Abstract
Background
US and international agencies have signaled their commitment to containing the HIV epidemic via early case identification and linkage to antiretroviral therapy (ART) immediately upon diagnosis. We forecast outcomes of this approach if implemented in Washington DC.
Methods
Using a mathematical model of HIV case detection and treatment, we evaluate combinations of HIV screening and ART initiation strategies. We define current practice as no regular screening program and ART at ≤350/μl, and test and treat as annual screening and ART upon diagnosis. Outcomes include life expectancy of HIV-infected persons and changes in the population time with transmissible HIV RNA. Data, largely from DC, include undiagnosed HIV prevalence 0.6%, annual incidence 0.13%, 31% test offer, 60% acceptance, and 50% linkage to care. Input parameters, including optimized ART efficacy, are varied in sensitivity analyses.
Results
Projected life expectancies, from an initial mean age 41 years, for current practice, test and treat, and test and treat with optimized ART are 23.9, 25.0, and 25.6 years. Compared to current practice, test and treat leads to a 14.7% reduction in time spent with transmissible HIV RNA in the next 5 years; test and treat with optimized ART results in a 27.2% reduction.
Conclusions
An expanded HIV test and treat program in Washington DC will increase life expectancy of HIV-infected patients but will have a modest impact on HIV transmission over the next five years and is unlikely to halt the HIV epidemic.
Summary
The CEPAC model shows a test and treat strategy in Washington DC would result in a substantial clinical impact to HIV-infected individuals. Results suggest a need to temper expectations regarding the extent to which test and treat will control the epidemic.
Keywords: HIV, Test and Treat, Washington DC, HIV screening
INTRODUCTION
Recent reports from the District of Columbia (DC) Department of Health describe an HIV epidemic in Washington comparable to that in East Africa [1, 2]. Three percent of adults in the US capital are known to be living with HIV; many more remain undiagnosed and unable to obtain either lifesaving care or counseling to reduce the spread of infection [1].
In response to the continuing spread of HIV in the US and internationally, both the US National Institutes of Health (NIH) and the World Health Organization (WHO) have committed to implementing and evaluating universal testing and treatment for prevention of HIV infection [3, 4]. This comprehensive approach aims to benefit infected individuals through early detection while decreasing their subsequent HIV transmission by lowering community levels of HIV RNA [5]. The approach is motivated by the persistent evidence that HIV prevention efforts to date have not controlled HIV transmission, with the view that combinations of interventions will be required to contain the epidemic [6]. A recent joint announcement from the Washington DC Department of Health and the NIH highlights a piloting of a Test-and-Treat strategy as part of a new, $26.4 million 2-year partnership [7].
This paper aims to assist in the evaluation of a test and treat strategy. We use a widely published computer model of HIV infection to project how program performance – including overall rates of test offer, acceptance, and linkage to care – will affect both individual patient and population-wide outcomes in Washington DC.
METHODS
Analytic Overview
We use the Cost-effectiveness of Preventing AIDS Complications (CEPAC) Model of HIV screening and treatment to evaluate the impact of alternative HIV screening and treatment strategies. We examine eight strategies, including current screening practice without regular screening and without ART (for comparison); no regular screening with ART initiation at CD4 ≤ 350/μl; and all six combinations of the following: 1) routine HIV screening once, every 3 years, or annually; and 2) ART starting at CD4 ≤ 350/μl or immediately upon diagnosis. We define current practice as no regular screening combined with ART at CD4 ≤ 350/μl; we define the test and treat strategy as annual screening with ART initiation at HIV diagnosis. In a final, ninth strategy, we examine the impact of test and treat under optimized ART regimens, with improved adherence and suppression efficacies higher than those reported in clinical trials.
For each strategy, we examine several scenarios of screening performance, characterized by different probabilities of test offer, test acceptance and linkage to care for newly-identified HIV cases. Model simulations result in projections of life expectancy in HIV-infected individuals as well as their mean CD4 count at detection. We also report the population impact of each strategy -- measured over a 5-year horizon -- by the amount of HIV-infected population time spent with a transmissible HIV RNA (>500 copies/ml).
The Cost-effectiveness of Preventing AIDS Complications (CEPAC) Model
The CEPAC model is a mathematical simulation model that projects the clinical course of HIV disease and the epidemiological trajectory of infection, based on alternative assumptions about the time of HIV detection and treatment initiation [8–11]. The model, composed of screening and disease modules, is used to consider how a program of accelerated detection and immediate ART might suppress HIV RNA among infected patients, thereby slowing the progress of their own HIV disease and decreasing its transmission to others.
The Screening Module
In the CEPAC model, HIV-infection may be detected or undetected. However, HIV-infected patients are only eligible for HIV-related care and ART upon successful disease detection and linkage to care. Without detection and linkage, infected patients progress with untreated HIV disease. The screening module is used to determine when and how HIV-infected patients become diagnosed, via one of three mechanisms: 1) “background” screening (as currently occurs in a variety of settings in the US); 2) development of an AIDS-related opportunistic infection; or 3) a routine HIV screening program. Current practice (without regular HIV screening) is defined as detection via mechanisms 1 and 2, but not 3. We assume that detection via mechanisms 1 and 2 leads invariably to HIV care, whereas there may be imperfect linkage and loss to follow-up among patients detected via mechanism 3. This leads to conservative estimates with respect to the value of a regular screening program. To describe the characteristics required for completion of a routine screening program, we define the performance index for a screening program as that program’s joint probability of test offer, test acceptance and linkage to care for each encounter.
Among those with HIV infection, demographic and clinical characteristics – including age, sex, CD4 count, and HIV RNA level – are defined as representative of prevalent HIV infection as reported from Washington DC. The CEPAC model determines if and when new cases of HIV infection occur based on user-specified incidence rates and the demographic characteristics of the Washington DC population. Further details of the screening module have been previously published [11–13].
The Disease Module
In the disease module, HIV-infected patients are characterized by health states defined by current CD4 count and HIV RNA; transition between health states occurs in monthly cycles. In the absence of HIV case detection and treatment, HIV-infected patients follow a trajectory of HIV RNA-dependent monthly CD4 decline, resulting in increased risk of opportunistic infections and HIV-related mortality [8, 10]. Patients also face risk of death from age-, sex- and race-adjusted background mortality [1, 14, 15].
Patients identified with HIV infection and successfully linked to care are eligible to start ART and opportunistic infection prophylaxis, if initiation criteria are met [16, 17]. We consider two ART initiation strategies: current practice with ART initiation at CD4 ≤ 350/μl; and ART at diagnosis, regardless of CD4 count. Successful ART leads to HIV RNA suppression (HIV RNA ≤ 500 copies/ml) and a concomitant CD4 increase at rates reported in clinical studies [18–24]. With treatment failure and HIV RNA rebound, a subsequent ART regimen is initiated. The model specifies four highly efficacious ART regimens followed by two late salvage regimens with poorer suppressive efficacies [18–24].
Calculating Community Viral Load and Transmissible HIV RNA
The model records each patient’s total HIV uninfected life-months as well as time spent in each HIV RNA stratum when infected (>100,000 copies/ml, 30,001–100,000 copies/ml; 10,001–30,000 copies/ml; 3,001–10,000 copies/ml; 501–3,000 copies/ml; 0–500 copies/ml). These values are aggregated over large numbers of individual patient simulations to project expected time spent with transmissible HIV RNA (i.e., >500 copies/ml or >3,000 copies/ml) over a defined time horizon (e.g five years) [25]. This distribution of total time in each HIV RNA stratum is defined as the “community viral load” [26].
Input Parameters (Table 1)
TABLE 1.
Model input parameters to examine an HIV test and treat strategy in Washington DC.
| Variable | Base Case Value (SD) | Range Examined | Reference |
|---|---|---|---|
| Baseline cohort characteristics | |||
| Undiagnosed HIV prevalence (%)* | |||
| Total | 0.6 | 0.2–3.0% | [1], [33] |
| Asymptomatic, chronic HIV+ | 0.49 | [11, 27] | |
| Symptomatic, chronic HIV+ | 0.1 | [11, 27] | |
| Acute, primary HIV infection | 0.008 | [11, 27] | |
| Annual HIV incidence (%) | 0.13 | 0.04–0.13 | Estimated, [32] |
| Age, mean years ± SD | 41 ± 10.3 | [1] | |
| Sex | [1] | ||
| Male (%) | 46.6 | ||
| Race/Ethnicity | [1] | ||
| White (%) | 36.0 | ||
| Black (%) | 55.6 | ||
| Hispanic (%) | 8.4 | ||
| Distribution of initial CD4, mean cells/μl (SD) | |||
| Acute, primary HIV infection‡ | 534 (164) | [31] | |
| Chronic HIV infection§ | 262 (70) | 183-332 | [1] |
| Baseline Cohort Characteristics | |||
| HIV RNA distribution in chronic HIV infection (%) | [35] | ||
| >100,000 copies/ml | 0.0 | ||
| 30,001–100,000 copies/ml | 25.7 | ||
| 10,001–30,000 copies/ml | 25.0 | ||
| 3,001–10,000 copies/ml | 25.2 | ||
| 501–3,000 copies/ml | 16.3 | ||
| <500 copies/ml | 7.7 | ||
| HIV testing protocols | |||
| Average background HIV test frequency | Every 5 years | [11] | |
| Sensitivity† (%) | 99.6 | [12] | |
| Specificity† (%) | 97.5 | [12] | |
| Test offer probability (%) | 31 | 30–100 | [29] |
| Test acceptance probability (%) | 60 | 30–100 | [29] |
| Probability of HIV-infected return for test results and linkage to care (%) | 50 | 50–100 | [29] |
SD: Standard deviation
Undiagnosed HIV infection is calculated from the reported HIV prevalence in Washington DC (3%) and multiplied by the CDC reported estimate of undiagnosed cases to diagnosed cases (21%) [1, 28, 33, 40]. Relative frequencies of acute, asymptomatic and symptomatic cases are calculated on a 12-year natural history timeline, assuming 2 months are spent with acute infection, 2 years with symptomatic disease (AIDS) and the remaining time with asymptomatic, chronic disease [11, 27].
Sensitivity and specificity refer to the characteristics of a single rapid test, not the confirmatory process; test sensitivity is assumed to be zero during the acute infection window period (approximately 2 months).
Starting CD4 cell count for incident cases
Starting CD4 cell count, on average, for prevalent cases
Population characteristics of the HIV-infected cohort are derived from the District of Columbia, HIV/AIDS Epidemiology Update 2008 [1]. We use the reported 3% diagnosed HIV prevalence, and apply recent Centers for Disease Control and Prevention (CDC) estimates – that 21% of all HIV-infected cases in the US are undiagnosed – to obtain an undiagnosed HIV prevalence of 0.6% [33]. We rely upon traditional models of infectious disease dynamics to estimate an HIV annual incidence of 0.13% [32]. The Washington DC population mean age is 41 years, 46.6% are male, 55.6% are Black; at simulation initiation, the prevalent HIV-infected undiagnosed population has a mean CD4 count of 262/μl. Background mortality rates reflect the demographics of the Washington DC population [1, 14, 15].
We assume that screening programs use rapid HIV testing (99.6% sensitivity, 97.5% specificity); reactive tests are followed by Western blot confirmation [12, 34]. In the base case, we use reported data from a successful Washington DC emergency department routine HIV screening experience where per encounter probabilities included: 31% test offer, 60% acceptance, and 50% linkage to care, resulting in a program-based performance index of 9.4% [29]. In sensitivity analysis, we also examine a hypothetical “optimistic” scenario of intensified efforts to scale up screening participation with 80% offer; 60% acceptance; and 80% linkage to care for those identified, resulting in a performance index of 38% (i.e., 80%*60%*80%). For comparative purposes, we additionally consider an “idealized” program with 90% offer, 90% acceptance, and 90% linkage to care (performance index = 73%).
Data on the natural history of disease, including CD4 count decline by HIV RNA stratum, and CD4-defined risks of opportunistic infections have been previously described [8, 9, 30, 35]. ART-eligible patients initiate a treatment regimen with efficacies representative of those reported in the literature [18–24]. Four sequential regimens, ranging in rates of virologic suppression at 24 weeks of 60–86%, are available, each resulting in a 100–190 cells/μl immunologic benefit at 48 weeks [18–20, 23, 24]. Upon exhaustion of these four highly effective regimens, two late salvage regimens are also available [21, 22]. Because a test and treat strategy may focus attention on adherence to improve ART efficacy, we also examined an “optimized ART” strategy by increasing virologic suppression rates of each regimen by 15% [36].
RESULTS
Base Case Results
Clinical Impact of Test and Treat on HIV-infected Individuals
Among prevalent HIV-infected cases, the CD4 counts at detection range from 162/μl with current practice to 180/μl with annual screening (Table 2a). Among those with incident infections, more frequent screening increases mean CD4 count at detection from 352/μl (current practice) to 388/μl (annual screening). In an HIV-infected population with mean age 41 years, per person life expectancy ranges from 23.9 years under current practice (no additional screen/ART at CD4 ≤ 350/μl) to 25.0 years with the test and treat strategy (annual testing/ART at diagnosis). With an ART starting criterion of CD4 ≤ 350/μl, increasing screening frequency from once to every 3 years to annually improves life expectancy in those HIV-infected by 0.08, 0.3, and 0.8 years, each compared to no regular screen. With identical screening frequencies, per person life expectancy increases are 0.3–0.4 years for ART at diagnosis versus ART at CD4 ≤ 350/μl.
TABLE 2a.
Base case results for screening and treatment strategies in Washington DC.
| Strategy (Performance index = 9.4%)* |
CD4 at detection (cells/μl) Prevalent cases | CD4 at detection (cells/μl) Incident cases | HIV-infected Life expectancy (years) | Total HIV- infected follow-up time at 5 yr|| | Follow-up time yrs (%) with HIV RNA >500 copies/ml (5yr) | % reduction in time with transmissible viral load (5yr)‡ | |
|---|---|---|---|---|---|---|---|
| Test frequency | ART initiation (CD4 count) | ||||||
| No regular screen | No ART | 162 | 352 | 15.8 | 8,850 | 8,150 (92.1%) | +25.6 |
| † No regular screen | ≤ 350/μl | 162 | 352 | 23.9 | 10,100 | 6,500 (64.3%) | -- |
| Once | ≤ 350/μl | 171 | 352 | 24.0 | 10,140 | 6,370 (62.8%) | −1.9 |
| Every 3 years | ≤ 350/μl | 173 | 366 | 24.2 | 10,140 | 6,320 (62.4%) | −2.5 |
| Annually | ≤ 350/μl | 180 | 388 | 24.7 | 10,180 | 6,170 (60.6%) | −4.9 |
| Once | At diagnosis | 171 | 352 | 24.3 | 10,150 | 5,860 (57.8%) | −9.6 |
| Every 3 years | At diagnosis | 173 | 366 | 24.6 | 10,160 | 5,780 (56.8%) | −11.0 |
| † Annually | At diagnosis | 180 | 388 | 25.0 | 10,210 | 5,530 (54.2%) | −14.7 |
| Annually | § Optimized ART at diagnosis | 180 | 388 | 25.6 | 10,280 | 4,720 (46.0%) | −27.3 |
Performance index is the per encounter joint probability of test offer*accept*linkage to care. In part a, the performance index is 9.4%, derived from test offer 31%, test acceptance 60%, and linkage to care 50% [29]. In part b, the performance index is 38%, derived from test offer 80%, test acceptance 60%, and linkage to care 80%. In part c, the performance index is 73%, derived from 90% test offer, test acceptance 90%, and linkage to care 90%. See methods for details.
We assume that the no regular screen and ART initiation at CD4 ≤ 350/μl strategy represents current practice. The test and treat strategy is represented by annual screen and ART upon diagnosis. These two strategies are presented in bold.
The percent reduction in time spent with HIV RNA >500 copies/ml is computed in comparison to current practice (i.e., the no regular screen and ART initiation at CD4 ≤ 350/μl strategy), as reported in the second row of the table.
ART efficacy is under “optimized ART” inflates, for all 6 ART regimens, virologic suppression by 15% above the base case (Table 1).
Follow-up time includes the entire HIV-infected population, both diagnosed and undiagnosed.
Community Viral Load and Transmissible HIV RNA
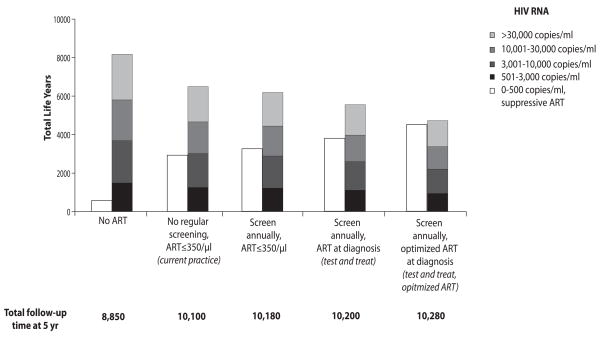
For the estimated 6,410 prevalent and incident cases expected over the next 5 years in Washington DC, the no regular screen/no ART strategy results in 8,150 life-years in the community with a transmissible viral load (HIV RNA >500 copies/ml, Table 2a, Figure 1). Current practice and test and treat lead to 6,500 and 5,530 life-years of transmissible viral load. Compared to current practice, test and treat decreases the proportion of time with transmissible viral load over a 5-year time horizon from 64.3% to 54.2% (Table 2a) and decreases the proportion of time with viral load over >3,000 copies/ml from 51.9% to 43.5% (data not shown). Over a 5-year horizon, the test and treat strategy, compared to current practice, offers a 14.7% reduction in overall population time spent with transmissible HIV RNA (Table 2a).
Figure 1.
Over a five-year time horizon in the base case, cumulative person-years spent at each viral load stratum (vertical axis) under five representative strategies (horizontal axis): no regular screen, no ART strategy; current practice; annual testing, ART at ≤350/μl; test and treat; and test and treat with optimized ART. For each strategy, all person-years spent with an HIV RNA >500 copies/ml are summed to create the colored bar. Within the colored bar, different shades represent total time spent within alternative HIV RNA strata. Total time spent on fully suppressive ART is indicated by the white bar. The sum of the colored and white bars yields the total HIV-infected life-years, reported at the bottom of each set.
Sensitivity Analyses
Test and Treat with Optimized ART
Test and treat with optimized ART increases projected life expectancy to 25.6 years, 0.6 years more than test and treat alone. Compared to current practice, test and treat with optimized ART leads to a 27.3% reduction in population time spent with transmissible infection over 5 years. (Table 2a, bottom; Figure 1).
Performance Index
Projected life expectancy and community viral load in the current practice strategy are unchanged as performance index varies. With 38% program performance, benefits to test and treat increase compared to the base case; projected life expectancy is 26.1 years, and 4,440 life-years (42.6% of time) are spent with transmissible HIV RNA (Table 2b). With 73% program performance, clinical and population benefits from test and treat improve; projected life expectancy is 26.6 years and, compared to current practice, there is a 43.9% reduction in overall population time spent with transmissible infection (Table 2c). Test and treat with optimized ART in this idealized scenario reduces time spent with transmissible HIV RNA by 65.1%, compared to current practice.
TABLE 2b.
Optimistic scenario
| Strategy (Performance index = 38%)* |
CD4 at detection (cells/μl) Prevalent cases | CD4 at detection (cells/μl) Incident cases | HIV-infected Life expectancy (years) | Total HIV- infected follow-up time at 5 yr|| | Follow-up time yrs (%) with HIV RNA >500 copies/ml (5yr) | % reduction in time with transmissible viral load (5yr)‡ | |
|---|---|---|---|---|---|---|---|
| Test frequency | ART initiation (CD4 count) | ||||||
| No regular screen | No ART | 162 | 352 | 15.8 | 8,870 | 8,160 (92.1%) | +25.8% |
| † No regular screen | ≤ 350/μl | 162 | 352 | 23.9 | 10,090 | 6,490 (64.3%) | -- |
| Once | ≤ 350/μl | 201 | 352 | 24.3 | 10,270 | 5,960 (58.1%) | −8.1 |
| Every 3 years | ≤ 350/μl | 204 | 401 | 25.1 | 10,290 | 5,820 (56.6%) | −10.3 |
| Annually | ≤ 350/μl | 220 | 449 | 25.8 | 10,400 | 5,450 (52.4%) | −16.0 |
| Once | At diagnosis | 201 | 352 | 24.6 | 10,300 | 5,420 (52.7%) | −16.4 |
| Every 3 years | At diagnosis | 204 | 401 | 25.4 | 10,310 | 5,110 (49.6%) | −21.2 |
| † Annually | At diagnosis | 220 | 449 | 26.1 | 10,410 | 4,440 (42.6%) | −31.7 |
| Annually | § Optimized ART at diagnosis | 220 | 449 | 29.2 | 10,490 | 3,320 (31.7%) | −48.8 |
Performance index is the per encounter joint probability of test offer*accept*linkage to care. In part a, the performance index is 9.4%, derived from test offer 31%, test acceptance 60%, and linkage to care 50% [29]. In part b, the performance index is 38%, derived from test offer 80%, test acceptance 60%, and linkage to care 80%. In part c, the performance index is 73%, derived from 90% test offer, test acceptance 90%, and linkage to care 90%. See methods for details.
We assume that the no regular screen and ART initiation at CD4 ≤ 350/μl strategy represents current practice. The test and treat strategy is represented by annual screen and ART upon diagnosis. These two strategies are presented in bold.
The percent reduction in time spent with HIV RNA >500 copies/ml is computed in comparison to current practice (i.e., the no regular screen and ART initiation at CD4 ≤ 350/μl strategy), as reported in the second row of the table.
ART efficacy is under “optimized ART” inflates, for all 6 ART regimens, virologic suppression by 15% above the base case (Table 1).
Follow-up time includes the entire HIV-infected population, both diagnosed and undiagnosed.
TABLE 2c.
Idealized scenario
| Strategy (Performance index = 73%)* |
CD4 at detection (cells/μl) Prevalent cases | CD4 at detection (cells/μl) Incident cases | HIV-infected Life expectancy (years) | Total HIV- infected follow-up time at 5 yr|| | Follow-up time yrs (%) with HIV RNA >500 copies/ml (5yr) | % reduction in time with transmissible viral load (5yr)‡ | |
|---|---|---|---|---|---|---|---|
| Test frequency | ART initiation (CD4 count) | ||||||
| No regular screen | No ART | 162 | 352 | 15.8 | 8,850 | 8,150 (92.0%) | +25.5 |
| † No regular screen | ≤ 350/μl | 162 | 352 | 23.9 | 10,100 | 6,500 (64.4%) | -- |
| Once | ≤ 350/μl | 234 | 352 | 24.6 | 10,450 | 5,490 (52.6%) | −15.4 |
| Every 3 years | ≤ 350/μl | 237 | 434 | 25.8 | 10,470 | 5,280 (50.4%) | −18.7 |
| Annually | ≤ 350/μl | 247 | 483 | 26.2 | 10,540 | 4,960 (47.1%) | −23.5 |
| Once | At diagnosis | 234 | 352 | 24.9 | 10,460 | 4,920 (47.0%) | −24.2 |
| Every 3 years | At diagnosis | 237 | 435 | 26.1 | 10,480 | 4,380 (41.8%) | −32.6 |
| † Annually | At diagnosis | 247 | 483 | 26.6 | 10,560 | 3,640 (34.5%) | −43.9 |
| Annually | § Optimized ART at diagnosis | 247 | 483 | 27.1 | 10,660 | 2,270 (21.3%) | −65.1 |
Performance index is the per encounter joint probability of test offer*accept*linkage to care. In part a, the performance index is 9.4%, derived from test offer 31%, test acceptance 60%, and linkage to care 50% [29]. In part b, the performance index is 38%, derived from test offer 80%, test acceptance 60%, and linkage to care 80%. In part c, the performance index is 73%, derived from 90% test offer, test acceptance 90%, and linkage to care 90%. See methods for details.
We assume that the no regular screen and ART initiation at CD4 ≤ 350/μl strategy represents current practice. The test and treat strategy is represented by annual screen and ART upon diagnosis. These two strategies are presented in bold.
The percent reduction in time spent with HIV RNA >500 copies/ml is computed in comparison to current practice (i.e., the no regular screen and ART initiation at CD4 ≤ 350/μl strategy), as reported in the second row of the table.
ART efficacy is under “optimized ART” inflates, for all 6 ART regimens, virologic suppression by 15% above the base case (Table 1).
Follow-up time includes the entire HIV-infected population, both diagnosed and undiagnosed.
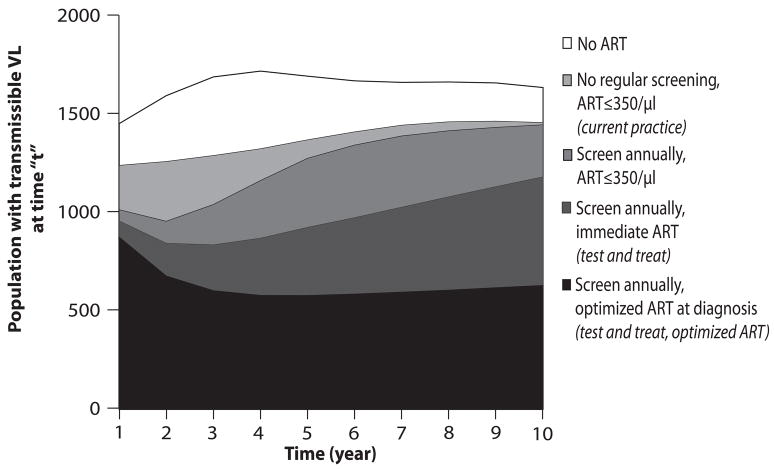
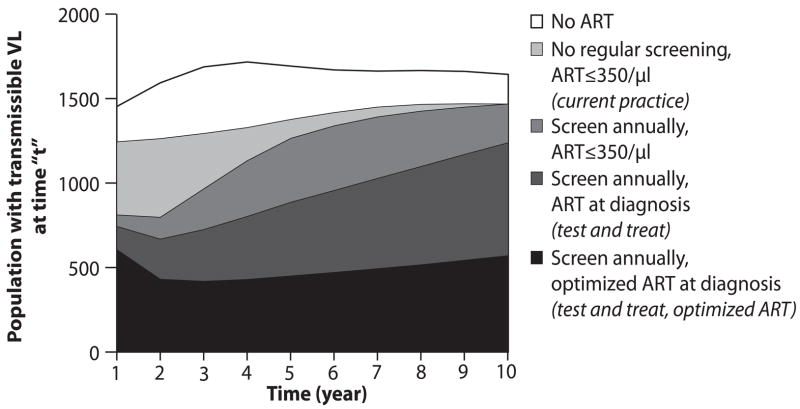
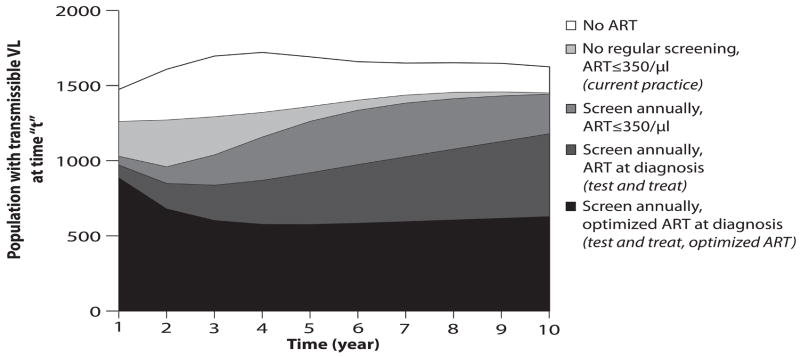
Alternative testing strategies, performance scenarios and ART efficacies change the rate at which total HIV transmissible life-years are lived (Figure 2). The area under each curve represents the cumulative time spent with transmissible viral load (>500 copies/ml), a proxy for overall transmission of infection in the population. More frequent testing with ART at diagnosis decreases life-years spent with HIV RNA >500 copies/ml; improvements in program performance and optimized ART also result in fewer total life-years with transmissible HIV RNA (Figure 2). With 73% program performance (2c), the area under the test and treat with optimized ART curve represents a substantial reduction in number of life-years lived within the cohort with potential for active viral transmission – a 65.4% reduction from current practice over 10 years.
Figure 2.
Rate of accrual of life years in the HIV-infected population (vertical axis) with a transmissible HIV RNA (>500 copies/ml) over a ten-year time horizon (horizontal axis). The height of the curve denotes the number of people with transmissible viral load living in the population at any moment in time. The area under each curve represents the community viral load burden. Five representative strategies are shown: no regular screen, no ART strategy (clear); current practice (black); annual testing, ART at ≤350/μl (gray); test and treat (hatched); and test and treat with optimized ART (dotted). Figures a-c represent each of these strategies under alternative assumptions about the participation index: base case, 9.4% (2a), optimistic, 38% (2b), and idealized, 73% (2c).
Other Sensitivity Analyses
In sensitivity analyses examining a less severe HIV epidemic (undiagnosed HIV prevalence 0.21% and 0.04% annual incidence) – similar to those in Miami and Philadelphia – the test and treat strategy offers a 14.7% reduction in time spent with transmissible HIV RNA over 5 years (Technical Appendix, Figure 1a–c) [37–39]. Analogous results are also achieved when the estimate of undiagnosed HIV infection in Washington DC is doubled (undiagnosed prevalence of 1.2%, 0.16% annual incidence) [28, 33, 40]. In sensitivity analyses varying the mean CD4 count of the undiagnosed HIV-infected population from 183/μl to 332/μl (Washington DC, 2002 and 2007), test and treat results in a 14.0% and 17.2% reduction in population time spent with transmissible infection, compared to current practice (Technical Appendix, Figure 2a–c) [1]. Near elimination of the HIV-infected population with transmissible viral load is achieved with monthly HIV screening, 100% program participation and linkage to care, and perfectly suppressive and durable ART efficacy (Technical Appendix Figure 3).
TA, Figure 1.
Rate of accrual of life years in the HIV-infected population (0.21% prevalence and 0.04% incidence) (vertical axis) with a transmissible HIV RNA (>500 copies/ml) over a ten-year time horizon (horizontal axis). The height of the curve denotes the number of people with transmissible viral load living in the population at any moment in time. The area under each curve represents the community viral load burden. Five representative strategies are shown: no regular screen, no ART strategy (clear); current practice (black); annual testing, ART at ≤350/μl (gray); test and treat (hatched); and test and treat with optimized ART (dotted). Figure a–c represents each of these strategies under alternative assumptions about the participation index: base case, 9.4% (TA1a), optimistic, 38% (TA1b), and idealized, 73% (TA1c).
TA, Figure 2.
Rate of accrual of life years in the HIV-infected population (mean CD4 at time of diagnosis, 332 cells/μl) (vertical axis) with a transmissible HIV RNA (>500 copies/ml) over a ten-year time horizon (horizontal axis). The height of the curve denotes the number of people with transmissible viral load living in the population at any moment in time. The area under each curve represents the community viral load burden. Five representative strategies are shown: no regular screen, no ART strategy (clear); current practice (black); annual testing, ART at ≤350/μl (gray); test and treat (hatched); and test and treat with optimized ART (dotted). Figure a–c represents each of these strategies under alternative assumptions about the participation index: base case, 9.4% (TA2a), optimistic, 38% (TA2b), and idealized, 73% (TA2c).
TA, Figure 3.
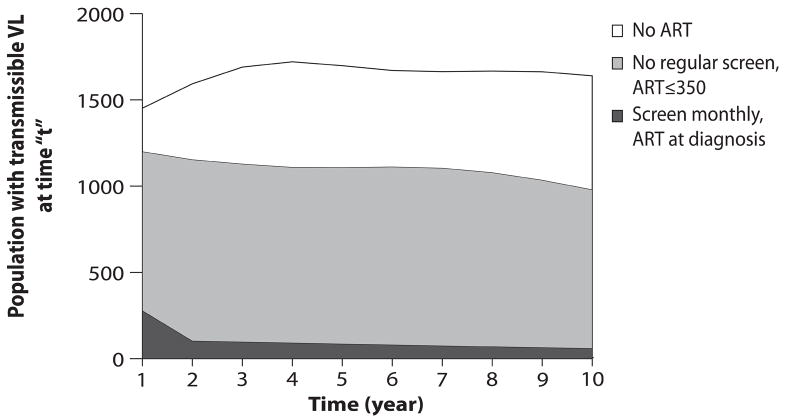
Rate of accrual of life years in the HIV-infected population (vertical axis) with a transmissible HIV RNA (>500 copies/ml) over a ten-year time horizon (horizontal axis) under the hypothetical scenario described by the following characteristics: monthly HIV test offered, test acceptance 100%, rate of linkage to HIV care among those identified 100%, monthly clinic visits, 100% viral suppression while on ART and perfect adherence. The height of the curve denotes the number of people with transmissible viral load living in the population at any moment in time. The area under each curve represents the community viral load burden. Three representative strategies are shown: no regular screen, no ART strategy (clear); no regular screen, ART at ≤350/μl (gray); and monthly testing, immediate ART (black).
DISCUSSION
Using data from Washington DC, one of the epicenters of the US HIV epidemic, we demonstrate that an intensive test and treat strategy can yield substantial benefits to individuals, improving HIV-infected life expectancy by up to 1.1 years compared to the current standard of care. Although most of the increase in life expectancy is achieved with improvements in testing frequency and coverage, an additional 4 months are likely added by ART initiation immediately upon diagnosis. And, compared to test and treat alone, further improvements in ART regimen efficacy may contribute an additional 7 months in life expectancy.
Beyond the clinical benefits to infected individuals, we find that test and treat may have quantifiable population benefits. A test and treat strategy may reduce overall life-years spent with transmissible HIV infection over the next 5 years by 15%. Any prevention intervention with the potential to decrease transmission by 15% and to produce substantial increases in individual HIV-infected life expectancy warrants further investigation. However, suggestions that a test and treat strategy might be sufficient to eradicate the HIV epidemic create public expectations that cannot be realized [5]. The transmission effect is the indirect result of viral suppression benefits that accrue most directly to the individual infected person. Therefore, prevention benefits result largely from earlier treatment initiation: providing ART upon diagnosis increases the number of transmissions averted over 5 years compared to frequent testing and guideline-concordant ART alone. Overall life-years with transmissible HIV RNA – and likely overall transmissions -- may be cut by almost one-quarter if test and treat could be combined with major efforts to improve ART adherence and rates of virologic suppression. This analysis highlights the interplay of the components of test and treat and their impact on HIV-infected individuals and the population. It also underscores that the success of any test and treat strategy hinges upon the process of successfully making HIV test offers, completing tests, linking infected patients to care and maximizing the effects of ART [41]. Numerous published programs that exemplify extraordinary efforts, testing large numbers of patients and identifying many new cases of HIV – even those beyond Washington DC -- have overall process success rates (from offer to acceptance to linkage to care) of only approximately 10% [29, 42]. These very low levels of participation will provide individual benefits to those identified, but will be inadequate to have a meaningful impact on the population. Although programs with extensive breadth (80% of the population offered) and depth (annual, or more frequent, testing), as illustrated by the optimistic scenario, may be challenging to achieve, such efforts could have a larger impact on population outcomes. Furthermore, improved ART efficacy -- likely attainable with currently available potent regimens, but beyond that even reported in trials -- is critical to effectively decrease transmission.
Like all model-based studies, this analysis is limited by the input data available. We derived input parameters from published sources, incorporating data from the Washington DC 2008 report whenever possible [1]. In the optimized and idealized scenarios, we intended to portray a very high level of program and ART performance; the results demonstrate, even under such optimism, the anticipated magnitude of population benefit achievable from a test and treat approach. To assess such population benefits, we report percent reduction in transmissible HIV RNA. The higher the initial HIV prevalence/incidence, the more the percent reduction translates into increased infections averted in absolute terms. The analysis of community viral load is restricted to prevalent and incident cases; to the extent that second- and third-generation HIV infections substantially contribute to community viral burden over a 5-year horizon, the benefits of test and treat may have been underestimated. Data continue to emerge on the benefits and risks of ART at CD4 counts >350/μl. Although this model excludes the benefits (and/or risks) of early ART on “non-AIDS”-related morbidities, such as cardiovascular and renal disease, the input parameters reflect the toxicity profiles of current treatment and therefore likely underestimate the benefits from earlier ART [43]. Natural history data used for this analysis may underestimate the proportion of hepatitis C co-infected patients in the urban DC population and thereby may over estimate HIV-infected life expectancy, in general [44]. Finally, we have excluded costs from this analysis. Cost-effectiveness results, in order to be methodologically sound, must be reported on a population-wide scale. As such, these results are more speculative with regard to future transmissions and detract from the prevention message (rather than the economic one) that lies at the heart of current debate over test and treat.
We find that dedicated efforts to address the HIV epidemic in Washington DC and in other heavily-affected US cities will substantially affect the survival of HIV-infected patients identified, averting many missed diagnoses and new AIDS cases. Moreover, earlier detection, linkage, and treatment of infected persons is likely to have a dramatic impact on secondary HIV transmission, reducing the number of new infections by as much as 15%. However, the success of such interventions hinges on careful attention to process and implementation – making frequent offers, securing high levels of consent, linking all detected cases to care, and initiating ART immediately. Even if future implementations greatly exceed the performance observed in recent, highly organized, well-financed programs, it is very unlikely that a test and treat strategy will stop the epidemic in Washington DC.
Acknowledgments
This research was funded by the National Institute of Allergy and Infectious Diseases (R37 AI042006, K24 AI062476, P30 AI060354), the National Institute of Mental Health (R01 MH065869, R01 MH073445) and the Doris Duke Charitable Foundation (Clinical Scientist Development Award). The funding sources had no role in the design, analysis, or interpretation of the study or in the decision to submit the manuscript for publication. Dr. Walensky had access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
All listed co-authors meet criteria for authorship. Specifically, the following contributions were made by the authors listed parenthetically: Conception and design (RPW, ADP, KAF); acquisition of data (RPW, BLM, EL); analysis and interpretation of data (all); drafting of the manuscript (RPW, ADP, KAF); critical revision of manuscript (all); statistical analysis (RW, EL, ERR); obtaining funding (RPW, ADP, KAF); administrative, technical, and material support (BLM, ERR); and supervision (RPW, ADP, KAF). No authors have financial conflicts to disclose.
The CEPAC US Investigators
We are indebted to the entire CEPAC team and investigators for their contributions, including Ingrid V. Bassett, Jessica Becker, John Chiosi, Jennifer Chu, Andrea Ciaranello, Kenneth A. Freedberg, Heather E. Hsu, Benjamin P. Linas, Sarah Lorenzana, Zhigang Lu, Bethany Morris, Farzad Noubary, Ji-Eun Park, Erin Rhode, Caroline Sloan, Callie A. Scott, Adam Stoler, Rochelle P. Walensky, Angela Wong (Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA); Elena Losina, Paul E. Sax (Brigham and Women’s Hospital, Boston, MA, USA); Sue J. Goldie, Marc Lipsitch, Chara Rydzak, George R. Seage III, Milton C. Weinstein (Harvard School of Public Health, Boston, MA, USA); Yazdan Yazdanpanah (Centre Hospitalier de Tourcoing, Tourcoing, France); April D. Kimmel, Bruce R. Schackman (Weill Cornell Medical College, New York, NY, USA); and A. David Paltiel (Yale University, New Haven, CT, USA).
Footnotes
These data have previously been presented at the 5th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention (IAS), July 19–22, 2009 in Cape Town, South Africa.
References
- 1.Government of the District of Columbia: Department of Health. [Accessed 18 March 2010];HIV/AIDS Epidemiology Update 2008. 2009 Available at: http://doh.dc.gov/doh/lib/doh/pdf/dc_hiv-aids_2008_updatereport.pdf.
- 2.UNAIDS. 2008 Report on the global AIDS epidemic. Geneva: Joint United Nations Programme on HIV/AIDS; 2008. [Accessed 21 March 2010]. Available at: http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_Global_report.asp. [Google Scholar]
- 3.Cover M. NIH Will Use Stimulus Funds to Test Feasibility of ‘Universal’ HIV Testing. [Accessed 22 March 2010];CNSNews.com. 2009 Available at: http://www.cnsnews.com/public/content/article.aspx?RsrcID=48963.
- 4.De Cock KM, Crowley SP, Granich RM, Williams BG. Preventing HIV transmission with antiretrovirals. Bull World Health Organ. 2009;87:488. doi: 10.2471/BLT.09.067330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373(9657):48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 6.Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300(5):520–9. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Department of Health and Human Services: National Institutes of Health. [Accessed 21 March 2010];NIH and D.C. Department of Health Team up to Combat District’s HIV/AIDS Epidemic. 2010 Available at: http://www.nih.gov/news/health/jan2010/niaid-12.htm.
- 8.Freedberg KA, Losina E, Weinstein MC, et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med. 2001;344(11):824–31. doi: 10.1056/NEJM200103153441108. [DOI] [PubMed] [Google Scholar]
- 9.Freedberg KA, Scharfstein JA, Seage GR, 3rd, et al. The cost-effectiveness of preventing AIDS-related opportunistic infections. JAMA. 1998;279(2):130–6. doi: 10.1001/jama.279.2.130. [DOI] [PubMed] [Google Scholar]
- 10.Walensky RP, Paltiel AD, Losina E, et al. The survival benefits of AIDS treatment in the United States. J Infect Dis. 2006;194(1):11–9. doi: 10.1086/505147. [DOI] [PubMed] [Google Scholar]
- 11.Paltiel AD, Weinstein MC, Kimmel AD, et al. Expanded screening for HIV in the United States--an analysis of cost-effectiveness. N Engl J Med. 2005;352(6):586–95. doi: 10.1056/NEJMsa042088. [DOI] [PubMed] [Google Scholar]
- 12.Paltiel AD, Walensky RP, Schackman BR, et al. Expanded HIV screening in the United States: effect on clinical outcomes, HIV transmission, and costs. Ann Intern Med. 2006;145(11):797–806. doi: 10.7326/0003-4819-145-11-200612050-00004. [DOI] [PubMed] [Google Scholar]
- 13.Walensky RP, Weinstein MC, Kimmel AD, et al. Routine human immunodeficiency virus testing: an economic evaluation of current guidelines. Am J Med. 2005;118(3):292–300. doi: 10.1016/j.amjmed.2004.07.055. [DOI] [PubMed] [Google Scholar]
- 14.Texas Department of State Health Services. [Accessed 7 July 2005];Texas Vital Statistics: Life Tables. 2005 Available at: http://www.tdh.state.tx.us/chs/vstat/latest/t24.HTM.
- 15.Arias E. United States Life Tables, 2002: National Vital Statistics Reports, Centers for Disease Control and Prevention, 2004. 2004 November 10;53(6) Report No. [PubMed] [Google Scholar]
- 16.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents - A Working Group of the Office of AIDS Research Advisory Council (OARAC) [Accessed 21 March 2010];Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2008 November 3; Available at: http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf.
- 17.Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58(RR-4):1–207. [PubMed] [Google Scholar]
- 18.Cooper DA, Steigbigel RT, Gatell JM, et al. Subgroup and resistance analyses of raltegravir for resistant HIV-1 infection. N Engl J Med. 2008;359(4):355–65. doi: 10.1056/NEJMoa0708978. [DOI] [PubMed] [Google Scholar]
- 19.Elzi L, Marzolini C, Furrer H, et al. Treatment modification in human immunodeficiency virus-infected individuals starting combination antiretroviral therapy between 2005 and 2008. Arch Intern Med. 2010;170(1):57–65. doi: 10.1001/archinternmed.2009.432. [DOI] [PubMed] [Google Scholar]
- 20.Gill VS, Lima VD, Zhang W, et al. Improved virological outcomes in British Columbia concomitant with decreasing incidence of HIV type 1 drug resistance detection. Clin Infect Dis. 2010;50(1):98–105. doi: 10.1086/648729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lalezari J, Goodrich J, DeJesus E, et al. Efficacy and safety of maraviroc plus optimized background therapy in viremic ART-experienced patients infected with CCR5-tropic HIV-1: 24-week results of a phase 2b/3 study in the US and Canada [abstract 104bLB]. 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA. 2007. [Google Scholar]
- 22.Nelson M, Arasteh K, Clotet B, et al. Durable efficacy of enfuvirtide over 48 weeks in heavily treatment-experienced HIV-1-infected patients in the T-20 versus optimized background regimen only 1 and 2 clinical trials. J Acquir Immune Defic Syndr. 2005;40(4):404–12. doi: 10.1097/01.qai.0000185314.56556.c3. [DOI] [PubMed] [Google Scholar]
- 23.Sax PE, Tierney C, Collier AC, et al. Abacavir-lamivudine versus tenofovir-emtricitabine for initial HIV-1 therapy. N Engl J Med. 2009;361(23):2230–40. doi: 10.1056/NEJMoa0906768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yazdanpanah Y, Fagard C, Descamps D, et al. High rate of virologic suppression with raltegravir plus etravirine and darunavir/ritonavir among treatment-experienced patients infected with multidrug-resistant HIV: results of the ANRS 139 TRIO trial. Clin Infect Dis. 2009;49(9):1441–9. doi: 10.1086/630210. [DOI] [PubMed] [Google Scholar]
- 25.Wood E, Kerr T, Marshall BD, et al. Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. BMJ. 2009;338:b1649. doi: 10.1136/bmj.b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montaner JS, Hogg R, Wood E, et al. The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet. 2006;368(9534):531–6. doi: 10.1016/S0140-6736(06)69162-9. [DOI] [PubMed] [Google Scholar]
- 27.Bartlett JG, Gallant JE. 2001–2002 Medical Management of HIV Infection. Baltimore: Johns Hopkins University, Division of Infectious Diseases; 2001. [Google Scholar]
- 28.Centers for Disease Control and Prevention. HIV prevalence estimates--United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;57(39):1073–6. [PubMed] [Google Scholar]
- 29.Brown J, Shesser R, Simon G, et al. Routine HIV screening in the emergency department using the new US Centers for Disease Control and Prevention Guidelines: results from a high-prevalence area. J Acquir Immune Defic Syndr. 2007;46(4):395–401. doi: 10.1097/qai.0b013e3181582d82. [DOI] [PubMed] [Google Scholar]
- 30.Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126(12):946–54. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 31.Walensky RP, Goldie SJ, Sax PE, et al. Treatment for primary HIV infection: projecting outcomes of immediate, interrupted, or delayed therapy. J Acquir Immune Defic Syndr. 2002;31(1):27–37. doi: 10.1097/00126334-200209010-00004. [DOI] [PubMed] [Google Scholar]
- 32.Rothman KJGS, Lash TL. Modern Epidemiology. 3. Lippincott Williams and Wilkins; 2008. [Google Scholar]
- 33.Campsmith M, Rhodes P, Hall I. Estimated prevalence of undiagnosed HIV infection: US, end of 2006 [abstract #1036]. 16th Conference on Retroviruses and Opportunitistic Infections; Montreal, QC, Canada. February 8–11, 2009.2009. [Google Scholar]
- 34.Centers for Disease Control and Prevention. Notice to Readers: Protocols for Confirmation of Reactive Rapid HIV Tests. MMWR Morb Mortal Wkly Rep. 2004;53(10):221–2. [Google Scholar]
- 35.Multicenter AIDS Cohort Study (MACS) Public Dataset: Release PO4. Springfield, VA: National Technical Information Service; 1995. [Google Scholar]
- 36.Simoni JM, Pearson CR, Pantalone DW, Marks G, Crepaz N. Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load. A meta-analytic review of randomized controlled trials. J Acquir Immune Defic Syndr. 2006;43 (Suppl 1):S23–35. doi: 10.1097/01.qai.0000248342.05438.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. HIV/AIDS Surveillance Supplemental Report: Reported number of persons living with HIV infection (non-AIDS), AIDS and total, by area of residence, as of December 2005. [Accessed 20 March 2010];2008 Available at: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/2008supp_vol13no3/pdf/table4.pdf.
- 38.United States Census Bureau. [Accessed 24 March 2010];Resident Population Estimates for the 100 largest U.S. counties. 2008 Available at: http://www.census.gov/popest/counties/CO-EST2008-07.html.
- 39.United States Census Bureau. [Accessed 24 March 2010];Annual Estimates of the Population for Incorporated Places over 100,000. 2008 Available at: http://www.census.gov/popest/cities/SUB-EST2007.html.
- 40.Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report: Cases of HIV Infection and AIDS in the United States and Dependent Areas, 2007. [Accessed 25 March 2010];2007 19 Report No. Available at: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/2007report/default.htm.
- 41.Walensky RP, Weinstein MC, Smith HE, Freedberg KA, Paltiel AD. Optimal allocation of testing dollars: the example of HIV counseling, testing, and referral. Med Decis Making. 2005;25(3):321–9. doi: 10.1177/0272989X05276955. [DOI] [PubMed] [Google Scholar]
- 42.White DA, Cheung PT, Scribner AN, Frazee BW. A Comparison of HIV Testing in the Emergency Department and Urgent Care. J Emerg Med. 2009 doi: 10.1016/j.jemermed.2009.04.068. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 43.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355(22):2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 44.Palella FJ, Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43(1):27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]