Summary
Objective
To estimate the extent to which varus malalignment, a source of abnormal intra-articular stresses in the medial tibiofemoral compartment and risk factor for progression of knee osteoarthritis (OA), may have diminished the structure-modifying benefit of doxycycline in knee OA.
Methods
Post hoc treatment group comparisons from a randomized, placebo-controlled trial of the effect of doxycycline (100 mg, twice daily) on medial joint space narrowing (JSN) in subgroups of varus and non-varus OA knees. Subjects (N=379 with x-ray follow-up) were obese 45–64 year old women with unilateral knee OA at baseline. JSN was measured manually in semiflexed anteroposterior radiographs acquired with standardized fluoroscopic positioning. The anatomic-axis angle was measured in each baseline radiograph and transformed to an estimate of the mechanical-axis angle (MAAest) using a validated regression equation. Knees with MAAest <178° were classified as varus.
Results
In our original comparison with placebo, doxycycline slowed the rate of medial JSN in OA knees by 38% at 16 months and by 33% at 30 months. Among non-varus OA knees, 16-mo JSN in the doxycycline group was 44% slower than in the placebo group (0.09 vs. 0.16 mm/yr, P=0.080), and 39% slower at month 30 (0.10 vs. 0.17 mm/yr, P=0.026). JSN in varus knees (0.20–0.27 mm/yr) was more rapid than in non-varus knees (P=0.083) and unaffected by doxycycline.
Conclusion
Varus malalignment negated the slowing of structural progression of medial compartment OA by doxycycline. To our knowledge, this is the first report documenting that static varus angulation can negate a pharmacologic structure-modifying effect.
Keywords: Structure modification, Knee osteoarthritis, Doxycycline, Varus malalignment
Introduction
While recent reviews have highlighted methodological advances that have increased the feasibility of clinical trials of disease-modifying osteoarthritis drugs (DMOADs) [1–3], the results of randomized controlled trials (RCTs) of drugs intended to slow the structural damage of OA have been inconsistent. One reason for this inconsistency may be that pharmacotherapy intended to slow the deterioration of articular cartilage does nothing to negate the effects of abnormal intra-articular stresses that are the prime force in the etiopathogenesis and progression of common garden-variety OA [4].
We have shown that, compared to placebo, treatment with doxycycline slowed the rate of radiographic joint space narrowing (JSN) in the medial compartment of knees with established OA [5]. Because varus malalignment increases loading of the medial tibiofemoral compartment [6] and, hence, the risk of OA progression [7], it may have attenuated the pharmacologic benefit of doxycycline. Herein we describe results of subgroup analyses from this trial to ascertain whether varus malalignment decreased the structure-modifying effect of doxycycline in knee OA.
Methods
All procedures for this RCT, including written informed consent, were approved by the Institutional Review Board (IRB) of Indiana University Purdue University Indianapolis, where the coordinating Center was located) and by IRBs at other participating institutions [5].
Subjects
The original sample of the doxycycline RCT consisted of 431 women recruited at 6 clinical centers in the United States. All were 45–64 years of age, in the upper tertile of sex-, age- and race-based norms for body mass index (BMI) and had unilateral knee OA (grade 2 or 3 by Kellgren and Lawrence criteria [8]) at baseline. Data for the present analysis were derived from 379 subjects (88% of those randomized) who underwent the interim (16-month) radiographic examination and 361 subjects (84% of those randomized) who completed the closeout (30-month) assessment.
Measurement of Joint Space Narrowing
The primary outcome of the RCT was radiographic joint space narrowing (JSN) in the medial tibiofemoral compartment. As previously reported [5], semiflexed anteroposterior (AP) knee radiographs were acquired at baseline and at months 16 and 30 according to Buckland-Wright’s protocol for fluoroscopically standardized radioanatomic positioning of the knee [9]. Quantitative JSN estimates were based on serial magnification-corrected measurements of minimum joint space width (JSW) obtained with a screw-adjustable calipers and graduated loupe according to the method described by Lequesne [10].
Measurement of malalignment
The anatomic-axis angle (AAA) in the frontal plane was measured by 1 of 2 readers (RC, SAM) in each baseline radiograph of the index (OA) knee, following the methods described by Kraus et al [11]. AAA was measured by goniometer along the medial aspect of the lower extremity. The AAA was formed by lines that began at points bisecting the widths of the proximal femur and distal tibia (~10 cm from the joint space) and converged at a point midway between the tibial spines. Inter-reader reproducibility (intra-class correlation) of AAA measurements from 36 randomly selected radiographs was 0.95.
Measurements of AAA were transformed into estimates of the mechanical-axis angle (MAAest) by use of the regression equation (MAAest=0.915*AAA+13.895, R2=0.77) validated by Hinman et al [12]. In their study, MAA estimated from AAA measured in knee radiographs accounted for 77% of variation in actual MAA measured in concurrent long-limb radiographs. Knees in the present study with MAAest<178° were classified as exhibiting varus angulation, by norms established by Moreland et al [13].
Analysis
Separate treatment group comparisons were performed on subgroups of varus and non-varus OA knees using the same mixed-effect (repeated measures) linear model employed in the original analysis of doxycycline effects [5]. To test whether the rates of medial compartment JSN in varus and non-varus subgroups were similar, we employed an additional repeated measures model that included all knees and a dichotomous indicator of varus angulation (MAAest < 178° vs. ≥178°) as a covariate. Terms were included in each model to account for Interactions between treatment group and visit, clinical center and varus malalignment (third model only). All comparisons were adjusted for baseline knee pain and JSW, visit (interim or closeout) and clinical center.
Results
Of the 379 subjects who underwent the interim (month 16) radiographic examination, the index (OA) knee exhibited varus angulation at baseline in 82 (22%). At closeout, 77 of the 361 index knees that were available for analysis (21%) had exhibited varus angulation in the baseline radiograph. As described previously [5], all subjects enrolled in the RCT were obese, middle-aged women with unilateral K&L grade 2–3 OA at baseline. Subgroups of subjects with varus and non-varus index knees were similar with respect to age, BMI, race and baseline knee pain. However, varus knees were significantly more likely than non-varus knees to have K&L grade 3 OA at baseline (64% vs. 32%, P<0.0001). Consistent with definitions of OA severity by K&L criteria [8], mean JSW in varus knees was significantly smaller at baseline than that in non-varus knees (2.77 mm vs. 3.90 mm, P<0.0001).
Table 1 contains a summary of non-annualized JSN in the doxycycline and placebo treatment arms among subgroups of varus and non-varus OA knees. In both treatment groups, over both intervals, varus knees exhibited a greater loss of JSW than non-varus knees (P=0.083 after adjustment for treatment group, baseline knee pain and JSW, visit, clinical center, and interactions between treatment group and both visit and clinical center).
Table 1.
Non-annualized medial compartment JSN (Mean ± SD, mm) by treatment group: results in varus and non-varus OA knees
| Varus OA Knees (MAAest <178°) |
Non-varus OA Knees (MAAest ≥178°) |
|||||
|---|---|---|---|---|---|---|
| Doxycycline | Placebo | P* | Doxycycline | Placebo | P* | |
| 16-mo JSN | 0.26 ± 0.39 | 0.36 ± 0.57 | 0.230 | 0.12 ± 0.42 | 0.21 ± 0.52 | 0.080 |
| N of knees | 37 | 45 | 151 | 146 | ||
| 30-mo JSN | 0.49 ± 0.64 | 0.55 ± 0.62 | 0.448 | 0.26 ± 0.58 | 0.42 ± 0.73 | 0.026 |
| N of knees | 35 | 42 | 146 | 138 | ||
Note: Estimated mechanical-axis angle (MAAest) was based on measurement of the anatomic-axis angle [12].
From mixed-effect (repeated measures) linear models adjusted for treatment group, baseline knee pain and JSW, visit (interim or closeout), clinical center, treatment group-clinical center interaction and treatment group-visit interaction.
Notably, group comparisons among varus knees showed similar rates of JSN in the two treatment groups, as reflected in average loss of minimum medial JSW at both month 16 (0.26 vs. 0.36 mm, P=0.230) and month 30 (0.49 vs. 0.55 mm, P=0.448). In contrast, among non-varus knees, the difference between treatment groups with respect to mean JSN favored doxycycline at both intervals. The mean difference between treatment groups was marginally significant as early as month 16 (0.12 vs. 0.21 mm, P=0.080) and unequivocally significant at month 30 (0.26 vs. 0.42 mm, P=0.026).
Discussion
Knee malalignment was not an exclusion criterion for the doxycycline trial, which we initiated in 1996, or for many other contemporaneous DMOAD trials in knee OA. Of 8 knee RCTs of DMOADs that we reviewed previously [3], only one [14] excluded subjects with “clinically important” axial deviation of the lower extremity. Although current protocols for RCTs of putative DMOADs now often exclude subjects with significant varus or valgus deformity on the presumption that the efficacy of the drug may be diminished in such patients, to our knowledge, this is the first report documenting that static varus angulation can negate a pharmacologic structure-modifying effect.
In our original comparison of outcomes in active treatment and placebo groups [5], oral doxycycline, 100 mg bid, slowed the annualized rate of medial JSN in OA knees by 38% at 16 months (P=0.027) and by 33% at 30 months (P=0.017). The post hoc subgroup analyses in the present study indicate, however, that doxycycline had no significant effect on the comparatively rapid rate of JSN in the medial tibiofemoral compartment of the varus knee (0.20–0.27 mm/yr). These results are in marked contrast to the outcomes in non-varus knees, in which doxycycline slowed JSN by 43% at month 16, and by 38% at month 30, relative to placebo (Table 1).
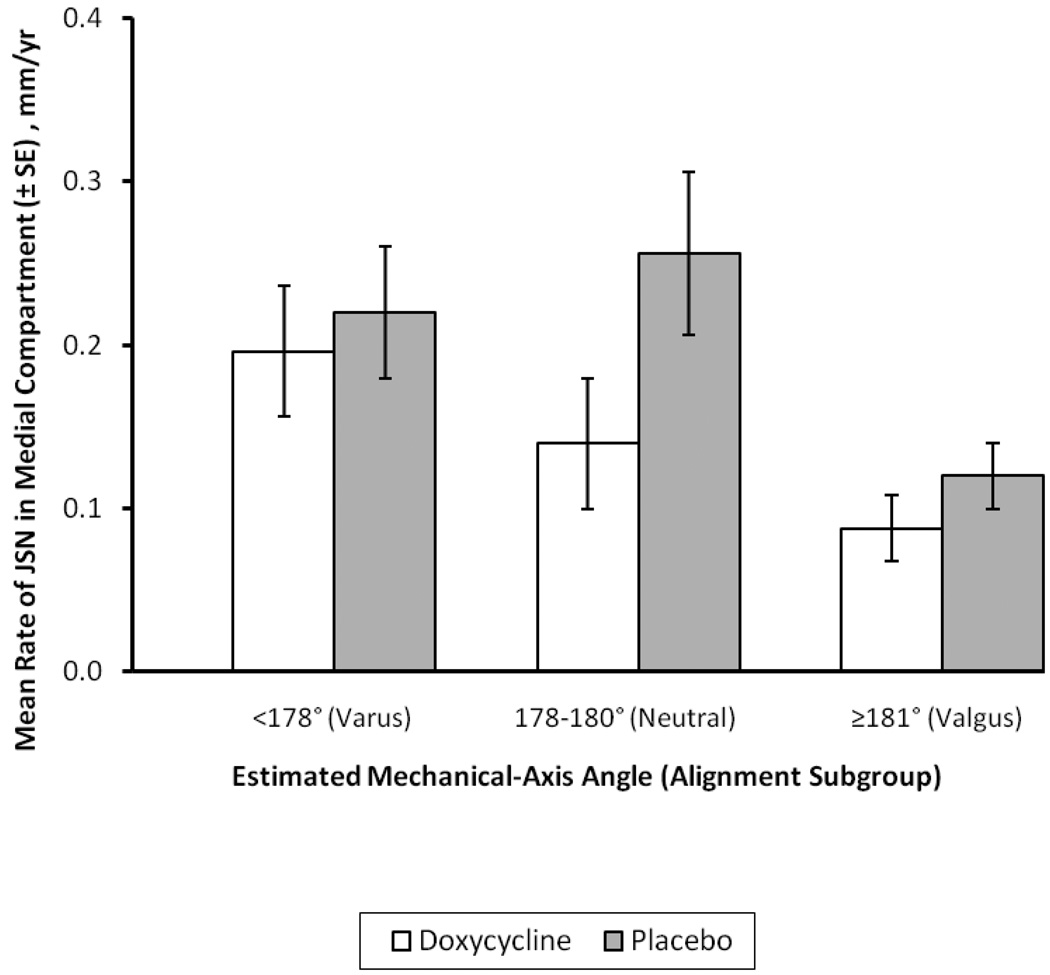
Our decision to combine for analysis knees with neutral or valgus angulation at baseline into a “non-varus” subgroup was intended to maximize the power of treatment group comparisons to elucidate how varus malalignment influenced the effect of doxycycline on the primary outcome, JSN in the medial tibiofemoral compartment. However, because of its propensity toward lateral progression of OA and a corresponding pseudo-widening of medial joint space, the valgus knee may have been ill-suited for the demonstration of a DMOAD effect on progression of JSN in the medial compartment. To explore the extent to which inclusion of subjects with valgus knees in the doxycycline RCT made it more difficult to demonstrate a significant DMOAD effect, we examined the annualized rate of medial-compartment JSN over 30 months (mean ±SE) in OA knees with varus, neutral or valgus angulation at baseline (Figure 1). Consistent with the results shown in Table 1, at 30 months the rate of JSN in the 35 varus knees of subjects treated with doxycycline was only 11% slower than that in 42 varus knees of subjects who received placebo. In contrast, in neutrally aligned knees (N = 37 and 49, respectively), doxycycline slowed the annual rate of medial JSN by nearly 50%, compared to placebo. Two findings are apparent in data from knees with valgus angulation (MAAest ≥181°) at baseline: The first is that, not surprisingly, the rate of medial JSN was much slower in valgus knees than in either varus or neutral knees. Second, despite less rapid JSN in the medial compartment, the mean rate of JSN in the 109 valgus knees of subjects treated with doxycycline was 25% slower than that in the 89 valgus knees of those receiving placebo.
Figure 1.
Mean rate (± SE) of medial-compartment joint space narrowing (JSN) over 30 months in varus, neutral and valgus knees: comparisons of knees treated with doxycycline and placebo. For varus, neutral and valgus knees, N’s in the doxycycline treatment group were 35, 37 and 109, respectively. For the placebo arm of the RCT, the corresponding N’s were 42, 49 and 89, respectively).
These data suggest that while the rapid medial-compartment JSN in varus knees was unaltered by treatment with doxycycline, the drug retarded JSN in valgus knees – although to an extent only about half as great as in neutrally aligned knees. Inclusion of valgus knees in the RCT did not preclude detection of a significant protective effect of doxycycline against medial JSN in either our original analysis of all OA knees [5] or in the current analysis of the non-varus subgroup.
This post hoc analysis has some limitations: First, restriction of enrollment in the doxycycline RCT to obese women may have limited the generalizability of any conclusions about the benefits of doxycycline as a DMOAD in the OA population at large. However, this narrow focus of the RCT did not compromise its value as a demonstration of the concept of structure-modification in knee OA – or of the present subgroup analysis as an exploration of the possible moderating effects of malalignment.
Second, the percentage of knees that were classified as having varus angulation at baseline (21–22%) was smaller than expected and may have limited our power to detect treatment effects in this subgroup. This low percentage is likely due, in part, to our exclusion of subjects with K&L grade 4 knee OA at baseline. In addition, a skew in the distribution of MAAest, away from the varus and toward the valgus end of the spectrum, may be a limitation inherent in the measurement of AAA with short (10–12 cm) segments of the tibial and femoral shafts [15] or a bias in the arithmetic transformation of AAA measurements into estimates of MAA [12]. However, the small (9%) difference in 30-month JSN between varus knees assigned to the doxycycline or placebo groups (Figure 1) suggests that the statistical power of treatment group comparisons among knees classified as varus in this analysis was not a significant limitation.
Third, malalignment itself is only a crude indicator of an adverse biomechanical environment, in which abnormal intra-articular stress drives the etiopathogenetic mechanisms that underlie joint breakdown in OA and may counteract attempts at physiologic or pharmacologic repair [4]. The evidence argues that it is not varus per se, or obesity, per se, that is detrimental to the knee, but the consequences of the resulting alteration in mechanical loading for the articular cartilage and subchondral bone. However, a recent report suggesting that leptin, which is produced by adipose tissue, protected against development of knee OA in markedly obese mice [16], suggests the story may be more complicated.
Varus-valgus malalignment, as measured in a full-limb radiograph or estimated in a knee film, is an indication of static malalignment, whereas a varus thrust visualized during gait reflects dynamic malalignment. Varus-valgus alignment is a key determinant of the peak adductor moment, a measure of the magnitude of the intrinsic compressive load on the medial tibiofemoral compartment in stance. Varus further increases the medial compartment load during gait; valgus increases stress in the lateral compartment [17]. Miyazaki et al [18] found that the peak adductor moment predicted x-ray progression in subjects with medial compartment OA.
Chang et al [19] reported that a visualized varus thrust was associated with a 4-fold increase in the likelihood of progression of medial tibiofemoral compartment OA. Among varus knees, the presence of a visualized thrust further increased the odds of progression of medial compartment JSN 3-fold. Thus, among OA knees with static varus malalignment, a subset that is that is characterized by the presence of a thrust appears to be at particularly high risk for OA progression. However, a thrust effect on progression (i.e., JSN) was apparent in knees with relatively mild varus alignment (<5°) but not in those with more severe varus. Although approximately 70% of the total force across the normal knee has been shown to act on the medial compartment during level walking [20], Chang et al [19] considered that with increasing varus malalignment, nearly 100% of the load is transmitted medially and hence, in the presence of higher degrees of varus, thrust has little additional impact. In any event, the possibility should be considered that dynamic, as well as static, loading of the knee might mitigate the structure-modifying effect of a DMOAD.
The significance of the present findings for the design of clinical trials of DMOADs should be viewed in light of published evidence [21] that argues powerfully that the underlying problem in common, garden-variety OA is a quantitative or qualitative abnormality in intra-articular stress, due to a variety of causes; and that if the abnormal stress is eliminated, the OA joint can heal, with marked structural and symptomatic improvement. We have suggested that if the abnormal stress on the OA joint is not corrected, the likelihood is small that a putative DMOAD will be efficacious [21].Our present data support that earlier suggestion. _We recommend that future clinical trials of pharmacologic or non-pharmacologic therapies aimed at disease modification control the extraneous effects of biomechanical factors (either through exclusion criteria or through measurement and stratified allocation to treatment groups). Furthermore, we suggest that DMOAD effects may be attenuated also by genetic or developmental abnormalities in joint shape [22,23] and by neuromuscular abnormalities (e.g., sarcopenia, proprioceptive defects) that impair micro-coordination and, like malalignment, can increase intra-articular stress.
Acknowledgement
This work was supported in part by grants from NIH/NIAMS (R01 AR044370 and R01 AR052740).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflict of Interest.
References
- 1.Hunter DJ, Hellio Le Graverand-Gastineau MP. How close are we to having structure-modifying drugs available? Med Clin North Am. 2009;93:223–234. doi: 10.1016/j.mcna.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Qvist P, Bay-Jensen AC, Christiansen C, Dam EB, Pastoureau P, Karsdal MA. The disease modifying osteoarthritis drug (DMOAD): Is it in the horizon? Pharmacol Res. 2008;58:1–7. doi: 10.1016/j.phrs.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Brandt KD, Mazzuca SA. Lessons learned from nine clinical trials of disease-modifying osteoarthritis drugs. Arthritis Rheum. 2005;52:3349–3359. doi: 10.1002/art.21409. [DOI] [PubMed] [Google Scholar]
- 4.Brandt KD, Dieppe P, Radin E. Commentary: Is it useful to subset “primary” OA? A critique based on evidence regarding the etiopathogenesis of osteoarthritis. Semin Arthritis Rheum. 2009;39:81–95. doi: 10.1016/j.semarthrit.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Brandt KD, Mazzuca SA, Katz BP, Lane KA, Buckwalter KA, Yocum DE, et al. Effect of doxycycline on progression of osteoarthritis. Arthritis Rheum. 2005;52:2015–2025. doi: 10.1002/art.21122. [DOI] [PubMed] [Google Scholar]
- 6.Tetsworth K, Paley D. Malalignment and degenerative arthropathy. Orthop Clin North Am. 1994;25:367–377. [PubMed] [Google Scholar]
- 7.Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop D. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. 2001;286:188–195. doi: 10.1001/jama.286.2.188. [DOI] [PubMed] [Google Scholar]
- 8.Kellgren JH, Lawrence JS. Radiographic assessment of osteoarthritis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckland-Wright JC, Macfarlane DG, Williams SA, Ward RJ. Accuracy and precision of joint space width measurements in standard and macroradiographs of osteoarthritic knees. Ann Rheum Dis. 1995;54:872–880. doi: 10.1136/ard.54.11.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lequesne M. Quantitative measurements of joint space during progression of osteoarthritis: chondrometry. In: Kuettner K, Goldberg V, editors. Osteoarthritic disorders. Rosemont, IL: American Academy of Orthopedic Surgeons; 1995. pp. 427–444. [Google Scholar]
- 11.Kraus VB, Vail TP, Worrell T, McDaniel G. A comparative assessment of alignment angle of the knee by radiographic and physical examination methods. Arthritis Rheum. 2005;52:1730–1735. doi: 10.1002/art.21100. [DOI] [PubMed] [Google Scholar]
- 12.Hinman RS, May RL, Crossley KM. Is there an alternative to the full-leg radiograph for determining knee joint alignment in osteoarthritis? Arthritis Rheum. 2006;55:306–313. doi: 10.1002/art.21836. [DOI] [PubMed] [Google Scholar]
- 13.Moreland J, Bassett L, Hanker G. Radiographic analysis of the axial alignment of the lower extremity. J Bone Joint Surg Am. 1987;69:745–749. [PubMed] [Google Scholar]
- 14.Jubb RW, Piva S, Beinat L, Dacre J, Gishen P. A one-year, randomised, placebo (saline) controlled clinical trial of 500–730 kDa sodium hyaluronate (Hyalgan©) on the radiological change in osteoarthritis of the knee. Int J Clin Pract. 2003;57:467–474. [PubMed] [Google Scholar]
- 15.Sheehy L, Felson D, Cooke T. Is the femoral shaft – tibial shaft angle a reliable substitute for the hip-knee-ankle angle in knee osteoarthritis? Osteoarthritis Cart. 2009;17:393. [Google Scholar]
- 16.Griffin TM, Hueber JL, Kraus VB, Guilak F. Extreme obesity due to impaired leptin signaling in mice does not cause knee osteoarthritis. Arthritis Rheum. 2009;60:2935–2944. doi: 10.1002/art.24854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma L, Dunlop DD, Cahue S, Song J, Hayes KW. Quadriceps strength and osteoarthritis progression in malaligned and lax knees. Ann Intern Med. 2003;138:613–619. doi: 10.7326/0003-4819-138-8-200304150-00006. [DOI] [PubMed] [Google Scholar]
- 18.Miyazaki T, Wada M, Kawahara H, Sato M, Baba H, Shimada S. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann Rheum Dis. 2002;61:617–622. doi: 10.1136/ard.61.7.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang A, Hayes K, Dunlop D, et al. Thrust during ambulation and the progression of knee osteoarthritis. Arthritis Rheum. 2004;50:3897–3903. doi: 10.1002/art.20657. [DOI] [PubMed] [Google Scholar]
- 20.Schipplein OD, Andriacchi TP. Interaction between active and passive knee stabilizers during level walking. J Orthop Res. 1991;9:113–119. doi: 10.1002/jor.1100090114. [DOI] [PubMed] [Google Scholar]
- 21.Brandt KD, Dieppe PA, Radin EL. Etiopathogenesis of osteoarthritis. Med Clin N Amer. 2009;93:1–24. doi: 10.1016/j.mcna.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Doherty M, Courtney P, Doherty S, Jenkins W, Maciewicz R, Muir K, et al. Nonspherical femoral head shape (pistol grip deformity), neck shaft angle, and risk of hip osteoarthritis. Arthritis Rheum. 2008;58:3172–3182. doi: 10.1002/art.23939. [DOI] [PubMed] [Google Scholar]
- 23.Cooke D, Scudamore A, Li J, Wyss U, Bryant T, Costigan P. Axial lower-limb alignment: comparison of knee geometry in normal volunteers and osteoarthritis patients. Osteoarthritis Cart. 1997;5:39–47. doi: 10.1016/s1063-4584(97)80030-1. [DOI] [PubMed] [Google Scholar]