Abstract
ADHD is characterized by inattention, hyperactivity, and disinhibition, including the inability to screen out distracting stimuli. Prepulse inhibition (PPI) of startle indexes a related gating process and is enhanced during attended compared to ignored stimuli. We predicted that PPI during attended stimuli would be enhanced by the stimulant methylphenidate (MPH) and that this effect would be moderated by baseline PPI. Children with ADHD (n = 36) completed a baseline day and a randomized, double-blind medication trial (placebo vs. sustained release MPH). Bilateral startle eyeblink EMG was measured during a tone discrimination task. MPH enhanced PPI during attended, but not during ignored stimuli. Extending findings that pretreatment functioning moderates stimulant effects on PPI, this effect tended to be inversely related to baseline PPI. These data fit with the clinical literature on ADHD and the hypothesis that MPH enhances interference control for important environmental stimuli.
Introduction
Attention-Deficit/Hyperactivity Disorder (ADHD) is characterized by impulsivity (e.g, trouble waiting turns, interrupting others), hyperactivity, and inattention (APA, 2000). According to a leading model, inhibitory control is theorized to be a core process in ADHD (Barkley, 1997). Nigg (2000) describes several facets of inhibitory control. These include behavioral (response) inhibition, or the inhibition of a prepotent response, and interference control, or the suppressed processing of a distracting stimulus to carry out a primary response.
An important prediction for any putative core process in ADHD is that deficits in that process should be reduced by effective treatments. Stimulants continue to be a leading treatment for ADHD (Zuvekas, Vitiello, & Norquist, 2006), and the stimulant methylphenidate (MPH) improves performance on measures of response inhibition (Lijffijt et al., 2006; Oosterlaan, Logan, & Sergeant, 1998; Tannock, Schachar, & Logan, 1995), and sustained attention (Losier, McGrath, & Klein, 1996; Riccio, Waldrop, Reynolds, & Lowe, 2001). The smaller literature on interference control is mixed, with some studies demonstrating that methylphenidate improves the screening out of distracting stimuli in ADHD (Scheres et al., 2003), whereas others do not (Langleben et al., 2006). Differences in the measurement of interference control may partially explain these mixed findings (Lansbergen, Kenemans, & van Engeland, 2007).
To elucidate the effect of methylphenidate on the controlled ability to screen out distracting stimuli, the present work employs an attentional modification of prepulse inhibition of startle paradigm. Prepulse inhibition (PPI), a decrease in the magnitude of the startle response when a weak, nonstartling stimulus is presented 30–500 ms before startle probe onset, is widely used to investigate early cognitive processes (Filion, Dawson, & Schell, 1998) and their psychopharmacology (Braff, Geyer, & Swerdlow, 2001a; Swerdlow, Weber, Qu, Light, & Braff, 2008). At these short-lead stimulus onset asynchronies (SOAs), PPI is thought to reflect partially automatic protection of processing of sensory stimuli (Graham, 1975). More generally, it has been conceptualized as a sensorimotor gating mechanism that serves a critical inhibitory function for sensory, cognitive, and motor output processing (Braff & Geyer, 1990).
Though basic PPI does not require conscious awareness, the effect is enhanced by directed attention (Filion et al., 1998). That is, PPI is enhanced during attended prepulses relative to ignored prepulses (e.g., Ashare, Hawk, & Mazzullo, 2007; Filion, Dawson, & Schell, 1993; Hawk, Yartz, Pelham, & Lock, 2003). Differences in these early inhibitory processes have been conceptualized as measures of controlled attention (Dawson, Schell, Swerdlow, & Filion, 1997) and selective inhibition (Hawk et al., 2003) that are central to the interference control construct.
Although passive PPI is not reduced in children (Castellanos et al., 1996; Ornitz, Hanna, & Traversay, 1992) or adults (Feifel, Minassian, & Perry, 2008; Hanlon, Karayanidis, & Schall, 2009) with ADHD compared to controls, preliminary evidence from a small sample of 9- to 12-year-old boys suggests that PPI during attended prestimuli is selectively diminished in ADHD (Hawk et al., 2003). Most germane to the present work is the finding that among the boys with ADHD, a .3mg/kg dose of MPH enhanced PPI during attended prestimuli, eliminating group differences.
This finding was consistent with the clinical effects of MPH, but was difficult to reconcile with extensive preclinical work on the psychopharmacology of PPI. Passive PPI is generally disrupted, or decreased, by dopamine (DA) agonists such as amphetamine and apomorphine (Geyer, Krebs-Thomson, Braff, & Swerdlow, 2001; Swerdlow, Geyer, & Braff, 2001). Therefore, the MPH findings in Hawk et al. (2003) contradict much of the animal literature since MPH, an indirect DA agonist, enhanced PPI during attended stimuli. However, there are at least two ways of incorporating the apparently incompatible data from ADHD. First, the effects of dopaminergic drugs on PPI may depend on baseline levels of PPI in humans (Bitsios, Giakoumaki, & Frangou, 2005; Swerdlow et al., 2003; Talledo, Sutherland Owens, Schortinghuis, & Swerdlow, 2009) and animals (e.g., Talledo et al., 2009). Specifically, the disruptive effects of DA agonists on passive PPI have been most robust among those with high levels of baseline PPI; among those with low baseline PPI, there are either no significant effects or trends towards PPI enhancement. Second, the effects observed by Hawk et al. (2003) may have been due to noradrenergic effects of MPH, a prediction consistent with recent evidence that noradrenergic drugs can increase PPI (Bakshi, Swerdlow, & Geyer, 1994; Gould, Rukstalis, & Lewis, 2005).
Clearly, conclusions from Hawk et al. (2003) were tentative. In addition to the inconsistency with much of the literature regarding the effects of DA agonists on PPI in rats, the sample was small and limited to boys. Furthermore, the sample had been chosen for a positive clinical response to MPH, limiting the generalizability of the findings. The present study attempted to more definitively test the effects of MPH on PPI during attended and ignored prepulses among a larger sample of both boys and girls with ADHD who were not pre-selected for clinical response to MPH. Importantly, the present study is one of a relatively small number to examine the controlled effects of a therapeutic agent on PPI among persons with psychopathology (Wynn et al., 2007) – a strikingly sparse literature given the hundreds of preclinical and clinical studies linking PPI abnormalities to psychopathology (Braff, Geyer, & Swerdlow, 2001b).
Based on our preliminary work, we predicted that MPH would selectively enhance PPI during attended stimuli but have no effect during ignored stimuli. We examined two therapeutic doses of MPH to provide initial data regarding the dose-response curve. Finally, based on evidence from non-clinical samples that drug effects on PPI depend in part on baseline levels of processing (Baschnagel & Hawk, 2008; Bitsios et al., 2005; Swerdlow et al., 2003), and given the marked heterogeneity in ADHD (Nigg, Willcutt, Doyle, & Sonuga-Barke, 2005), we predicted that MPH enhancement of PPI during attended stimuli would be strongest among those with poor gating during attended tones at baseline.
Methods and Materials
Participants
Participants were 36 9- to 12-year-old children (8 females) diagnosed with ADHD. Sample characteristics are listed in Table 1. Participants were recruited from a University psychiatric clinic and pediatricians’ offices. Parents were remunerated with money; children were rewarded with toys and gift cards.
Table 1.
Sample characteristics, mean (SD).
| Age, mean (SD) | 10.5 (1.1) |
| Gender (male:female) | 28:8 |
| Ethnicity | |
| Caucasian | 80% |
| African-American | 17% |
| Mixed race | 3% |
| WISC Full Scale IQ, mean (SD) | 102 (13) |
| WJ Test of Achievement, mean (SD) | |
| Letter-Word Identification | 103 (9) |
| Calculation | 104 (11) |
| Spelling | 104 (12) |
| DBD rating scale | |
| Hyp/Imp, mean (SD) | |
| Parent report | 13 (6) |
| Teacher report | 10 (7) |
| Inattentive, mean (SD) | |
| Parent report | 16 (6) |
| Teacher report | 13 (7) |
| CBCL, mean T-score (SD) | |
| Attention Problems | 68 (9) |
| Externalizing Problems | 61 (9) |
| Internalizing Problems | 57 (9) |
| Comorbid diagnoses (percent subjects) | |
| ODD:CD | 47%:17% |
Note. These values represent the total score of the items within each subtype domain on the DBD rating scale.
Children were excluded based on the following criteria: Full Scale IQ below 80; history of seizures, neurological disorders, and other medical problems contraindicating psychostimulant treatment; current use of non-ADHD psychotropic medications; history or concurrent diagnosis of pervasive developmental disorder or psychosis; and sensory problems that would make it difficult to complete the task.
Diagnostic Assessment
All participants had a DSM-IV (American Psychiatric Association, 2000) diagnosis of ADHD based on a structured interview with the primary caregiver (Diagnostic Interview Schedule for Children Version IV (DISC-IV); Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000). In addition, parents and teachers completed the Disruptive Behavior Disorder (DBD) rating scale (Pelham, Fabiano, & Massetti, 2005; Pelham, Gnagy, Greenslade, & Milich, 1992) and the Impairment Rating Scale (Fabiano et al., 2006) to document the presence of functionally impairing ADHD symptoms in multiple realms. Regarding subtype, 61% were Combined, 31% were Predominantly Inattentive, and 8% were Predominantly Hyperactive/Impulsive. Additionally, 64% of the sample met criteria for Oppositional Defiant Disorder and 17% for Conduct Disorder. Standardized measures of intellectual ability and achievement included the Vocabulary and Block Design subtests from the Wechsler Intelligence Scale for Children - Fourth Edition (WISC-IV; Kaplan, Fein, Kramer, Delis, & Morris, 2004). Table 1 presents descriptive statistics for diagnostic symptom, intellectual, and achievement variables.
Setting
The Summer Research Camp was held from 7:30 am to 5pm Monday through Friday. On Monday through Thursday cohorts of 5 children completed a variety of computerized tasks measuring neurocognitive processes implicated in ADHD, classroom periods, and recreational activities. Task order was randomized between participants but remained consistent for each child. The current report is limited to tone discrimination task described below. Children earned points for task participation and appropriate behavior. These points were exchanged for toys and gift cards at the end of each day.
Medication Assessment
After an initial baseline day, each child participated in a 3-day double-blind, placebo-controlled medication assessment. Two doses of long-acting osmotic release oral system methylphenidate (OROS-MPH; Concerta®) were employed. One provided equivalent effects to TID IR MPH at .3mg/kg dose, producing a total daily dose of .9mg/kg; the other dose was equivalent to TID IR MPH at .6mg/kg/dose, producing a total daily dose of 1.8mg/kg. As per current dosing guidelines by the American Academy of Child and Adolescent Psychiatry (Greenhill et al., 2002; Pliszka, 2007), these represent medium to large doses, respectively (c.f., Pliszka, 2007). OROS-MPH was selected to ensure consistent blood levels of MPH over the 8-hour testing period. Most (81%) participants were taking stimulant medication at the time of the study or had prior exposure to stimulants. Participants discontinued any stimulant medication at least 24 hours prior to participation; participants taking atomoxetine completed a 1-week washout period. To promote reasonable tolerability of the medication in subjects who were stimulant naïve or previously prescribed only very low doses (<.4 mg/kg/day), thirteen children were order restricted so that they received the .3 mg/kg TID-equivalent dose prior to receiving the .6 mg/kg TID-equivalent dose. Among those who were not order restricted, six possible drug orders were counterbalanced across participants.
Medication was administered when the child arrived in the morning, 90 minutes prior to the initial cognitive task. Two prior studies have established that OROS-MPH significantly separates from placebo in this timeframe (Pelham et al., 2001; Swanson et al., 2003). Pelham et al. (2003) also established that a 20% higher OROS-MPH dose produces comparable effects to a TID Immediate-Release MPH dose over a 12 hour period (i.e., 18mg of OROS-MPH = 15mg of IR MPH dosed as 5mg TID). We used similar procedures as this study to calculate matching doses of OROS-MPH. Doses ranged from 18 to 90 mg (dose was capped at 90 mg for safety reasons). By using combinations of commercially-available doses (we used 18, 27, and 36 mg) and providing each child with either two or three capsules each morning (number of capsules remained constant for a given child), we were able to provide most within 4.5 mg OROS-MPH of the ideal weight-based dose up to the 90 mg maximum (no medium doses were affected by the maximum, but 9 children would have received high doses greater than 90 mg [median = 99 mg] were it not for this cap). The mean of the medium dose was 40 mg OROS-MPH (SD = 9.2) and the mean of the high dose was 76.5 mg OROS-MPH (SD = 13.2).
Adverse events were rated daily by camp counselors and parents using the Pittsburgh Side Effect Rating Scale, which inquires about common side effects seen with stimulants (rated none to severe) (Pelham, 1993). Blood pressure and pulse were also measured daily during times of peak medication effects; no child had marked elevation of either cardiovascular parameter. Any subject reporting significant distress or exhibiting marked side effects was evaluated by the study nurse or physician.
Apparatus
VPM 10.5 software (Cook, Atkinson, & Lang, 1987) running on a Pentium-class computer (Gateway; North Sioux City, S.D.) controlled the presentation of tone prestimuli and startle probes and sampled the eyeblink EMG. Stimulus parameters were based on earlier work (Filion et al., 1993; Hawk et al., 2003). Startle probes were 50-ms, 100-dB(A) bursts of white noise with near-instantaneous rise/fall times, and prestimuli were 5- and 8-s, 70-dB(A), 400- and 1200-Hz tones with 25-ms rise/fall times. The prepulse tones and startle probes were presented via a Soundblaster 64 AWE Gold sound card, amplified with an Optimus SA-155 (Radio Shack; Fort Worth, TX) stereo receiver, and played through matched Telephonics TDH49-P headphones. Ambient background noise was approximately 55 dB(A).
The eyeblink startle response was measured electromyographically from orbicularis oculi, using TDE-23 Ag/AgCl surface electrodes (Med Associates, East Fairfield, VT) placed about 1 cm below the pupil and outer canthus of each eye. EMG was amplified by Grass Instruments bioamplifiers (7P3/7DA; West Warwick, OH) with a bandpass of 10–500 Hz. Amplifier output was fed to the A/D converter of a Scientific Solutions (Solon, OH) Lab Master DMA interface, which sampled the amplified EMG at 1000 Hz from 50 ms before until 300 ms after startle probe onset.
Procedure
All procedures were approved by the University at Buffalo Children and Youth IRB. Parents provided written consent for participation in the study and assent to participate was obtained from all children. Children were tested individually in a sound-attenuated booth. A cover story of going into space to intercept and decode messages from space was employed to engage children in the setting and the task. Children earned up to 100 points for following the rules, including: 1) follow directions, 2) stay in your assigned area, 3) use material and possessions appropriately, and 4) try your best. Children were also informed that, following a warning, they would lose 25 points per rule violation. Behavior was well-maintained, with only 2 children losing any points.
Sensors were attached for measurement of bilateral eyeblink EMG. Two tone series were presented over headphones to ensure consistent pitch and length discrimination. After training, 5 habituation probes were presented and the following instructions were provided based on whether the child was told to respond to high or low pitched tones:
During the mission, you’ll begin to hear some tones like the ones you’ve already heard. Some will be high, and some will be low. Most will be of the regular length, but some will be longer-than-usual. Like I mentioned before, we think that we’ll be closer to understanding the message once we know more about the LOW tones that are longer than usual. So, pay attention to the LOW tones. You can ignore the HIGH tones. Every time there is a longer than usual LOW tone, I want you to click the LEFT mouse button. Remember to wait until right after the tone to click the mouse and to press it only if the tone was a LOW one and longer-than-usual.
Now, there’s a bonus for astronauts who do this well. If you “mark” all of the LOW, longer-than-usual tones, then you’ll get a 500 point bonus! If you miss one or click the mouse when there wasn’t a longer-than-usual LOW tone, you’ll lose 50 points, so then you would have 450 points. If you did this twice, then you would only have 400 points…and so on.
Then, participants completed 60 trials (3 blocks of 20 trials, separated by brief breaks) of a tone discrimination paradigm modeled after that of Dawson and colleagues (e.g., Filion et al., 1993; see; Hawk et al., 2003). Tones were of two pitches (400 and 1200 Hz); within each pitch one-third were longer-than-usual (8s vs. 5s). Bilateral startle eyeblink EMG responses to probes presented at 120-, 180-, and 4500-ms prepulse-probe stimulus onset asynchronies (SOAs) were assessed on 75% of trials. The two early SOAs assessed short-lead prepulse inhibition, whereas the 4500-ms SOA assessed long-lead prepulse facilitation. The remaining 25% of trials were equal numbers of no-startle trials and trials containing intertrial interval (ITI) startle probes. Following tone offset, participants had a 3-s window in which to respond to the tone via computer mouse. Performance measures, such as correct hits and false alarms as well as reaction time data were recorded via E-Prime software (Psychology Software Tools, Pittsburgh, PA). Children were informed of their performance and given their points at the end of each session.
Data Reduction and Analysis
A total of 50 children were run in one or more sessions but were excluded due to an equipment problem resulting in EMG artifact (n=4), missing multiple testing sessions (n=4, namely due to reported nervousness regarding the testing), or outlying or missing data following data reduction, as outlined below (n=6), resulting in 36 participants with usable data.
As in recent work (Ashare et al., 2007), startle responses were digitally integrated off-line (rectified, low-pass filtered with a 50-ms time constant, and high-pass filtered with 30 Hz cutoff; van Boxtel, Boelhouwer, & Bos, 1998) and scored using the computer program of Balaban et al. (1986), and trials were also excluded on the basis of excessive baseline range if 1) baseline range exceeded 5 µV or 2) baseline range was greater than 3 µV and response magnitude was < 5 times the baseline range. Using these criteria, approximately 4.0% of trials on both eyes were excluded. If baseline range was between 0 and 1 µV and the response magnitude was less than the baseline range, the magnitude was set to 0.
All available trials were used to compute average eyeblink EMG magnitude for each SOA (120, 180 ms) × Attend (attended pitch vs. ignored pitch) × Day × Eye cell of the design. These eyeblink EMG magnitude subject averages were used to compute percent inhibition, relative to the magnitude on probe-alone (ITI startle) trials ([(Mprepulse_trials − MITI_trials)/(MITI_trials)] × −100). A parallel series of analyses were conducted on percent prepulse facilitation (PPF) at the long-lead 4500-ms SOA.1 For each condition, an average was considered an outlier when it was greater than three times the interquartile range above the 75th percentile (as in our previous work; Baschnagel & Hawk, 2008). For subjects with no outlying averages, percent modification scores were averaged across right and left eyes. If outliers were evident at only one eye, then data for the other eye were retained for analyses. If data for both eyes contained outlying values then the participant’s data were excluded from analyses (n=6, as noted above).
Data analyses were repeated measures ANOVAs with drug as a within-subjects factor, except where noted. For drug effects, orthogonal contrasts of medication, comparing placebo v. active drug (average of .3 mg/kg and .6 mg/kg), and dose, comparing the .3 mg/kg v. .6 mg/kg, were employed. Sex and drug order were tested in preliminary models, but because neither significantly moderated the findings, both were removed from the final models. For percent PPI, SOA (120, 180), and attend (attended vs. ignored) were additional within-subjects factors. Exploratory analyses consider the possible roles of ADHD subtype, comorbid externalizing disorder (ODD/CD), and previous treatment with stimulant medication (stimulant naïve n = 7).
Based on recent work suggesting that baseline processing may be important in evaluating medication effects on PPI (Baschnagel & Hawk, 2008; Bitsios et al., 2005; Swerdlow et al., 2003; Talledo et al., 2009), we employed regression analyses to examine the role of baseline (Monday) startle modification in predicting the effect of placebo v. active drug on PPI. Separate analyses were conducted for attended and ignored prestimuli such that in each model the drug effect on PPI was regressed on PPI during attended or ignored prestimuli during the baseline day, respectively. Because 6 children did not complete the entire baseline session, this analysis is based on n=30.
The number of responses to longer-than-usual attended tones (i.e., “hits”; Max=10) was the primary performance outcome. False alarms were examined in a parallel ANOVA.
Results
ITI Startle magnitude
Methylphenidate did not affect ITI startle magnitude, ps > .2, Placebo v. MPH mean difference = .68 µV (S.E. = 1.2); Dose mean difference = 1.4 µV (S.E. = 1.1).
Percent prepulse inhibition
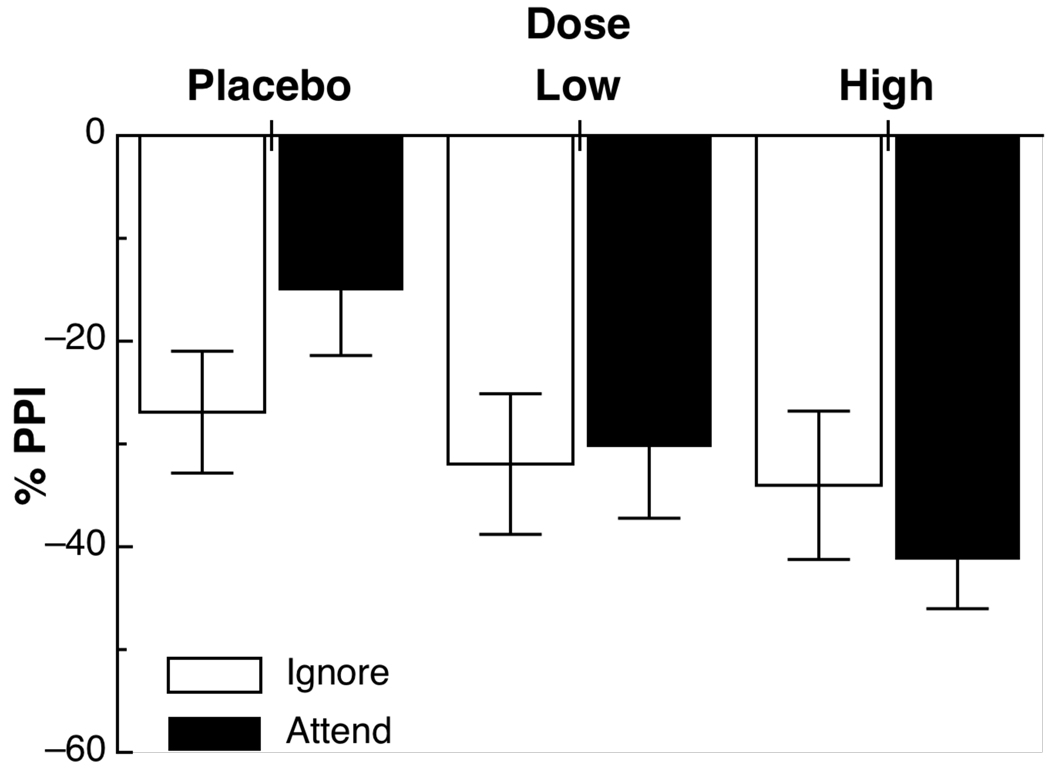
Figure 1 depicts the mean percent prepulse modification for ignored and attended prestimuli (averaged across SOAs), for placebo and both active doses of methylphenidate. Although there was a significant main effect of methylphenidate on prepulse inhibition, Placebo v. MPH F(1,35) = 7.6, p < .01, d = .46, this effect was influenced by the attentional nature of the prepulse, Placebo v. MPH × Attend interaction, F(1,35) = 5.2, p < .05. Follow-up tests revealed that MPH enhanced PPI compared to placebo during attended tones, p < .02, d = .61, but not during ignored tones, p = .46, d = .17. There was little evidence of dose separation, .3 mg/kg vs. .6 mg/kg dose and Dose × Attend Fs(1,35) < 2.0, ps > .2. There were no significant differences between the 120- and 180-ms SOAs, Fs < 1.
Figure 1.
Mean (SE) percent prepulse inhibition for all Dose × Attend conditions.
In exploratory analyses, neither ADHD subtype (combined vs. inattentive; hyperactive/impulsive was not included because n=3) nor the presence of comorbid externalizing disorders (ODD and CD combined) moderated the Dose × Attend interactions, all Fs < 1. However, previous treatment tended to moderate the effect of MPH on PPI to attended and ignored tones, Treatment History × Placebo v. Active MPH × Attend interaction, F(1,34) = 3.0, p = .1 and Treatment History × .3 mg/kg vs .6 mg/kg Dose × Attend interaction, F(1,34) = 5.1, p < .05. Among those with a history of stimulant treatment, the high dose (mean PPI = 43%, S.E. = 5.7%) appeared to be necessary to demonstrate a statistically significant increase in PPI during attended stimuli, .6 mg/kg v. Placebo, p < .01 and .6 mg/kg v. .3 mg/kg Dose, p = .06; .3 mg/kg v. Placebo, p = .18; Placebo mean = 19% (S.E. = 7%), .3 mg/kg mean = 34% (S.E. = 8%). In contrast, among the small number of children who were stimulant naïve, both active doses (.3 mg/kg mean = 25%, S.E. = 16%; .6 mg/kg mean = 29%, S.E. = 11%) significantly enhanced PPI to attended tones compared to placebo (mean = 11%, S.E. = 13.4), both ps < .05. However, there was no further enhancement from .3 mg/kg to .6 mg/kg, p = .8. There were no significant differences for ignored stimuli, all ps > .28, with PPI ranging from 21% to 37%.
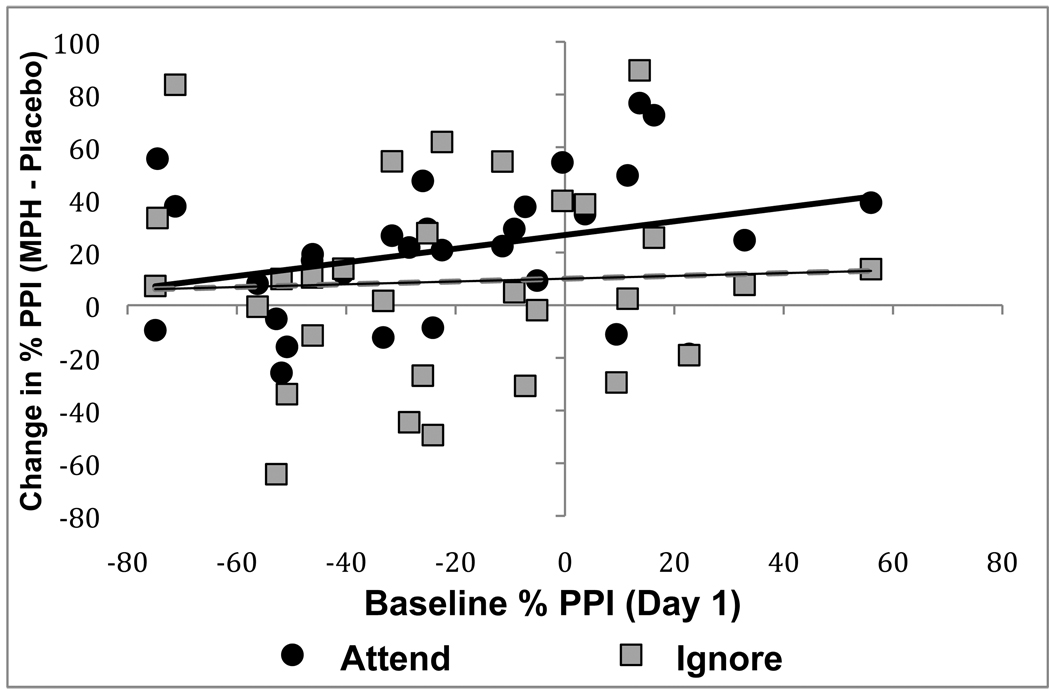
Consistent with our hypothesis regarding moderation of medication effects by baseline PPI, lower baseline PPI during attended tones tended to predict greater increases with MPH relative to placebo during attended tones, F(1,29) = 3.4, p = .08, β = −.35 (see Figure 2). However, baseline PPI during ignored tones was not related to the effect of MPH on PPI during these stimuli, F < 1, β = −.03.
Figure 2.
The effect of MPH v. Placebo on PPI of startle during attended and ignored prepulses as a function of baseline PPI.
Task performance
Hits, or correct responses to longer-than-usual attended tones, were greater during active drug compared to placebo (mean = 7.7, S.E = .4), Placebo vs Active drug F(1,35) = 7.8, p < .01; this seemed to be due in part to a marginally significant increase in hits with the .6 mg/kg dose (mean = 9.1, S.E. = .25) compared to the .3 mg/kg dose (mean = 8.4, S.E. = .4), .3 mg/kg vs. .6 mg/kg F(1,35) = 3.7, p = .06. The number of false alarms to short attended tones was reduced during active drug compared to placebo (mean = 2.4, S.E. = .6), Placebo vs Active drug F(1,35) = 4.5, p < .05 and tended to be reduced during the .6 mg/kg dose (mean = 1.5, SE = .5) compared to the .3 mg/kg dose (mean = 2.3, S.E. = .7), F(1,35) = 3.1, p = .09. There were no discernable effects of MPH on false alarms to ignored tones, ps > .4.
Discussion
Based on theories of ADHD (Nigg, 2000) and preliminary evidence regarding PPI during attended and ignored stimuli in ADHD (Hawk et al., 2003), we predicted that stimulant medication, a frontline treatment for ADHD, would selectively enhance PPI during attended stimuli. The ability to suppress processing of a distracting stimulus that may prevent one from carrying out a primary response, or interference control, is theorized to be part of a core inhibitory process in ADHD (Nigg, 2000). Although PPI is generally thought to reflect a partially automatic sensorimotor gating process (Braff et al., 2001a; Graham, 1975), PPI is enhanced when attention is directed towards a relevant stimulus. This may reflect the ability to protect attention to task-relevant stimuli from interference caused by the task-irrelevant startle probe (Filion et al., 1993). Consequently, we tested school-aged children with ADHD in a paradigm in which some tone prepulses were to be attended whereas others could be ignored under conditions of acute administration of placebo and two different doses of the stimulant methylphenidate (MPH) in a double-blind, randomized study.
Consistent with predictions, MPH enhanced PPI among children with ADHD, but only when they were engaged in effortful cognitive processing (i.e., directing their attention to relevant tones); PPI during ignored tones was not affected by medication. The effect size of MPH compared to placebo on PPI during attended stimuli was moderate, d = .61. Behaviorally, stimulant treatment also tended to reduce errors of omission and commission, though these effects were weaker, likely because of the relatively low task difficulty in the present paradigm.
We have previously observed a beneficial effect of 0.3 mg/kg immediate-release MPH on PPI in a relatively homogeneous sample of boys with a documented positive response to ongoing stimulant treatment (Hawk et al., 2003). The present study replicates and extends our earlier findings. The present sample is more than twice as large as the original study, and it is more heterogeneous and generalizeable with respect to important demographics and clinical characteristics, namely sex and stimulant treatment history, respectively. Regarding sex, because ADHD is diagnosed 3–5 times more frequently among boys than girls (see Arnold, 1996), we recruited a sample to match this distribution but was not powered to detect sex effects; hence the null results in supplementary analyses of sex effects are not taken as convincing evidence of the absence of such effects.
Regarding stimulant treatment history, it was important to recruit a sample that was more varied in clinical response to stimulant treatment. In the current study, a small number of children (n=7) had never taken stimulant medication before completing this short-term study. Results of exploratory analyses of stimulant-naïve compared to children with a history of stimulant treatment suggested that the findings of Hawk et al. (2003) were not driven by “responder” status. Although the higher dose of MPH (nearest OROS-MPH equivalent of .6 mg/kg MPH IR t.i.d.) enhanced PPI to during attended tones regardless of stimulant treatment history, the lower dose (nearest equivalent of .3 mg/kg t.i.d.) significantly enhanced PPI during attended tones only among children who were naïve to stimulant medication. Although the sample size of stimulant naïve children was quite small and the results should be interpreted cautiously, the findings are certainly not consistent with speculation that MPH effects on PPI would be present only among those with a positive clinical response and ongoing stimulant treatment. Overall, it appears that acute MPH improves PPI during attended prestimuli among a relatively broad range of children (and perhaps adults; see Hanlon et al., 2009) with ADHD.
Together, these data suggest that MPH alleviates deficits in the ability to suppress distracting stimuli in ADHD. Although typically described as a measure of early selective attention (e.g., Dawson et al., 1997) or selective inhibition (e.g., Hawk et al., 2003), PPI during the tone discrimination paradigm may reflect interference control, which incorporates both attention and inhibition constructs (Barkley, Murphy, & Fischer, 2007). Evidence regarding the construct validity of PPI and its attentional modification is needed (see, e.g., Filion, Kelly, & Hazlett, 1999) in both adults and children, and we will directly address this issue in a larger sample of children with ADHD.
At present, there are mixed results regarding the effects of MPH on interference control. For example, Scheres and colleagues (2003) found that MPH marginally enhanced performance on the Flanker task but had no effect on Stroop interference. However, Langleben et al. (2006) found that MPH reduced interference on a Stroop task in children with ADHD. One possibility for understanding these mixed findings is that there are key moderators at play. The present work focused on one such moderator that is also relevant to the broader PPI and psychopharmacology literature, baseline startle modulation.
Work with nonclinical samples in passive PPI paradigms suggests that the degree, and even the direction, of medication effects on PPI may be a function of the amount of PPI observed at baseline (Baschnagel & Hawk, 2008; Bitsios et al., 2005; Swerdlow et al., 2003; Talledo et al., 2009). The present data extend this to those with psychopathology. Specifically, supplemental analyses suggested that the MPH enhancement of PPI during attended tones was strongest for those who exhibited poorest PPI to attended tones during the baseline session. Importantly, as Swerdlow et al. (2008) noted, the presence of an actual baseline session is critical for demonstrating such effects while ruling out regression to the mean (c.f., Baschnagel & Hawk, 2008, for a more limited statistical approach). Given the heterogeneity of disorders such as ADHD and schizophrenia, not to mention healthy controls, true baselines seem well worth the trouble in studies of treatment effects on PPI. Of course, the finding observed here was only marginally statistically significant, and replication of the effect in ADHD will be important.
The baseline effect also provides one perspective on how the present findings fit in with the broader literature on the psychopharmacology of PPI. That is, although DA agonists such as amphetamine and apomorphine typically disrupt passive PPI (Braff et al., 2001b; Swerdlow et al., 2001), this may depend upon where participants fall along the PPI continuum. Specifically, several studies of adults now suggest that the disruptive effects of DA agonists on passive PPI are greater among those with high levels of baseline PPI. At the other end of the spectrum, persons with low baseline PPI exhibit trends towards improvement in PPI (e.g., Bitsios et al., 2005; Talledo et al., 2009). Children with ADHD show reduced PPI during attended tones, compared to typically developing children (Hawk et al., 2003), perhaps putting them in a range that is consistent with improved PPI with DA agonists. Even more generally consistent with the baseline perspective is the present finding that the enhancement of PPI with MPH during attended tones appears to is inversely related to the amount of PPI exhibited during attended tones in a true baseline session. Such individual differences in baseline PPI may, in turn, reflect variation in resting DA tone (Bitsios & Giakoumaki, 2005; Roussos et al., 2008; Talledo et al., 2009).
Alternatively, the fact that MPH also prevents reuptake of norepinephrine may be important for interpreting the present findings. Animal studies suggest that PPI is also sensitive to noradrenergic regulation (Swerdlow, Bongiovanni, Tochen, & Shoemaker, 2006). Both clonidine, an alpha-2 agonist (Sallinen, Haapalinna, Viitamaa, Kobilka, & Scheinin, 1998; Swerdlow et al., 2006) and atomoxetine, a selective NE-reuptake inhibitor used to treat ADHD (Gould et al., 2005) enhance passive PPI in animals. Of course, it is difficult to generalize across paradigms and species. Future research should examine more selective therapeutic agents in order to better elucidate the relative contributions of dopamine and norepinephrine to PPI enhancement in ADHD.
The differences between the passive (no task) paradigms used in most pharmacologic studies, particularly in non-humans, and the active (tone discrimination) PPI paradigm used here are important to consider in efforts to integrate the literature. Passive PPI is not reduced in ADHD (Castellanos et al., 1996; Ornitz et al., 1992), and a similar pattern has been observed for ignored stimuli in an active paradigm (Hawk et al., 2003). However, results from the two paradigms are not necessarily interchangeable. ‘Ignored’ stimuli must be processed to determine that they can be ignored, and even subsequent ‘ignoring’ may involve active processes that influence PPI (e.g., Filion & Poje, 2003; Hawk, Pelham, & Yartz, 2002; c.f., Jennings, Schell, Filion, & Dawson, 1996; Thorne, Dawson, & Schell, 2005). Analogues to active paradigms are just beginning to emerge (e.g., Baschnagel, Hawk, Colder, & Richards, 2007; see Li, Du, Li, Wu, & Wu, 2009, for a review; Roskam & Koch, 2006). Ultimately, research using both active and passive paradigms in both species will be necessary to fully elucidate the psychological and neurobiological processes in PPI and its top-down regulation.
Stepping back from task parameters, the present data add to a very small but important literature regarding controlled medication effects on PPI among persons with mental disorders. This literature is important because many of the hundreds of human and animal studies of PPI over the past few decades have been aimed at modeling and understanding psychological and neurobiological processes involved in psychopathology. The small but growing literature on baseline effects discussed above suggests that it will be quite important to actually include studies of those with the relevant psychopathology. Yet, the literature on controlled within-subjects medication studies of patient populations is remarkably small, including just one schizophrenia study (see review by Swerdlow et al., 2008; Wynn et al., 2007) and now two studies of ADHD (Hawk et al., 2003; present study). Though such studies are challenging to complete, they are critical for realizing the full translational promise of PPI.
At the clinical end of the spectrum, the present findings are consistent with, but insufficient for testing directly, the hypothesis that MPH exerts its therapeutic effects, at least in part, by enhancing the ability to screen out distracting stimuli and attend to relevant stimuli. We will soon test this mediational hypothesis in a larger sample.
In sum, acute treatment with the stimulant methylphenidate enhanced PPI of startle, but only during attended/task-relevant stimuli, among children with ADHD. This effect tended to be greatest among those children with the weakest baseline PPI, extending preclinical dimensional work on the psychopharmacology of PPI. The present data fit nicely within the literature on the effects of stimulants on attention and inhibition. Further work is needed to elucidate the neurobiological basis, construct validation, and clinical implications of stimulant enhancement of PPI during attended prestimuli in ADHD.
Acknowledgments
The authors thank Brian Gangloff, Sarah Spencer, Rebecca Mazzullo, Amanda Krol, Michael Strand, Jacob Via, and Joyce Mixson for assisting with data collection and providing comments on an earlier draft. The authors thank Mark Kutgowski for assisting with computer programming. We also express our gratitude to the children and families who participated in this study. This research was supported by grants R01MH069434 from the National Institute of Mental Health (LWH, PI) and by F31DA024532 (to RLA) from the National Institute on Drug Abuse.
Footnotes
Because analyses involving PPI were primary, PPF results are summarized here. As expected long-lead PPF was significantly greater during attended compared to ignored stimuli, F(1,35) = 9.0, p < .01. Although this attentional modification of long-lead PPF was significant under active medication (means = 104% and 43% for attended and ignored, respectively; p < .01), but not under placebo (60% and 38%, p = .18), the interaction was marginal, F(1,35) = 3.0, p = .09.
Financial Disclosures
All authors report no biomedical financial interest or potential conflicts of interest.
References
- American Psychiatric, A. Diagnostic and statistical manual of mental disorders (DSM-IV-TR) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Arnold LE. Sex differences in ADHD: Conference summary. Journal of Abnormal Child Psychology. 1996;24(5):555–569. doi: 10.1007/BF01670100. [DOI] [PubMed] [Google Scholar]
- Ashare RL, Hawk LW, Mazzullo RJ. Motivated attention: Incentive effects on attentional modification of prepulse inhibition. Psychophysiology. 2007;44(6):839–845. doi: 10.1111/j.1469-8986.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi VP, Swerdlow NR, Geyer MA. Clozapine antagonizes phencyclidine-induced deficits in sensorimotor gating of the startle response. J Pharmacol Exp Ther. 1994;271(2):787–794. [PubMed] [Google Scholar]
- Balaban MT, Losito BDG, Simons RF, Graham FK. Off-line latency and amplitude scoring of the human reflex eye blink with Fortran IV [abstract] Psychophysiology. 1986;23:612. [Google Scholar]
- Barkley Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Murphy KR, Fischer M. ADHD in Adults: What the Science Tells Us. New York: Guilford; 2007. [Google Scholar]
- Baschnagel JS, Hawk LW, Jr, Colder CR, Richards JB. Motivated attention and prepulse inhibition of startle in rats: using conditioned reinforcers as prepulses. Behavioral neuroscience. 2007;121(6):1372–1382. doi: 10.1037/0735-7044.121.6.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baschnagel JS, Hawk LW., Jr The effects of nicotine on the attentional modification of the acoustic startle response in nonsmokers. Psychopharmacology (Berl) 2008;198(1):93–101. doi: 10.1007/s00213-008-1094-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitsios P, Giakoumaki SG. Relationship of prepulse inhibition of the startle reflex to attentional and executive mechanisms in man. International Journal of Psychophysiology. 2005;55:229–241. doi: 10.1016/j.ijpsycho.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Bitsios P, Giakoumaki SG, Frangou S. The effects of dopamine agonists on prepulse inhibition in healthy men depend on baseline PPI values. Psychopharmacology (Berl) 2005;182(1):144–152. doi: 10.1007/s00213-005-0056-x. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Archives of General Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001a;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001b;156(2–3):234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Fine EJ, Kaysen D, Marsh WL, Rapoport JL, Hallett M. Sensorimotor gating in boys with Tourette's syndrome and ADHD: preliminary results. Biol Psychiatry. 1996;39(1):33–41. doi: 10.1016/0006-3223(95)00101-8. [DOI] [PubMed] [Google Scholar]
- Cook EWI, Atkinson LS, Lang KG. Stimulus control and data acquisition for IBM PCs and compatibles. Psychophysiology. 1987;24:726–727. [Google Scholar]
- Dawson ME, Schell AM, Swerdlow NR, Filion DL. Cognitive, clinical, and neurophysiological implications of startle modification. Attention and orienting: Sensory and motivational processes. 1997:257–279. [Google Scholar]
- Fabiano GA, Pelham JWE, Waschbusch DA, Gnagy EM, Lahey BB, Chronis AM, et al. A Practical Measure of Impairment: Psychometric Properties of the Impairment Rating Scale in Samples of Children With Attention Deficit Hyperactivity Disorder and Two School-Based Samples. Journal of Clinical Child & Adolescent Psychology. 2006;35(3):369–385. doi: 10.1207/s15374424jccp3503_3. [DOI] [PubMed] [Google Scholar]
- Feifel D, Minassian A, Perry W. Prepulse inhibition of startle in adults with ADHD. J Psychiatr Res. 2008 doi: 10.1016/j.jpsychires.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filion DL, Dawson ME, Schell AM. Modification of the acoustic startle-reflex eyeblink: A tool for investigating early and late attentional processes. Biological Psychology. 1993;35:185–200. doi: 10.1016/0301-0511(93)90001-o. [DOI] [PubMed] [Google Scholar]
- Filion DL, Dawson ME, Schell AM. The psychological significance of human startle eyeblink modification: A review. Biological Psychology. 1998;47:1–43. doi: 10.1016/s0301-0511(97)00020-3. [DOI] [PubMed] [Google Scholar]
- Filion DL, Kelly KA, Hazlett EA. Behavioral Analogies of Short Lead Interval Startle Inhibition. In: Dawson ME, Schell AM, Bohmelt AH, editors. Startle Modification: Implications for neuroscience, cognitive science, and clinical science. New York: Cambridge University Press; 1999. pp. 269–283. [Google Scholar]
- Filion DL, Poje AB. Selective and nonselective attention effects on prepulse inhibition of startle: a comparison of task and no-task protocols. Biological Psychology. 2003;64(3):283–296. doi: 10.1016/s0301-0511(03)00077-2. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156(2):117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Rukstalis M, Lewis MC. Atomoxetine and nicotine enhance prepulse inhibition of acoustic startle in C57BL/6 mice. Neuroscience Letters. 2005;377(2):85–90. doi: 10.1016/j.neulet.2004.11.073. [DOI] [PubMed] [Google Scholar]
- Graham FK. The more or less startling effects of weak prestimulation. Psychophysiology. 1975;12:238–248. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Greenhill LL, Pliszka S, Dulcan MK, Bernet W, Arnold V, Beitchman J, et al. American Academy of Child and Adolescent Psychiatry. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry. 2002;41(2 Suppl):S26–S49. doi: 10.1097/00004583-200202001-00003. [DOI] [PubMed] [Google Scholar]
- Hanlon M-C, Karayanidis F, Schall U. Intact sensorimotor gating in adult attention deficit hyperactivity disorder. The International Journal of Neuropsychopharmacology. 2009;12(05):701–707. doi: 10.1017/S1461145708009711. [DOI] [PubMed] [Google Scholar]
- Hawk LW, Jr, Yartz AR, Pelham WE, Jr, Lock TM. The effects of methylphenidate on prepulse inhibition during attended and ignored prestimuli among boys with attention-deficit hyperactivity disorder. Psychopharmacology (Berl) 2003;165(2):118–127. doi: 10.1007/s00213-002-1235-7. [DOI] [PubMed] [Google Scholar]
- Hawk LWJR, Pelham WEJR, Yartz AR. Attentional modification of short-lead prepulse inhibition and long-lead prepulse facilitation of acoustic startle among preadolescent boys. Psychophysiology. 2002 May;39:333–339. doi: 10.1017/s0048577201393071. 2002. [DOI] [PubMed] [Google Scholar]
- Jennings PD, Schell AM, Filion DL, Dawson ME. Tracking early and late stages of information processing: Contributions of startle eyeblink reflex modification. Psychophysiology. 1996;33:148–155. doi: 10.1111/j.1469-8986.1996.tb02118.x. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Fein D, Kramer J, Delis D, Morris R. WISC-IV Integrated. San Antonio, TX: The Psychological Corporation. 2004 [Google Scholar]
- Langleben DD, Monterosso J, Elman I, Ash B, Krikorian G, Austin G. Effect of methylphenidate on Stroop Color-Word task performance in children with attention deficit hyperactivity disorder. Psychiatry Research. 2006;141(3):315–320. doi: 10.1016/j.psychres.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Lansbergen MM, Kenemans JL, van Engeland H. Stroop interference and attention-deficit/hyperactivity disorder: a review and meta-analysis. Neuropsychology. 2007;21(2):251–262. doi: 10.1037/0894-4105.21.2.251. [DOI] [PubMed] [Google Scholar]
- Li L, Du Y, Li N, Wu X, Wu Y. Topñdown modulation of prepulse inhibition of the startle reflex in humans and rats. Neuroscience and Biobehavioral Reviews. 2009 doi: 10.1016/j.neubiorev.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Lijffijt M, Kenemans JL, Wal A, Quik EH, Kemner C, Westenberg H, et al. Dose-related effect of methylphenidate on stopping and changing in children with attention-deficit/hyperactivity disorder. European Psychiatry. 2006;21(8):544–547. doi: 10.1016/j.eurpsy.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Losier BJ, McGrath PJ, Klein RM. Error Patterns on the Continuous Performance Test in Non-Medicated and Medicated Samples of Children With and Without ADHD: A Meta-Analytic Review. Journal of Child Psychology and Psychiatry. 1996;37(8):971–987. doi: 10.1111/j.1469-7610.1996.tb01494.x. [DOI] [PubMed] [Google Scholar]
- Nigg JT. On Inhibition/Disinhibition in Developmental Psychopathology: Views From Cognitive and Personality Psychology and a Working Inhibition Taxonomy. Psychological Bulletin. 2000;126(2):220–246. doi: 10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJS. Causal Heterogeneity in Attention-Deficit/Hyperactivity Disorder: Do We Need Neuropsychologically Impaired Subtypes? Biological Psychiatry. 2005;57(11):1224–1230. doi: 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Oosterlaan J, Logan GD, Sergeant JA. Response Inhibition in AD/HD, CD, Comorbid AD/HD+ CD, Anxious, and Control Children: A Meta-analysis of Studies with the Stop Task. The Journal of Child Psychology and Psychiatry and Allied Disciplines. 1998;39(03):411–425. [PubMed] [Google Scholar]
- Ornitz EM, Hanna GL, Traversay J. Prestimulation-Induced Startle Modulation in Attention-Deficit Hyperactivity Disorder and Nocturnal Enuresis. Psychophysiology. 1992;29(4):437–451. doi: 10.1111/j.1469-8986.1992.tb01717.x. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Fabiano GA, Massetti GM. Evidence-based assessment of attention deficit hyperactivity disorder in children and adolescents. J Clin Child Adolesc Psychol. 2005;34(3):449–476. doi: 10.1207/s15374424jccp3403_5. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Greenslade KE, Milich R. Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:210–218. doi: 10.1097/00004583-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Pelham WE., Jr Pharmacotherapy for Children with Attention-Deficit Hyperactivity Disorder. School Psychology Review. 1993;22(2):199–227. [Google Scholar]
- Pliszka S. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(7):894. doi: 10.1097/chi.0b013e318054e724. [DOI] [PubMed] [Google Scholar]
- Riccio CA, Waldrop JJM, Reynolds CR, Lowe P. Effects of Stimulants on the Continuous Performance Test (CPT): Implications for CPT Use and Interpretation. J Neuropsychiatry Clin Neurosci. 2001;13(3):326–335. doi: 10.1176/jnp.13.3.326. [DOI] [PubMed] [Google Scholar]
- Roskam S, Koch M. Enhanced prepulse inhibition of startle using salient prepulses in rats. International Journal of Psychophysiology. 2006;60(1):10–14. doi: 10.1016/j.ijpsycho.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Roussos P, Giakoumaki SG, Rogdaki M, Pavlakis S, Frangou S, Bitsios P. Prepulse inhibition of the startle reflex depends on the catechol O-methyltransferase Val158Met gene polymorphism. Psychological Medicine. 2008;38(11):1651–1658. doi: 10.1017/S0033291708002912. [DOI] [PubMed] [Google Scholar]
- Sallinen J, Haapalinna A, Viitamaa T, Kobilka BK, Scheinin M. Adrenergic alpha 2C-Receptors Modulate the Acoustic Startle Reflex, Prepulse Inhibition, and Aggression in Mice. Journal of Neuroscience. 1998;18(8):3035. doi: 10.1523/JNEUROSCI.18-08-03035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres A, Oosterlaan J, Swanson J, Morein-Zamir S, Meiran N, Schut H, et al. The Effect of Methylphenidate on Three Forms of Response Inhibition in Boys with AD/HD. Journal of Abnormal Child Psychology. 2003;31(1):105–120. doi: 10.1023/a:1021729501230. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Swerdlow N, Geyer M, Braff D. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001;156(2):194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Bongiovanni MJ, Tochen L, Shoemaker JM. Separable noradrenergic and dopaminergic regulation of prepulse inhibition in rats: implications for predictive validity and Tourette Syndrome. Psychopharmacology (Berl) 2006;186(2):246–254. doi: 10.1007/s00213-006-0374-7. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Wasserman LC, Talledo JA, Casas R, Bruins P, Stephany NL. Prestimulus modification of the startle reflex: relationship to personality and physiological markers of dopamine function. Biological Psychology. 2003;62(1):17–26. doi: 10.1016/s0301-0511(02)00090-x. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology. 2008;199(3):331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talledo JA, Sutherland Owens AN, Schortinghuis T, Swerdlow NR. Amphetamine effects on startle gating in normal women and female rats. Psychopharmacology. 2009;204(1):165–175. doi: 10.1007/s00213-008-1446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock R, Schachar R, Logan G. Methylphenidate and cognitive flexibility: Dissociated dose effects in hyperactive children. Journal of Abnormal Child Psychology. 1995;23(2):235–266. doi: 10.1007/BF01447091. [DOI] [PubMed] [Google Scholar]
- Thorne GL, Dawson ME, Schell AM. Attention and prepulse inhibition: the effects of task-relevant, irrelevant, and no-task conditions. International Journal of Psychophysiology. 2005;56(2):121–128. doi: 10.1016/j.ijpsycho.2004.11.006. [DOI] [PubMed] [Google Scholar]
- van Boxtel A, Boelhouwer AJ, Bos AR. Optimal EMG signal bandwidth and interelectrode distance for the recording of acoustic, electrocutaneous, and photic blink reflexes. Psychophysiology. 1998;35(6):690–697. [PubMed] [Google Scholar]
- Wynn JK, Green MF, Sprock J, Light GA, Widmark C, Reist C, et al. Effects of olanzapine, risperidone and haloperidol on prepulse inhibition in schizophrenia patients: A double-blind, randomized controlled trial. Schizophrenia Research. 2007;95(1–3):134–142. doi: 10.1016/j.schres.2007.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuvekas SH, Vitiello B, Norquist GS. Recent Trends in Stimulant Medication Use Among US Children. Vol. 163. Am Psychiatric Assoc.; 2006. pp. 579–585. [DOI] [PubMed] [Google Scholar]