. Molecular weight markers are indicated in kilodaltons. (C) Cells were seeded in a 24-well plate, washed, and incubated with increasing concentrations of the purified fusion proteins (TRX–TGF-α: 2, 20, 200 ng/mL; TRX–HB-EGF: 3, 30, 300 ng/mL). EGF (10 ng/mL) was used as a positive control. After a 10-min long incubation, the cells were lysed, and cleared extracts immunoblotted (IB) with an antiphosphotyrosine (P-Tyr) antibody. (D) A scheme presenting the domain structure of a generic GPI-fusion protein comprising the HER2’s signal peptide (SP), the EGF-like domain of TGF-α (or HB-EGF), an HA-peptide tag, and a GPI-lipid anchor. Residue numbers refer to TGF-α. Cysteine residues of the EGF-like domain are highlighted. (E) Cells were seeded in a 24-well plate, washed, and coincubated with or without TGF-α (5 ng/mL), and an antiserum from TGF-α-immunized mice (0.2, 2, 4, 6, 8, and 10 μL of serum diluted in 120 μL total; control: 10 μL serum from a naive animal). An antiphosphotyrosine mAb was used to detect phosphorylated EGFRs.
Generation of an Antagonistic Antibody Directed Against the EGF-Like Domain of TGF-α.
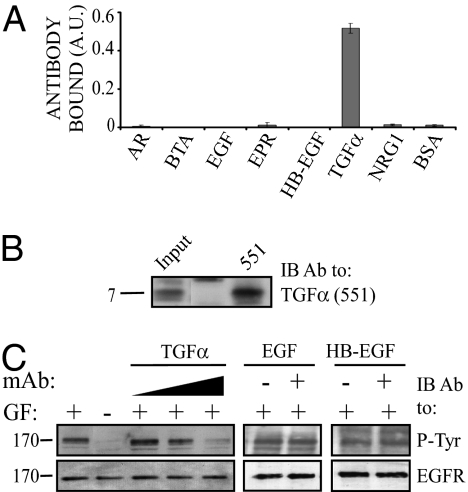
Following four injections of the TRX–TGF-α fusion protein, sera were obtained from mice and examined for antiligand responses. To facilitate screening of antisera and hybridoma supernatants, we established a CHO cell line that expresses the EGF-like domain of TGF-α at the plasma membrane. For this purpose, the EGF-like domain was fused to a signal peptide, an HA-peptide tag, and a GPI (glycosyl phosphatidylinositol) anchor motif, which is responsible for lipid-based anchoring at the plasma membrane (Fig. 1D). Antisera of immunized mice were tested for their ability to inhibit ligand-induced EGFR phosphorylation (Fig. 1E). Subsequently, the spleens of two mice were used to establish hybridomas, which were screened for their ability to recognize cell surface-exposed GPI–TGF-α fusion protein. To functionally characterize a selected anti–TGF-α mAb, denoted mAb-551, we tested its specificity using an ELISA assay. This assay confirmed binding to TGF-α but not to six other ligands we tested (Fig. 2A). Furthermore, the anti–TGF-α mAb could immunopercipatate a commercial preparation of TGF-α (Fig. 2B) and specifically inhibit TGF-α–induced EGFR phosphorylation (Fig. 2C). Notably, neither EGF- nor HB-EGF–induced activation of EGFR were inhibited. Taken together, these results establish suitability of mAb-551 for attempts to intercept TGF-α-mediated autocrine loops.
Fig. 2.
Specificity and functional tests of an anti–TGF-α mAb. (A) The 96-well plates were coated with the indicated ligands (0.1 ng/mL) and then incubated for 3 h with an anti–TGF-α mAb-551. Thereafter, wells were incubated for 2 h with an anti-mouse antibody conjugated to HRP, followed by a 30-min incubation with ATBS. Signals were determined using an ELISA reader (set at 420 nm). (B) Anti-TGFα mAb-551 antibody was used to immunopercipitate TGF-α (200 ng; Sigma) using beads conjugated to anti-mouse Fc antibodies. Immune-complexes were blotted with mAb-551. A molecular weight marker (7 kDa) is indicated. (C) Cells were seeded in a 24-well plate, and after 10 h of incubation they were washed and incubated for 10 min with or without the indicated ligands, and with increasing concentrations of an anti–TGF-α mAb (15, 80, and 160 μg/mL). Thereafter, cells were lysed and cleared extracts immonublotted with antibodies to phosphotyrosine or to EGFR.
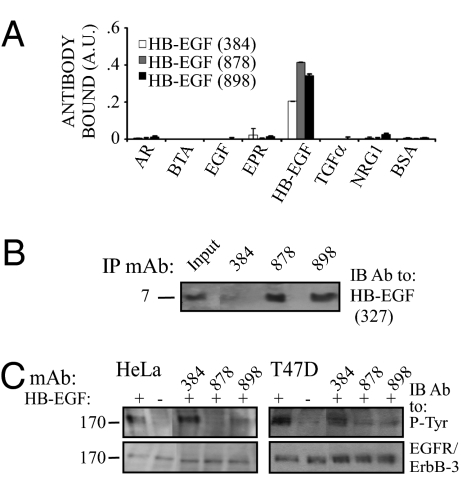
Generation of an Antagonistic Antibody Directed Against the EGF-Like Domain of HB-EGF.
To enable combination treatments, we similarly generated mAbs to another major growth-stimulating ligand, HB-EGF. Using three mAbs to HB-EGF and an immobilized HB-EGF, we confirmed antigen specificity, along with absence of cross-reactivity (Fig. 3A). In the next step, we confirmed the ability of two positive hybridoma clones to immunoprecipitate a commercial preparation of HB-EGF (Fig. 3B). In addition, by testing HB-EGF-induced tyrosine phosphorylation in HeLa and in T47D mammary cancer cells, we concluded that the three mAbs to HB-EGF variably inhibited ligand-induced receptor activation (Fig. 3C). These functional assays selected mAb-898 for further studies in vitro and in animals.
Fig. 3.
Specificity and functional tests of anti HB-EGF mAbs. (A) The 96-well plates were coated with the indicated ligands, incubated with anti–HB-EGF mAbs, and processed as in Fig. 2A. (B) The indicated mAbs were used to immunopercipitate HB-EGF (Sigma) using beads conjugated to anti-mouse Fc antibodies. Immune-complexes (or purified HB-EGF; Input) were immunoblotted with mAb-327 to HB-EGF. A molecular weight marker is indicated. (C) Sparse monolayers of HeLa or T47D cells were washed and coincubated for 10 min with HB-EGF (3 ng/mL) and the indicated anti–HB-EGF mAbs. Thereafter, cells were lysed and cleared extracts immunoblotted with an anti-phosphotyrosine, an anti-EGFR (HeLa) or an anti ErbB-3 mAbs (T47D).
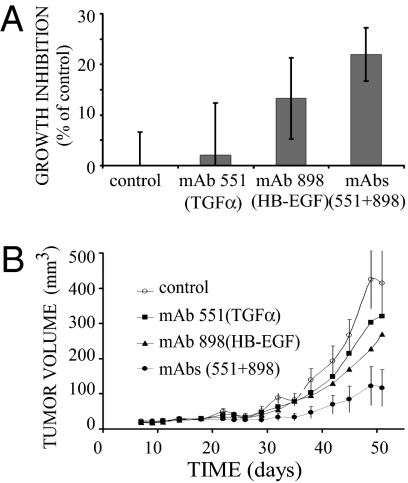
Growth-Inhibitory Activities of Individual Antibodies and Their Combination.
The availability of antagonistic mAbs to TGF-α and to HB-EGF, along with the finding that BxPC3 pancreatic tumor cells secrete both ligands (Table 1), prompted us to test our working hypothesis, assuming that a combined treatment targeting two autocrine loops would inhibit tumorigenic growth of BxPC3 cells. To this end, we quantified in vitro proliferation of BxPC3 cells in the presence of each mAb alone, or a combination of the two antibodies. The results, presented in Fig. 4A, demonstrate that the combination of two antibodies reached statistical significance compared with the untreated control (P value of 1.23 × 10−11) or to the effect elicited by each antibody alone (P value for TGF-α: 5.01 × 10−6; P value for HB-EGF: 4.54 × 10−3). It is notable that mAb-898 to HB-EGF, when singly applied, elicited a reproducible and statistically significant inhibitory effect (P value of 7.85 × 10−6), but the effect of mAb-551 to TGF-α reached no statistical significance.
Fig. 4.
A combination of anti–TGF-α and anti–HB-EGF mAbs effectively inhibits growth of human pancreatic cancer cells, both in vitro and in animals. (A) BxPC3 cells (2 × 104) were seeded in a 96-well plate and allowed to adhere overnight, before the addition of anti–TGF-α or anti–HB-EGF mAbs (each at 15 μg/mL), or a mixture of both mAbs (each at 15 μg/mL). Inhibition of cell growth was determined in hexaplicates after 96 h using the MTT method. Averages ± SD are shown. The experiment was repeated twice. (B) Female nude mice were inoculated s.c. with BxPC3 cells (2 × 106). Once tumors became palpable (5–7 d), mice were randomized and i.p. injected with saline (control), an anti–TGF-α, or an anti–HB-EGF mAb (each at 250 μg per injection), or with a combination of both mAbs (each at 250 μg). Mice were treated with mAbs on days 9, 16, 20, 23, 26, 30, 33, 37, 40, and 44. The control group included 12 mice and each treatment group included 8 mice. Average volumes ± SD are shown.
Next, we checked in vivo the efficacy of a combined treatment, using xenografts of the pancreatic BxPC3 cells. Cells were injected s.c. and allowed to grow until palpable tumors appeared. The mice were then treated twice a week with either mAb or with the combination of anti–TGF-α and anti–HB-EGF mAbs (Fig. 4B). Remarkably, the combination of mAbs led to statistically significant enhancement of growth inhibition (P value of 0.009; calculated for the comparison of the effect of the combination of mAbs with control). We note that the in vivo activity of the pair of mAbs was more profound than in vitro, probably because of impacts on the stroma or immune cells. It is also noteworthy that proliferation rates of another pancreatic tumor cell line (MiaPaCa-2) and a lung cancer line (H1437) were also inhibited in vivo by the pair of mAbs, but monitoring the body weights of all mice we treated revealed no consistent effects of the mAbs or the combination. Hence, we concluded that combining antibodies to two growth factors induces a strong growth-inhibitory effect, which is associated with no apparent toxicity.
Combination of Antigrowth Factor Antibodies Sensitizes Tumors to Chemotherapy.
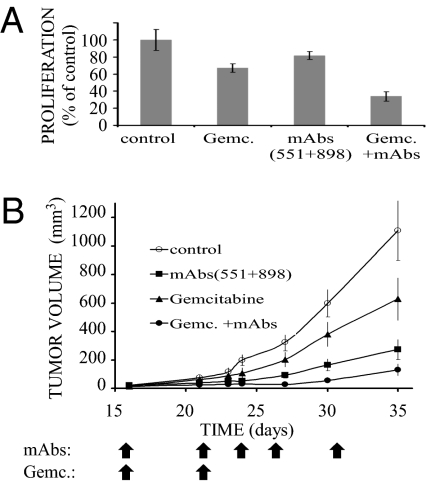
Our working hypothesis assumes that self-produced growth factors play essential roles in evolvement of resistance of pancreatic and other tumors to chemo- and radiotherapy. Hence, it is conceivable that blocking such autocrine loops will block escape mechanisms and resensitize tumors to the toxic effects of conventional therapies. As an initial test of this scenario, we examined in vitro the combined effect of two mAbs and gemcitabine, the mainstay chemotherapeutic drug of advanced pancreatic tumors (24). The results, presented in Fig. 5A, verify the ability of a mixture of antibodies to TGF-α and HB-EGF to augment the growth inhibitory effect of gemcitabine on cultured BxPC3 cells. Hence, our next experiment examined in animals the effect of a triple combination. Cells were injected s.c. and allowed to grow until palpable tumors appeared. Thereafter, the mice were treated twice (days 16 and 21) with gemcitabine (150 mg/kg body weight), and with or without the two mAbs. The results presented in Fig. 5B demonstrate that two injections of gemcitabine resulted in >85% inhibition of tumor growth, but repeated injections of a mixture of two mAbs consistently augmented the cytotoxic effects of the chemotherapeutic agent.
Fig. 5.
A combination of chemotherapy and two mAbs to growth factors effectively inhibits pancreatic cancer cells, both in vitro and in animal. (A) BxPC3 cells (2 × 104) were treated as in Fig. 4A, except that gemcitabine (0.5 ng/mL) was used, either alone or in combination with a mixture of anti–TGF-α and anti–HB-EGF mAbs (each at 10 μg/mL). Averages and SDs (bars) of hexaplicates are shown. The experiment was repeated twice. (B) Female nude mice (6 wk old) were s.c. inoculated with 2 × 106 BxPC3 cells. Once tumors became palpable (5–7 d), mice were randomized into groups. The first group (8 mice) was i.p. injected with a mixture of mAbs to TGF-α and to HB-EGF (each at 120 μg per injection; arrows). A second group (11 mice) was injected i.p. on days 16 and 21 with gemcitabine (150 mg/kg body weight; arrows), and a third group (6 mice) was treated with a combination of gemcitabine and mAbs. The control group (11 mice) was similarly treated with saline. Averages ± SD (bars) of tumor volume are shown.
In summary, by concentrating on BxPC3 pancreatic tumor cells that maintain several autocrine loops involving EGFR and at least two ligands, TGF-α and HB-EGF, we provide evidence supporting the notion that autocrine loops help tumors to evade the cytotoxic action of chemotherapeutic drugs like gemcitabine. If verified in additional tumor models, these observations offer a scenario of individualized cancer therapy. Accordingly, the autocrine loops operating in a specific tumor are first characterized using immunological or other assays. In the next step, antibodies capable of blocking the specific growth factors are combined with chemotherapy in a way that sensitizes tumors to cytotoxicity and delays onset of chemoresistance.
Discussion
The work presented here offers a general strategy for effective blockade of the tumorigenic action of ErbB-specific ligands, taking into account ligand multiplicity, tumor-specific distinct repertoires of ligands, and the roles played by such growth factors in frequent emergence of resistance to chemotherapy. Chemoresistance is a multifactorial phenomenon and a major clinical obstacle, which obliterates successful treatment of cancer patients. Under chemical stress, or upon irradiation, tumor cells overexpress several growth factors, including fibroblast growth factors, the macrophage growth factor (CSF-1), and members of the EGF family (25). Moreover, up-regulation of EGF-like ligands is considered an inherent part of the cellular response to growth factors, which establishes an auto-stimulatory feedback loop (26). Accordingly, tumors driven by dysregulated ErbB proteins, such as brain tumors expressing the EGFRvIII mutant (27) or RAS-transformed cells, which secrete large amounts of TGF-α and HB-EGF, may also display sensitivity to the triple drug combination we propose.
Motivated by a working hypothesis that attributes chemoresistance to secretion of several distinct EGF-like ligands, we designed an experimental three-step therapeutic strategy and tested it in animals. In the first step, the repertoire of EGF-like ligands secreted by an individual tumor is determined using PCR or paraffin-embedded tissue microarrays (28), which enables the next step, namely tailoring a combination of mAbs specific to the respective growth factors. In the last step, the selected mixture of antibodies is combined with a chemotherapeutic agent representing the mainstay of the respective clinical indication. For example, the study we reported focused on a pancreatic cancer cell line, hence we used gemcitabine, a chemotherapeuitc agent often used to treat pancreatic tumors (29). Although the clinical feasibility of the protocol we propose here is a matter of future studies, it is worthwhile noting that a combination of chemotherapy with an anti-VEGF antibody, bevacizumab, is clinically effective in colorectal (22) and in other carcinomas, and antibodies to another member of the VEGF family, placenta growth factor, showed promising results in animal models (30).
Several recent reports interested in mechanisms underlying evolvement of resistance to ErbB-targeted therapies (e.g., mAbs and TKIs), have identified EGF-like ligands as potential targets for treatments aimed at delaying the onset of resistance. For example, trastuzumab-resistant breast cancer cells exhibited higher levels of EGFR/ErbB-1, TGF-α, HB-EGF, and NRG (18). Similarly, it was shown that several chemoresistant cell lines were more sensitive to gefetinib, an anti–ErbB-1 TKI, and this correlated with altered ligand expression, as well as with constitutive phosphorylation of ErbB-2/HER2 and ErbB-3 (31). Another study characterized mRNA expression of EGF-like ligands before and following treatment of mammary tumor cells with an EGFR-specific TKI, and correlated resistance with ligand expression (32). Taken together, these studies predict that resistance to antibodies and TKIs directed at ErbB proteins is because of production of autocrine growth factors; hence, the combination protocol we propose may prevent not only chemoresistance, but also resistance to molecular targeted therapies.
In summary, we envision a tailor-made strategy for cancer treatment, which combines with chemotherapy two or more antibodies to tumor-specific autocrine growth factors. Accurate determination of the autocrine growth factors specific to each tumor is crucial for successful delay of resistance. Future studies will examine other types of carcinomas, along with antibodies to additional EGF-like peptides. Likewise, we are interested in the possibility raised by some recent observations (33, 34) that the treatment we envision may overcome not only resistance to chemo- and molecular-targeted therapy, but also to radiotherapy.
Materials and Methods
Materials and Cells.
Growth factors were from PeproTech Asia. NiNTA beads were from Novagen. ATBS [2,2'-Azino-bis (3-ethylbenzthiozoline-6-sulfonic acid)] and MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] were purchased from Sigma. Duo-set kits were from R&D Systems. Female athymic NCr-nude mice and female BALB/c mice were purchased from Harlen. Monoclonal antibodies to EGFR and ErbB-3 were generated in our laboratory. HRP-conjugated anti-mouse antibody was from Jackson Immuno Research Laboratories. Human cancer cell lines were purchased from the American Type Culture Collection. All animal procedures were approved by the Institute’s Review Board.
Cloning and Expression of EGF-Like Ligands in Bacteria and in Mammalian Cells.
EGF-like domains were cloned into the pET32b vector, and expressed as C-terminal TRX fusion proteins with a Factor Xa cleavage site flanking the N-terminal residue of the EGF-like domain. The fusion proteins were expressed in Escherichia coli (BL21) using standard procedures. Following sonication, cleared extracts were transfered to pre-equilibrated NiNTA beads. The beads were washed and then eluted with 300 mM immidazole. Construction of fusion proteins comprising a GPI motif was performed in two steps. The first PCR was performed on the GPI signal of the rat contactin-1. The 5′ primer introduced a NsiI cleavage site, which was followed by an HA tag, and the 3′ primer introduced a NotI site. The product was cloned into the pIRES-Hyg vector using NsiI and NotI restriction enzymes. The second step employed overlapping PCR. The first reaction used the signal peptide of HER2 as a template, and a 3′ primer that included the 5′ sequence of the respective EGF-like domain. The second PCR employed the respective EGF-like domain as a template, and a 5′ primer that included the 3′ end of the HER2 signal peptide. The products of both reactions served as templates for another PCR. The final PCR product was cloned into pIRES-Hyg-GPI, by using BamHI and NsiI cleavage sites. To establish clones of CHO cells, we transfected the corresponding pIERS-Hyg using Lipofectamine (Invitrogen) and selected clones using hygromycin (2 μg/mL).
Generation of Monoclonal Antibodies.
Five Female BALB/c mice (3 mo old) were injected s.c. and into the foot pad with 30 μg protein in complete Freund's adjuvant (Tifco). Three weeks later, a second injection was performed in incomplete Freund's adjuvant. This injection was followed by three to five injections at intervals of 3 wk. A month after the last boost, the two mice with the highest titer received two more injections on two consecutive days. Four days after the last boost, cells from each spleen were fused with 20 × 106 NS0/1 myeloma line as described (35). Following fusion, cells were distributed into 96-well plates, at concentration of 2 × 104 viable myloma cells per well. Hybrid cells were selected for growth in the presence of HAT. Positive hybrid cultures were weaned out of HAT, cloned and recloned in limiting dilution.
In Vitro Tests of mAb Binding to EGF-Like Ligands.
The 96-well plates were coated with the indicated ligands and incubated for 3 h at 37 °C. Plates were washed twice and blocked with 1% albumin for 1 h at 37 °C, followed by a 3-h long incubation at room temperature with an antibody (1 μg/mL) or with saline. Thereafter, wells were incubated for 120 min with an anti-mouse HRP antibody, followed by incubation with ATBS (Sigma). Signals were determined using an ELISA reader (420 nm).
Determination of Ligand Concentration in Conditioned Medium.
Human cancer cell lines (1 × 106) were seeded in 10-cm plates, covered with 8 mL medium, and incubated for 4 d. Media were then collected and ligand quantified using the DuoSet ELISA kit (R&D Systems).
Cell Proliferation Assays.
Cells were plated on 96-well plates (2,000 cells per well) in hexaplicates. Proliferation was measured after 24, 48, and 72 h using the MTT method. MTT was added to the wells, and 2 h later the cells were dissolved in 4 mM HCl (in isopropanol), and absorbance was determined at 570 nm.
Determination of Antitumor Activity of mAbs in Animals.
Female atymic NCr-nude mice (6 wk old) were inoculated s.c. with 2 × 106 human cancer cells. Once tumors became palpable (5–7 d), mice were randomized into groups and injected i.p. at the indicated time points with a mAb, chemotherapy, or various combinations. Tumor volumes were monitored twice a week.
Acknowledgments
We thank members of our group for insightful comments. Y.Y. is the incumbent of the Harold and Zelda Goldenberg Professorial Chair. This work is supported by research grants from the US National Cancer Institute (CA072981), the Israel Science Foundation, Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the M.D. Moross Cancer Institute, and the Marc Rich Foundation for Education, Culture, and Welfare (the Linda de Picciotto Program).
Footnotes
The authors declare no conflict of interest.
References
- 1.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 2.Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res. 2003;284:2–13. doi: 10.1016/s0014-4827(02)00105-2. [DOI] [PubMed] [Google Scholar]
- 3.Falls DL. Neuregulins: Functions, forms, and signaling strategies. Exp Cell Res. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 4.Klapper LN, et al. The ErbB-2/HER2 oncoprotein of human carcinomas may function solely as a shared coreceptor for multiple stroma-derived growth factors. Proc Natl Acad Sci USA. 1999;96:4995–5000. doi: 10.1073/pnas.96.9.4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kochupurakkal BS, et al. Epigen, the last ligand of ErbB receptors, reveals intricate relationships between affinity and mitogenicity. J Biol Chem. 2005;280:8503–8512. doi: 10.1074/jbc.M413919200. [DOI] [PubMed] [Google Scholar]
- 6.Jorissen RN, et al. Epidermal growth factor receptor: Mechanisms of activation and signalling. Exp Cell Res. 2003;284:31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 7.Barozzi C, et al. Relevance of biologic markers in colorectal carcinoma: A comparative study of a broad panel. Cancer. 2002;94:647–657. doi: 10.1002/cncr.10278. [DOI] [PubMed] [Google Scholar]
- 8.Thøgersen VB, et al. A subclass of HER1 ligands are prognostic markers for survival in bladder cancer patients. Cancer Res. 2001;61:6227–6233. [PubMed] [Google Scholar]
- 9.Grandis JR, Chakraborty A, Zeng Q, Melhem MF, Tweardy DJ. Downmodulation of TGF-alpha protein expression with antisense oligonucleotides inhibits proliferation of head and neck squamous carcinoma but not normal mucosal epithelial cells. J Cell Biochem. 1998;69:55–62. [PubMed] [Google Scholar]
- 10.Wang F, et al. Heparin-binding EGF-like growth factor is an early response gene to chemotherapy and contributes to chemotherapy resistance. Oncogene. 2007;26:2006–2016. doi: 10.1038/sj.onc.1209999. [DOI] [PubMed] [Google Scholar]
- 11.Eckstein N, et al. Epidermal growth factor receptor pathway analysis identifies amphiregulin as a key factor for cisplatin resistance of human breast cancer cells. J Biol Chem. 2008;283:739–750. doi: 10.1074/jbc.M706287200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Britten CD. Targeting ErbB receptor signaling: A pan-ErbB approach to cancer. Mol Cancer Ther. 2004;3:1335–1342. [PubMed] [Google Scholar]
- 13.Weiner LM, Borghaei H. Targeted therapies in solid tumors: Monoclonal antibodies and small molecules. Hum Antibodies. 2006;15(3):103–111. [PubMed] [Google Scholar]
- 14.Ishikawa N, et al. Increases of amphiregulin and transforming growth factor-alpha in serum as predictors of poor response to gefitinib among patients with advanced non-small cell lung cancers. Cancer Res. 2005;65:9176–9184. doi: 10.1158/0008-5472.CAN-05-1556. [DOI] [PubMed] [Google Scholar]
- 15.Zhou BB, et al. Targeting ADAM-mediated ligand cleavage to inhibit HER3 and EGFR pathways in non-small cell lung cancer. Cancer Cell. 2006;10:39–50. doi: 10.1016/j.ccr.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valabrega G, et al. TGFalpha expression impairs Trastuzumab-induced HER2 downregulation. Oncogene. 2005;24:3002–3010. doi: 10.1038/sj.onc.1208478. [DOI] [PubMed] [Google Scholar]
- 17.Engelman JA, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 18.Ritter CA, et al. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res. 2007;13:4909–4919. doi: 10.1158/1078-0432.CCR-07-0701. [DOI] [PubMed] [Google Scholar]
- 19.Bianchi S, et al. ErbB-receptors expression and survival in breast carcinoma: A 15-year follow-up study. J Cell Physiol. 2006;206:702–708. doi: 10.1002/jcp.20535. [DOI] [PubMed] [Google Scholar]
- 20.Karamouzis MV, Badra FA, Papavassiliou AG. Breast cancer: The upgraded role of HER-3 and HER-4. Int J Biochem Cell Biol. 2007;39:851–856. doi: 10.1016/j.biocel.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 21.Shih T, Lindley C. Bevacizumab: An angiogenesis inhibitor for the treatment of solid malignancies. Clin Ther. 2006;28:1779–1802. doi: 10.1016/j.clinthera.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Hurwitz H, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 23.Van Zoelen EJ, Stortelers C, Lenferink AE, Van de Poll ML. The EGF domain: Requirements for binding to receptors of the ErbB family. Vitam Horm. 2000;59:99–131. doi: 10.1016/s0083-6729(00)59005-0. [DOI] [PubMed] [Google Scholar]
- 24.Mackenzie RP, McCollum AD. Novel agents for the treatment of adenocarcinoma of the pancreas. Expert Rev Anticancer Ther. 2009;9:1473–1485. doi: 10.1586/era.09.109. [DOI] [PubMed] [Google Scholar]
- 25.Harari PM, Wheeler DL, Grandis JR. Molecular target approaches in head and neck cancer: Epidermal growth factor receptor and beyond. Semin Radiat Oncol. 2009;19:63–68. doi: 10.1016/j.semradonc.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Citri A, Yarden Y. EGF-ERBB signalling: Towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 27.Ramnarain DB, et al. Differential gene expression analysis reveals generation of an autocrine loop by a mutant epidermal growth factor receptor in glioma cells. Cancer Res. 2006;66:867–874. doi: 10.1158/0008-5472.CAN-05-2753. [DOI] [PubMed] [Google Scholar]
- 28.Abdeen A, et al. Correlation between clinical outcome and growth factor pathway expression in osteogenic sarcoma. Cancer. 2009;115:5243–5250. doi: 10.1002/cncr.24562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heinemann V. Gemcitabine: Progress in the treatment of pancreatic cancer. Oncology. 2001;60:8–18. doi: 10.1159/000055290. [DOI] [PubMed] [Google Scholar]
- 30.Fischer C, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 31.Servidei T, Riccardi A, Mozzetti S, Ferlini C, Riccardi R. Chemoresistant tumor cell lines display altered epidermal growth factor receptor and HER3 signaling and enhanced sensitivity to gefitinib. Int J Cancer. 2008;123:2939–2949. doi: 10.1002/ijc.23902. [DOI] [PubMed] [Google Scholar]
- 32.Ferrer-Soler L, et al. An update of the mechanisms of resistance to EGFR-tyrosine kinase inhibitors in breast cancer: Gefitinib (Iressa) -induced changes in the expression and nucleo-cytoplasmic trafficking of HER-ligands (Review) Int J Mol Med. 2007;20:3–10. [PubMed] [Google Scholar]
- 33.Bianco C, et al. Enhancement of antitumor activity of ionizing radiation by combined treatment with the selective epidermal growth factor receptor-tyrosine kinase inhibitor ZD1839 (Iressa) Clin Cancer Res. 2002;8:3250–3258. [PubMed] [Google Scholar]
- 34.Raben D, Helfrich BA, Chan D, Johnson G, Bunn PA., Jr ZD1839, a selective epidermal growth factor receptor tyrosine kinase inhibitor, alone and in combination with radiation and chemotherapy as a new therapeutic strategy in non-small cell lung cancer. Semin Oncol. 2002;29(1, Suppl 4):37–46. doi: 10.1053/sonc.2002.31521. [DOI] [PubMed] [Google Scholar]
- 35.Eshhar Z, Ofarim M, Waks T. Generation of hybridomas secreting murine reaginic antibodies of anti-DNP specificity. J Immunol. 1980;124:775–780. [PubMed] [Google Scholar]