Abstract
To elucidate cellular mechanisms of sex-related differences in fat distribution, we determined body fat distribution (dual-energy X-ray absorptiometry and single slice abdominal computer tomography), adipocyte size, adipocyte number, and proportion of early-differentiated adipocytes (aP2+ CD68−) in the stromal vascular fraction (SVF) in the upper- and lower-body of normal-weight healthy men (n=12) and premenopausal women (n=20) (age: 18–49 y, BMI: 18–26 kg/m2). Women had more subcutaneous and less visceral fat than men. The proportion of early-differentiated adipocytes in the subcutaneous adipose tissue SVF of women was greater than in men (p = 0.01), especially in the femoral depot, although in vitro adipogenesis, as assessed by PPARγ expression, was not increased in femoral preadipocytes cultured from women compared with men. In women, differentiation of femoral preadipocytes was less than that of abdominal subcutaneous preadipocytes (p = 0.04) and femoral subcutaneous preadipocytes tended to be more resistant to TNFα-induced apoptosis (p=0.06). Thus, turnover and utilization of the preadipocyte pool may be reduced in lower vs. the upper body fat in women. Collectively, these data indicate that the microenvironment, rather than differences in inherent properties of preadipocytes between genders, may explain the gynoid obesity phenotype and higher percent body fat in women compared to men.
Keywords: body composition, adipocyte, macrophage, fat distribution, obesity, preadipocyte, adipogenesis, gender
INTRODUCTION
Fat accumulation in the upper body as well as visceral depots and ectopic sites is associated with insulin resistance and obesity-related metabolic abnormalities (1). Preferential lower body subcutaneous fat gain seems to have a protective effect against the unfavorable consequences of obesity (2–4). This suggests a link between fat distribution and metabolic health. Understanding the mechanisms of regional fat mass expansion may facilitate developing strategies for modulating fat distribution and influencing whole body metabolism. Men and women differ fundamentally with respect to adiposity and fat distribution. Women store more fat in subcutaneous areas, especially in the gluteal and femoral depots, while men accumulate fat preferentially in upper-body and visceral compartments (5). These sex-related differences, which are readily apparent in normal-weight men and women, may predispose to a spectrum of fat distribution phenotypes with obesity (6, 7).
One approach to understand sex-related differences in fat distribution is to examine the cellular composition of different fat depots. Fat tissue turns over throughout life (8, 9), with new fat cells developing from their progenitors, preadipocytes. Fat mass is determined by both adipocyte hypertrophy and hyperplasia, which depend on preadipocyte proliferation, differentiation, and apoptosis (10). We found that aP2+CD68− cells in the stromovascular fraction (SVF) of adipose tissue range from cells with no detectable lipid to those that contain multiple small (<10 μm in diameter) lipid droplets (11), comprising a pool of early-differentiating adipocytes. To investigate mechanisms of differences in fat distribution between men and women, we determined the number and size of mature adipocytes, the proportions of early-differentiating adipocytes in the SVF, and cell kinetic properties of preadipocytes in abdominal subcutaneous and femoral depots of normal-weight men and women.
METHODS AND PROCEDURES
Study design and body composition
Twenty premenopausal women and twelve men with a body mass index (BMI) <25 kg/m2 were included in this study. Participants could not be using corticosteroids or thyroid hormone replacement. Four women were using oral contraceptives, which do not appear to have effects on adipose tissue lipolysis (12). The original studies were approved by the Mayo Clinic Institutional Review Board.
After obtaining informed consent, the volunteers came to the Mayo General Clinical Research Center on the morning after an overnight fast. the women were permitted to participate in any phase of their menstrual cycle, largely because we have not seen the phase of the menstrual cycle as a confounding factor for adipose fatty acid storage (13) or release (14). Total body fat and body fat distribution were assessed using dual energy x-ray absorptiometry (DXA) using a region of interest software option and single slice computed tomography (CT) of the abdomen at the L2–L3 interspace (15). Adipose tissue biopsies were taken from the lateral periumbilical region of the abdominal subcutaneous and lateral thigh areas using needle aspiration under local anesthesia.
Adipose cellularity
Processing of adipose tissue
Adipose tissue was digested in HEPES buffer (0.1mol/L HEPES, 0.12mol/L NaCl, 0.05mol/L KCl, 0.005mol/L glucose, 1.5% w/v BSA, 1mmol/L CaCl2; pH 7.4) containing 1 mg/ml collagenase (Sigma Type II C-6885) at 37°C in a shaking (100 cycles per minute) water bath for 45–60 min. After centrifugation, the top layer containing adipocytes was separated and the cellular pellet was reconstituted in erythrocyte lysis buffer (0.154 M NH4Cl, 10 mM KHCO3, 1 mM EDTA) for 5 min at room temperature. The isolated fat cells were used to measure cell size and the SV cells were cytospun on a slide for immunocytochemistry and/or cultured for assessment of in vitro adipogenesis. After 18 hours (during which no replication occurs) cultures were trypsinized and replated. Confluent cultures were expanded by passaging 5 – 7 times before being frozen. These procedures yield essentially pure preadipocyte populations that are free of macrophages and endothelial cells by morphology and assaying cell type markers by real time PCR (16, 17).
Adipocyte size and number
Fat cell size was measured using digital photomicrographs with an automated software program by determining the areas of at least 300 adipocytes and calculating the mean lipid content per adipocyte (18). Regional adipocyte number was calculated by dividing regional fat mass by the mean lipid content per adipocyte corrected for the density of triolein.
Immunofluorescence to determine the proportion of early-differentiated adipocytes in the SVF
To quantify early-differentiated adipocytes, we stained cytospun SVF using a rabbit anti-recombinant mouse aP2 (a gift from Dr. Bernlohr, University of Minnesota). To account for macrophages that may express aP2, we co-stained with a mouse anti-human CD68 (KP1, DAKOCytomation Corp, Carpinteria, CA). The binding of primary antibodies was visualized by incubation with fluorescently-labeled secondary antibodies, as previously described (11). We took images of fluorescently-labeled cells on a LSM510 Confocal Microscope and counted at least 1000 nuclei (blue) with KS-400 image analysis software (Carl Zeiss, Inc., Oberkochen, Germany), after which the number of single-stained cells (aP2+CD68− [green] and aP2−CD68+ [red]) and dual-stained cells (aP2+CD68+, green and red) were counted manually. Thus, the percent early-differentiated adipocytes (aP2+ CD68− cells) and macrophages (aP2−CD68+ plus aP2+CD68+ cells) from the SVF were calculated.
Preadipocyte cell kinetics
Replication of preadipocyte clones
We followed a procedure described in detail elsewhere (16). Briefly, frozen preadipocytes were thawed and plated at a density of 50 cells/96 well plate in plating medium without phenol containing 10% FBS (ensuring that wells are seeded at most with one cell 99% of the time). After 2 weeks, colonies were evident and by 3 weeks, some were near confluence. Medium was aspirated and 100 μl of a lysis buffer (0.5N NH4OH, 0.1% Triton X-100) were added to each well. The plates were shaken and frozen at −80° C for at least 16 h to ensure complete lysis. The plates were thawed at room temperature and 50 μl of lysis buffer and 50 μl of a 2X stock of CyQuant dye (Molecular Probes, Inc., Eugene, OR) were added to each well. Contents of the wells were mixed and incubated for 10 min at room temperature in the dark. Florescence measurements at excitation and emission wavelengths of 485 and 535, respectively, were compared to standard curves using known concentrations of DNA.
Paracrine effect of regional adipose tissue on replication of preadipocyte cultures
Thawed abdominal subcutaneous and femoral preadipocytes from 3 men and 3 women were combined and plated in duplicate in 6-well plates and grown in medium containing 10% FBS until they were 50–60% confluent Cells were serum starved for 24 h followed by inserting trans-well inserts containing 50 mg of abdominal or femoral adipose obtained from 3 non-obese men and 3 women (BMI <25 kg/m2). Control cultures were grown in the same medium without adipose tissue-containing inserts. After 5 days, DNA was measured using a CyQuant kit as mentioned above.
Apoptotic index
Confluent cultures grown in plating medium containing 10% FBS were treated with 0, 10, or 50 ng/ml recombinant human TNFα for 4 h. Cells were stained with bisbenzimide and examined for fragmented and/or condensed nuclei, morphological features of apoptosis, by fluorescence microscopy (19). The mean percentage of such nuclei relative to the all of the nuclei in at least 4 fields was used as an apoptotic index.
Preadipocyte differentiation and Real Time PCR
The expression of the key transcription factors that regulate adipogenesis, PPARγ and C/EBPα, was used as an index of differentiation. Abdominal and femoral subcutaneous preadipocytes were differentiated in duplicate for 15 days in medium (without serum) enriched with 0.1 μM dexamethasone, 0.5 μM insulin, 0.2 nM triiodothyronine, 0.5 μM roziglitazone, 20 μM fetuin, antibiotics, and 540 μM methylisobutylxanthine (20).
Total RNA from the differentiated cultures was extracted with Trizol (Invitrogen, Carlsbad, CA). For exclusion of genomic DNA, 10 μg of total RNA was treated with DNase I (Ambion, Austin, TX) for 1 hour at 37°C. RNA integrity and quality was assessed by electrophoresis. 1 μg of RNA was reverse-transcribed into cDNA using a Taqman One-Step RT-PCR kit (#4309169, Applied Biosystems, Foster City, CA) in 100 μl reaction mixture. Real-time PCR was carried out using TaqMan Fast Universal PCR Master Mix 2x in a 7500 Fast Real Time PCR System (Applied Biosystems). In brief, 10 μl of Fast PCR Master Mix were combined with 5 μl of cDNA, 1 μl of the appropriate TaqMan single gene assay (PPARγ: Applied Biosystems catalog # Hs00234592; C/EBPα: catalog #Hs00269972), and 4 μl water. Following an initial 95°C incubation for 20 seconds, PCR was carried out for 40 cycles at 95°C for 3 seconds and 60°C for 30 seconds. RNA was analyzed by relative quantification using TATA box binding protein RNA as an internal control (5′-FAM/3′-MGB probe, Applied Biosystems, # 4333769F).
Statistical analysis
Data were analyzed using JMP statistical software version 5.1 and SAS statistical software version 9.1.3 (Cary, NC). Means ± 1 standard deviation (SD) are shown.
Differences in anthropometric characteristics between men and women were tested using Student’s or Wilcoxon Sign-Rank t tests; the latter for data that were not normally distributed. Sex-related differences in regional adipose cell characteristics were tested by two-way ANOVA using depot, sex, and the sex × depot interaction as fixed effects. Tukey adjustment was used in pair-wise comparisons in instances of significant sex × depot interactions. The apoptotic response to treatment with increasing doses of TNFα was tested by 3-way ANOVA using depot, sex, dose, and the interactions among them as fixed effects. Because four of the women were using oral contraceptive hormones, we tested whether adipocyte size, number of mature and early-differentiated adipocytes, and PPARγ expression differed from the rest of the female participants by performing two-way analysis of variance (ANOVA) using depot, usage of oral contraceptives, and the usage of oral contraceptives, × depot interaction as fixed effects. Because there were no significant oral contraceptive effects, the subsequent analyses included all women. The relationships between the percent of early-differentiated adipocytes in the SVF and relative lower-body fat accumulation were analyzed by using simple linear regression models. We used the logarithmic transformation of the measurements of visceral CT area to account for its curvilinear relation with the relative number of regional early-differentiated adipocytes. The level for statistical significance in all tests was set at p <0.05.
RESULTS
Body composition and adipocyte size
Subject characteristics are provided in Table 1. The expected sex differences in body composition, including regional fat mass, were found. Femoral adipocytes were larger (p = 0.0005) than abdominal adipocytes in both men and women (Table 2).
Table 1.
Anthropometric characteristics of participants
| Variable | Men (n = 12) | Women (n = 20) | P value |
|---|---|---|---|
| Age, y | 28.9 (8.7) | 30.3 (8.1) | 0.6 |
| Body weight, kg | 77.4 (7.7) | 58.9 (6.9) | 0.0001 |
| BMI kg/m2 | 23.8 (2.1) | 23.7 (5.3) | 0.9 |
| Total fat | |||
| Mass, kg | 14.1 (3.1) | 17.7 (3.9) | 0.01 |
| Percent of body weight | 19.0 (3.7) | 32.5 (5.8) | 0.0001 |
| Lower-body fat | |||
| Mass, kg | 5.4 (1.4) | 7.2 (1.6) | 0.003 |
| % of total fat mass | 37.2 (4.2) | 41.1 (4.1) | 0.1 |
| Upper-body subcutaneous fat | |||
| Mass, kg | 7.2 (1.6) | 9.4 (2.2) | 0.006 |
| Percent of total fat mass | 52.5 (3.2) | 53.2 (4.0) | 0.2 |
| Visceral fat | |||
| Mass, kg | 1.4 (0.7) | 1.0 (0.7) | 0.1 |
| % of total fat mass | 10.1 (3.3) | 5.6 (3.2) | 0.0007 |
| CT area, cm2 | 49.4 (26.4) | 26.5 (18.3) | 0.007 |
Values are mean (SD). BMI – body mass index; CT – computed tomography.
Table 2.
Adipose cellularity and preadipocyte kinetics in normal-weight men (n=12) and women (n=20).
| Abdominal |
Femoral |
p value |
|||||
|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Sex | Depot | Sex × Depot | |
| Adipocyte size, μg lipid/cell | 0.44 (0.17) | 0.46 (0.13) | 0.55 (0.24) | 0.67 (0.15) | 0.08 | 0.0005 | 0.2 |
| Subcutaneous adipocyte number, 109 | 18.3 (6.5) | 21.5 (6.5) | 11.3 (5.2) | 11.1 (2.8) | 0.09 | 0.008 | 0.051 |
| Immature adipocytes (aP2+CD68−), % of SV cells | 27 (6) | 31 (14) | 19 (6) | 30 (10) | 0.01 | 0.11 | 0.3 |
| Macrophages (aP2+/−CD68+), % of SV cells | 0.6 (1.1) | 0.7 (1.0) | 0.4 (0.5) | 1.0 (1.3) | 0.3 | 0.8 | 0.4 |
| Percent rapidly proliferating preadipocyte clones | 67 (13) | 69 (12) | 72 (6) | 63 (21) | 0.66 | 0.95 | 0.49 |
| Paracrine effect of adipose tissue on proliferation, % change in DNA | 76 (50) | 57 (21) | 110 (73) | 104 (31) | 0.74 | 0.10 | 0.76 |
| PPARγ, AU × 104 | 1.6 (0.5) | 1.8 (0.8)a | 1.6 (0.4) | 1.0 (0.3)b | 0.23 | 0.047 | 0.04 |
| PPARγ, % of total PPARγ mRNA* | 50 (9) | 63 (10)a | 50 (9) | 37 (10)b | 1.0 | 0.001 | 0.0007 |
| C/EBPα, AU × 104 | 2.1 (0.8) | 1.9 (0.7) | 1.8 (0.4) | 1.7 (1.1) | 0.60 | 0.48 | 0.92 |
| C/EBPα, % of total C/EBPα mRNA* | 52 (6) | 56 (13) | 48 (6) | 44 (13) | 1.0 | 0.04 | 0.34 |
| TNFα-induced apoptotic cells, % of cultured preadipocytes** | 7.4 (4.2) | 8.3 (4.8) | 7.9 (5.6) | 7.5 (4.2) | 0.85 | 0.62 | 0.06 |
Values are mean (SD);
indicate a difference (p <0.05) by using Tukey adjustment;
mRNA (AU) in one depot/mRNA in both depots from each subject X 100 percent;
mean (SD) of the apoptotic response to treatment with increasing doses of TNFα determined by 3-way ANOVA using depot, sex, dose, and the interactions among them as fixed effects.
Early-differentiated adipocytes
The fraction of SV cells that were early-differentiated adipocytes (aP2+CD68− cells) was ~10% greater in women than men in the abdominal subcutaneous and ~35% in the femoral depot (p = 0.01) (Table 2).
Macrophages
In these normal weight adults ≤1% of stromovascular cells were found to express macrophage markers (aP2−CD68+ plus aP2+CD68+ cells). We found no differences in the fraction of SV cells that were macrophages between men and women for each adipose site or between adipose depots within each group (Table 2).
Cell kinetic properties of preadipocytes
Proliferation
There were no differences in preadipocyte proliferation between abdominal subcutaneous and femoral preadipocytes, as assessed by the percent of rapidly proliferating clones (Table 2). Co-culture of fresh abdominal subcutaneous adipose tissue with abdominal and femoral preadipocytes revealed that, compared to control conditions, there was significant stimulation of proliferation (p = 0.03). The differences between sexes (p = 0.74) and between abdominal and femoral tissue (p = 0.10) were not statistically significant.
Adipogenesis
The relative expression of adipogenic markers C/EBPα and PPARγ was less in femoral than in cultured abdominal subcutaneous preadipocytes (Table 2). The between-depot differences in PPARγ mRNA were accounted for mainly by differences among depots in women (sex × depot interaction, p = 0.04).
Apoptosis
Treatment of preadipocyte cultures with increasing doses of TNFα (0, 10, or 50 ng/ml) induced a significant (p <0.0001) dose-dependent increase in the percent of apoptotic cells. Analysis of the responsiveness of preadipocytes to the maximal dose of TNFα (50 ng/ml) indicated a tendency of the femoral preadipocytes in women to have lower susceptibility to apoptosis compared to subcutaneous abdominal preadipocytes (sex × depot interaction, p = 0.06).
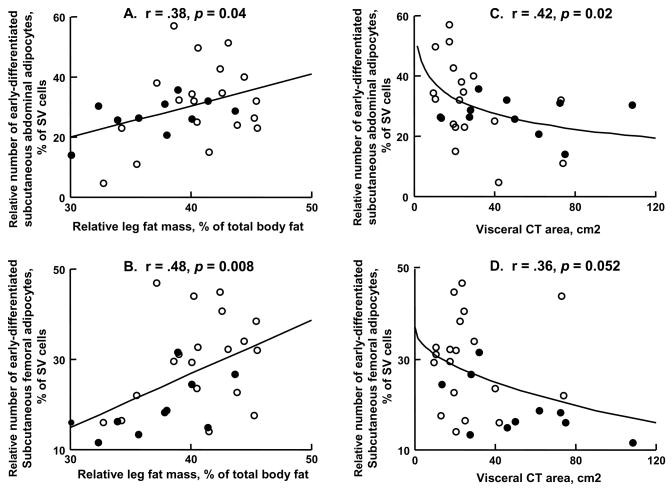
Relationships between relative number of regional early-differentiated adipocytes and fat distribution
There was a significant positive association between the tendency towards a gynoid fat distribution (lower-body fat/total body fat) and the proportion of total SVF cells that were early-differentiated adipocytes; this was true for both abdominal and femoral depots (Figure 1A, B). In contrast, the relative number of early-differentiated subcutaneous abdominal adipocytes was negatively associated with visceral CT area; there was a trend for a similar relationship between visceral CT area and femoral early-differentiated adipocytes (Figure 1C, D). The same trend was not seen if the percent early-differentiated adipocytes in SVF was plotted vs. percent body fat, indicating that the visceral fat finding was not merely a reflection of total body fat.
Figure 1.
The relationships between regional adiposity and the percent of adipose tissue stromovascular (SV) cells that are early-differentiated adipocytes for men (closed circles) and women (open circles) are depicted. The regression lines depicted are from univariate correlation analysis including all participants. The visceral CT area (cm2) were logarithmically transformed in order to linearize the data for regression analysis. A, B: Percent of total body fat present in the lower-body is plotted vs. subcutaneous abdominal and femoral early-differentiated adipocytes, respectively. C, D: Visceral CT area is plotted versus subcutaneous abdominal and femoral early-differentiated adipocytes, respectively.
DISCUSSION
We studied regional adiposity, adipocyte cellularity, and cell kinetics of preadipocytes in normal weight men and women to identify potential cellular mechanisms causing gender differences in regional fatness. In addition to the expected sex differences in regional adiposity (6, 7), we found sex differences in the regional expression of adipogenic markers C/EBPα and PPARγ (Table 2), but little in the way of differences in preadipocyte proliferation and borderline differences in the sensitivity of preadipocytes to TNFα-induced apoptosis.
Cells in the SVF of adipose tissue that express aP2, but not CD68, are capable of proliferation and differentiation into mature adipocytes (11). We had originally termed these cells “committed preadipocytes” (11). However, it has subsequently been reported that preadipocytes constitute the majority of the CD34+ CD31− sub-fraction from the SV cells (21). We performed an exploratory flow cytometry of SVF isolated from liposuction using antibodies against aP2 and CD34 and found only a very small (0.3%) fraction of dual stained CD34+aP2+ cells (data not shown). Thus, we believe that the aP2+CD68− population in the SVF is better considered to be early-differentiated (immature) adipocytes.
Our main finding is that there was a greater abundance of early-differentiated adipocytes in the SVF in women compared to men, most notably in the femoral depot (~35%). The number of early-differentiated adipocytes reflects the balance between the formation of new immature adipocytes (early differentiation) and their disappearance from the SVF. The latter can occur via further differentiation to mature adipocytes, dedifferentiation to more primitive preadipocytes, or apoptosis. The recruitment of new immature adipocytes is controlled by the available preadipocyte pool, which itself is regulated by the balance between preadipocyte proliferation, apoptosis, and recruitment of preadipocytes from uncommitted stem cells. To gain insight as to whether there are sex differences in some of these processes, we measured expression of PPARγ and C/EBPα (key transcription factors involved in differentiation) in in vitro-differentiated preadipocyte cultures, as well as preadipocyte proliferation and apoptosis. We did not find gender differences in the amount of C/EBPα mRNA, preadipocyte proliferation, or in the susceptibility of preadipocytes to apoptotic stimuli. Taken together, these data suggest that mechanisms other than solely inherent gender-dependent differences in preadipocytes are responsible for the larger population of early-differentiated adipocytes in women.
Our other interesting finding is the lower PPARγ mRNA abundance in cultured femoral compared to abdominal subcutaneous preadipocytes in women but not in men. With respect to differences among fat depots, this is consistent with a previous report showing less differentiation of femoral compared to abdominal preadipocytes in obese women (22). However, with respect to differences between men and women, these differences are opposite to those anticipated if purely inherent properties of preadipocytes dictated gender differences in fat cell size, since femoral fat cells are larger in women than men. PPARγ is reported to mediate adipocyte hypertrophy (23) and lower PPARγ would be consistent with smaller, not larger, femoral adipocytes. Possible mechanisms causing this could include gender-dependent differences in the microenvironment of different fat depots, rather than in inherent properties of preadipocytes from males vs. females. To address this possibility, we co-cultured fat tissue from different depots from men and women with preadipocytes. While the fat tissue stimulated preadipocyte proliferation, it did not bring out gender-dependent differences. Further studies are warranted to elucidate potential mechanisms causing gender-dependent differences in fat cell function.
One possibility is that estrogens or androgens have distinct effects on the preadipocytes or fat cells from different depots. Clinical and epidemiological studies strongly suggest a major role for sex steroid hormones in the determination of anatomical specificities of fat distribution in humans (24). Sex-steroid nuclear receptors that bind androgens, estrogens, and progesterone, as well as membrane estrogen receptors, have been found in preadipocytes or stromovascular cells in both men and women (25–27), suggesting that preadipocytes are targets for sex hormones in both sexes. Moreover, an inhibiting effect of testosterone (28) on adipogenesis has been demonstrated, as opposed to a stimulatory effect of estrogens and progestins (29, 30). Theoretically, regional differences in microenvironment regarding androgens or estrogens could account for sex differences in preadipocyte cell dynamics or regional fat cell size, and fat cell number. Alternatively, the responsiveness of subcutaneous preadipocytes from upper- and lower-body fat depots in men and women to sex steroids may vary. Therefore, testing the responsiveness of subcutaneous preadipocytes from upper- and lower-body fat depots in men and women to treatment with sex steroids is necessary to proof/refute whether there are ‘intrinsic’ differences in preadipocyte dynamics between men and women.
Another possibility is that gender-dependent differences among fat depots in innervation, circulation, or other microenvironmental factors not sustained following removal of fat tissue from the subjects could contribute. If microenvironmental factors that persist after isolation of fat biopsies were responsible (for example, differences in numbers of lymphocytes, macrophages, endothelial cells, or other cell types in fat tissue fragments), we might expect to have found distinct effects of biopsies from females vs. males in our co-culture studies, but we did not. Yet another explanation could be differences between men and women in susceptibility of femoral adipocytes to apoptosis, since apoptosis plays a role in determining adipocyte size (31, 32). Future studies of depot- and sex-dependent susceptibility of adipocytes to apoptosis would be necessary to test this.
Femoral preadipocytes tended to be more resistant to TNFα-induced apoptosis than abdominal subcutaneous preadipocytes in women. This, together with reduced differentiation capacity, suggests potentially slower cell turnover of preadipocytes in the lower body in women compared to the upper-body. If true, this may lead to a lower rate of utilization of the available preadipocyte and stem cell pools and could contribute to the proclivity for expansion of leg fat stores in women.
We previously measured in vitro preadipocyte differentiation in a small number of obese women with upper- and lower-body fat distribution phenotypes (n=3 per group) (33). These data suggested that preadipocytes from obese women with gynoid fat distribution (lower-body obesity) differentiated more readily than those from women with upper-body obesity. In this study, we measured the relative number of early-differentiated adipocytes, which is regulated, in part, by preadipocyte differentiation. We found that indices of gynoid and android fat distribution (percent fat in the leg and visceral CT area) were correlated with the relative number of early-differentiated adipocytes in normal weight men and women. A gynoid fat distribution was positively associated with the number of early-differentiated adipocytes whereas visceral fat accumulation was negatively correlated with the number of early differentiated adipocytes in the subcutaneous abdominal depot. This is consistent with the hypothesis that a greater capacity for adipogenesis in subcutaneous fat reduces ectopic (visceral) fat accumulation and predisposes towards greater relative leg fat mass. Prospective studies of the relationship between subcutaneus adipogenic potential and regional fat gain during intentional weight gain will be needed to adequately test this hypothesis, however.
In summary, we found that: 1) the availability of early-differentiated adipocytes in lower-body fat differs markedly between normal weight, healthy men and women, 2) preadipocyte differentiation markers differ between upper- and lower-body depots in women, and 3) inherent differences between men and women in preadipocyte capacities for replication and differentiation, in response to serum containing medium and serum free adipogenic cocktail containing insulin, dexamethasone, and thyroid hormone, did not explain gender-related variation in regional fat distribution. Taken together, these findings implicate: 1) gender-dependent regional variation in microenvironmental conditions, 2) regional variation in preadipocyte responses to sex steroids, and/or 3) gender-dependent differences among depots in fat cell removal, as potential causes of differences in fat distribution between men and women.
Acknowledgments
Supported by grants DK45343 (MDJ), DK50456(MDJ), AG13925 (JLK), and RR00585 (MDJ) from the U.S. Public Health Service and by the Mayo Foundation, the Noaber Foundation, the Ted Nash Long Life Foundation, and the Robert and Arlene Kogod Center on Aging. The authors thank Caleb Morris, Deborah Harteneck, Jessica Eastman, and Carol Siverling from Mayo Clinic for technical assistance.
Footnotes
DISCLOSURE STATEMENT
Authors have nothing to disclose.
References
- 1.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 2.Snijder MB, Zimmet PZ, Visser M, Dekker JM, Seidell JC, Shaw JE. Independent and opposite associations of waist and hip circumferences with diabetes, hypertension and dyslipidemia: the AusDiab Study. Int J Obes Relat Metab Disord. 2004;28:402–9. doi: 10.1038/sj.ijo.0802567. [DOI] [PubMed] [Google Scholar]
- 3.Van Pelt RE, Jankowski CM, Gozansky WS, Schwartz RS, Kohrt WM. Lower-body adiposity and metabolic protection in postmenopausal women. J Clin Endocrinol Metab. 2005;90:4573–8. doi: 10.1210/jc.2004-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snijder MB, Dekker JM, Visser M, et al. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care. 2004;27:372–7. doi: 10.2337/diacare.27.2.372. [DOI] [PubMed] [Google Scholar]
- 5.Jensen MD. Adipose tissue and fatty acid metabolism in humans. J R Soc Med. 2002;95 (Suppl 42):3–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Kuk JL, Lee S, Heymsfield SB, Ross R. Waist circumference and abdominal adipose tissue distribution: influence of age and sex. Am J Clin Nutr. 2005;81:1330–4. doi: 10.1093/ajcn/81.6.1330. [DOI] [PubMed] [Google Scholar]
- 7.Schreiner PJ, Terry JG, Evans GW, Hinson WH, Crouse JR, 3rd, Heiss G. Sex-specific associations of magnetic resonance imaging-derived intra-abdominal and subcutaneous fat areas with conventional anthropometric indices. The Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1996;144:335–45. doi: 10.1093/oxfordjournals.aje.a008934. [DOI] [PubMed] [Google Scholar]
- 8.Bertrand HA, Masoro EJ, Yu BP. Increasing adipocyte number as the basis for perirenal depot growth in adult rats. Science. 1978;201:1234–5. doi: 10.1126/science.151328. [DOI] [PubMed] [Google Scholar]
- 9.Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–7. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Kirkland JL, Hollenberg CH. Varying capacities for replication of rat adipocyte precursor clones and adipose tissue growth. J Clin Invest. 1989;83:1741–6. doi: 10.1172/JCI114075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tchoukalova YD, Sarr MG, Jensen MD. Measuring committed preadipocytes in human adipose tissue from severely obese patients by using adipocyte fatty acid binding protein. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1132–40. doi: 10.1152/ajpregu.00337.2004. [DOI] [PubMed] [Google Scholar]
- 12.Jensen MD, Levine J. Effects of oral contraceptives on free fatty acid metabolism in women. Metabolism. 1998;47:280–4. doi: 10.1016/s0026-0495(98)90257-8. [DOI] [PubMed] [Google Scholar]
- 13.Uranga AP, Levine J, Jensen M. Isotope tracer measures of meal fatty acid metabolism: reproducibility and effects of the menstrual cycle. Am J Physiol Endocrinol Metab. 2005;288:E547–55. doi: 10.1152/ajpendo.00340.2004. [DOI] [PubMed] [Google Scholar]
- 14.Heiling VJ, Jensen MD. Free fatty acid metabolism in the follicular and luteal phases of the menstrual cycle. J Clin Endocrinol Metab. 1992;74:806–10. doi: 10.1210/jcem.74.4.1548345. [DOI] [PubMed] [Google Scholar]
- 15.Shadid S, Jensen MD. Effects of pioglitazone versus diet and exercise on metabolic health and fat distribution in upper body obesity. Diabetes Care. 2003;26:3148–52. doi: 10.2337/diacare.26.11.3148. [DOI] [PubMed] [Google Scholar]
- 16.Tchkonia T, Tchoukalova YD, Giorgadze N, et al. Abundance of two human preadipocyte subtypes with distinct capacities for replication, adipogenesis, and apoptosis varies among fat depots. Am J Physiol Endocrinol Metab. 2005;288:E267–77. doi: 10.1152/ajpendo.00265.2004. [DOI] [PubMed] [Google Scholar]
- 17.Tchkonia T, Lenburg M, Thomou T, et al. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol Endocrinol Metab. 2007;292:E298–307. doi: 10.1152/ajpendo.00202.2006. [DOI] [PubMed] [Google Scholar]
- 18.Tchoukalova YD, Harteneck DA, Karwoski RA, Tarara J, Jensen MD. A quick, reliable, and automated method for fat cell sizing. J Lipid Res. 2003;44:1795–801. doi: 10.1194/jlr.D300001-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Myers JN, Tabas I, Jones NL, Maxfield FR. Beta-very low density lipoprotein is sequestered in surface-connected tubules in mouse peritoneal macrophages. J Cell Biol. 1993;123:1389–402. doi: 10.1083/jcb.123.6.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauner H, Petruschke T, Russ M, Rohrig K, Eckel J. Effects of tumour necrosis factor alpha (TNF alpha) on glucose transport and lipid metabolism of newly-differentiated human fat cells in cell culture. Diabetologia. 1995;38:764–71. doi: 10.1007/s001250050350. [DOI] [PubMed] [Google Scholar]
- 21.Sengenes C, Lolmede K, Zakaroff-Girard A, Busse R, Bouloumie A. Preadipocytes in the human subcutaneous adipose tissue display distinct features from the adult mesenchymal and hematopoietic stem cells. J Cell Physiol. 2005;205:114–22. doi: 10.1002/jcp.20381. [DOI] [PubMed] [Google Scholar]
- 22.Hauner H, Entenmann G. Regional variation of adipose differentiation in cultured stromal-vascular cells from the abdominal and femoral adipose tissue of obese women. Int J Obes. 1991;15:121–6. [PubMed] [Google Scholar]
- 23.Kubota N, Terauchi Y, Miki H, et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- 24.Mayes JS, Watson GH. Direct effects of sex steroid hormones on adipose tissues and obesity. Obes Rev. 2004;5:197–216. doi: 10.1111/j.1467-789X.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- 25.Joyner JM, Hutley LJ, Cameron DP. Estrogen receptors in human preadipocytes. Endocrine. 2001;15:225–30. doi: 10.1385/ENDO:15:2:225. [DOI] [PubMed] [Google Scholar]
- 26.O’Brien SN, Welter BH, Mantzke KA, Price TM. Identification of progesterone receptor in human subcutaneous adipose tissue. J Clin Endocrinol Metab. 1998;83:509–13. doi: 10.1210/jcem.83.2.4561. [DOI] [PubMed] [Google Scholar]
- 27.Joyner J, Hutley L, Cameron D. Intrinsic regional differences in androgen receptors and dihydrotestosterone metabolism in human preadipocytes. Horm Metab Res. 2002;34:223–8. doi: 10.1055/s-2002-32144. [DOI] [PubMed] [Google Scholar]
- 28.Singh R, Artaza JN, Taylor WE, Gonzalez-Cadavid NF, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology. 2003;144:5081–8. doi: 10.1210/en.2003-0741. [DOI] [PubMed] [Google Scholar]
- 29.Roncari DA, Van RL. Promotion of human adipocyte precursor replication by 17beta-estradiol in culture. J Clin Invest. 1978;62:503–8. doi: 10.1172/JCI109153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lacasa D, Garcia E, Agli B, Giudicelli Y. Control of rat preadipocyte adipose conversion by ovarian status: regional specificity and possible involvement of the mitogen-activated protein kinase-dependent and c-fos signaling pathways. Endocrinology. 1997;138:2729–34. doi: 10.1210/endo.138.7.5246. [DOI] [PubMed] [Google Scholar]
- 31.Alkhouri N, Gornicka A, Berk MP, et al. Adipocyte apoptosis: A link between obesity, insulin resistance and hepatic steatosis. J Biol Chem. 2009 doi: 10.1074/jbc.M109.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.West M. Dead adipocytes and metabolic dysfunction: recent progress. Curr Opin Endocrinol Diabetes Obes. 2009;16:178–82. doi: 10.1097/med.0b013e3283292327. [DOI] [PubMed] [Google Scholar]
- 33.Tchoukalova Y, Koutsari C, Jensen M. Committed subcutaneous preadipocytes are reduced in human obesity. Diabetologia. 2007;50:151–7. doi: 10.1007/s00125-006-0496-9. [DOI] [PubMed] [Google Scholar]