Abstract
Acute lymphoblastic leukemia (ALL) with translocation t(4;11) is found in 60–85% of infants with ALL and is often refractory to conventional chemotherapeutics after relapse. Using the t(4;11) ALL line SEM, we evaluated chemotherapy resistance in NOD.CB17-Prkdcscid/J mice. SEM cells were injected into the tail vein and engraftment was monitored by flow cytometry. Once engraftment was observed, mice were injected intraperitoneally with phosphate-buffered saline (PBS), or vincristine (0.5 mg/kg body weight) three times per week for 4 weeks (n = 8 per group). The level of P-glycoprotein in SEM cells was increased 3-fold by vincristine treatment compared to PBS-treated mice. Survival curves showed that leukemia cell growth was initially delayed by vincristine treatment, but the mice eventually succumbed to disease. These data describe a novel inducible model for investigating multidrug resistance mechanisms in high risk t(4;11) ALL.
Keywords: leukemia, translocation, P-glycoprotein, multidrug resistance
1. Introduction
Acute lymphoblastic leukemia (ALL) with chromosomal translocation t(4;11) is found in 60–85% of infants with ALL, 2% of children, and 3–6% of adults with this disease [1,2]. This leukemia is a high-risk leukemia since it is particularly resistant to conventional chemotherapeutics upon relapse. Commonly used chemotherapeutics for treating high-risk ALL include vincristine, prednisone, doxorubicin, cytarabine, methotrexate [3]. Multidrug resistance (MDR) is an important factor in the development of drug-resistant cells during chemotherapy that results in a poorer prognosis. A key MDR gene that has been extensively studied is P-glycoprotein (PgP), encoded by the mdr1 gene [4]. PgP is an energy dependent, transmembrane efflux pump with specificity for a broad range of substrates that include Vinca alkaloids (vincristine), taxanes, actinomycin D, and anthracyclins [5]. Multidrug resistance protein-1 (MRP1) and lung resistance protein (LRP) constitute two non-PgP-mediated mechanisms of MDR [6].
The severe-combined immunodeficient (SCID) mouse and, more recently, the nonobese diabetic × SCID (NOD/SCID) cross have been useful as mouse models for evaluation of different chemotherapeutic agents against leukemia [7–12]. The SCID background is well known for the absence of T and B lymphocyte populations and eliminates a T-cell mediated rejection of xenografted leukemia cells. The NOD background presents reduced natural killer lymphocyte activity and absence of circulating complement that further increase engraftment efficiency. Leukemia in this mouse model mimics the human disease and engraftment sites are bone marrow, spleen, and liver, with significant numbers of cells in the blood [13].
Several cell lines have been established from patients with ALL carrying the t(4;11) chromosomal translocation. The SEM leukemia line was established from a 5 year old female with relapsed t(4;11) ALL and has been useful for evaluating alternative treatment strategies for this disease [14]. In a preliminary study, we found that vincristine treatment initially delayed SEM cell growth in NOD/SCID mice. However, the SEM cells subsequently grew out and the mice died from leukemia during the vincristine treatment regimen. We hypothesized that the mechanism of re-growth of the SEM leukemia cells was due to increased resistance of the cells to vincristine. In this study, we describe a novel inducible model for the investigation of multi-drug resistance in high-risk t(4;11) ALL in vivo. The objective of the study is to verify whether P-glycoprotein, product of the mdr1 gene, is elevated in these cells after treatment with vincristine. This model should prove useful for mechanistic studies of multi-drug resistance in this high-risk leukemia that may lead to more efficacious treatment strategies.
2. Methods and Materials
2.1. Cells and reagents
SEM is an established cell line from a patient diagnosed with high-risk pre-B ALL containing the chromosomal translocation t(4;11)(q21;q23) [14]. The cells were grown at 37°C, 5% CO2 in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Sigma, St. Louis, Mo.), 50 IU/ml penicillin, 50 µg/ml streptomycin, 0.25 µg/ml amphotericin B, 1 mM sodium pyruvate, and 2 mM L-glutamine (Invitrogen). For injection into mice, SEM cells were harvested, washed 2× in Dulbecco’s PBS without Ca2+ or Mg+ (Sigma), and resuspended at a final concentration of 50 × 106 cells/ml in PBS for injections.
Vincristine was purchased from Sigma. Phycoerythrin-cyanin 7 (PE-Cy7)-conjugated anti-human CD19, allophycocyanin-Cy7 (APC-Cy7) conjugated anti-mouse CD45, fluorescein isothiocyanate (FITC)-conjugated anti-human PgP, and FITC-conjugated mouse IgG2b were purchased from Becton Dickinson (San Jose, CA).
2.2. Immunodeficient mice
Sixteen 6 wk old female NOD.CB17-Prkdcscid/J mice were purchased from the Jackson Laboratory (Bar Harbor, ME, common name NOD/SCID). Mice were housed and handled under pathogen-free conditions at the University of California, Davis vivarium in a temperature controlled environment with a 12 h light-dark cycle. Mice were given sterile food and water ad libitum. Mice were weighed once per week. At the age of 8 weeks, each mouse was injected with 5 × 106 SEM cells (100 µl volume) through the tail vein using a 1 cc syringe equipped with a 30 gauge needle (Becton Dickinson). All experimental procedures were approved by the University of California, Davis Institutional Animal Care and Use Committee.
2.3 Detection of leukemia cell engraftment
To monitor engraftment of the leukemia cells, approximately 25–30 µl of blood was collected from the tail artery of each mouse beginning 2 weeks after tail vein injection. Red blood cells were lysed using PharmLyse (Becton Dickinson) according to the manufacturer’s recommendation and the peripheral blood mononuclear cells (PBMCs) were stained with PE-Cy7 conjugated anti-human CD19 and APC-Cy7 conjugated anti-mouse CD45. Incubations of the cells with antibodies were performed at room temperature for 20 min. The cells were washed in PBS containing 0.1% BSA and 7 mm sodium azide (Sigma) and then fixed in 1% paraformaldehyde (Sigma) before analysis. The stained cells were analyzed on a FACSCanto fluorescence-activated cell sorter (FACS) using FACSDiva software (Becton Dickinson). Each analysis of peripheral blood cells was performed using appropriate scatter gates to exclude cellular debris and aggregated cells. As a negative control for engraftment, peripheral blood mononuclear cells were prepared from immunodeficient mice (from a previous study) that had not been injected with leukemia cells and were frozen at −80°C in 10% dimethylsulfoxide (DMSO), 90% fetal bovine serum until use. The frozen PBMCs were quickly thawed and washed in PBA to remove the DMSO. As a positive control for CD19+ cells, the frozen PBMCs from non-engrafted mice were spiked with SEM cells cultured in vitro. Both the negative control cells and PBMCs spiked with cultured SEM cells were stained with the antibodies described above and used to set the gates for CD19+ cells. Thirty thousand events were collected for each sample. Positive engraftment was established when the proportion of human CD19+ cells reached 0.5 to 1% in the murine PBMC population.
2.4 Vincristine treatmemt
Vincristine was dissolved in sterile PBS and stored at −20°C until use. Vincristine was tested against SEM cells in vitro to confirm toxicity. SEM cells were treated with a final concentration of 2 µg/ml vincristine or an equivalent amount of PBS as a control. After 48 h, cell death was measured by lysing the cells in a hypotonic solution containing 1 mg/ml sodium citrate, 0.1% Triton X-100, and 50 µg/ml propidium iodide (PI, Sigma) and analyzing the resulting nuclei by flow cytometry. The extent of cell death (percent) was determined by measuring the fraction of nuclei that contained sub-diploid DNA content [15]. Twenty thousand events were collected for each sample stained with propidium iodide.
Once engraftment of leukemia was observed in the peripheral blood, mice were randomly separated into control or vincristine treatment groups (n = 8 per group). Mice were treated three times per week by intraperitoneal injection with PBS (control group) or vincristine at a concentration of 0.5mg/kg body weight. The treatment period was four weeks. The approximate volume per injection was between 80 – 120 µl depending upon the weight of the mouse. During the treatments, the blood from each mouse was monitored for growth of the leukemia cells twice in the first week and weekly afterward. Body weights were obtained weekly in order to adjust the quantity of vincristine per animal. For statistical comparisons between treatment groups, the event free survival (EFS) was calculated beginning with the initiation of PBS or vincristine injections. An event was defined as overt clinical illness necessitating euthanization, which included greater than 20% weight loss, lethargy, severe weakness, or inability to reach food or water for 24h.
2.5 Analysis of P glycoprotein (PgP) expression
For analysis of PgP expression in mice, blood was collected from each mouse when the proportion of human CD19+ cells reached 3–5 % of the PBMC population. Red blood cells from each mouse were lysed as described above and the PBMCs were stained with PE-Cy7- conjugated anti-human CD19 and FITC-conjugated anti-human PgP (Becton Dickinson). Cells were stained with FITC-conjugated mouse IgG2b (Becton Dickinson) as an isotype control. Forty thousand events were collected for each sample. Appropriate scatter gates were used to exclude cellular debris and aggregated cells. Cultured SEM cells were also stained with FITC-labeled isotype control or FITC-anti-human PgP to determine the level of protein on the surface of these cells grown under in vitro conditions. Thirty thousand events were collected on the flow cytometer for each sample of cultured SEM cells.
2.6 Statistical analysis
All statistical analyses were performed with GraphPad software (GraphPad Software, Inc., San Diego, CA) and the data were displayed as arithmetic means ± standard error (SE). Kaplan-Meier survival curves were used to determine differences in EFS by log-rank test. P values were obtained using two-tailed t-tests for evaluation of significant differences for percent of CD19+ cells, and PgP expression between vincristine-treated and control mice. The confidence interval for significant differences was set at 95%.
3. Results
3.1. PgP is not expressed in the SEM line in culture
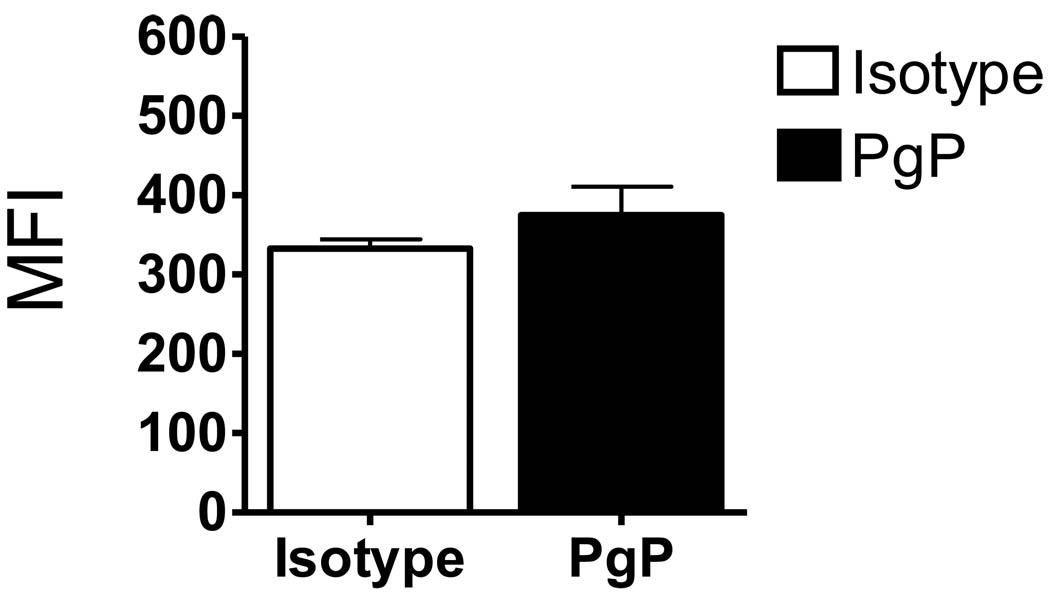
In a preliminary study, we found the growth of the SEM cells in the NOD/SCID mice treated with vincristine was reduced or delayed 7–10 days compared to the control mice, but the cells eventually grew and the mice died from leukemia 4–5 wk after treatment began (data not shown). We hypothesized that at least a part of the mechanism by which the SEM cells grew during vincristine treatment was due to an increased resistance to vincristine. Since vincristine is a target of the multi-drug resistant pumping mechanism, we analyzed whether P glycoprotein, the protein product of mdr1 gene, was elevated in these cells. Vincristine was first tested against SEM cells grown in culture to ensure that the drug was toxic to the leukemia cell line. After 48 h, greater than 95% of SEM cells were dead (data not shown) indicating vincristine was toxic to these cells in vitro. The presence of PgP on the cell surface of cultured SEM cells was determined by staining with anti-PgP antibody or isotype control immunoglobulin, with subsequent analysis by flow cytometry. The difference in mean fluorescence intensity between cells stained with isotype and PgP antibody was not statistically significant for SEM cells harvested from in vitro culture conditions (Fig. 1).
Figure 1.
SEM cells do not express PgP under in vitro culture conditions. SEM cells grown in culture were stained with FITC-conjugated mouse IgG2b (isotype control) or FITC-conjugated anti-PgP antibody and analyzed by flow cytometry. MFI, mean fluorescence intensity. Data represent means ± SE for three independent experiments.
3.2 PgP expression is induced by vincristine treatment in vivo
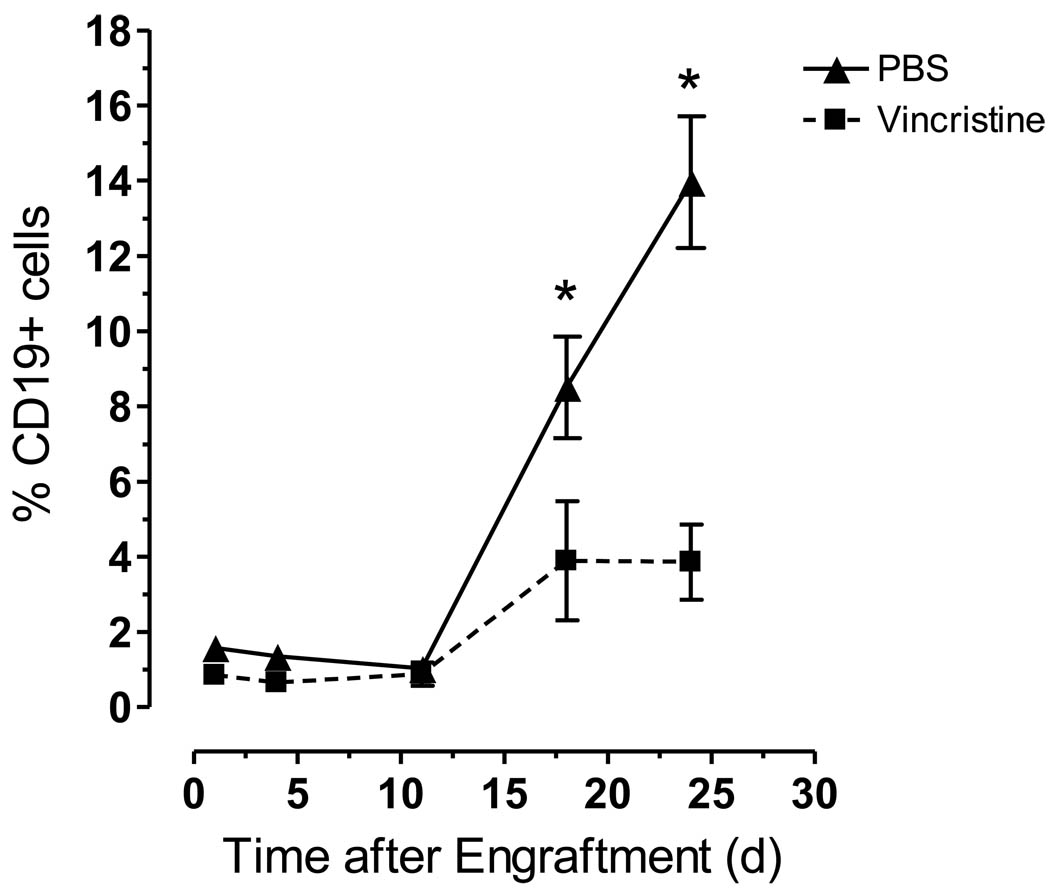
Sixteen NOD/SCID mice were injected with SEM leukemia cells. By monitoring for the presence of human CD19+SEM cells in the peripheral blood of these mice, 100% engraftment of the leukemia cells was observed 15–18 days after injection into the tail vein. Once engraftment was observed, the mice were treated with PBS (n = 8) or vincristine at a concentration of 0.5 mg/kg body weight (n = 8) three times per week. The percent of human CD19+ cells increased significantly in the PBS-treated compared to the vincristine-treated mice over a treatment period of 24 days (P < 0.05, Fig. 2). By day 24, the number of living mice in the PBS-treated and vincristine-treated groups was 5 and 7, respectively. Three of the 5 mice in the PBS-treated group were euthanized on day 24 after blood was collected to determine leukemia burden.
Figure 2.
Vincristine initially reduces the leukemia burden in NOD/SCID mice compared to control animals. Sixteen NOD/SCID mice were engrafted with SEM leukemia cells and treated intraperitoneally with either PBS (control mice, n = 8) or vincristine (0.5mg/kg body weight, n = 8) three times per week for 4 weeks. The percentage of human CD19+ cells in the mouse peripheral blood was monitored by staining the mononuclear cells with APC-Cy7 conjugated anti-mouse CD45 and PE-Cy7 conjugated anti-human CD19, and analyzing the cells by flow cytometry. Asterisks indicate significant differences in %CD19+ cells from PBS and vincristine-treated mice (P < 0.05).
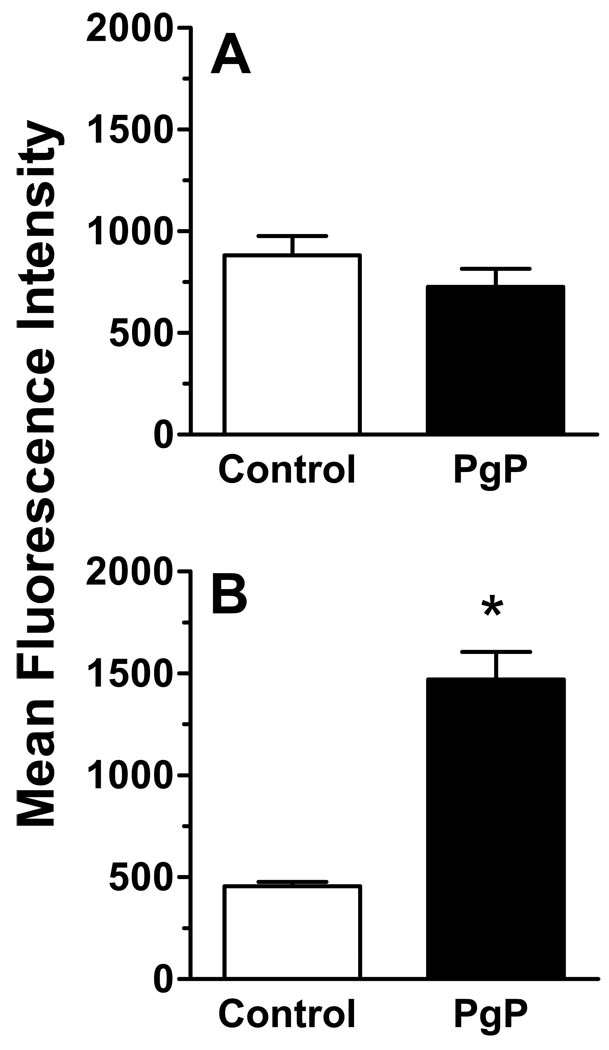
PgP expression was measured on the cell surface when the mean percent of human CD19+ cells reached 3–5 % of the PBMC population in each group, to allow enough cells to be collected and statistically analyzed by flow cytometry. Therefore, the level of PgP was measured in the PBS-treated mice and vincristine-treated mice on days 13 and 18 after the beginning of treatment, respectively. Fig. 3 shows the expression levels of PgP on the cell surface of SEM cells from the control mice (A) and the vincristine-treated mice (B). The difference in mean fluorescence intensity between cells stained with isotype and PgP antibody was not significant for SEM cells from the PBS- treated mice (Fig. 3A). However, in the vincristine-treated mice, the mean fluorescence intensity of the PgP staining was approximately 3-fold greater than in cells stained with the isotype control antibody (Fig. 3B), indicating that PgP expression was induced by in vivo treatment of the mice with vincristine (P < 0.05). The difference in staining intensity for the isotype control IgG2b in Fig. 3A and B was likely due to nonspecific binding of the isotype in experiments performed on two different experimental days. Hemolysis of samples during collection of small volumes and the required lysis of red blood cells before staining could contribute to nonspecific background staining.
Figure 3.
Vincristine treatment induces expression of PgP on the cell surface of SEM leukemia cells in NOD/SCID mice. Sixteen NOD/SCID mice were engrafted with SEM leukemia cells and treated as described in Figure 2. Peripheral blood mononuclear cells were isolated and stained with PE-Cy7-conjugated anti-human CD19 and FITC-conjugated anti-P-glycoprotein or FITC-conjugated isotype control antibody. Staining was performed when the %CD19+ cells were 3–5% of the murine PBMC population (day 13 for the PBS group, day 18 for the vincristine group). The cells were gated on the CD19 population and the levels of PgP were analyzed by flow cytometry. MFI, mean fluorescence intensity. Asterisks indicate significant differences between the MFI of isotype control stained cells and PgP stained cells (P < 0.05).
3.3 Survival of mice is briefly enhanced by vincristine treatment
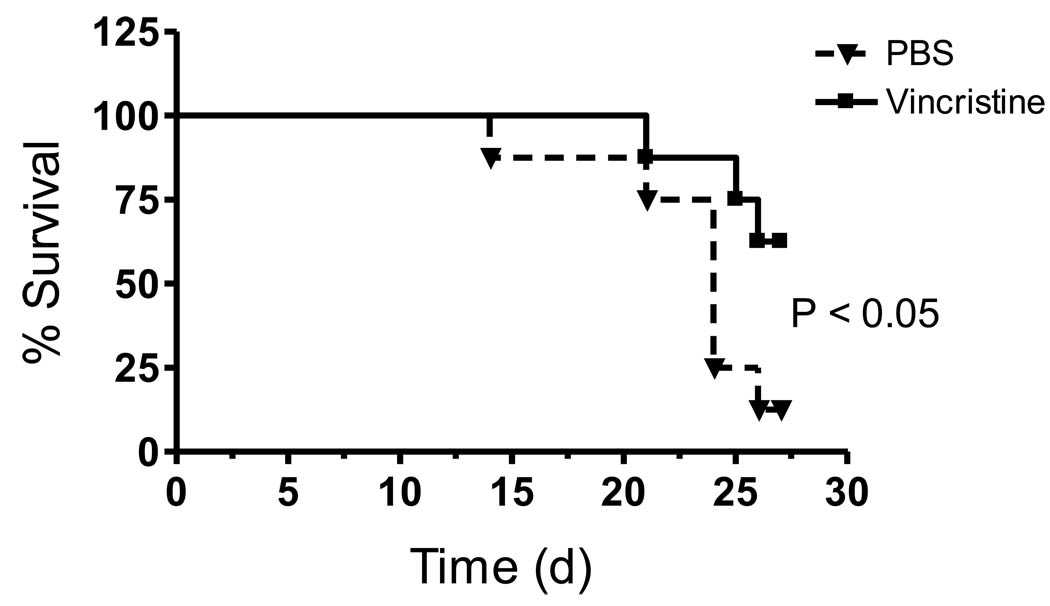
The mice were monitored for clinical illness for the entire treatment period of four weeks. Survival curves showed that 62.5% of mice receiving the vincristine were still alive compared to 12.5% of the PBS-treated mice by day 27 of the 4 week treatment period (P < 0.05, Fig. 4). . However, mean body weights for the PBS- and vincristine-treated mice remained equivalent for each week throughout the study. Both groups of mice began to lose weight one week after engraftment was established and the animals progressively lost weight during the treatment period. At 10 weeks of age (2 weeks after injection of leukemia cells and just prior to reaching positive engraftment levels), the PBS-treated mice weighed 21.29 ± 0.44 g and vincristine-treated mice weighed 21.11 ± 0.89 g (maximum weight). By 13 weeks of age, the PBS- and vincristine-treated mice weighed 17.76 ± 0.88 and 18.15 ± 0.51, respectively.
Figure 4.
Vincristine treatment increases survival of NOD/SCID mice, but does not induce remission. Sixteen NOD/SCID mice were engrafted with SEM leukemia cells and treated as described in Figure 2. Mice were euthanized when they displayed overt clinical illness, which included greater than 20% weight loss, lethargy, severe weakness, or inability to reach food or water for 24h. Differences in survival between the PBS- and vincristine-treated mice were determined by log-rank test (P < 0.05).
4. Discussion
To our knowledge, this is the first report of an inducible in vivo model for multidrug resistance in human high-risk ALL with chromosomal translocation t(4:11). MDR is often studied by generating MDR-resistant sublines from cultured cells and either studying these sublines in vitro or injecting the resistant clonal cells into immunodeficient mice. Resistant sublines have been utilized to study MDR in many types of cancers in vivo, including T-cell leukemia, Burkitt’s lymphoma, and promyelocytic leukemia [16–19]. The SEM cell line used for this study was established in culture from a relapsed patient with ALL containing the translocation t(4;11) [14], and treatment of the patient may have induced an initial MDR state prior to removal of the cells and establishment of the cell line. However, without selective pressure, these cells lost PgP expression and were sensitive to vincristine in vitro. Upon engraftment into NOD/SCID mice and subsequent treatment with vincristine, SEM cells increased expression of the PgP protein and likely other MDR transport proteins, and resulted in reversion to a drug resistant phenotype. A delay in leukemia cell growth in the mice and increased survival were observed after vincristine treatment compared to control, but all of the mice succumbed to disease. This model represents an inducible system that is superior to injecting already resistant sublines and may be used to evaluate step-wise in vivo mechanisms involved in the development of or reversion to a multidrug resistant state in these cells.
The greatest obstacle for successful chemotherapy against leukemia is the development of cells that are resistant to the therapeutic agents. Olson et al. [20] recently showed that prognosis of pediatric ALL patients was not significantly dependent upon overexpression of the MDR efflux pumps PgP, MRP or LRP in newly diagnosed patients. PgP was upregulated or overexpressed in only about 2% (5 of 295) of the patient samples taken at the time of diagnosis and the small number did not allow statistical analysis of a correlation between PgP expression and survival. Lack of correlation of PgP, MRP-1, and LRP expression and therapeutic success against pediatric ALL at diagnosis was also shown by others [21–23]. However, Styczynski et al. [24] showed patients with relapsed ALL had a strong trend toward adverse impact of PgP, MRP1, and LRP expression on prognosis. These data suggest that these efflux and transport systems play an important role in the greater lack of success of conventional chemotherapeutics used for patients with relapsed ALL. It should be noted that the studies referenced above did not specifically differentiate the subtypes of ALL in the analysis groups, so the actual impact of PgP expression on the prognosis and overall survival in patients with high-risk t(4;11) ALL is not clear, whether at diagnosis or after relapse.
Vincristine is a neurotoxic therapeutic agent that can induce cumulative peripheral neuropathy and epileptic seizures and is usually administered once per week to the human patient [25]. We did not observe overt signs of intolerance to the multiple dosing of vincristine (3× per week), such as weakness, palsy, or changes in behavior when compared to the condition of the PBS-treated mice. However, the mice in both groups showed similar changes in weight before and after engraftment of the leukemia cells. By day 25, the average percent of CD19+ SEM cells was 13.96 ± 1.75 for PBS-treated mice compared to 3.86 ± 1.0 for vincristine-treated mice. Since the leukemia burden was significantly higher in the PBS-treated mice which would make these mice more ill, the lack of a difference in weight loss between the two groups may reflect some toxicity induced by the vincristine treatment.
Palucka et al. [26] showed that leukemic cells from patients at relapse engrafted more rapidly in SCID mice than cells from newly diagnosed patients. SEM cells engrafted in the NOD/SCID mice approximately 2 weeks after injection into the tail vein, whereas Leim et al. [10] showed that biopsy material took an average of 43 ± 5 days for engraftment to occur. The rapid engraftment of SEM cells and reversion to chemotherapy resistance after vincristine treatment provides a useful model for the study of MDR in high-risk leukemia without the need to obtain patient biopsy material. This model is also relatively uncomplicated and provides an important means for developing novel treatment strategies that overcome MDR mechanisms in this often fatal disease.
Acknowledgments
This study was supported by a grant from National Institutes of Health, National Cancer Institute, grant number 1R21CA122117-01. The study sponsors had no role in study design, collection, analysis, or interpretation of data, writing the manuscript, or decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
No conflict of interest for any of the authors.
References
- 1.Faderl S, Kantarjian HM, Talpaz M, Estrov Z. Clinical significance of cytogenetic abnormalities in adult acute lymphoblastic leukemia. Blood. 1998;91:3995–4019. [PubMed] [Google Scholar]
- 2.Greaves MF, Wiemels J. Origins of chromosome translocations in childhood leukaemia. Nature Rev. 2003;3:1–11. doi: 10.1038/nrc1164. [DOI] [PubMed] [Google Scholar]
- 3.Nachman JB, Sather HN, Sensel MG, Trigg ME, Cherlow JM, Lukens JN, Wolff L, Uckun FM, Gaynon PS. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N. Engl. J. Med. 1998;338:1663–1671. doi: 10.1056/NEJM199806043382304. [DOI] [PubMed] [Google Scholar]
- 4.Ueda K, Cornwell MM, Gottesman MM, Pastan I, Roninson IB, Ling V, Riordan JR. The mdr1 gene, responsible for multidrug-resistance, codes for P-glycoprotein. Biochem. Biophys. Res. Commun. 1986;141:956–962. doi: 10.1016/s0006-291x(86)80136-x. [DOI] [PubMed] [Google Scholar]
- 5.Gottesman M, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu. Rev. Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 6.Krishnamachary N, Center MS. The MRP gene associated with a non-P-glycoprotein multidrug resistance encodes a 190-kDa membrane bound glycoprotein. Cancer Res. 1993;53:3658–3661. [PubMed] [Google Scholar]
- 7.Kamel-Reid S, Letarte M, Sirard C, Doedens M, Grunberger T, Fulop G, Freedman MH, Phillips RA, Dick JE. A model of human acute lymphoblastic leukemia in immune-deficient SCID mice. Science. 1989;246:1597–1600. doi: 10.1126/science.2595371. [DOI] [PubMed] [Google Scholar]
- 8.Uckun FM. Severe combined immunodeficient mouse models of human leukemia. Blood. 1996;88:1135–1146. [PubMed] [Google Scholar]
- 9.Lock RB, Liem N, Farnsworth ML, Milross CG, Xue C, Tajbakhsh M, Haber M, Norris MD, Marshall GM, Rice AM. The nonobese diabetic/severe combined immunodeficient (NOD/SCID) mouse model of childhood acute lymphoblastic leukemia reveals intrinsic differences in biologic characteristics at diagnosis and relapse. Blood. 2002;99:4100–4108. doi: 10.1182/blood.v99.11.4100. [DOI] [PubMed] [Google Scholar]
- 10.Liem NLM, Papa RA, Milross CG, Schmid MA, Tajbakhsh M, Choi S, Ramirez CD, Rice AM, Haber M, Norris MD, MacKenzie KL, Lock RB RB. Characterization of childhood acute lymphoblastic leukemia xenograft models for the preclinical evaluation of new therapies. Blood. 2004;103:3905–3914. doi: 10.1182/blood-2003-08-2911. [DOI] [PubMed] [Google Scholar]
- 11.Steele JP, Clutterbuck RD, Powles RL, Mitchell PL, Horton C, Morilla R, Catovsky D, Millar JL. Growth of human T-cell lineage acute leukemia in severe combined immunodeficiency (SCID) mice and non-obese diabetic SCID mice. Blood. 1997;90:2015–2019. [PubMed] [Google Scholar]
- 12.Baersch G, Mollers T, Hotte A, Dockhorn-Dworniczak B, Rube C, Ritter J, Jurgens H, Vormoor J. Good engraftment of B-cell precursor ALL in NOD-SCID mice. Klin. Padiatr. 1997;209:178–185. doi: 10.1055/s-2008-1043947. [DOI] [PubMed] [Google Scholar]
- 13.Borgmann A, Baldy C, von Stackelberg A, Beyermann B, Fichtner I, Nurnberg P, Henze G. Childhood ALL blasts retain phenotypic and genotypic characteristics upon long-term serial passage in NOD/SCID mice. Pediatr. Hematol. Oncol. 2000;17:635–650. doi: 10.1080/08880010050211349. [DOI] [PubMed] [Google Scholar]
- 14.Greil J, Gramatzki M, Burger R, Marschalek R, Peltner M, Trautmann U, Hansen-Hagge TE, Bartram CR, Fey GH, Stehr K, Beck J. The acute lymphoblastic leukemia cell line SEM with t(4;11) chromosomal rearrangement is biphenotypic and responsive to interleukin-7. Br. J. Haematol. 1994;86:275–283. doi: 10.1111/j.1365-2141.1994.tb04726.x. [DOI] [PubMed] [Google Scholar]
- 15.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Meth. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 16.Hill AB, Beck WT, Trent JM. Cytogenetic and molecular characterization of tumors in nude mice derived from a multidrug-resistant human leukemia cell line. Cancer Res. 1988;48:393–398. [PubMed] [Google Scholar]
- 17.Liu C, Lambert JM, Teicher BA, Blätler WA, O’Connor R. Cure of multidrug-resistant human B-cell lymphoma xenografts by combinations of anti-B4-blocked ricin and chemotherapeutic drugs. Blood. 1996;87:3892–3898. [PubMed] [Google Scholar]
- 18.Cucco C, Calabretta B. In vitro and in vivo reversal of multidrug resistance in a human leukemia-resistant cell line by mdr1 antisense oligodeoxynucleotides. Cancer Res. 1996;56:4332–4337. [PubMed] [Google Scholar]
- 19.Van de Vrie W, Marquet RL, Stoter G, De Bruijn EA, Eggermont AMM. In vivo model systems in P-glycoprotein-mediated multidrug resistance. Crit. Rev. Clin. Lab Sci. 1998;35:1–57. doi: 10.1080/10408369891234165. [DOI] [PubMed] [Google Scholar]
- 20.Olson DP, Taylor BJ, La M, Sather H, Reaman GH, Ivy SP. The prognostic significance of P-glycoprotein, multidrug resistance-related protein 1 and lung resistance protein in pediatric acute lymphoblastic leukemia: a retrospective study of 295 newly diagnosed patients by the Children’s Oncology Group. Leuk. Lymph. 2005;46:681–691. doi: 10.1080/10428190500032612. [DOI] [PubMed] [Google Scholar]
- 21.van den Heuvel-Eibrink MM, Sonneveld P, Pieters R. The prognostic significance of membrane transport-associated multidrug resistance (MDR) proteins in leukemia. Int. J. Clin. Pharmacol. Ther. 2000;38:94–110. doi: 10.5414/cpp38094. [DOI] [PubMed] [Google Scholar]
- 22.Plasschaert SL, Vellenga E, De Bont E, Van der Kolk D, Veerman A, Sluiter W, Daenen S, de Vries E, Kamps WA. High functional P-glycoprotein activity is more often present in T-cell acute lymphoblastic leukaemic cells in adults than in children. Leuk. Lymph. 2003;44:85–95. doi: 10.1080/1042819021000040288. [DOI] [PubMed] [Google Scholar]
- 23.den Boer ML, Pieters T, Kazemier KM, Rottier MM, Zwann CM, Kaspers GJ, Janka-Schaub G, Henze G, Creutzig U, Scheper RJ, Veerman AJ. Relationship between major vault protein/lung resistance protein, multidrug resistance-associated protein, P-glycoprotein expression, and drug resistance in childhood leukemia. Blood. 1998;91:2092–2098. [PubMed] [Google Scholar]
- 24.Styczynski J, Wysocki M, Debski R, Czyzewski K, Kolodziej B, Rafinska B, Kubicka M, Koltan S, Koltan A, Pogorzala M, Kurylak A, Olszewska-Slonina D, Balwierz W, Juraszewska E, Wieczorek M, Olejnik I, Krawczuk-Rybak M, Kuzmicz M, Kowalczyk J, Stefaniak J, Badowska W, Sonta-Jakimczyk D, Szczepanski T, Matysiak M, Malinowska I, Stanczak E. Predictive value of multidrug resistance proteins and cellular drug resistance in childhood relapsed acute lymphoblastic leukemia. J. Cancer Res. Clin. Oncol. 2007;133:875–893. doi: 10.1007/s00432-007-0274-1. [DOI] [PubMed] [Google Scholar]
- 25.Sioka C, Kyritsis AP. Central and peripheral nervous system toxicity of common chemotherapeutic agents. Cancer Chemother. Pharmacol. 2009;63:761–767. doi: 10.1007/s00280-008-0876-6. [DOI] [PubMed] [Google Scholar]
- 26.Palucka AK, Scuderi R, Porwit A, Jeha S, Gruber A, Bjorkholm M, Beran M, Pisa P. Acute lymphoblastic leukemias from relapse engraft more rapidly in SCID mice. Leukemia. 1996;10:558–563. [PubMed] [Google Scholar]