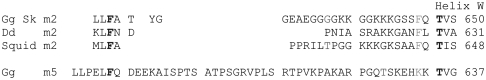Abstract
In order to understand the mechanism of muscle contraction at the atomic level, it is necessary to understand how myosin binds to actin in a reversible way. We have used a novel molecular dynamics technique constrained by an EM map of the actin-myosin complex at 13-Å resolution to obtain an atomic model of the strong-binding (rigor) actin-myosin interface. The constraining force resulting from the EM map during the molecular dynamics simulation was sufficient to convert the myosin head from the initial weak-binding state to the strong-binding (rigor) state. Our actin-myosin model suggests extensive contacts between actin and the myosin head (S1). S1 binds to two actin monomers. The contact surface between actin and S1 has increased dramatically compared with previous models. A number of loops in S1 and actin are involved in establishing the interface. Our model also suggests how the loop carrying the critical Arg 405 Glu mutation in S1 found in a familial cardiomyopathy might be functionally involved.
Keywords: cryoelectron microscopy, myosin II, myosin V
The binding of myosin to actin can be weak or strong. The affinity, which changes over 5 orders of magnitude, is controlled by ATP binding to the myosin head at a position remote from the actin binding site. ATP binding produces “weak binding.” In the absence of ATP the binding is “strong.” The mechanism of this interaction and its control by ATP is central to an understanding of muscle contraction (1). Thus we need to know the structure of the strong-binding state of myosin to actin in atomic detail. Attempts to crystallize the complex have been unsuccessful. However, incubating myosin heads (subfragment 1, S1) with filamentous actin (f-actin) produces “decorated actin” in which each myosin head binds to the actin filament in the strong-binding or “rigor” configuration. Decorated actin may be used as a model of the actin-myosin “strong-binding” mode. Cryoelectron microscopy and 3D reconstructions of actin decorated with myosin II have produced a density map at 13-Å resolution (2). Combining this with structural data from the head of myosin V, which can be crystallized in the strong-binding myosin conformation (3), gave a near-atomic view of the actin-myosin rigor interaction (4). The actin binding site comprises parts of the upper and lower 50-K domains that are separated by a cleft in the weak-binding form. The cleft closes on strong binding. However, the interface appears incomplete: Some additional local refolding of myosin and actin appears to be necessary. We have now used constrained molecular dynamics—molecular dynamics flexible fitting (MDFF) (5) to fit atomic models into the interface using the EM density map as a constraint. In MDFF the gradient of the density is used as an extra force field. It appears that, in addition to the elements of the upper and lower 50-K domains recognized in the earlier study, part of the flexible loop 2 between the upper and lower 50-K domains in S1 and the actin-DNase I binding loop in actin form essential parts of the interface. Furthermore, loop 4 in S1 (sequence 364–380) located close to the cardiomyopathy loop (sequence 400–418) and loop 3 (sequence 567–577) move in toward the actin binding interface, suggesting contacts with two actin monomers. Similar molecular dynamics studies were undertaken previously without EM restraints (6). However, compared with the previous study our studies indicate about twice the area of interaction.
Results
The First Run with Unconstrained Coordinates.
As starting coordinates for f-actin we used PDB ID code 2ZWH derived from X-ray fiber diffraction data (7). For myosin II we used the crystallographic coordinates 2MYS (8). MD trajectories are often more stable with crystallographically derived coordinates. Since the coordinates of f-actin were the output from an MD process, we replaced each of the two main domains of the actin monomer coordinates with the best overlaid coordinates from 1J6Z for each domain (9) taking over just the DNAse I binding loop and C terminus from 2ZWH. This ensures good secondary structure for the starting coordinates. Moreover, the 2MYS coordinates needed to be modified by removing the added methyl groups from the surface lysines [2MYS crystal structure was solved with methylated surface lysines (8)] and by adding the missing side chain coordinates to the light chain Cα coordinates (this has no effect on the actin-myosin interface). Initially, the atomic coordinates were fitted to the EM density as rigid bodies by least squares. Subsequently an MD trajectory was run for 5 ns. The 2MYS coordinates of myosin II are in the postpower stroke weak-binding form, whereas the EM density data are derived from the rigor-like strong-binding form (4). Therefore, we anticipated quite big changes on running an MD simulation.
Indeed, under the influence of the strong-binding (rigor) EM density the myosin II coordinates deform to become very much like 1W8J of myosin V (10), which is already in the strong-binding form. The actin binding cleft closes and the β-sheet (Fig. 1B) takes on the more twisted configuration shown by myosin V. In addition, part of loop 2 (see Fig. 1B) initially inserted as random coil was moved into the actin-myosin interface (residues 633–640 and 643–646).
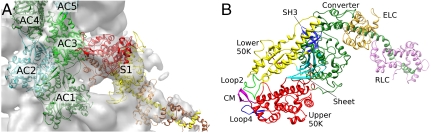
Fig. 1.
(A) Starting structure of the actin-S1 complex with five actin monomers (AC1–AC5) and S1. The colors in the S1 structure indicate the upper 50-K domain (red), the lower 50-K domain (orange), the remaining parts including the converter and the SH3-domain and the lever arm (yellow) and the two light chains (brown) for the unconstrained MD runs as described in the text. Also shown is the EM density (gray surface). (B) Important segments and loops of S1, which are often referred to in the text. ELC: essential light chain, RLC: regulatory light chain. Generated with Chimera (11).
The final MDFF calculations were carried out in the absence of water because embedding the acto-S1 complex in a water box showed the same overall features such as the closing of the actin binding cleft. We found that calculations in vacuum were sufficient in order to uncover the main structure segments and loops that participate in the acto-S1 interaction. The map force scaling was set to 0.2 in order to avoid it dominating the MD simulation. The final model used five actin monomers to allow the major S1-binding actin monomer (AC3; see Fig. 1A for an explanation) all possible interactions with neighboring actins. The EM density force was not applied to the lever arm in S1 (residues 795–843), the regulatory light chain and the essential light chain. The results are shown in Fig. 2.
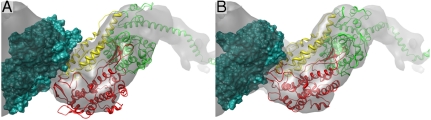
Fig. 2.
(A) The starting structure of the acto-S1 complex as described in the text, embedded in the EM map. (B) The converged acto-S1 complex after 1-ns MD run in MDFF. It can be seen that the upper 50-K domain closes. Both pictures show the main S1-binding actin monomer as surface representation in dark green, and S1 is shown as a ribbon. Only the motor domain and the beginning of the lever arm are shown—color code as in Fig. 1B. The orientation is looking along the helix axis from the + end of decorated actin. Pictures generated with Chimera (11).
The first runs displayed a number of problems including a tendency to shift the structure toward the actin helix axis caused by a small but significant radial fall off of the EM density. Also the limited resolution of the EM density map resulted in strong gradients near the edges of the molecule, which caused some unrealistic bending of structures. Moreover, parts of loop 2 were pushed into the actin-myosin interface rather indiscriminately.
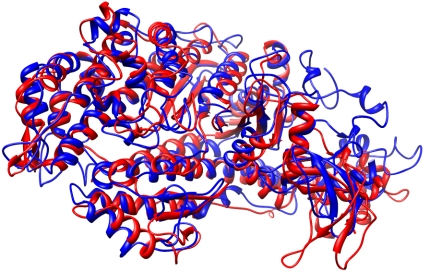
Nevertheless, the final model we obtained showed a good match with the myosin V motor domain structure (10). The overlay shown (Fig. 3) was calculated in a highly extensible program for interactive visualization and analysis of molecular structures, called Chimera (11), based on secondary structure elements, showing the converged myosin II structure in blue and the myosin V structure in red. The actin binding cleft in myosin II closed and the β-sheet took on the more twisted conformation found in myosin V that is thought to represent the strong-binding conformation of S1 (12).
Fig. 3.
Comparison of the converged structure of myosin II in blue and myosin V (chain A of 1W8J) in red shown as secondary structure cartoons. The actin binding cleft in myosin II has closed during the refinement and the β-sheet has become more twisted. Generated with Chimera (11).
The rmsd between the starting model and the converged model was slightly more than 7 Å after 5 ns of MD simulation, using backbone atoms of five actin monomers and S1 for the RMSD calculation (Fig. S1, SI Text). Even though the MDFF runs converged after 1–2 ns, the refinements were extended to over 5 ns to ensure convergence.
Modeling Myosin II in the Strong-Binding Form.
To counter the problems described in the previous section we first generated starting coordinates of myosin II in the strong-binding form based as far as possible on crystallographically derived coordinates. Then harmonic constraints were applied to areas not close to the interface to prevent unwarranted movement.
Previous studies (12) showed that myosin V without bound nucleotide is in the strong-binding form. As described above, unconstrained refinement of myosin II starting in the weak-binding state transforms into a myosin V strong-binding (rigor-like) state when exposed to the map force (see Fig. 3). Thus myosin V provides a good template for modeling myosin II in its strong-binding state. The head of myosin II can be divided into three domains (upper 50-K, lower 50-K, and N-C domain) each of which is very similar in structure to the corresponding domain in myosin V. The 50-K upper, 50-K lower, and N-C-terminal domain of myosin II weak binding and myosin V strong binding can be matched separately onto one another with high coincidence (SI Text). It transpires that two rotational transformations of the individual domains suffice to bring the whole structure of myosin II into good coincidence with myosin V. By using these transformations to generate starting coordinates we are able to preserve the crystallographic quality of the data.
For myosin V we used chain A of 1W8J (10). The two molecules were brought to similar orientations by overlaying the lower 50-K domains (Fig. S2, SI Text). Then the upper 50-K domain of myosin II weak binding may be fitted to myosin V strong binding by a 22° rotation of the upper 50-K domain around an axis that runs approximately along the β-sheet and is roughly at right angles to the helix axis.
The N-C-terminal domain may be treated in the same way. Superposition of the N-C-terminal domain from myosin II (yellow, Fig. S3, SI Text) on myosin V (apple green, Fig. S3, SI Text) is achieved by a rotation of 8.1° around an axis that runs close to strand 3 of the β-sheet. Because this axis lies close to the domain boundary, the rotation amounts to an 8.1° bending of the β-sheet. The converter domain carries the long light chain bearing lever arm of myosin. The orientation of the converter domain achieved by the process outlined above leads to a positioning of the lever arm that is in excellent agreement with the EM density map.
The two rotations described above produced a model of myosin 2 in the strong-binding form (Figs. S4 and S5, SI Text).
Adding the Missing Loops.
Loop 1.
Loop 1 in myosin II (199–219) is disordered as in most myosin structures. The residues from 205–215 are missing in 2MYS (8) and thus were model built and inserted as a random coil and were not exposed to the map force or harmonic constraints.
Loop 2.
Loop 2, between the upper and lower 50-K domains, is disordered in most myosin S1 structures. It is missing in 2MYS from residues 627–646. However, initial studies described above showed that at least some of loop 2 is involved in the actin-myosin interface. The resolution of the map is too low to build the missing structure without initial modeling. We have constructed the part of loop 2 that appears to be involved in actin binding by hand using the EM map and crystallographic data from dictyostelium myosin II as a guide.
Loop 2 is of variable length and is rich in lysine and glycine residues. At the C terminus of the loop (647 in skeletal myosin II; 635 in myosin V) is an invariant threonine at the N terminus of helix W (649–667). The eight residues on the N-terminal side of the threonine are conserved in myosin II. They are quite different in myosin V (loop 2 is also much longer) and indeed in all other myosins. The N terminus of loop 2 is marked by an invariant phenylalanine. Dictyostelium discoideum myosin II (Dd) is unusual because the loop 2 region is short—it has only 16 residues. The whole of loop 2 is visible in 2AKA (13) and shows a β-hairpin. The form taken may be indicative of a preferred fold of the polypeptide chain. Therefore, we have built an extended polypeptide chain (working from C to N) toward the actin surface from the beginning of the W helix into a type-2 β-bend. After the bend an extended polypeptide runs away from the actin surface toward the invariant phenylalanine that marks the N terminus of loop 2. This configuration lies entirely within that part of the EM density that was empty in earlier fits. With the given build, the Phe 646 that is invariant in myosin II interacts with the Pro 602 of the “strut” (14), which is common to all myosin II (see Fig. 4). This may be a stabilizing interaction. Furthermore, on strong binding of skeletal myosin II to actin, the sequence between Gly 634 and Gln 647 would become an ordered part of the actin binding site, in which case all the trypsin-sensitive lysine residues would become unavailable for tryptic cleavage. This would provide a nice explanation of the protection of loop 2 from tryptic attack when myosin is bound to actin (15). Murphy and Spudich (16) also found that changes in loop 2 altered the affinity of S1 for actin both in the absence and presence of nucleotide. Chaussepied (17) proposed that residues 633–642 in S1 are already essential for the weak-binding state of the acto-S1 complex.
Fig. 4.
Loop 2 sequences (after ref. 25). Three selected myosin II sequences and one myosin V are shown: Gg Sk—skeletal myosin II, Dd—Dictyostelium myosin II, squid myosin II (26), and Gg m5—chicken brain myosin V. The ends of loop 2 are shown in bold type. The C terminus of loop 2 ends in helix W. The sequence numbers of the last listed amino acid are shown on the right. Note that loop 2 in myosin V is considerably longer than in myosin II. Myosin IIs have a conserved phenylalanine (red) not present in myosin V. Loop 2 is generally not visible in crystal structures of myosin S1. The exception is Dd, which is short and ordered in two crystal structures.
Loop 3.
Loop 3 was completed by inserting the three missing residues 572–574. It is located in the lower 50-K domain of S1 from residues 567–577 and moves toward AC1 (first actin monomer; see Fig. 1A) during the MDFF refinement. This possible contact was already suggested by an earlier EM reconstruction (18).
We refer to myosin II matched onto myosin V and with loops rebuilt as described as “myo2-5.”
Refinement of Myo2-5.
We used myo2-5 as a starting model for several MD runs in MDFF with slightly altered parameters. In addition to the secondary structure preserving energy function described in the Materials and Methods, we added harmonic constraints to several areas of actin and S1. The harmonic constraints were applied to the Cα atoms of the designated areas in the molecules and were slightly altered for the three different runs in MDFF. The scaling of the density force from the EM map was also changed. Parameters are shown in Table S1 (SI Text). The MD runs in MDFF were carried out for 5 ns for models 1 and 2 where the density force was set to 0.1 and for 3 ns for model 3 where the density force was set to 0.2.
For all three models we calculated the acto-S1 contact surface and the potential intermolecular H bonds and potential electrostatic interaction. The surface areas are shown in Table 1. S1 not only makes major contacts with the actin monomer (AC3) but also with the monomer (AC1) below the major actin monomer in the actin helix (see Fig. 1A). The measurements of the contact surface area are in good agreement with previous results (19). The solvent accessible surface areas were calculated with visual molecular dynamics (VMD) (20) using the “measure sasa” routine (20). The sasa-parameter “sradius,” which is the additional value in Å added to the atomic radius in order to scan the solvent accessible area, was set to 1.4 Å.
Table 1.
Contact surfaces of the actin-myosin models
| Myosin2-5 |
Model 1 |
Model 2 |
Model 3 |
|
| Contact surface AC3-S1 (Å2) | 945.0 | 1,385.0 | 1,429.0 | 1,295.0 |
| Contact surface AC1-S1 (Å2) | 242.0 | 595.0 | 587.0 | 358.0 |
| Total contact surface (Å2) | 1,187.0 | 1,980.0 | 2,016.0 | 1,653.0 |
Surface areas are listed separately for interactions between S1 and the major actin (AC3) and the actin located underneath the major actin (AC1). Surface is given in Å2.
Furthermore, to regularize the result produced by the unconstrained refinement of the DNase I binding loop, the rather similar crystallographic coordinates of the loop from the actin:DNase I crystal structure 1ATN (21) were inserted into the actin coordinates. This enhanced the interface with S1 by moving the DNase I binding loop into the S1 contact area as is described in the following section. All three models converged to a similar result with the same loops and segments being involved in the actin-S1 interface.
The details of the residues involved in contacts between actin and S1 varied with the altered loop and harmonic constraint conditions, thereby providing an estimate of the accuracy of the method. The rms deviations of the three models from each other ranged between 1.0 and 1.1 Å. From the initial MYS2 coordinates of myosin II used in the unconstrained first runs, the values ranged between 4.7 and 4.9 Å (the rmsd values were determined by selecting the backbone atoms of significant secondary structure elements of the 50-K upper and 50-K lower domains, resulting in 165 residues). In the next section we describe the details of model 1 where the nucleotide binding pocket of S1 was preserved by applying strong harmonic constraints to the P loop and switch 1 (these already had the geometry of the strong-binding state because myosin II had been matched onto myosin V).
The rmsd of the actin structure from the initial starting coordinates was 1.3 Å, mainly resulting from the regions that make actin-S1 contacts and actin-actin contacts. The rmsd of the heavy chain of S1 from the initial starting coordinates was 2.6 Å.
The Actin-Myosin Interface of Model 1.
The refinement of the acto-S1 complex using MDFF (5) shows strong interactions not only from S1 to AC3 (the major actin binding site) but also from S1 to AC1, which increases the contact surface and probably stabilizes the strong-binding state. Fig. 5 shows the binding site of the acto-S1 complex in stereo.
Fig. 5.
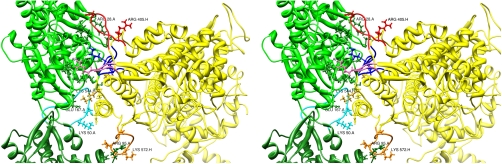
Stereo view of the contacts of the acto-S1 complex of model 1. AC1 is shown in dark green, AC3 in light green, and S1 in yellow. Loop 2 is highlighted in dark blue, cardiomyopathy loop in red, loop 4 in pink, and loop 3 in orange. The DNase I binding loop in actin is highlighted in cyan. The orientation is approximately at right angle with the actin filament axis. The labels show residue type, residue number, and chain identifier (A for actin, H for S1). Generated with Chimera (11).
Contacts of S1 with AC3.
The binding of S1 to AC3 (see Fig. 1A) shows a stable contact surface with S1 of 1,385 Å2 as shown in Table 1. The contact surface indicates several potential electrostatic interactions and H bonds. The interaction between the lower 50-K domain and actin described in ref. 4—helix and bulge 528–544 (S1) and segments 349–353 and 146–149 in AC3–is maintained. Potential candidates for H bonds between S1 and AC3 are mainly found in the loops of S1, namely, loop 2, loop 4, and the cardiomyopathy loop (see Fig. 1B). Furthermore, the loop 541–545 (S1) shows possible H bonds to AC3.
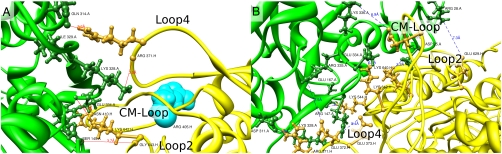
Fig. 6A shows the major potential H bonds between S1 and AC3. In addition, two lysines in the modeled loop 2 show possible interactions from Lys 640 (S1) to Asp 25 (AC3) and from Lys 642 (S1) to Glu 334 (AC3). Loop 4 running from 366 to 377 in S1 in the form of a β-hairpin also shows a number of potential electrostatic interactions with residues in AC3. Fig. 6B displays the contact area of AC3 and S1 with potential electrostatic interactions.
Fig. 6.
(A) Possible H bonds between S1 (yellow) and AC3 (green) with loop 4, the cardiomyopathy loop, and at the lower part of loop 2. The position of Arg 405 is shown in cyan as van der Waals surface pointing away from actin (see text). (B) Electrostatic interactions between S1 (yellow) and the major S1 binding actin monomer (AC3) in green. The main interacting partners in S1 come from loop 2, loop 4, and the cardiomyopathy loop (CM-Loop). The labels show residue type, residue number, and segment (A for actin, H for S1). Generated with Chimera (11).
The cardiomyopathy loop makes a direct electrostatic interaction via Glu 411 (S1) to Lys 336 (AC3). Other hydrophobic interactions also occur. The critical Arg 405 Gln mutation (identical with the Arg 403 Gln mutation in human cardiac myosin), which occurs in a severe familial hypertrophic cardiomyopathy and results in a decreased actin-activated myosin ATPase (22), is not in the interface: It faces away from actin. However, it may make a salt bridge with Glu 631 in the now structured part of loop 2. Therefore, its affect on the actin-myosin association is probably via a stabilization of the folded conformation of loop 2. Liu and co-workers (6) found in a MD simulation of the acto-S1 structure of Holmes et al. (2) that Lys 415 in S1 forms a stable H bond with Glu 334 in AC3. In our refinement we did not find this salt bridge. However, the participating atoms are only 6 Å away from each other. Instead we found a H bond from Asn 410 of S1 to Tyr 337 in AC3. They also found possible contacts of loop 4 to actin (they called it “C-Loop”). Our results are in broad agreement with the findings of the earlier MD simulation (6).
Furthermore, Onishi et al. (23) performed mutations of three regions in heavy meromyosin (HMM) by phosphorylation of residues 546–548 (hydrophobic region), residues 407 and 409 and 412–414 in the cardiomyopathy loop, and finally residues 652 and 653 in loop 2. They all have significantly lower actin-activated ATPase in HMM or even completely extinguish it. We found all those mutations in the contact surface of acto-S1 with possible contacts to actin residues (see Table 2).
Table 2.
Possible electrostatic interactions and H bonds between S1 and AC3 and AC1 (see Fig. 1 for explanation)
| Potential electrostatic interactions | Potential H bonds | ||
| AC3 – S1 | AC1 – S1 | AC3 – S1 | AC1 – S1 |
| ASP24 – GLU629 | LYS50 – GLU576 | GLY23 N – LYS637 O | LYS50 NZ – ASN552 OD1 |
| ASP25 – LYS640 | ARG95 – GLU576 | ASP24 N – GLY635 O | ARG95 NE – PRO570 O |
| ARG28 – GLU629 | GLU99 – LYS569 | LYS328 NZ – ARG371 O | ARG95 NH1 – ALA575 O |
| ARG147 – GLU373 | TYR337 OH – ASN410 OD1 | ARG95 NH2 – LYS572 O | |
| GLU167 – LYS544 | SER348 OG – LYS637 O | GLY46 O – LYS553 NZ | |
| ASP311 – ARG371 | THR351 N – PRO529 O | GLY48 O – LYS553 N | |
| LYS328 – GLU372 | THR351 OG1 – PRO529 O | GLN49 OE1 – SER549 OG | |
| GLU334 – LYS642 | GLY23 O – GLY638 N | TYR91 O – ALA571 N | |
| ARG335 – GLU372 | ARG28 O – ASN410 ND2 | ||
| LYS336 – GLU411 | SER145 O – GLY643 N | ||
| GLU167 O – LYS544 NZ | |||
| GLN314 OE1 – ARG371 NH1 | |||
| ILE329 O – ARG371 NH2 | |||
| GLU334 O – LYS642 NZ | |||
Contacts of S1 with AC1.
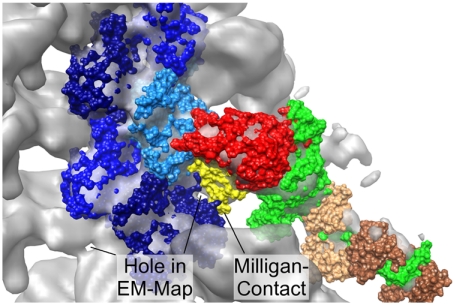
S1 also makes significant contacts with AC1 (see Fig. 1A). Milligan (18) suggested that there might be an interaction between loop 3 (567–577 in S1) and actin. This was also suggested by Mornet et al. (15) by zero-cross-linking experiments. Furthermore, Blanchoin et al. (24) also favored the binding of S1 to two actin monomers in their kinetic studies. The EM map (2) used in the present paper shows the same features as the Milligan map: a hole in the map and a “finger” below interacting with actin. Fig. 7 shows model 1 with five actin monomers and S1 in the color code as in Fig. 1B in a surface representation plus the EM map. The hole in the surface representation matches the surface of the model rather well. We refer to this feature as the “Milligan contact.” The model of Liu and collegues (6) could not account for the Milligan contact.
Fig. 7.
Surface representation of the model 1 and the EM map at 13-Å resolution (orientation: at right angles to the helix axis). The cutoff level of the map is chosen in order to show the hole in the density surrounded by regions of AC1 that bind to S1. Color code as in Fig. 1B, ELC in light brown, RLC in dark brown. Generated with Chimera (11).
The initial MD trajectory moved the DNase I binding loop away from the configuration given in 2ZWH to a configuration similar to that found in the crystal structure of the actin-DNase I complex 1ATN (21). Replacing the DNase I binding loop in actin with the original loop from the crystal structure revealed an interaction of the DNase I binding loop with S1. During the subsequent MD trajectory the crystal configuration was modified, but it retained the general shape of the DNase I binding loop. It showed strong interactions with S1 particularly through 44–49 (AC1) with S1 543–554 (S1).
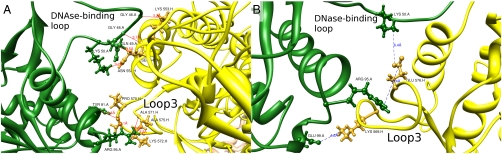
We also found candidate H bonds between AC1 and S1 in the S1 helix 543–554 to the DNase I binding loop in actin and from loop 3 in S1 to residues Tyr 91 and Arg 95 in actin. Possible H bonds are shown in Fig. 8A.
Fig. 8.
(A) Potential H-bond contacts between S1 (yellow) and AC1 (green). (B) Potential electrostatic contacts between AC1 (green) and S1 (yellow). The labels show residue type, residue number, and chain identifier (A for actin, H for S1). Generated with Chimera (11).
Fig. 8B shows the potential electrostatic interactions of AC1 with S1. Lys 50 located in the DNase I binding loop in actin shows a weak interaction with Glu 576 from loop 3 in S1. However, the major contacts are established by the Milligan contact from Glu 576 (S1) to Arg 95 (AC1) and Lys 569 (S1) to Glu 99 (AC1).
Table 2 shows all potential electrostatic interactions and potential H bonds between AC1 and S1 and AC3 and S1 during the MDFF refinement.
Discussion
MDFF (5) trajectories run on an actin-myosin complex using the EM map at 13-Å resolution (2) as a constraint showed the cleft-open weak-binding state (myosin 2 postrigor) changing into a structure very similar to the cleft-closed strong-binding state (myosin 5 rigor) (Fig. 3). Moreover, the results showed that part of the disordered loop 2 was involved in the actin-myosin interface. The MDFF method in the unconstrained run showed that the actin binding cleft, which is open in myosin II, has to close into a myosin V-like structure in order to account for the strong-binding state. However, the procedure distorted some regions near the boundaries of the map and a falloff of radial density in the EM map produced a force pushing all structures 1–2 Å toward the helix axis. Therefore details of the unconstrained run are uncertain and are not further discussed. In order to rectify these deficiencies, we took as a starting structure a modified myosin 2 modeled on the crystallographic structure of myosin 5. In addition, loop 2 in S1 and the DNase-binding loop in actin (AC1) were rebuilt as described in the text. For stability, harmonic constraints were applied to selected areas not involved in the interface. Three MDFF trajectories with varying starting parameters led to similar structures for the interface. The generated interface is extensive and stereochemically plausible.
We found that myosin S1 interacts with two adjacent actin molecules. In addition to the established interactions between the lower 50-K domain and actin described in ref. 4, most of loop 2 is involved in establishing the association of S1 and AC3. Furthermore, the cardiomyopathy loop participates in the acto-S1 interaction.
We also found substantial contacts between AC1 and S1. The DNase-binding loop showed strong contacts with S1 residues. Moreover, the MDFF refinement moved loop 3 toward the actin helix axis resulting in substantial support of an additional site predicted by Milligan (18).
Our results are also in good agreement with the findings by Liu and colleagues (6). However, the contacts from loop 2 to actin differ as the part of loop 2 that makes contacts with AC3 in our acto-S1 structure was modeled in a different way to be buried in the actin-S1 contact surface. There is also a discrepancy of the buried solvent accessible surface area. Whereas in our structure the total contact surface of AC1-S1 and AC3-S1 is 1980 Å2, Liu and colleagues measure only 463 Å2. But they also favor loop 2, loop 4, and the cardiomyopathy loop in S1 as important segments to establish contacts with actin. Their MD simulations could not take account for the Milligan contact as shown in our acto-S1 structure that was driven by the map force during the MDFF refinement.
The resulting acto-S1 model was achieved by using harmonic constraints in the regions of actin and S1, which do not contribute to the acto-S1 and also actin-actin interface. The reason was that the resolution is too low to let all of the structure move in the molecular dynamics process using MDFF. However, it seems to be very likely that actin would not behave like a rigid body upon S1 binding, resulting in at least small movements of loops and segments in order to enhance the interface. Our actin-S1 model also shows some small movements of actin segments in the interface region. Further details would require higher resolution of the EM map.
Our results reveal additional acto-S1 interactions that provide a plausible model for the strong-binding state of S1 to actin. Since the EM map has a resolution of only 13 Å, which is too low to show atomic structure, the details of the present model need to be refuted or substantiated by additional experiments. Fortunately, the additional potential contacts suggest numerous biochemical experiments.
Materials and Methods
MDFF (5) is a MD simulation with each atom being subjected to an additional force derived from the gradient of the EM density. The EM density map from Holmes (2) was used to provide the constraining force field. This procedure results in atoms being moved into higher density areas of the EM map. In addition, we added another energy term to preserve secondary structure elements as an option in MDFF. Thus the total energy function we used is
where UMD is the MD energy function, UEM is the energy function corresponding to a force derived from the gradient of the EM density, and USS the energy function used to preserve the secondary structure elements. A scaling factor adjusted by trial and error was applied locally to UEM in order to avoid dominance of the force resulting from the EM density map.
Calculations were carried out both in vacuum and in a water box in which the protein complex had been embedded. The box was set up to have periodic boundary conditions. The MD runs were carried out until the rmsd between the refined structures and the initial coordinate set reached a stable value. We carried out 12 MD runs in vacuum and 3 MD runs in a water box. Convergence was generally reached after 1 to 2 ns of simulation depending on the parameter settings. The following parameters were changed during the refinements:
The map force scaling, varying between 0.1 and 0.5,
simulation using 2 actin monomers or 5 actin monomers,
different start configurations of loop 2,
strength of constraints used to preserve secondary structure elements,
harmonic constraints—applied to selected areas.
Supplementary Material
Acknowledgments.
We thank Dr. Elizabeth Villa for her support to set up a preliminary version of MDFF and the fruitful discussions with her on the manuscript. We also thank Dr. Anne Houdusse for her valuable comments on the manuscript.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1003604107/-/DCSupplemental.
References
- 1.Geeves MA, Fedorov R, Manstein DJ. Molecular mechanism of actomyosin-based motility. Cell Mol Life Sci. 2005;62:1462–1477. doi: 10.1007/s00018-005-5015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holmes KC, Angert I, Kull FJ, Jahn W, Schroder RR. Electron cryo-microscopy shows how strong binding of myosin to actin releases nucleotide. Nature. 2003;425:423–427. doi: 10.1038/nature02005. [DOI] [PubMed] [Google Scholar]
- 3.Coureux PD, et al. A structural state of the myosin V motor without bound nucleotide. Nature. 2003;425:419–423. doi: 10.1038/nature01927. [DOI] [PubMed] [Google Scholar]
- 4.Holmes KC, Schroder RR, Sweeney HL, Houdusse A. The structure of the rigor complex and its implications for the power stroke. Philos Trans R Soc London Ser B. 2004;359:1819–1828. doi: 10.1098/rstb.2004.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trabuco LG, Villa E, Mitra K, Frank J, Schulten K. Flexible fitting of atomic structures into electron microscopy maps using molecular dynamics. Structure. 2008;16:673–683. doi: 10.1016/j.str.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu YM, Scolari M, Im W, Woo HJ. Protein-protein interactions in actin-myosin binding and structural effects of R405Q mutation: A molecular dynamics study. Proteins. 2006;64:156–166. doi: 10.1002/prot.20993. [DOI] [PubMed] [Google Scholar]
- 7.Oda T, Iwasa M, Aihara T, Maeda Y, Narita A. The nature of the globular- to fibrous-actin transition. Nature. 2009;457:441–445. doi: 10.1038/nature07685. [DOI] [PubMed] [Google Scholar]
- 8.Rayment I, et al. Three-dimensional structure of myosin subfragment-1: A molecular motor. Science. 1993;261:50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- 9.Otterbein LR, Graceffa P, Dominguez R. The crystal structure of uncomplexed actin in the ADP state. Science. 2001;293:708–711. doi: 10.1126/science.1059700. [DOI] [PubMed] [Google Scholar]
- 10.Coureux PD, Sweeney HL, Houdusse A. Three myosin V structures delineate essential features of chemo-mechanical transduction. EMBO J. 2004;23:4527–4537. doi: 10.1038/sj.emboj.7600458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pettersen EF, et al. UCSF chimera—A visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 12.Sweeney HL, Houdusse A. The motor mechanism of myosin V: Insights for muscle contraction. Philos Trans R Soc London Ser B. 2004;359:1829–1841. doi: 10.1098/rstb.2004.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reubold TF, Eschenburg S, Becker A, Kull FJ, Manstein DJ. A structural model for actin-induced nucleotide release in myosin. Nat Struct Biol. 2003;10:826–830. doi: 10.1038/nsb987. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki N, Ohkura R, Sutoh K. Insertion or deletion of a single residue in the strut sequence of Dictyostelium myosin II abolishes strong binding to actin. J Biol Chem. 2000;275:38705–38709. doi: 10.1074/jbc.M001966200. [DOI] [PubMed] [Google Scholar]
- 15.Mornet D, Bertrand R, Pantel P, Audemard E, Kassab R. Structure of the actin-myosin interface. Nature. 1981;292:301–306. doi: 10.1038/292301a0. [DOI] [PubMed] [Google Scholar]
- 16.Murphy CT, Spudich JA. The sequence of the myosin 50–20 K loop affects Myosin’s affinity for actin throughout the actin-myosin ATPase cycle and its maximum ATPase activity. Biochemistry. 1999;38:3785–3792. doi: 10.1021/bi9826815. [DOI] [PubMed] [Google Scholar]
- 17.Chaussepied P. Interaction between stretch of residues 633-642 (actin binding-site) and nucleotide binding-site on sekeletal myosin subfragment-1 heavy-chain. Biochemistry. 1989;28:9123–9128. doi: 10.1021/bi00449a025. [DOI] [PubMed] [Google Scholar]
- 18.Milligan RA. Protein-protein interactions in the rigor actomyosin complex. Proc Natl Acad Sci USA. 1996;93:21–26. doi: 10.1073/pnas.93.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones S, Thornton JM. Principles of protein-protein interactions. Proc Natl Acad Sci USA. 1996;93:13–20. doi: 10.1073/pnas.93.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humphrey W, Dalke A, Schulten K. VMD: Visual molecular dynamics. J Mol Graphics. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 21.Kabsch W, Mannherz HG, Suck D, Pai EF, Holmes KC. Atomic structure of the actin:DNase I complex. Nature. 1990;347:37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- 22.Roopnarine O, Leinwand LA. Functional analysis of myosin mutations that cause familial hypertrophic cardiomyopathy. Biophys J. 1998;75:3023–3030. doi: 10.1016/S0006-3495(98)77743-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onishi H, Mikhailenko SV, Morales MF. Toward understanding actin activation of myosin ATPase: The role of myosin surface loops. Proc Natl Acad Sci USA. 2006;103:13897–13897. doi: 10.1073/pnas.0601595103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanchoin L, Fievez S, Travers F, Carlier MF, Pantaloni D. Kinetics of the interaction of myosin subfragment-1 with G-actin. Effect of nucleotides and DNaseI. J Biol Chem. 1995;270:7125–7133. doi: 10.1074/jbc.270.13.7125. [DOI] [PubMed] [Google Scholar]
- 25.Yengo CM, Sweeney HL. Functional role of loop 2 in myosin V. Biochemistry. 2004;43:2605–2612. doi: 10.1021/bi035510v. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y, et al. Rigor-like structures from muscle myosins reveal key mechanical elements in the transduction pathways of this allosteric motor. Structure. 2007;15:553–564. doi: 10.1016/j.str.2007.03.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.