Abstract
Epidermal growth factor receptor (EGFR)-specific monoclonal antibodies predominantly inhibit colorectal cancer (CRC) growth by interfering with receptor signaling. Recent analyses have shown that patients with CRC with mutated KRAS and BRAF oncogenes do not profit from treatment with such antibodies. Here we have used the binding domains of cetuximab and pantitumumab for constructing T cell-engaging BiTE antibodies. Both EGFR-specific BiTE antibodies mediated potent redirected lysis of KRAS- and BRAF-mutated CRC lines by human T cells at subpicomolar concentrations. The cetuximab-based BiTE antibody also prevented at very low doses growth of tumors from KRAS- and BRAF-mutated human CRC xenografts, whereas cetuximab was not effective. In nonhuman primates, no significant adverse events were observed during treatment for 3 wk at BiTE serum concentrations inducing, within 1 d, complete lysis of EGFR-overexpressing cancer cells. EGFR-specific BiTE antibodies may have potential to treat CRC that does not respond to conventional antibodies.
Keywords: bispecific antibody, cetuximab, panitumumab, immunotherapy
Epidermal growth factor receptor (EGFR) type 1 (referred to hereafter as EGFR) is a validated target for therapy of colorectal cancer (CRC). Small molecule tyrosine kinase inhibitors as well as EGFR-blocking monoclonal antibodies, such as cetuximab (Erbitux) and panitumumab (Vectibix), are marketed for the treatment of patients with CRC (1, 2). Recent analyses showed that patients with tumors mutated in KRAS and BRAF genes do not profit from an increased overall survival following treatment with cetuximab or panitumumab (3). Cetuximab, a human/mouse chimeric IgG1 antibody, has been shown to lyse EGFR-expressing lung cancer cells by antibody-dependent cellular cytotoxicity (4), but the impact of mutated KRAS and BRAF proteins on overall survival suggests that receptor inhibition is the dominant mode of cetuximab action, at least in patients with CRC.
BiTE antibodies are designed to transiently connect T cells with cancer cells for initiation of redirected target cell lysis (5). Regular IgG1 antibodies cannot engage T cells because these lack Fcγ receptors as needed for interacting with antibodies. BiTE antibodies are composed of two flexibly linked single-chain antibodies, one binding to CD3 on T cells and the other to a surface antigen on the target cell. Many publications have analyzed the mode of BiTE antibody action and characterized antitumor activity in cell culture and various xenograft models (6). Most studies characterized BiTE antibodies targeting CD19 on B cell malignancies (7, 8), or EpCAM on adenocarcinoma (9). The CD19/CD3–bispecific BiTE antibody blinatumomab (MT103) has shown high response rates at very low doses in patients with non-Hodgkin lymphoma (10) and B-precursor acute lymphoblastic leukemia (11), providing clinical proof of concept for the principle of antibody-based T cell engagement. Key features of BiTE antibodies are a conditional activation of T cells depending on the presence of target cells (12), induction of serial lysis by T cells (13), effective formation of cytolytic synapses (14), and high potency of redirected lysis (15). Treatment of mice with BiTE antibodies for several weeks does not trigger substantial internalization of CD3 or lead to T cell anergy (16).
Here we have generated new BiTE antibodies based on minimal binding domains of monoclonal antibodies with specificity for EGFR. EGFR-specific BiTE antibodies based on variable domains of therapeutic monoclonal antibodies cetuximab and panitumumab triggered a very effective redirected lysis of KRAS- and BRAF-mutated CRC lines. The cetuximab-based BiTE antibody was also highly efficacious at microgram-per-kilogram doses in two different xenograft models, whereas cetuximab had no or very little impact on tumor growth even on prolonged treatment with 50-mg/kg doses. In macaque monkeys, serum levels of the cetuximab-based BiTE antibody could be safely maintained for 3 wk that would, in vitro, support complete cancer cell lysis by macaque T cells within 24 h. T cell engagement by BiTE antibodies has the potential to overcome limitations of conventional monoclonal antibodies, and may have a therapeutic window based on differential recognition of EGFR-overexpressing cancer cells.
Results
Bispecific Binding and T cell Activation by BiTE Antibodies Based on Cetuximab and Panitumumab.
For construction of EGFR-specific BiTE antibodies, cDNAs encoding variable domains of chimeric monoclonal antibody cetuximab and of human antibody panitumumab were procured by gene synthesis according to published sequences. Variable domains of anti-EGFR antibodies were genetically fused by linker sequences and connected by a second linker with the variable domains of a human single-chain antibody specific for CD3 of human and nonhuman primate origin. Cetuximab- and panitumumab-based BiTE antibodies (C-BiTE and P-BiTE antibodies, respectively) were stably expressed in CHO cells and monomeric 55–60-kDa polypeptides purified to homogeneity from cell culture supernatants as described for other BiTE antibodies (17).
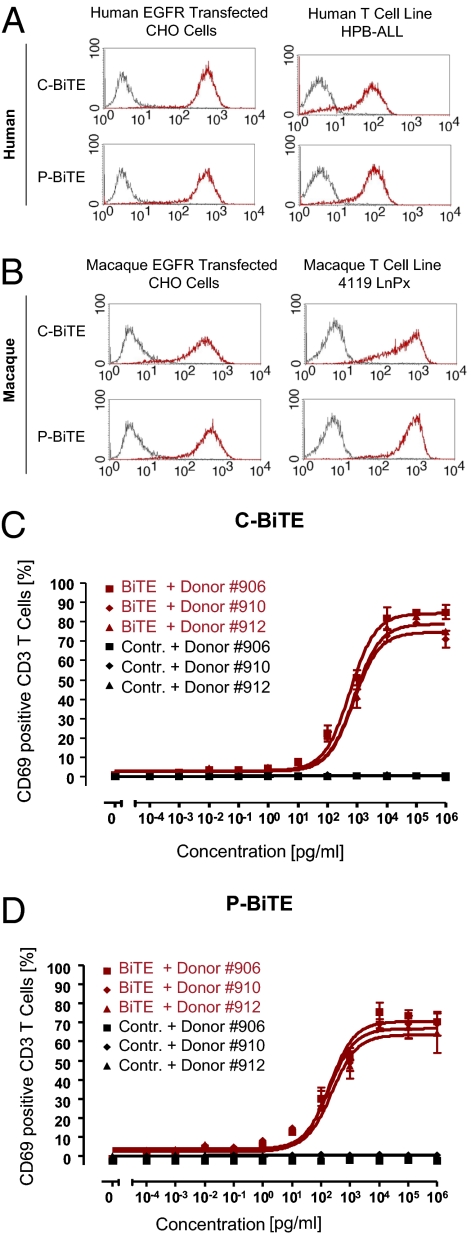
C- and P-BiTE antibodies produced robust and similar binding signals in FACS analysis with CHO cell lines stably expressing EGFR of either human (Fig. 1A) or cynomolgus monkey (Macaca fascicularis) origin (Fig. 1B). Likewise, C- and P-BiTE antibodies bound with similar strength to CD3 on the human T cell line HPB-ALL (Fig. 1A), and to CD3 of the Rhesus monkey (Macaca mulatta) T cell line 4119 LnPx (Fig. 1B).
Fig. 1.
Characterization of cetuximab- and panitumumab-based BiTE antibodies. Cross-reactivity of C- and P-BiTE antibodies between CHO cells transfected with human (A) and macaque antigen (B) was assessed by FACS analysis. Each panel shows FACS histograms analyzing binding of a control antibody with irrelevant specificity and respective BiTE antibodies at 5 μg/mL The x axis shows the mean fluorescence intensity. T cell activation in response to C-BiTE (C) or P-BiTE (D) was investigated by surface expression of immediate/early T cell activation surface marker CD69 on CD3+ cells using FACS analysis. A BiTE dose–response analysis testing three human T cell donors with C- or P-BiTE, or a control BiTE antibody to CD3 and to an irrelevant hapten antigen, was performed. Mean values and SDs of triplicate determinations are shown.
In the presence of EGFR-positive target cells, both C- and P-BiTE antibodies induced, in a dose-dependent fashion, de novo expression of the early T cell activation marker CD69 on resting peripheral T cells derived from human peripheral blood mononuclear cells (PBMCs; Fig. 1 C and D). T cells isolated from three different human donors showed a very similar dose–response relationship. A control BiTE antibody binding to an irrelevant antigen but sharing the anti–CD3-binding arm with C- and P-BiTE antibodies did not trigger T cell activation (Fig. 1 C and D). During the 15-h incubation period, the majority of T cells (65–80%) became newly activated by C- and P-BiTE antibodies, which is consistent with their polyclonal activation. Half-maximal activation of T cells by C-BiTE was seen at approximately 1 ng/mL, and for P-BiTE at 0.1 ng/mL.
Potent Redirected Lysis of KRAS- and BRAF-Mutated Cancer Cells by Cetuximab- and Panitumumab-Based BiTE Antibodies.
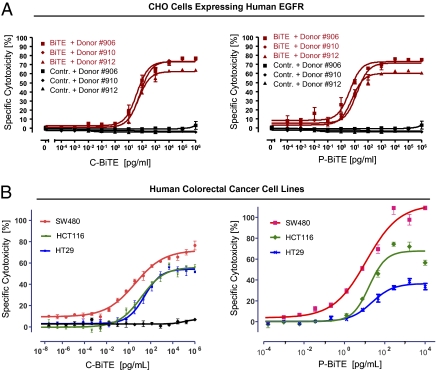
A CHO cell line stably expressing human EGFR was used in a FACS-based assay to determine the cytotoxic activity of C- and P-BiTE antibodies against EGFR-expressing cells with a presumed nonmutated background. The principle of the FACS-based assay is shown in Fig. S1. C- and P-BiTE antibodies both mediated a robust redirected lysis by human T cells of the hamster cell line expressing the human EGFR target antigen (Fig. 2A). The mean concentrations for half-maximal lysis by three different donor T cells were 55 ± 8 pg/mL for C-BiTE and 8.5 ± 3.2 pg/mL for P-BiTE (Table 1). These values were lower than EC50 values for T cell activation determined in the same assay (Fig. 1 C and D), indicating that only a fraction of T cells needed to be activated for potent redirected lysis. The potency difference between C- and P-BiTE antibodies in terms of both T cell activation and cytotoxic activity may be a result of recognition of different epitopes on EGFR, as has been observed for other BiTE antibodies (18).
Fig. 2.
Redirected lysis of human EGFR-expressing CHO cells and three human CRC cell lines by BiTE antibodies based on cetuximab and panitumumab. Dose-dependent redirected lysis of CHO cells transfected with human EGFR (A) or of the three human CRC cells lines SW480, HCT116, and HT29 (B) by C-BiTE (Left) and P-BiTE (Right) was assessed by an FACS-based cytotoxicity assay (A) or a chromium release assay (B). A control BiTE antibody binding to CD3 and an irrelevant hapten antigen was used in A and the left panel of B using HCT116 cells. For A, the three T cell donors were identical, whereas different T cell donors were used in B for C- and P-BiTE. The E:T ratio in all experiments was 10:1. Mean values and SDs of triplicate determinations are shown.
Table 1.
Potency of redirected lysis by cetuximab- and panitumumab-based BiTE antibodies
| Potency of redirected lysis EC50, pg/mL |
||||
| Experiment no. | Effector cells | Target cells | C-BiTE | P-BiTE |
| 1 | Unstimulated CD3-selected human T cells (n = 3 donors) | Human EGFR expressing CHO cells | 55 ± 8 | 8.5 ± 3.2 |
| 2 | Unstimulated human CD3-selected T cells | SW480 human CRC cell line (KRAS-mutated) | 3.8 | 12 |
| 3 | Unstimulated human CD3-selected T cells | HCT116 Human CRC cell line (KRAS-mutated) | 14 | 17 |
| 4 | Unstimulated human CD3-selected T cells | HT29 Human CRC cell line (BRAF-mutated) | 27 | 23 |
| 5 | Stimulated Cynomolgus PBMC (n = 9 donors) | KATO III human gastric carcinoma cell line | 39 ± 44 | ND |
| 6 | Stimulated human PBMC (n = 3 Donors) | KATO III human gastric carcinoma cell line | 41 ± 45 | ND |
| 7 | Rhesus T cell line 4119 LnPx | Cynomolgus EGFR-expressing CHO cells | 20 | ND |
| 8 | Unstimulated Cynomolgus PBMC | Cynomolgus EGFR-expressing CHO cells | 570/134 | ND |
Results from various cytotoxicity assays are shown. Potency of redirected lysis is given as EC50 values for cell lysis in pg/mL Various effector and target cell combinations were tested to compare the cytotoxic potential of the C- and P- BiTE antibodies. The 51-chromium release assay (2 –4) used an E:T ratio of 10:1 and an assay duration of 18 h, the FACS-based cytotoxicity assay (1, 5–8) an E:T ratio of 10:1 and an assay period of 24 h. Experiments 2, 3 and 4 used T cells from the same human PBMC donors. ND, not determined.
The cytotoxic activity of C- and P-BiTE antibodies against mutated human CRC cells was assessed by using SW480, HCT116, and HT-29 lines, which harbor mutations in KRAS or BRAF oncogenes and showed resistance against growth inhibition by cetuximab (19). Mutations were confirmed for the HT-29 and HCT116 cell lines used (Fig. S2). C- and P-BiTE antibodies mediated redirected lysis of all three CRC lines with similar potency (Fig. 2B). The six EC50 values for redirected lysis were in a narrow range between 3.8 and 27 pg/mL, corresponding to 0.07 and 0.48 pM of BiTE antibodies (Table 1). C- and P-BiTE antibodies were more active against the mutated cancer cell lines than against the EGFR-transfected CHO cell line, indicating that KRAS or BRAF mutations of CRC lines did not negatively impact the sensitivity of CRC cells for lysis by redirected T cells. During the 18-h incubation, the highest degree of lysis was reached with SW480 cells (Fig. 2B). Assay results obtained with or without base line correction for spontaneously lysed target cells are shown in Fig. S3.
Efficacy of a Cetuximab-Based BiTE Antibody in KRAS- and BRAF-Mutated Xenograft Models.
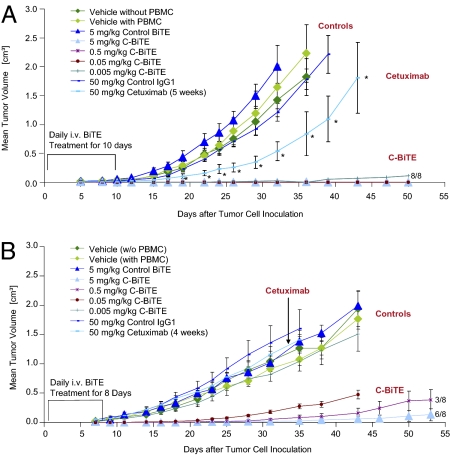
The KRAS-mutated CRC line HCT116 and BRAF-mutated line HT-29 was used to establish xenograft tumor models in immunodeficient NOD/SCID mice. Human T cells were supplied to mice in the form of nonstimulated PBMCs mixed with the respective tumor cells at a ratio of 2:1 before inoculation. As shown in Fig. 3 A and B, human PBMCs did not significantly interfere with the outgrowth of HCT116 and HT-29 tumors in the absence of BiTE antibodies. Likewise, the vehicle alone, a control BiTE antibody binding to an irrelevant hapten antigen given at 5 mg/kg for 8 d (HT-29) or 10 d (HCT116), or an isotype IgG1 control antibody given twice weekly at 50 mg/kg for 4 (HT-29) or 5 wk (HCT116), did not significantly alter tumor outgrowth in either mouse xenograft model. Only C-BiTE and cetuximab were compared in animals because the human IgG1 antibody cetuximab can engage Fcγ receptor–bearing effector cells against EGFR-positive target cells, whereas the human IgG2 panitumumab cannot. Only cetuximab therefore allowed for a direct comparison of antibody-dependent cellular cytotoxicity to T cell engagement by the C-BiTE.
Fig. 3.
Antitumor activity of C-BiTE antibody and parental cetuximab in two human CRC xenograft mouse models Tumor cell doses were determined upfront, allowing a rapid and reproducible outgrowth of tumors from HCT116 (A) or HT29 human CRC cell lines (B) in NOD/SCID mice. HT-29 (2× 106 cells per inoculum) or human colon carcinoma cell line HCT116 (5× 106 per inoculum) were injected s.c. together with PBMCs from healthy human donors at an E:T cell ratio of 1:2 in the right dorsal flank of female NOD/SCID mice (n = 8 per group). As indicated, four different control conditions were tested for their influence on tumor outgrowth: vehicle in the absence, and vehicle, isotype IgG1, and a control BiTE antibody binding to CD3 and an irrelevant hapten antigen in the presence of human PBMCs. Cetuximab was intraperitoneally administered twice a week starting on d 1 at 50 mg/kg for 4 (HT29) or 5 wk (HCT116), whereas C-BiTE was daily administered by i.v. bolus starting on d 1 only for the first 8 d (HT29) or 10 d (HCT116). Tumor growth was determined by external caliper measurements, and tumor volumes were calculated using a standard hemiellipsoid formula (Eq. 2). Values represent mean tumor size (in cm3) ± SEM (n = 8 per group). Growth retardation by cetuximab in (A) was statistically significant for all time points labeled by an asterisk (P < 0.05). For HCT116 xenografts, all BiTE responses were highly significant, and at all four doses tested, all eight mice did not develop tumors. For HT29, all but the lowest dose of 0.005 mg/kg resulted in a statistically significant inhibition of tumor growth (Student t test).
In both xenograft models, C-BiTE showed a highly potent inhibition of tumor outgrowth. In the HCT116 model, all four i.v.-administered BiTE doses—0.005, 0.05, 0.5, or 5 mg/kg, given daily for the first 10 d after tumor cell inoculation—completely inhibited tumor growth in all 32 mice for the entire observation period of 50 d. In two mice from the 0.005 mg/kg–treated group, small nodules were measured during the in-life phase, but no tumor cells were found within nodules at necropsy. In the HT-29 model, C-BiTE showed a dose-dependent prevention of tumor growth in six of eight, three of eight, and zero of eight mice at 5, 0.5, or 0.05 mg/kg/d, respectively. At the 0.05-mg/kg dose, tumor growth was still significantly delayed in all mice, whereas the 0.005-mg/kg/d dose was as ineffective as the control BiTE antibody.
In both xenograft models, the parental monoclonal antibody cetuximab was administered i.p. at 50 mg/kg twice weekly for 4 (HT-29) or 5 wk (HCT116). The monoclonal antibody significantly delayed tumor growth by approximately 1 wk in the HCT116 model (Fig. 3A), whereas it was ineffective in the HT-29 model (Fig. 3B). Hence, a T cell-engaging BiTE format could restore antitumor activity of cetuximab against KRAS- or BRAF-mutated CRC xenografts despite loss of bivalent target binding and absence of an Fcγ portion.
Tolerability of Cetuximab- and Panitumumab-Based BiTE Antibodies in Nonhuman Primates.
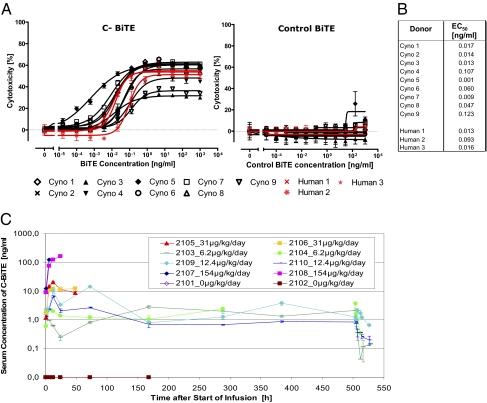
To further qualify the cynomolgus monkey as a relevant species for toxicology testing, PBMC from nine cynomolgus monkeys were compared with PBMC from three human donors for potency of redirected lysis of EGFR-expressing human carcinoma line KATO III by the C-BiTE antibody. PBMCs were prestimulated with anti-CD3/anti-CD28 antibodies and IL-2 to maximize cytotoxic T cell activity and to reduce donor variation resulting from differences in the immune status of individuals. As shown in Fig. 4 A and B, EC50 values for cancer cell lysis by nine monkey PBMC preparations ranged from 0.001 to 0.123 ng/mL (mean, 0.043 ± 0.045 ng/mL), whereas it ranged from 0.013 to 0.093 ng/mL for the three human donor PBMCs (mean, 0.041 ± 0.045 ng/mL). This difference was insignificant, indicating that stimulated human and macaque PBMCs were of comparable activity against the same target cell line, and that C-BiTE–mediated redirected lysis by human and monkey T cells may have the same potential for inducing side effects.
Fig. 4.
Validation of cynomolgus monkey as relevant animal models to assess the tolerability of C- and P-BiTE. PBMCs were prepared from nine cynomolgus monkey and three human donors and T cells therein tested for redirected lysis of EGFR-expressing human gastric carcinoma cell line KATO III in a FACS-based cytotoxicity assay at an E:T ratio of 10:1 and incubation period of 24 h. (A) Dose–response analysis was used to obtain EC50 values for comparing the efficacy of individual donor T cells. Because of limited blood supply from monkeys, PBMCs were cultured and T cells therein expanded by anti-CD3/anti-CD28/IL-2 treatment for 3 d to obtain sufficient effector T cells for dose–response analyses. A control BiTE antibody binding to human and Cynomolgus CD3 and to an irrelevant hapten antigen was tested in the right panel. (B) EC50 values determined in cytotoxicity assays for monkey and human T cells and mean values. (C) Serum concentrations of C-BiTE dose levels during continuous i.v. infusion up to the maximum infusion period of 3 wk. The lower limit of quantification was 0.5 ng/mL Animal test numbers and dose levels are shown in the insert. Sera from animals that did not receive the C-BiTE did not give a signal in the C-BiTE ELISA.
A study was performed in cynomolgus monkeys to explore whether concentrations required for maximal redirected lysis of EGFR-expressing target cells can be safely reached in vivo, and to identify potential target organ toxicity of EGFR-specific BiTE antibodies. As performed in clinical trials with CD19- and EpCAM-specific BiTE antibodies, C-BiTE antibody was administered to cynomolgus monkeys by continuous i.v. infusion. A 3-wk treatment was performed because this period was required for development of skin toxicity in cynomolgus monkeys treated with cetuximab. Groups of two monkeys received none (vehicle control) or 6.2, 12.4, 31, or 154 μg/kg/d of C-BiTE, or 0.8 μg/kg/d of P-BiTE as outlined in Table 2. Initial dosing of C-BiTE was based on pharmacokinetic and efficacy data obtained with the similarly potent CD19/CD3–bispecific BiTE antibody blinatumomab in patients with lymphoma or leukemia, as well as on the potency of redirected lysis seen in in vitro assays in stimulated monkey PBMCs (Fig. 4 A and B and Table 1).
Table 2.
Determination of dose levels for treatment of Cynomolgus monkeys with cetuximab- and panitumumab-based BiTE antibodies
| Human equivalent dose |
|||||
| Dose group | Treatment | Multiples of human therapeutic MT103 dose | μg/patient | μg/kg | Cynomolgus equivalent dose, μg/kg /d |
| 1 | Vehicle Control | 0 | 0 | 0 | 0 |
| 2 | Cetuximab BiTE | 1× | 120 | 2 | 6.2 |
| 3 | Cetuximab BiTE | 2× | 240 | 4 | 12.4 |
| 5 | Cetuximab BiTE | 5× | 600 | 10 | 31 |
| 4 | Cetuximab BiTE | 25× | 3,000 | 50 | 154 |
| 6 | Panitumumab BiTE | 3× | 375 | 6.25 | 0.8 |
The test doses for C-BiTE in monkeys were based on those of the similarly potent CD19/CD3-bispecific antibody blinatumomab (MT103) showing clinical activity in lymphoma patients (10). With blinatumomab, a total dose of 30 μg per patient showed first responses, and 120 μg per patient (i.e., 2 μg/kg/d) partial and complete tumor regression in >90% of patients. Allometric correction of 2 μg/kg/d translates into a dose of 6.2 μg/kg/d for Cynomolgus monkeys as a starting dose. A lower dose was selected for panitumumab-based BiTE antibody because the molecule consistently showed a higher activity than C-BiTE in assays when T cells from the same donor were used as effector cells (Table 1).
Serum levels of C-BiTE increased in a dose-linear fashion, suggesting that the antibody was not significantly sequestered by EGFR expressed on normal monkey tissues (Fig. 4C). Assay qualification data are shown in Fig. S4. Maximum serum concentrations reached were 3.25 ±0.45 ng/mL at 6.2 μg/kg/d, 10.44 ± 4 ng/mL at 12.4 μg/kg/d, 16.5 ± 4 ng/mL at 31 μg/kg/d, and 142.4 ±19 ng/mL at 154 μg/kg/d. Dose linearity of maximum serum concentration values can be assumed with a regression coefficient of 0.99. Serum steady-state levels remained fairly constant for the respective infusion periods of as long as 3 wk (Fig. 4C). As expected, serum concentrations of P-BiTE after administration of 0.8 μg/kg/d remained lower than the lower limit of quantification of the assay of 0.5 ng/mL.
C-BiTE doses of 6.2 or 12.4 μg/kg/d were well tolerated for the entire 3-wk infusion period, suggesting that serum levels as required for complete lysis of cancer cells in vitro can be safely reached in macaque monkeys. Adverse events were mild and transient and, unlike with cetuximab, no skin toxicity was observed after 3 wk of infusion. Clinical findings during treatment with C-BiTE at 6.2 or 12.4 μg/kg/d were minimal and consisted of a slight and transient increase in body temperature within 24 h after the start of infusion in three of four animals. Laboratory findings were leukopenia after 1 wk in both animals at 12.4 μg/kg/d, a slight and transient increase in hepatobiliary parameters (bilirubin, alkaline phosphatase, alanine aminotransferase) in both animals at 12.4 μg/kg/d, and effects on the liver enzyme alanine aminotransferase in one animal dosed at 6.2 μg/kg/d.
At the higher doses of 31 and 154 μg/kg/d, severe signs of toxicity were observed within 56 h after the start of infusion, leading to termination of animals for welfare reasons. Histopathological analysis of the animals receiving C-BiTE at 31 or 154 μg/kg/d showed signs of liver and kidney toxicity, which may be a result of redirected lysis of cells expressing low levels of EGFR in these organs. Additionally, animals in both high-dose groups showed increased levels of inflammatory cytokines in serum (i.e., TNF-α, IFN-γ, IL-6, IL-5, and IL-2), as presumably released by T cells encountering EGFR-positive cells (Fig. S5). Histopathological changes including lymphocyte infiltration and cell death were noted in all tissues known to express EGFR, i.e., salivary glands, liver, stomach, small intestine, colon, rectum, kidneys, adrenal glands, ureter, urinary bladder, prostate, and epididymides. Increased lymphocyte infiltration into EGFR-positive tissues was also observed at the well tolerated dose levels of 6.2 or 12.4 μg/kg/d, but could not be quantified because of technical reasons.
Two monkeys were treated with P-BiTE for 3 wk at a dose level of 0.8 μg/kg/d. The P-BiTE dose was eightfold lower than that the lowest C-BiTE dose to take the higher potency of P-BiTE into account, which was seen in the in vitro cytotoxicity assay (Table 1). Treatment with the P-BiTE was well tolerated, with no side effects revealed except for some infiltration of inflammatory cells in EGFR-positive organs.
Discussion
The present study shows that conversion of EGFR-specific monoclonal antibodies cetuximab and panitumumab into T cell–engaging BiTE antibodies is technically feasible and that the BiTE technology can overcome resistance of KRAS- and BRAF-mutated CRC lines to the therapeutically used parental antibodies. The simplest explanation for the latter is that T cell–engaging BiTE antibodies do not rely on inhibition of EGFR signaling but use the receptor tyrosine kinase as mere surface anchor for attachment of cytotoxic T cells. This function of BiTE antibodies is not expected to be affected by mutations of downstream components of the EGFR pathway that obviate the cancer cell's dependence on the EGFR surface receptor for delivery of growth-promoting signaling. The high potency of EGFR-specific BiTE antibodies suggests that monovalent binding of BiTE antibodies at very low concentrations does not cause substantial down-modulation of the EGFR receptor. Receptor-independent signaling by mutated KRAS, BRAF, or PI3-kinase, or from loss of PTEN, may obviate the need of cancer cells to express high levels of EGFR. However, no reduction in EGFR expression levels was observed when multiple WT and mutated CRC lines were compared (19). We therefore expect that BiTE antibodies are active against a wide range of CRC cells with diverse mutations in the EGFR pathway.
EGFR is widely expressed on normal tissues (20) in which the receptor is expected to be accessible to its ligands and to antibodies alike. These two features may be responsible for adverse events observed with cetuximab and panitumumab in patients and nonhuman primates (21). For instance, dose-related skin lesions were observed with cetuximab in all treated cynomolgus monkeys down to the human equivalent dose, which were occasionally accompanied by other epithelial toxicities such as conjunctivitis, reddened and swollen eyes, and signs of intestinal disturbance. In a pivotal toxicology study with cetuximab (22), half the monkeys in the high-dose group died as a result of the treatment, which caused lesions of the skin, tongue, nasal cavity, and esophagus. The pathology of skin lesions was interpreted as cetuximab-induced maturation defects of the epidermis. Notably, the EGFR-specific BiTE antibody based on cetuximab did not induce any skin lesions during a 3-wk treatment, a time period sufficient for development of skin toxicities in cetuximab-treated monkeys. Although the 3-wk exposure may have been still too short for the development of skin lesions with C-BiTE treatment, it seems more likely that skin reactions are caused by receptor blockade at saturating doses of cetuximab, which cannot be accomplished by the well tolerated dose levels of the BiTE antibody.
C-BiTE at doses of 6.2 and 12.4 μg/kg/d was well tolerated by monkeys. These two dose levels were equivalent to, or even higher than, those of the anti-CD19 BiTE antibody blinatumomab, which led to a high rate of partial and complete tumor responses in patients with lymphoma (10) and led to molecular remissions in B-ALL patients.† In in vitro assays, blinatumomab and C-BiTE show similar EC50 values of redirected target cell lysis. Moreover, the well tolerated serum concentrations of C-BiTE between 1 and 10 ng/mL was at or exceeding concentrations for more than 90% redirected lysis of EGFR-expressing cancer cells in a 24-h assay by prestimulated cynomolgus effector T cells. Taken together, these observations make it likely that C-BiTE has a therapeutic window in patients with cancer, which derives from a differential expression of the EGFR target on cancerous versus normal tissues. A therapeutic window of this nature must also be assumed for the clinically used antibodies cetuximab and panitumumab.
At the toxic dose levels of C-BiTE of 31 and 154 μg/kg/d, we observed adverse reactions as a result of cytokine release and to damage of EGFR-expressing organs by redirected T cells. The infiltration of inflammatory cells in EGFR-expressing tissues of cynomolgus monkeys has been observed at all dose levels, and may therefore not cause adverse events per se but rather reflect an increased motility and extravasation of T cells. As for cetuximab, the toxicities seen with C-BiTE in cynomolgus monkeys validate this primate species as being relevant for future nonclinical safety and dose-finding studies. EGFR-specific BiTE antibodies must be carefully escalated in patients to dose levels that maximally affect EGFR-overexpressing cancer cells but minimally impact normal epithelial cells expressing EGFR at a lower level.
In summary, we show that (i) the EGFR receptor tyrosine kinase is a suitable BiTE target, i.e., it can be used to effectively engage T cells for redirected lysis of EGFR-expressing cancer cells; (ii) BiTE antibodies can use EGFR as drug target even if downstream mutations have destroyed its utility as drug target for signal inhibitory monoclonal antibodies; (iii) cetuximab- and panitumumab-based antibodies are among the most potent BiTE antibodies ever studied in carcinoma cells, with EC50 values for lysis in the subpicomolar range; and (iv) although the therapeutic window for EGFR-specific BiTE antibodies in a nonhuman primate model appeared to be narrow and tolerable doses were associated with symptoms, the same is true for conventional EGFR antibodies as are used in routine cancer therapy.
Materials and Methods
C- and P-BiTE antibodies were constructed by recombinant DNA technology and produced in supernatants from stably transfected CHO cells. Cell culture-based assays were used to determine T cell activation and redirected target cell lysis by C- and P-BiTE antibodies. Either unstimulated or pre-stimulated human T cells from healthy donors were used as effector cells. A variety of EGFR-expressing cancer cells were used as target cells. Lysis was studied by release of 51-chromium from pre-loaded target cells, and T cell activation by fluorescence-activated cell sorting.
The anti-tumor activity of C-BiTE against subcutaneous xenografts with human cancer cell lines was investigated in immunodeficient NOD/SCID mice. Unstimulated human T cells were mixed with cancer cells prior to inoculation. Tumor growth was determined by external caliper measurement.
The pharmacokinetic analysis of C- and P-BiTE in cynomolgus monkeys used an electrochemiluminesence-based detection technology for determining serum concentrations. The toxicology study in primates was conducted in accordance with the guiding principles in the care and use of animals (http://www.the-aps.org).
Further experimental details on construction, expression and purification of C- and P-BiTE antibodies, their dose-response analysis for T cell activation and redirected target cell lysis, and in-vivo efficacy analyses of C- and P-BiTE antibodies in xenograft mouse models are given in SI Materials and Methods. Likewise, details on how pharmacokinetic analyses in serum of cynomolgus monkeys were performed and how the toxicology study in primates was conducted are found in SI Materials and Methods.
Supplementary Material
Footnotes
Conflict of interest statement: All authors are full-time employees of Micromet.
*This Direct Submission article had a prearranged editor.
Topp M, et al., 14th Congress of the European Hematology Association, June 4–7, 2009, Berlin, abstr 482.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1000976107/-/DCSupplemental.
References
- 1.Peeters M, Balfour J, Arnold D. Review article: panitumumab—a fully human anti-EGFR monoclonal antibody for treatment of metastatic colorectal cancer. Aliment Pharmacol Ther. 2008;28:269–281. doi: 10.1111/j.1365-2036.2008.03717.x. [DOI] [PubMed] [Google Scholar]
- 2.Rivera F, Vega-Villegas ME, López-Brea MF. Cetuximab, its clinical use and future perspectives. Anticancer Drugs. 2008;19:99–113. doi: 10.1097/CAD.0b013e3282f23287. [DOI] [PubMed] [Google Scholar]
- 3.Karapetis CS, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 4.Kurai J, et al. Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines. Clin Cancer Res. 2007;13:1552–1561. doi: 10.1158/1078-0432.CCR-06-1726. [DOI] [PubMed] [Google Scholar]
- 5.Baeuerle PA, Reinhardt C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res. 2009;69:4941–4944. doi: 10.1158/0008-5472.CAN-09-0547. [DOI] [PubMed] [Google Scholar]
- 6.Baeuerle PA, Reinhardt C, Kufer P. BiTE: A new class of antibodies that recruit T cells. Drugs Future. 2008;33:137–147. [Google Scholar]
- 7.Dreier T, et al. Extremely potent, rapid and costimulation-independent cytotoxic T-cell response against lymphoma cells catalyzed by a single-chain bispecific antibody. Int J Cancer. 2002;100:690–697. doi: 10.1002/ijc.10557. [DOI] [PubMed] [Google Scholar]
- 8.Dreier T, et al. T cell costimulus-independent and very efficacious inhibition of tumor growth in mice bearing subcutaneous or leukemic human B cell lymphoma xenografts by a CD19-/CD3- bispecific single-chain antibody construct. J Immunol. 2003;170:4397–4402. doi: 10.4049/jimmunol.170.8.4397. [DOI] [PubMed] [Google Scholar]
- 9.Brischwein K, et al. MT110: A novel bispecific single-chain antibody construct with high efficacy in eradicating established tumors. Mol Immunol. 2006;43:1129–1143. doi: 10.1016/j.molimm.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 10.Bargou R, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 11.Topp M, et al. Treatment with anti-CD19 BiTE antibody blinatumomab (MT103 / MEDI-538) is able to eliminate minimal residual disease (MRD) in patients with B-precursor acute lymphoblastic leukemia (ALL): First results of an ongoing phase II study. Blood. 2008;112(ASH Annual Meeting Abstracts):672. abstr 1926. [Google Scholar]
- 12.Brischwein K, et al. Strictly target cell-dependent activation of T cells by bispecific single-chain antibody constructs of the BiTE class. J Immunother. 2007;30:798–807. doi: 10.1097/CJI.0b013e318156750c. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann P, et al. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct. Int J Cancer. 2005;115:98–104. doi: 10.1002/ijc.20908. [DOI] [PubMed] [Google Scholar]
- 14.Offner S, Hofmeister R, Romaniuk A, Kufer P, Baeuerle PA. Induction of regular cytolytic T cell synapses by bispecific single-chain antibody constructs on MHC class I-negative tumor cells. Mol Immunol. 2006;43:763–771. doi: 10.1016/j.molimm.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Mølhøj M, et al. CD19-/CD3-bispecific antibody of the BiTE class is far superior to tandem diabody with respect to redirected tumor cell lysis. Mol Immunol. 2007;44:1935–1943. doi: 10.1016/j.molimm.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 16.Amann M, et al. Antitumor activity of an EpCAM/CD3-bispecific BiTE antibody during long-term treatment of mice in the absence of T-cell anergy and sustained cytokine release. J Immunother. 2009;32:452–464. doi: 10.1097/CJI.0b013e3181a1c097. [DOI] [PubMed] [Google Scholar]
- 17.Lutterbuese R, et al. Potent control of tumor growth by CEA/CD3-bispecific single-chain antibody constructs that are not competitively inhibited by soluble CEA. J Immunother. 2009;32:341–352. doi: 10.1097/CJI.0b013e31819b7c70. [DOI] [PubMed] [Google Scholar]
- 18.Bluemel C, et al. Epitope distance to the target cell membrane and antigen size determine the potency of T cell-mediated lysis by BiTE antibodies specific for a large melanoma surface antigen. Cancer Immunol Immunother. 2010 doi: 10.1007/s00262-010-0844-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jhawer M, et al. PIK3CA mutation/PTEN expression status predicts response of colon cancer cells to the epidermal growth factor receptor inhibitor cetuximab. Cancer Res. 2008;68:1953–1961. doi: 10.1158/0008-5472.CAN-07-5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yano S, et al. Distribution and function of EGFR in human tissue and the effect of EGFR tyrosine kinase inhibition. Anticancer Res. 2003;23(5A):3639–3650. [PubMed] [Google Scholar]
- 21.Zhang W, Gordon M, Lenz HJ. Novel approaches to treatment of advanced colorectal cancer with anti-EGFR monoclonal antibodies. Ann Med. 2006;38:545–551. doi: 10.1080/09546630601070812. [DOI] [PubMed] [Google Scholar]
- 22.European Medicines Agency Summary of product characteristics (Erbitux) 2009 Available at http://www.ema.europa.eu/humandocs/PDFs/EPAR/erbitux/H-558-PI-en.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.