Abstract
Background
Adenoviral directed enzyme prodrug therapy is a promising approach for head and neck cancer gene therapy. Challenges with this approach however are transient gene expression and dissemination of viruses to distant organs.
Methods
We used recombinant silk-elastinlike protein copolymer (SELP) matrices for intratumoral delivery of adenoviruses containing both thymidine kinase-1, and luciferase genes in a nude mice model of JHU-022 head and neck tumor. Hydrogels made from two SELP analogues (47K and 815K) with similar silk to elastinlike block ratios but different block lengths were studied for intratumoral viral delivery. Tumor bearing mice were followed up for tumor progression and luciferase gene expression concomitantly for five weeks. Polymer’s safety was evaluated through body weight change, blood count, liver and kidney functions in addition to gross and microscopic histological examination.
Results
SELP 815K analogues efficiently controlled the duration and extent of transfection in tumors for up to 5 weeks with no detectable spread to the liver. About five-fold greater reduction in tumor volume was obtained with matrix-mediated delivery compared to intra-tumoral injection of adenoviruses in saline. SELP matrix proved safe in all injected mice compared to control group.
Conclusion
SELP- controlled gene delivery approach could potentially improve the anticancer activity of virus-mediated gene therapy while limiting viral spread to normal organs.
Keywords: Gene therapy, Silk-elastinlike polymers, Head and neck tumors, Adenovirus, Hydrogel
1. Introduction
Head and neck squamous cell carcinoma (HNSCC) is among the most ominous cancers with about 48,000 new cases diagnosed annually in the US [1]. The 5-year survival rate is less than 40% for advanced HNSCC with current therapeutic modalities. Present therapy includes radical surgical procedures coupled with radiotherapy in early stages with possibility of cure, while chemotherapy is usually utilized in advanced stages. Surgery typically results in significant patient morbidity and associated disfigurement. Radiotherapy also causes substantial adverse effects, specifically mandible and laryngeal cartilage radionecrosis, soft tissue fibrosis and mucosal atrophy, pain, and xerostomia. The local and regional biology of HNSCC and the consistently poor treatment outcomes with conventional therapies in advanced stages present a significant need for innovative treatments including gene therapy approaches [2,3].
Gene-directed enzyme prodrug therapy (GDEPT) using adenovirus with thymidine kinase (Ad-Tk) in conjunction with ganciclovir (GCV) is efficacious in tumor management [4]. Therapeutic efficacy of Ad-Tk is mediated by the conversion of an inactive prodrug (GCV) to a phosphorylated cytotoxic agent in the presence of the thymidine kinase (Tk-1) transgene, producing a local anticancer effect that is further augmented by a strong “bystander effect” [5]. Unfortunately, transient expression levels of Tk-1 have limited the progress of these therapeutic vectors in the clinic. In addition, studies have shown that >90% of intravenously administered adenovirus is removed from circulation by the liver; as a result repeated administration of high doses of antigenic adenovirus are needed for therapeutic effect [6,7]. Viral uptake by the liver can result in hepatotoxicity due to transfection of liver cells and expression of the therapeutic gene [8]. Localized adenoviral delivery can diminish clearance of therapeutic virus by liver cells and thus reduce hepatotoxicity associated with systemic injections. Controlled delivery of adenoviral particles using polymeric biomaterials takes advantage of both the high transfection efficiency provided by the adenovirus and the biocompatibility and control over release provided by polymers.
Various polymers have been investigated for controlled delivery of viruses. These polymers are by and large either synthesized by chemical synthetic strategies [9,10] or are of natural origin [11]. Chemical synthesis generally results in polymers with limited control over sequence of monomers and molecular weight. This in turn results in diminished control over virus release and physicochemical and biological fate of the polymers. In addition presence of solvent residues can adversely affect virus bioactivity and biocompatibility. On the other hand, natural polymers are difficult to modify. Using recombinant DNA techniques to produce protein polymers, the disadvantages of traditional synthetic or naturally derived polymers can be minimized. Compared to chemically synthesized matrices made from random copolymers [10], SELP copolymers have precise composition, sequence and length, do not contain organic solvents and monomer residues, and can allow precise systematic structure-function relationships. Relative to naturally occurring polymers such as collagen, it is possible to modify the structure of SELPs by controlling the sequence of the monomers at the genetic level. The versatile structure of SELPs, their compatibility with the aqueous environment for the viability of bioactive agents, and the ability to control sequence and properties at the molecular level are clear advantages of this system over other polymeric matrices for viral gene therapy. For example, in contrast to particle-based delivery systems which are potentially subject to migration from the injection site [9], SELP hydrogels are resistant to migration.
We have postulated that recombinant polymer technology enables systematic correlation of polymer structure with function in gene therapy applications [12,13]. One family of recombinant polymers are silk-elastinlike protein polymers (SELPs) that are composed of silkworm silk and human elastin motifs to produce recombinant peptide repeats of silklike (GAGAGS) and elastinlike (GVGVP) units [14]. These motifs confer to the polymer selected characteristics of silk and elastin, namely crystallization, gel formation, elasticity, and biocompatibility. Depending on structure and concentration, SELP solutions will irreversibly self-assemble at 37°C to form dense physically cross-linked hydrogels. This enables SELP solutions to be easily mixed with bioactive agents at room temperature and injected minimally invasively using needles or catheters to form hydrogels at body temperature [15]. Recombinant DNA techniques enable variation of the sequence and ratio of structural motifs of SELP’s in a precise manner, allowing control over the hydrogel network, controlled release, transfection, and biodegradation for localized matrix-mediated adenoviral gene delivery [16–19].
Previously we had shown that matrix-mediated adenovirus delivery by SELP-47K, a copolymer with tandem repeats containing 4 silk units, 7 elastin units, and 1 elastin modified with a lysine (K) substitution, prolongs and localizes transfection in animal models of head and neck cancer [19]. We further demonstrated that systematic structural variations in SELP sequence and composition using recombinant techniques enables control over viral release [20], physicochemical properties [21], and transfection efficiency [18]. Of the SELP analogs SELP-47K, and SELP-815K, which has double the number of silk and elastin units per monomer than SELP-47K, showed robust hydrogel formation [21] and when mixed with adenoviruses containing marker genes prolonged and increased transfection in head and neck tumors in mice [18]. In this paper, we evaluate the influence of SELP structure and composition on the efficacy and safety of tumor therapy. An adenovirus construct of thymidine kinase and luciferase (Ad-Luc-Tk) (Figure 1) was used to simultaneously measure the transfection efficiency, biodistribution, and antitumor effect of the therapeutic virus delivered with and without SELP polymers as a function of time, polymer and virus concentration. Safety of SELP hydrogels as matrix-mediated delivery systems for gene therapy was also evaluated.
Figure 1.

The structural map of the recombinant adenovirus; both antitumor effect and bio-luminescence imaging were obtained in the same set of animals utilizing this construct. I: Human Ad5 sequence (wt 1–458; includes 5’ L-ITR and packaging signal). II: CMV-HSV tK Poly A-CMV-Luc-PolyA. III: Human Ad5 sequences (wt 351335935; E3 region deleted, includes 3’ R-ITR), E3 deletion: 28587–30464.
2. Materials and Methods
2.1 Materials
Two SELP copolymers were used for this study. SELP-47K with a molecular weight of 69,814 Da was obtained as a 12 wt% solution from Protein Polymer Technologies, Inc. (San Diego, CA, USA). SELP-815K with a molecular weight of 65,374 Da was biosynthesized and characterized as described previously [20]. Replication defective human adenovirus (Ad) Type 5 with E1/E3 deletion, under the control of the CMV promoter, was purchased from Vector Biolabs (Philadelphia, PA, USA). The adenovirus construct (Ad-Luc-Tk) contained two genes, the firefly luciferase (Luc) reporter gene and the thymidine kinase-1 (TK-1) therapeutic gene. Construction of the Ad-Luc-Tk dual vector was carried out by incorporation of herpes virus TK plasmid pORF-HSVtk into human Ad-type 5 DUAL-LUC-CCM. Positive constructs having forward: 3.3Kb/4.0Kb, and reverse 2.1Kb/5.2Kb were identified with EcoRI digestion. Products were then obtained through transfection of human kidney 293T cells. JHU-022 oral cavity cancer cell line was a kind gift from Professor David Sidransky of John Hopkins University [22]. For bioimaging, luciferin was purchased from Gold Biotech (St. Louis, MO, USA). For establishing xenografts, JHU-022 cells were cultured in Roswell Park Memorial Institute (RPMI 1640) medium containing 0.1 mg/mL streptomycin, 100 IU/mL penicillin, and 10% fetal calf serum (Gibco, Carlsbad, CA, USA) in a humidified atmosphere with 5% CO2 at 37°C. Ganciclovir was purchased from Sigma-Aldrich (Saint Louis, MO) and reconstituted in sterile saline.
2.2 Animals and tumor model
Six week old female athymic (nu/nu) mice were obtained from Charles River Laboratories, Davis, CA and were used in accordance with the Institutional Animal Care and Use Committee (IACUC) of the University of Utah. Mice were anesthetized using 4% isoflurane mixed with oxygen, and then head and neck cancer xenografts were established by subcutaneously injecting 2×106 JHU-012 cells suspended in 200 µl phosphate buffer saline (PBS) bilaterally on the flank of each mouse. Tumors were allowed to grow for two weeks to reach average dimensions of 7 × 7 mm, then treatment commenced.
2.3 Study Design
To evaluate the influence of polymer composition on therapeutic efficacy and toxicity of adenoviral-containing SELP hydrogels in JHU-022 HNSCC mouse model, SELP-47K, and -815K at 4 wt%, were selected based on previous observations of increased and prolonged transfection with these compositions [18,19]. Ad-Luc-Tk dual gene construct was used to detect gene transfection efficiency, duration of transgene expression, and biodistribution, through bioluminescence due to luciferase gene insert. In addition, therapeutic or toxic effect due to Tk gene in conjunction with GCV was simultaneously monitored. Adenovirus at two viral loads, V1 (1×108) and V2 (2×108) PFU were used. As the desired tumor size was attained, groups of animals each of 4 mice of total 8 tumors were randomly selected. Control groups were injected with saline, GCV, V1, V2, SELP-47K, or SELP-815K. Treatment groups received V1+GCV, V2+GCV, SELP-47K V1+GCV, SELP-47K V2+GCV, SELP-815K V1+GCV, or SELP-815K V2+GCV. The control groups in addition to providing reference values for comparison of the antitumor effect of SELP mediated gene-directed GDEPT, served to evaluate safety of SELP injection. Treatment groups were used to evaluate both the biodistribution and anticancer efficacy of the GDEPT system in conjunction with SELP.
2.4 Anticancer activity of SELP mediated gene-directed GDEPT
As tumor size reached average diameter of 7×7 mm, mice were anesthetized using 4% isoflurane mixed with oxygen. Tumors were grasped dorsally with forceps and Ad-Luc-Tk was administered intratumorally in 4 wt% (final polymer concentration) SELP-47K, SELP-815K, or in physiological saline. Virus-polymer solutions were prepared by thawing SELP and virus stocks and mixing them gently with physiological saline. Ad-Luc-Tk was given at two dosages: V1=1×108 PFU, and V2=2×108 PFU. GCV was administered intraperitoneally on a daily basis to the animals receiving the Ad-Luc-Tk treatments at a dose of 25 mg/kg for 10 total injections beginning the day of intratumoral injection. Tumor size was measured with digital calipers biweekly and the estimated tumor volume (V) was calculated using the longitudinal cross section (L) and transverse cross section (W) according to the formula, V = (L×W2)/2 expressed in mm3. The day of starting treatments was designated day 0.
2.5 Bioluminescent imaging of gene expression in vivo
Imaging was performed biweekly up to 5 weeks after intratumoral injection. For imaging, animals were injected intraperitoneally with 200 µl of 15 mg/mL luciferin solution. Twenty-five minutes after the luciferin injection, mice were anesthetized using 4% isoflurane mixed with oxygen and placed in the imaging chamber of the Xenogen IVIS100 Bioluminescent Imager (Caliper Life Sciences, Hopkinton, MA). Peak luminescence was obtained at 30 minutes post injection of luciferin at one minute exposures. Animals were imaged dorsally at 30 minutes and ventrally at 35 minutes to visualize the localization and the relative expression level of luciferase in the tumor and liver, respectively. Image data and luminescent counts were analyzed by Living Image 2.2 software (Caliper LifeSciences, Hopkinton, MA) coupled to the bioluminescent imaging system.
2.6 Safety of SELP-mediated delivery
SELP -47K and SELP-815K (4 wt%) without adenovirus were administered intratumorally as described in Section 2.4. Animal weight was measured and recorded biweekly for 5 weeks, at which time the animals were euthanized by CO2 asphyxiation. For blood collection, 23 G needles were inserted into the inferior vena cava, and 0.7 ml blood was collected into heparinized 1 ml syringes, and then transferred to heparinized Vaccutainer tubes for blood count and blood chemistry analysis. Whole blood counts used 20 µl of collected blood sample to measure RBCs, WBCs, platelets and hemoglobin, using a Heska CBC-Diff blood counting machine (Heska, Loveland, CO). The remaining blood sample from each animal was centrifuged at 2,000 rpm for 6 minutes to separate plasma. ALT, AST, albumin, urea (BUN), creatinine, and total bilirubin were measured using an automated DRI-CHEM 4000 (Heska, Loveland, CO). Major organs (heart, liver, kidney, lung and spleen) were removed from each mouse at necropsy, weighed, and stored in 10% formalin. These tissues were subsequently embedded in paraffin and histological slides prepared and stained with hematoxylin & eosin (H&E).
2.7 Statistical analysis
Student’s t-test was used to analyze the significance of outcome difference between control groups and test groups. P value of ≤ 0.05 was considered statistically significant.
3. Results
3.1 in vivo Antitumor Activity of SELP mediated GDEPT
As shown in Figure 2A, all Ad-Luc-Tk treated groups experienced marked tumor suppression of more than 80% compared to the control group. It is interesting that SELP injection without virus could result in tumor size reduction in both SELP-47K and SELP-815K, though not statistically significant. Figure 2B, a subset of the data shown in Figure 2A, shows the antitumor effect of virus administered in saline (V2, 2×108 PFU) and when viruses were administered in 4 wt% gels (V2 in the respective SELPs). The anticancer response is higher at all time points for Ad-Luc-Tk administered in SELP-815K compared to SELP-47K or Ad-Luc-Tk in saline. At day 19, the SELP 815K-V2 group had a 5.76 fold greater tumor size reduction compared to the virus in saline group (p=0.029). This difference continued through day 33, when the tumor size reduction was still 4.68 times greater than the virus in saline (p<0.01). Animals treated with adenovirus in saline (V1 and V2) started to relapse by day 22, while increase in tumor size did not occur until day 28 in the SELP-47K groups and did not occur at all for the duration of the study in SELP-815K groups. In contrast to both plain Ad-Luc-Tk and SELP-47K matrices, animals treated with viruses administered in SELP-815K continued to exhibit antitumor inhibition with five of eight tumors in SELP-815K V2 treated mice measuring only the initial volume of SELP injected and hence considered as cured 5 weeks after the single dose administration of the adenovirus carrying the Tk therapeutic gene.
Figure 2.
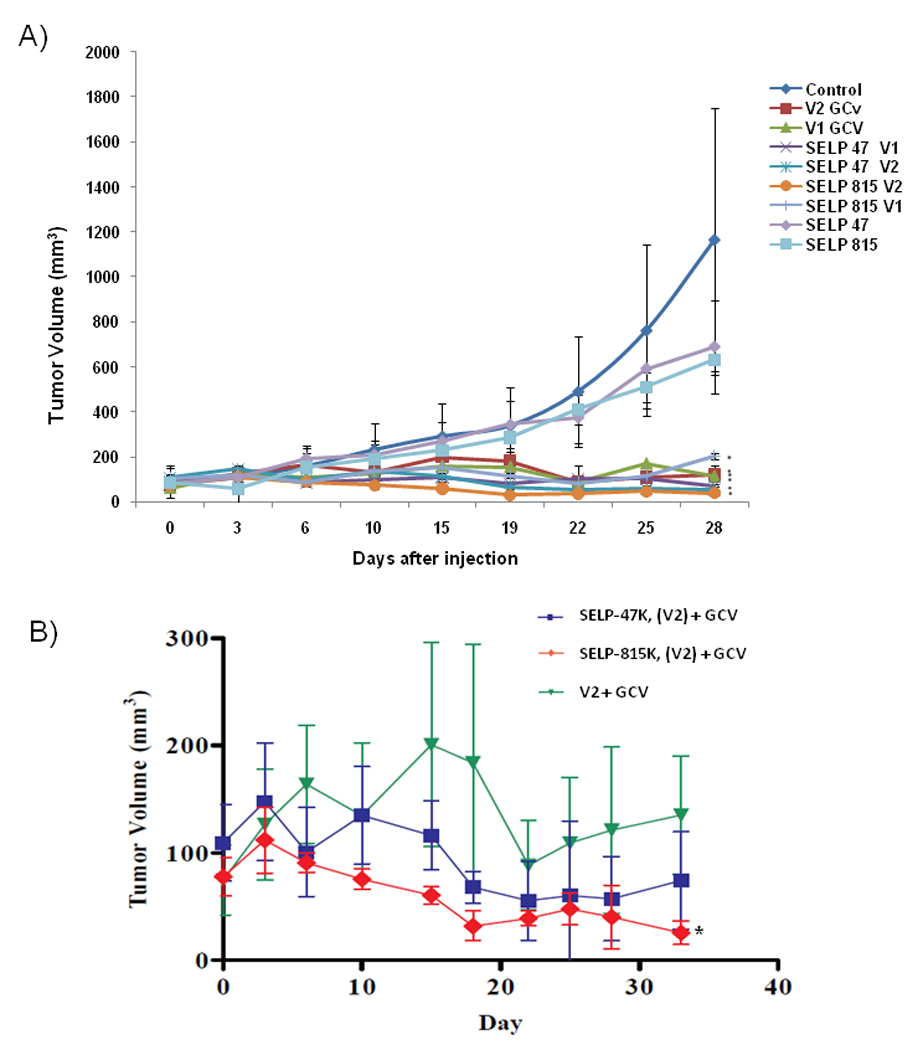
Antitumor efficacy of adenoviruses injected intratumorally in nude mice in conjunction with intraperitoneal GCV. Each data point shows the mean tumor volume of the group with the standard error. A) Therapeutic adenovirus doses (Ad-Luc-Tk) V1 = 1×108 PFU and V2 = 2×108 PFU, were injected alone or in 4 wt % SELP-47K or SELP-815K polymers, *P < 0.05 compared to control group (normal saline). B) Subset of data from 2A to show differences in antitumor effect between animals injected with 2×108 PFU Ad-Luc-Tk in SELP (-47K, and -815K with GCV) to 2×108 PFU Ad-Tk-Luc (V2-GCV) injected in saline, *P < 0.05 compared to V2.
3.2 Expression of Ad-Luc-Tk in treatment groups
Images of luciferase expression 7 days after intratumoral injection are shown in Figure 3. Luciferase expression in animals injected with virus in SELP-815K (top left) was confined to the tumor site although it was less intense compared to animals injected with virus in saline (top right) which shows a diffuse pattern of expression beyond the border of the tumor. However, this diffuse pattern may be a product of the intense expression seen at this time point. Furthermore, in the group injected with adenovirus in saline, only two of four animals showed luciferase expression on Day 7 compared to all animals in the SELP-815K group. On day 22, all animals injected with virus in SELP’s still expressed luciferase and had expression confined to the tumor site (Figure 3, middle left and right), compared to only one animal injected with virus in saline expressing luciferase (Figure 3, lower left). Interestingly, as shown in the ventral view on day 22 (Figure 3, lower right), 50% of animals treated with virus in saline showed clear liver dissemination of the virus as evidenced by intense luciferase expression in the liver, while none of the animals treated with virus in SELPs showed any evidence of liver expression. Virus is released at a slower rate from SELP hydrogels and disseminates to more distant organs slower and to a lesser degree than virus injected in saline, resulting in a lower level of expression in the liver.
Figure 3.

Dorsal view of bioluminescence imaging of gene expression in nude mice of 2×108 PFU Ad-Luc-Tk delivered by: SELP-815K at one week (top left) compared to same dose of virus in saline at one week (top right); SELP-815K after 3 weeks (dorsal view-middle left) and ventral view (middle right), compared to virus in saline (dorsal view lower left and ventral view lower right).
3.3 Safety of SELP treatment
In addition to gene transduction and anticancer efficacy, we evaluated the safety of SELP hydrogels injected intratumorally without virus in the nude mouse model of human JHU-022 head and neck cancer. We followed the animal weight throughout the study duration. All animals gained weight throughout the study suggesting no overt toxicity of any element of the treatment system components (Supplementary Figure S1). We observed no statistical differences in the blood count values, kidney function, or liver function from control mice and SELP injected groups (Table 1). In addition, no abnormalities in organ weight at necropsy (Supplementary Table S2) were noted for SELP injected groups, nor were there any histological abnormalities in the heart, liver, kidney, lung and spleen. Similar findings were observed for viral treated groups with and without SELP (data not shown).
Table 1. Blood count, kidney and liver functions of nude mice administered interatumorally with SELPs*.
Measurement of blood parameters and proteins representing kidney and liver functions in nude mice. No statistical difference was found among groups or between groups and control animals. Measurements are the averages of 4 values ± SD. Each group of four animals was injected with 50 µl volume intratumorally; physiological saline (control), SELP-47K 4% in saline, and SELP-815K 4% in saline.
| Group | Blood Parameter | Kidney Function | Liver function | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hemoglobin g/dL |
White blood cell count 103 cells/mL |
Platelet count 103 /mm3 |
Creatinine mg/dL |
BUN mg/dL |
Total Protein g/dL |
GOT U/L |
GPT U/L |
Bilirubin mg/dL |
|
| Control | 14.76±0.31 | 4.38±0.74 | 336±34.1 | 0.34±0.05 | 29.9±1.4 | 4.98±0.10 | 87±5 | 21±1 | 0.63±0.10 |
| SELP-47K | 13.38±0.15 | 6.25±1.67 | 139.0±7.1 | 0.45±0.15 | 25.2±1.7 | 5.13±0.22 | 73±6 | 31±6 | 0.90±0.14 |
| SELP-815K | 13.30±0.71 | 5.40±1.78 | 187.0±24 | 0.30±0.06 | 22.4±1.7 | 4.73±0.41 | 81±13 | 28±8 | 0.95±0.21 |
No significant difference between control group and SELPs treated groups were found. Measurements are the average of 4 values ± SD. Each group of four animals was injected with 50µL volume interatumorally; physiological saline (control), SELP-47K 4% in saline, and SELP-815K 4% in saline.
Discussion
GDEPT has been widely studied as a strategy to increase the sensitivity of cancer cells to apoptosis induced by prodrugs [4]. Introduction of the thymidine kinase gene using an adenoviral vector provides the tumor cell with the capacity for localized activation of the prodrug ganciclovir, thereby restricting production of the toxic drug metabolite to the tumor tissue. GDEPT can impart a strong bystander cytotoxic response because of the diffusion of activated drug metabolites from a transduced tumor cell into neighboring, naïve tumor cells that do not express the prodrug activation enzyme. In contrast to other methods of cancer gene therapy, GDEPT does not require the genetic modification of each individual tumor cell. This approach results in marked efficacy in vitro with chemo-resistant tumor cells such as glioma cell lines.
A major limitation of viral gene therapy however is the rapid dissemination of the vectors from the tumor site resulting in suboptimal therapeutic response. Transgene expression from adenovirus delivered from chemically synthesized polymers intratumorally has increased but these systems have been unable to prolong duration. PLGA microspheres and poloxamer systems have been used to increase transfection of adenoviral vectors [9,10,23]. Along with SELP, polymeric adenovirus delivery systems have shown increased transfection at the injection site with limited transfection away from the target tissue. However, SELP also has shown increased duration of transfection for a period of weeks, prolonging the release of adenovirus [15]. Viral vectors that reach the circulation have the potential to illicit a strong immune response that can be harmful to the host. Finally, viral vectors in circulation are eventually removed by the liver where high coxsackie adenovirus receptors (CAR) on hepatocytes surface are expressed resulting in liver toxicity upon administration of the prodrug. The demonstrated tumor suppression data (Figure 2B) supports the hypothesis that SELP-mediated delivery localizes gene expression while limiting off-target hepatic dissemination and expression and leads to a more pronounced antitumor effect. Ways by which the superior efficacy of SELP-mediated adenoviral gene therapy can be further enhanced need to be examined.
Marker Ad-LacZ when delivered with SELP-815K in previous work showed prolonged and up to fifty times enhanced gene expression in the same nude mouse model of head and neck tumor xenograft [18]. Expression with the SELP-815K group was also pronounced compared to intratumoral delivery of Ad-Luc with SELP-47K [18]. SELP-815K has longer silk and elastin blocks in each monomer repeat compared to SELP-47K, although the ratio of silk to elastin blocks and polymer molecular weights are the same. This provides evidence that in addition to the ratio of silk and elastin units, the sequence of these units in the polymer chain influences the physicochemical properties, transfection efficiency, and antitumor efficacy of the resulting hydrogels when loaded with a viral gene carrier. We hypothesized that the release rate from each hydrogel is affected by the pore size related to the number of silk and elastin building blocks, leading to higher transfection efficiency when adenovirus is released from SELP-815K. The more controlled release of SELP-815K leads to a higher residence time of the virus within the tumor and overall prolonged and higher transfection efficiency. Based on the above observations [18], in this study we evaluated the influence of polymer structure on efficacy. The onset of relapse (marked by the increase in average tumor size in each group) was later in SELP groups compared to tumors treated with virus in saline. This demonstrates the advantages of sustained release from the SELP matrix. As tumor cells are eliminated, new tumor cells are transfected by the sustained release of virus, leading to sustained anticancer effect in the SELP treatment groups.
Viral localization in parallel with the antitumor effect of GDEPT from SELP-815K matrices was evaluated by bioluminescence imaging. Bioluminescent imaging has the advantage that gene expression can be monitored in the same animal over the study period without the need to sacrifice to recover tumor and liver tissue for measurement of expression. We measured the transfection level and the extent of localization of the luciferase gene. By using a novel adenovirus construct encoding for both therapeutic gene (Ad-Tk) and a bioluminescent imaging agent (Luc), we are able to report simultaneous anticancer efficacy and distribution data using one cohort of animals (Figures 2–3).
An anticancer effect related to the transcription of Tk delivered in a localized fashion in conjunction with intraperitoneal GCV was produced (Figure 2). At the higher adenovirus dose, each group experienced a reduction in tumor size from the peak size. Consistent with increased transfection efficiency observed previously [18], adenoviruses released from SELP-815K sustained tumor size reduction to a higher extent compared to SELP-47K and adenovirus only groups. The effectiveness of the SELP matrices in controlling the release of adenoviral vector as a function of luciferase gene expression at various time points was monitored. Gene expression was evaluated to determine how much spread and diffusion from the tumor site occurred under each treatment condition. SELP matrices localize expression in the tumor area (Figure 3). SELP815K treatment groups did not show detectable levels of expression in the liver. This is consistent with previous observations [18] that high tumor to liver expression ratios were observed when Ad-LacZ was delivered with SELP-815K.
An important concern for matrix-mediated gene delivery is the safety of the biomaterial used. SELPs are under investigation in clinical trials as bulking agents for management of urinary incontinence [24]. Their safety and biocompatibility as matrices for in vivo gene delivery need to be carefully examined. We evaluated the safety of the SELPs in the tumor mouse model using conventional clinical chemistry parameters and body weight for the duration of study. Significant differences from control and normal values could not be detected in body weight, blood counts, kidney function, liver function, or organ weight at necropsy (Table 1, Figure S1, Table S2). These data demonstrate the safety of locally injected SELP in nude mice. It should be mentioned that the immunodeficiency of this model did not allow the possible immune response against SELP to be assessed. If an immune reaction to SELP occurs, it is likely that it will be localized at the injection site, where it may have an advantageous antitumor effect. These assumptions need to be validated however in an immunocompetent mouse model. The purpose of the present study was to evaluate the influence of SELP-mediated delivery and polymer structure on therapeutic efficacy of GDEPT in treatment of HNSCC in a readily available model. Further detailed studies are needed to establish the efficacy, long-term safety, biodegradation and biological fate of SELP-mediated adenoviral gene delivery.
Taken as a whole, we have demonstrated that SELPs prolong viral gene expression and boost antitumor effect of Ad-Tk in conjunction with GCV for treatment of head and neck tumors in nude mice. Structure – function relationships suggest that SELP-815K increases the level of gene expression and antitumor efficacy compared to other SELP analogs, while minimizing dissemination to distant organs such as liver. Short-term studies in nude mice suggest that SELPs are safe when injected intratumorally in nude mice. The properties of the hydrogels show promise for matrix-mediated head and neck cancer gene therapy. Future directions of this research include modifications of the polymer structure to control biodegradation and viral release in response to the tumor microenvironment as well as evaluating the safety and efficacy of SELP-mediated adenoviral gene therapy in immunocompetent animals.
Supplementary Material
Change in animal body weight during the course of the study. No statistical difference was found among groups or between groups and control animals. G, ganciclovir; V1,108 PFU Ad-Luc-Tk; V2, 2×108 PFU Ad-Luc-Tk, SELP-47K, SELP-815K, and control (normal saline).
Acknowledgements
This research was supported by the National Institutes of Health grant (R01-CA107621) and the Utah Science Technology and Research (USTAR) Initiative.
References
- 1.Detailed Guide: Oral Cavity and Oropharyngeal Cancer. [Date accessed: 06/29/09];American Cancer Society. 2009 Online Article http://www.cancer.org/docroot/CRI/CRI_2_3x.asp?dt=60. [Google Scholar]
- 2.Deshpande AM, Wong DT. Molecular mechanisms of head and neck cancer. Expert Rev Anticancer Ther. 2008;8(5):799–809. doi: 10.1586/14737140.8.5.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chin D, Boyle GM, Theile DR, Parsons PG, Coman WB. Molecular introduction to head and neck cancer (HNSCC) carcinogenesis. Br J Plast Surg. 2004;57(7):595–602. doi: 10.1016/j.bjps.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Goebel EA, Davidson BL, Graham SM, Kern JA. Tumor reduction in vivo after adenoviral mediated gene transfer of the herpes simplex virus thymidine kinase gene and ganciclovir treatment in human head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg. 1998;119(4):331–336. doi: 10.1016/S0194-5998(98)70073-7. [DOI] [PubMed] [Google Scholar]
- 5.Li S, Tokuyama T, Yamamoto J, Koide M, Yokota N, Namba H. Bystander effect-mediated gene therapy of gliomas using genetically engineered neural stem cells. Cancer Gene Ther. 2005;12:600–607. doi: 10.1038/sj.cgt.7700826. [DOI] [PubMed] [Google Scholar]
- 6.Kuzmin AI, Finegold MJ, Eisensmith RC. Macrophage depletion increases the safety, efficacy and persistence of adenovirus-mediated gene transfer in vivo. Gene Ther. 1997;4:309–316. doi: 10.1038/sj.gt.3300377. [DOI] [PubMed] [Google Scholar]
- 7.Worgall S, Wolff G, Falck-Pedersen E, Crystal RG. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum. Gene Ther. 1997;8:37–44. doi: 10.1089/hum.1997.8.1-37. [DOI] [PubMed] [Google Scholar]
- 8.Mahasreshti PJ, Kataram M, Wang MH, et al. Intravenous delivery of adenovirus-mediated soluble FLT-1 results in liver toxicity. Clin Cancer Res. 2003;9(7):2701–2710. [PubMed] [Google Scholar]
- 9.Beer SJ, Matthews CB, Stein CS, Ross BD, Hilfinger JM, Davidson BL. Poly (lacticglycolic) acid copolymer encapsulation of recombinant adenovirus reduces immunogenicity in vivo. Gene Ther. 1999;5:740–746. doi: 10.1038/sj.gt.3300647. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Liu S, Li CY, Yuan F. A novel method for viral gene delivery in solid tumors. Cancer Res. 2005;65(17):7541–7545. doi: 10.1158/0008-5472.CAN-05-1112. [DOI] [PubMed] [Google Scholar]
- 11.Chandler LA, Doukas J, Gonzalez AM, Hoganson DK, Gu DL, Ma C, et al. FGF2-Targeted adenovirus encoding platelet-derived growth factor-B enhances de novo tissue formation. Mol Ther. 2000;2(2):153–160. doi: 10.1006/mthe.2000.0102. [DOI] [PubMed] [Google Scholar]
- 12.Megeed Z, Ghandehari H. Genetically engineered protein-based polymers: potential in gene delivery. In: Amiji M, editor. Polymeric Gene Delivery: Principles and Applications. Boca Raton, FL: CRC Press; 2005. pp. 489–507. [Google Scholar]
- 13.Hatefi H, Megeed Z, Ghandehari H. Recombinant polymer-protein fusion: a promising approach towards efficient and targeted gene delivery. Journal of Gene Medicine. 2006;8:468–476. doi: 10.1002/jgm.872. [DOI] [PubMed] [Google Scholar]
- 14.Cappello J, Crissman J, Dorman M, et al. Genetic engineering of structural protein polymers. Biotechnol Prog. 1990;6(3):198–202. doi: 10.1021/bp00003a006. [DOI] [PubMed] [Google Scholar]
- 15.Dandu R, Ghandehari H. Delivery of bioactive agents from recombinant polymers. Prog in Polym Sci. 2007;32:1008–1030. [Google Scholar]
- 16.Megeed Z, Haider M, Li D, O'Malley BW, Jr, Cappello J, Ghandehari H. In vitro and in vivo evaluation of recombinant silk-elastinlike hydrogels for cancer gene therapy. J Control Release. 2004;94(2–3):433–445. doi: 10.1016/j.jconrel.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 17.Hatefi A, Cappello J, Ghandehari H. Adenoviral gene delivery to solid tumors by recombinant silk-elastinlike protein polymers. Pharm Res. 2007;24(4):773–779. doi: 10.1007/s11095-006-9200-5. [DOI] [PubMed] [Google Scholar]
- 18.Gustafson J, Greish K, Frandsen J, Cappello J, Ghandehari H. Silk-elastinlike recombinant polymers for gene therapy of head and neck cancer: From molecular definition to controlled gene expression. J Control Release. 2009;140(3):256–261. doi: 10.1016/j.jconrel.2009.05.022. 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greish K, Gustafson J, Frandsen J, Cappello J, Ghandehari H. Silk-elastinlike protein polymer hydrogels for localized adenoviral gene therapy of head and neck tumors. Biomacromolecules. 2009;10(8):2183–2188. doi: 10.1021/bm900356j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dandu R, Cappello J, Ghandehari H. Characterization of structurally related adenovirus-laden silk-elastinlike hydrogels. Journal of Bioactive and Compatible Polymers. 2008;23:5–19. [Google Scholar]
- 21.Dandu R, Cresce AV, Briber R, Dowell P, Cappello J, Ghandehari H. Silk-elastinlike protein polymer hydrogels: Influence of monomer sequence on physicochemical properties. Polymer. 2009;50:366–374. [Google Scholar]
- 22.Li D, Duan L, Freimuth P, O’Malley BW., Jr Variability of Adenovirus Receptor Density Influences Gene Transfer Efficiency and Therpeutic Response in Head and Neck Cancer. Clinical Cancer Research. 1999;5:4175–4181. [PubMed] [Google Scholar]
- 23.Feldman LJ, Pastore CJ, Aubailly N, Kearney M, Chen D, Perricaudet M, Steg PG. Improved efficiency of arterial gene transfer by use of poloxamer 407 as a vehicle for adenoviral vectors. Gene Therapy. 1997;4:189–198. doi: 10.1038/sj.gt.3300382. [DOI] [PubMed] [Google Scholar]
- 24.Protein Polymer Technologies. [accessed July 2009]; www.ppti.com. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Change in animal body weight during the course of the study. No statistical difference was found among groups or between groups and control animals. G, ganciclovir; V1,108 PFU Ad-Luc-Tk; V2, 2×108 PFU Ad-Luc-Tk, SELP-47K, SELP-815K, and control (normal saline).


