Abstract
The use of electrocorticography (ECoG) with etiologically realistic epilepsy models promises to facilitate the discovery of better anti-epileptic drugs (AEDs). However, this novel approach is labor intensive, and must be optimized. To this end, we employed rostral parasaggital fluid percussion injury (rpFPI) in the adolescent rat, which closely replicates human contusive closed head injury and results in posttraumatic epilepsy (PTE). We systematically examined variables affecting the power to detect antiepileptic effects by ECoG and used a non-parametric bootstrap strategy to test several different statistics, study designs, statistical tests, and impact of non-responders. We found that logarithmically transformed data acquired in repeated-measures experiments provided the greatest statistical power to detect decreases in seizure frequencies of pre-clinical interest with just 8 subjects and with up to ~40% non-responders. We then used this optimized design to study the antiepileptic effects of acute exposure to halothane, and chronic (1 week) exposures to carbamazepine (CBZ) and valproate (VPA) one month post-injury. While CBZ was ineffective in all animals, VPA induced, during treatment, a progressive decrease in seizure frequency in animals primarily suffering from non-spreading neocortical seizures, but was ineffective in animals with high frequency of spreading seizures. Halothane powerfully blocked all seizure activity. The data show that rpFPI and chronic ECoG can conveniently be employed for evaluation of AEDs, suggest that VPA may be more effective than CBZ to treat some forms of PTE and support the theory that pharmacoresistance may depend on the severity of epilepsy. The data also demonstrate the utility of chronic exposures to experimental drugs in preclinical studies and highlight the need for greater attention to etiology in clinical studies of AEDs.
Keywords: posttraumatic epilepsy, neocortical epilepsy, trauma, screening, in vivo, EEG, partial seizure, pharmacoresistance, fluid percussion injury, remission, bootstrap
INTRODUCTION
Numerous anti-epileptic drugs (AEDs) have been introduced over the last 30 years, but the proportion of patients with poor seizure control has remained stable at ~30% (Duncan et al., 2006; French, 2007). In addition, no agent has been identified to cure epilepsy (Temkin, 2009). Thus, many epilepsy patients require chronic administration of AEDs, and suffer from varying side effects that negatively impact quality of life (Gilliam et al., 2004). This failure to broaden AED efficacy significantly has led to doubts about the models most commonly employed in AED development (White, 2002, 2003; Stables et al., 2002; Kwan and Brodie, 2003; Schmidt and Rogawski, 2002; Löscher and Schmidt, 2004). These include acute seizure models, in which anti-epileptic activity is assessed by the acute inhibition of chemically- or electrically-induced behavioral seizures in otherwise healthy non-epileptic animals, that may not capture the mechanisms most important for precipitation (and control) of acquired chronic spontaneous recurrent seizures (CSRSs) in humans. In addition, acute assays are poorly suited to detect anti-epileptic effects that develop slowly, over days-to-weeks, such as the time-dependent increase in the efficacy of valproate (VPA; Löscher and Hönack, 1995) or as might be expected with drugs targeting pro-epileptic inflammatory mechanisms (Vezzani and Granata, 2005). The use of epileptic, rather than normal, animals has been recommended to improve the performance of preclinical testing (Meldrum, 2002; White, 2002, 2003). Because the pace of therapy development may largely depend on the fidelity of animal models to the human condition (Sloviter, 2005), recent efforts have focused on the development of acquired CSRS models closely reproducing brain insults known to be epileptogenic in humans, and that are thus likely to recruit mechanisms of ictogenesis and epileptogenesis that are relevant to the corresponding human syndrome: stroke (Kelly et al., 2001; Kharlamov et al., 2003), head injury (D’Ambrosio et al., 2004, 2005), early-life febrile seizures (Dube et al., 2006; Oakley et al., 2009), and hypoxia-ischemia (Williams and Dudek, 2007; Klein et al. 2010). Another limitation of current approaches to AED discovery is a reliance on behavioral endpoints of seizure activity, especially clonic behavior (D’Ambrosio and Miller, 2010). In humans, not all epileptic seizures are accompanied by overt or distinctive behavioral output, such as convulsions. Human complex partial seizures (CPSs), which are characterized by altered cognitive state, represent a much greater challenge for pharmacological treatment than other seizure types (Juul-Jensen, 1986; Mattson et al., 1996; Semah et al., 1998). CPSs often have very subtle behavioral manifestations and, depending on the location of the focus and the spread of the seizure, may be associated with normal-looking but unconscious automatic behavior, staring, memory lapses, muscle twitching, or other impairments in normal function that may go unrecognized by patients and physicians alike (Blum et al., 1996; Tatum et al., 2001). The behavioral correlate of spontaneous CPSs induced by etiologically realistic injuries in animals is similarly subtle (D’Ambrosio et al., 2009) and, thus, difficult to track accurately. Electrocorticography (ECoG), in contrast, permits a sensitive and reliable measurement of seizure frequency and duration that is independent from ictal behavioral output, and is suitable for the extended monitoring required to detect anti-epileptic effects in models with CSRSs. An approach that combines etiologically relevant models of epilepsy, ECoG monitoring, and chronic treatments is, thus, better positioned to identify more effective therapies for epilepsies now refractory to treatment. However, this combined approach is costly and fraught with technical challenges. First, the chronic nature of these models results in significant housing costs because animals must be housed for weeks to months in order to develop epilepsy and evaluate the treatment. In addition, chronic electrodes must be implanted with precision and within sturdy headsets to avoid artifactual damage to the neocortex (D’Ambrosio et al., 2009) and to minimize losses over time. Second, the chronic administration of drugs required to reliably assess slowly developing antiepileptic activities is often complicated by metabolic autoinduction (Löscher, 2007). Thus, dosing protocols capable of maintaining stable therapeutic drug levels for the duration of the study must be developed empirically, and often are labor intensive, requiring multiple daily doses for days to weeks. Third, the frequency of CSRSs is typically highly variable, both among animals and over time (Arida et al., 1999; Bethmann et al., 2007; Grabbenstatter and Dudek, 2008), and a complete model of acquired epilepsy would produce pharmacoresistant subjects. These two factors increase the group sizes needed to reach statistically significant conclusions. Finally, in the absence of highly sensitive and specific seizure-detection software, the manual analysis of rat video-ECoG recordings is laborious and requires considerable expertise. Therefore, a systematic investigation of optimized study designs and data analysis strategies is needed to identify protocols that minimize data collection, labor and costs, while maximizing the power to detect anti-epileptic effects of preclinical interest.
Here we adapt the rostral parasaggital fluid percussion injury (rpFPI) model of posttraumatic epilepsy (PTE) in the adolescent rat to the identification of antiepileptic activity. In humans, the risk of traumatic brain injury is highest in the young (Kraus and McArthur, 2000), and rpFPI is mechanically identical to human contusive closed head injury, reproduces key histopathological and pathophysiological sequelae (Thomson et al., 2005), and reliably results, within weeks after injury, in a high incidence of PTE with frequent partial seizures that are readily detected by ECoG (D’Ambrosio et al., 2004, 2005, 2009). Before progressing to a dual pathology several months post-injury (D’Ambrosio et al., 2005), rpFPI-induced PTE first appears as frontal-lobe neocortical partial seizures (D’Ambrosio et al., 2004, 2009) that differ from typical motor seizures currently used for AED screening but are similar to corresponding human frontal-lobe seizures from both the electrical (Williamson and Spencer, 1986; Bancaud and Talairach, 1992; D’Ambrosio et al., 2009), and behavioral standpoint (Williamson et al., 1985; Williamson and Spencer, 1986; Bancaud and Talairach, 1992; Lüders et al., 1992). Similar frontal-lobe partial seizures are also observed in the rat chronically after photothrombotic stroke (Kelly et al., 2001). In addition, rpFPI-induced epilepsy incorporates all the risk factors for pharmacoresistant epilepsy that were summarized by French (2007) for the human: epilepsy is acquired (Semah et al., 1998; Hitiris et al., 2005; Callaghan et al., 2007), it is associated with dual pathology (Semah, 1998), it is induced before adulthood (Berg et al., 2001), and it presents with frequent seizures (Collaborative group for the study of epilepsy, 1992). Thus, rpFPI in the adolescent rat is a particularly promising model for pharmacological studies of CSRSs and seems well-suited to help identify novel classes of antiepileptics.
To optimize the use of the model, we employed a data-driven non-parametric bootstrap strategy to examine the performance of raw and transformed ECoG-based CSRS frequency data in the detection of defined anti-epileptic effects using different experimental designs and statistical tests, under varying conditions of data variance and treatment responsiveness. Bootstrap methods are well-suited to conduct power analyses (Efron and Tibshirani, 1994) and, in contrast to parametric power analyses, they permit both comparison of the performance of different statistics, regardless of their underlying distributions, and evaluation of the impact of non-responsive subjects on statistical power. Our results provide guidelines useful in minimizing animal use and costs, and demonstrate that modest numbers of rpFPI-injured subjects provide good statistical power to detect preclinically interesting reductions in seizure frequency even in the presence of non-responders. We then employ the optimized protocol to test the sensitivity of neocortical partial seizures to chronic exposures to carbamazepine (CBZ) and valproic acid (VPA), and acute exposures to halothane. Our results demonstrate that, while halothane abolishes all CSRSs, PTE five weeks after rpFPI is insensitive to carbamazepine (CBZ) and controlled by valproate (VPA) in only ~40% of the animals. A comparison of the PTE syndrome in VPA-induced responders vs non-responders provides support for the recent hypothesis that links pharmacoresistance to the severity of epilepsy, and suggests VPA may be more effective than CBZ on CSRSs that appear in the first weeks to months after contusive closed head injury in humans.
MATERIALS AND METHODS
Power Analyses
Monte Carlo methods were used to estimate the power to detect specified reductions in seizure frequency using several statistics and study designs, under various experimental conditions (Fig. 1A; Table 1). ECoG-based CSRS data were used, as detailed below, to provide the best available estimate of the distribution of seizure frequencies in epileptic rpFPI rats. Experimental group sizes, the ranges of group members’ pre-treatment frequencies of seizure, the magnitude of the anti-epileptic effect on individuals’ seizure frequencies, and the proportion of experimental subjects that would respond to treatment were specified for each simulated experiment. Statistics (e.g. mean seizure frequency) were computed and compared for each simulated experiment and the performance of each experimental/analytical protocol was assessed on the basis of 105 repetitions. These bootstrap analyses utilized seizure frequency data from 35 identically injured and untreated FPI-epileptic rats exhibiting seizure frequencies compatible with inclusion in the drug-testing studies (see below) and from which we had obtained three separate 24-hr video-ECoG recordings during post injury week 4, corresponding to ~43% of that week. The frequency of posttraumatic CSRS in rpFPI rats varies from rat to rat, and also from measurement-to-measurement in individual rats. In order to model both components of variability closely on experimental observations with minimal distributional assumptions, modeled seizure frequencies were based on the range of seizure frequencies observed for each individual rat. In simulated experiments, subjects were chosen randomly with replacement from the 35 reference animals. A subject’s seizure frequency was always determined as the mean of 2 uniformly distributed random numbers drawn from within that subject’s observed frequency range, reflecting our experimental practice of averaging seizure frequency over at least two (usually three), 24-hr recordings per week. Drug treatment effects on responders were simulated by reducing each randomly selected frequency value by a specified proportion. Non-responders were simulated by setting the treatment effect to zero. Drug effects reported throughout refer to decreases in seizure frequency in responding animals, not to group averages. To examine their reliability, all power analyses were conducted with data drawn, as described above, from the observed seizure frequency range and from ranges extended by 25% and 50%. Since extending the range by 25% yielded power estimates, as expected, in between those obtained with the observed and 50% extended ranges, data from the 25%-extended range are omitted from presentation for clarity. This bootstrap strategy was used to evaluate and compare the performance of different study designs, statistics and statistical tests. For unpaired comparisons of independent groups of treated versus untreated subjects (Table 1, IIIa–b), we examined the performance of raw (f5) and logarithmically transformed (log10(f5)) seizure frequency data compared using either the Mann-Whitney U test or Welch’s independent groups t-test for groups with potentially unequal variances. These unpaired experiments were simulated by randomly selecting N subjects for control and N subjects for treatment. A frequency value was obtained from each subject’s range and each responder’s frequency was reduced to simulate a specified treatment effect. For simulations of treatment effects in repeated-measures experiments (Table 1, IIIc–h), we examined the performance of pre-treatment minus treatment differences of raw (f4–f5), logarithmically transformed (log10(f4)−log10(f5) = log10(f4/f5)) seizure frequency data, and proportional differences ((f4–f5)/f4) in seizure frequency. These experiments were simulated by randomly selecting N subjects, obtaining a pre-treatment and treatment frequency from each subject’s range and reducing responders’ treatment frequencies to simulate a specified treatment effect. The performance of unpaired comparisons (Table 1, IIIc–e) of effective (log10(f4)−log10(f5)) versus sham (log10(f4)−log10(untreated f5)) treatments was examined using the Mann-Whitney U test and Welch’s independent groups t-test. For this purpose, paired pre-treatment minus treatment differences were obtained from two independent groups of subjects, each simulated as for paired comparisons in repeated measures experiments but with anti-epileptic effect set to zero (sham) or to a non-zero value. Paired comparisons of pre-treatment minus treatment seizure frequencies (Table 1, IIIf–h) were evaluated in single treated groups using either a Wilcoxon matched pairs rank sum test or a paired t-test.
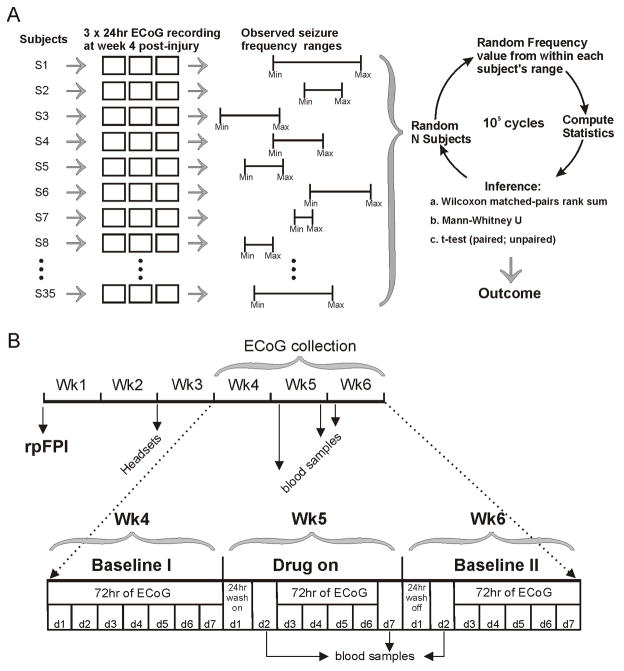
Figure 1. Methods.
A) Schematic of the algorithm employed to implement the non-parametric bootstrap power analysis. Three 24hr ECoG recordings (hollow squares) were obtained from each of 35 different injured epileptic rats (subjects), and used to describe the reference population. For each subject, the 3 recordings defined the range of seizure frequency (Min, Max), which differed from rat to rat. The bootstrap was implemented by randomly selecting N subjects to represent a group, randomly choosing a frequency from within each subject’s frequency range, computing statistics and then comparing them (see methods). B) Schematic of the study design for the determination of the antiepileptic effects of CBZ and VPA on rpFPI-induced PTE. Recording headsets (Headsets) were implanted two weeks after rostral parasaggital FPI (rpFPI). Video/ECoG recordings were obtained on post-injury weeks 4–6 before, during and after treatment on week 5. Blood was drawn for determination of plasma levels of VPA or CBZ after at least 24 hours wash-on time, and immediately before the first and after the last ECoG recording on week 5 (Drug on), and 24hrs after cessation of drug administration. An additional blood sample was then collected after 24 hours off the drug to confirm wash-out.
Table 1.
Variables investigated by bootstrap power analyses
| I) Study design: |
|
| II) Statistical Test: |
|
| III) Seizure frequency statistics: |
|
| IV) Seizure frequency range: |
|
| V) Proportion of non-responders: | 0%, 12.5%, 25%, 37.5%, 50% |
| VI) Drug effect: | seizure frequency decrease ranging 0%–100% |
| VII) Group size: | ranging 5–30 |
| VIII) Power |
f4 and f5 indicate a subject’s seizure frequency at week 4 (before drug) and 5 (on drug) post-injury, respectively.
Surgical Procedures
All procedures were approved by the University of Washington Institutional Animal Care and Use Committee. All reagents were purchased from Sigma (St. Louis, MO) unless otherwise noted. Rostral parasaggital fluid percussion injury has been described in detail previously (D’Ambrosio et al., 2004; 2005). Briefly, 32–36 day old male Sprague-Dawley rats (Charles Rivers, Hollister, CA) were anesthetized with 4% halothane, intubated and mechanically ventilated on 1.5% halothane and 30% O2 and air. Core temperature was maintained at 37°C with a heat pad. A 3 mm burr hole was drilled 2 mm posterior to bregma and 3 mm from the midline over the right convexity. An 8 ms pressure pulse of 3.25–3.5 atm was delivered through the FPI device (Scientific Instruments, University of Washington) and measured by a transducer (Measurement Specialties, Hampton, VA). After a 10-second pause in breathing upon injury, the animal was re-connected to the ventilator. Sham-injured animals underwent the same procedure but the pressure pulse was generated with the stopcock of the FPI device closed. The mortality rate from acute posttraumatic complications was ~11%. Injured animals had righting times in excess of 10 minutes. Acute mortality rate was ~11%. Epidural electrodes were implanted as detailed previously (D’Ambrosio et al., 2004, 2005). Two weeks after rpFPI, epidural electrodes (stainless-steel screws of ф =1 mm) were implanted through guiding craniotomies (~ф =0.75 mm). To prevent artifactual epileptiform discharges, particular care was taken to avoid brain compression or damage to the underlying dura (D’Ambrosio et al., 2009). The ECoG montage consisted of five epidural electrodes: one was placed midline in the frontal bone and used as reference, while two electrodes per parietal bone were placed at coordinates bregma 0 mm and −6.5 mm, 4 mm from the midline. All electrodes were connected through insulated wire to a gold-plated pin in a plastic pedestal, and the entire assembly was cemented onto the skull with dental acrylic. Osmotic pumps (Durect, Cupertino, CA) were loaded, primed and implanted subcutaneously. Animals were anesthetized with halothane, the pump assembly was inserted into the subcutaneous pocket formed by an incision in the skin on the animal’s back, and the skin was sutured.
Drug administration
Carbamazepine (CBZ) was dissolved in a DMSO/propylene glycol/ethanol (42.5:42.5:15) vehicle and loaded identically into 2 pumps to deliver the drug in 3 dosage steps: 4 mg*kg−1*hr−1 for 1.5 days, 8 mg*kg−1*hr−1 for 1 day, and 12 mg*kg−1*hr−1 for 4.5 days. Pilot studies demonstrated that a fixed dose of CBZ (4 mg*kg−1*hr−1) failed to maintained therapeutic levels for more than a day, consistent with autoinduction of CBZ metabolism. In contrast, a stepped-dosing protocol maintained stable therapeutic plasma levels for a week (Fig. 7F). Pump reservoirs were filled to provide CBZ at the highest dose (12 mg*kg−1*hr−1), and two additional CBZ solutions, in concentrations and volumes to permit administration at 4 and 8 mg*kg−1*hr−1 were loaded into PE tubing, separated by small air bubbles to prevent mixing. This tubing was connected to the pumps to provide the stepped dosing regimen required for stable serum [CBZ]. Valproate (VPA) was dissolved in saline and loaded into a single osmotic minipump to deliver 480 mg*kg−1*day−1 (10.3 ul/hr) for a week. The pump was implanted immediately after intraperitoneal administration of a loading dose of 100 mg*kg−1 in saline. Pilot studies showed that this protocol achieved steady plasma levels over the course of a week. Consistent with the TD50 reported by White et al. (1998), in a pilot study chronic administration of VPA at 600 mg*kg−1 day−1 resulted in ECoG slowing in some animals during apparently normal waking behavior. Thus, 480 mg*kg−1*day−1 was selected to avoid toxicity during chronic administration.
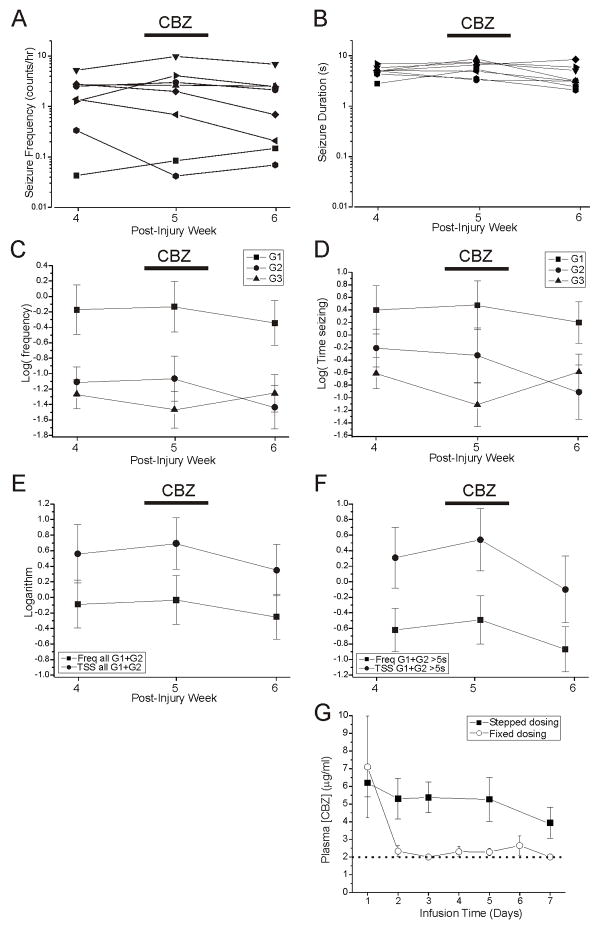
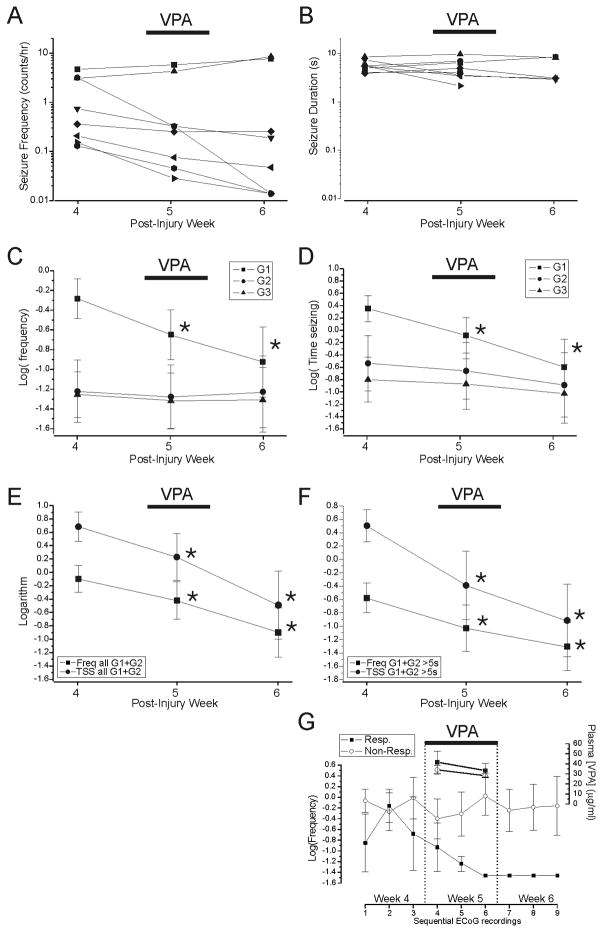
Figure 7. Lack of effect of carbamazepine on rpFPI-induced PTE 5 weeks after injury.
A) Plots of mean seizure frequency in 8 individual rats before (week 4), during (week 5) and after (week 6) administration of CBZ via s.cu. osmotic minipump. B) Plots of mean duration of all seizures in the same rats before, during and after CBZ administration. Data in A–B are on a logarithmic scale. The mean logarithms of the frequencies of G1, G2 and G3 seizures recorded before during and after CBZ treatment is shown in C, while the mean logarithm of the time spent seizing is shown for each seizure type in D. E) Activity of the neocortical focus as demonstrated by the mean logarithms of the frequencies (Freq) and times spent in G1 and G2 seizures (TSS) before during and after CBZ treatment. Displayed data include all (longer than 0.8s) detectable seizures. F) Activity of the neocortical focus demonstrated as in (E), but including only seizures longer than 5s. Note that the analyses displayed in (E) and (F) are similar but the greater number of events included in (E) may result in enhanced sensitivity. G) The results of pharmacokinetic pilot studies of CBZ are shown. The 3-dose stepped dosing protocol by osmotic minipump used in this study (4 → 8 → 12 mg*kg−1*day−1; filled squares) maintained stable blood levels of CBZ over a week (N=6). Conversely, infusion of CBZ at a fixed dose (4mg*kg−1*day−1; hollow circles) could not maintain therapeutic blood levels (N=7). Dotted line indicates the detection threshold for CBZ.
Drug Testing
The drug-testing protocol (Fig. 1B) was specifically designed to evaluate the effects of AED on neocortical partial seizures. Since these seizures predominate when their frequency reaches a plateau by 1 month post-injury (D’Ambrosio et al., 2004, 2005), the effects of AEDs were examined 4–6 weeks after rpFPI. Testing commenced on post-injury week 4, when over 90% of injured rats were projected to be epileptic, and continued through week 6. Three 24hr video-ECoG recordings (~72hr/week for each rat) were acquired before (week 4), during (week 5) and after (week 6) drug treatment. Blood withdrawals for determination of plasma levels of drugs were scheduled and conducted to minimize possible stress effects on seizures. Blood was withdrawn (8am–12noon) from the tail veins of anesthetized animals, at least 24h prior to ECoG recording, on days 2 and 7 of week 5 (on drug) and day 2 of week 6, after cessation of drug administration. Plasma concentrations of CBZ and VPA were determined with a Synchron System drug calibrator (Beckman Coulter, Fullerton, CA) by the clinical pharmacology laboratory at Harborview Medical Center (Seattle, WA). Drug-treated animals recorded for 48 hrs or more on each week of the study were retained for analysis. This approach was chosen to minimize data collection/analysis costs, while fully sampling any circadian seizure clustering and permitting a degree of robustness to technical problems (eg. noise, headset loss) that sometimes limit the amount of data that can be collected. A seizure frequency threshold was enforced to minimize the spurious occurrence of seizure-free observation periods in epileptic animals with low seizure frequency.
Calculations in which seizures were considered to occur randomly in a homogeneous Poisson process, showed a 0.05 probability of observing no seizures (k=0) during any observation period (τ) with a seizure frequency (λ) of at least 3 events per period:
For k = 0 and T = 1, P(0,events during T) = e−λ1 (λ1)0/0! = e−λ, and
For P(0,events during T) = 0.05, ln(0.05) = −λ, and λ = 3.
Thus, animals with pre-treatment seizure frequencies < 3 events/72hr did not enter the study.
Neuropathology
Coronal sections taken near the sites of electrode implantation at Bregma 0 and −6.5 mm were immunostained for glial fibrillary acidic protein (GFAP) using a standard ABC technique and developed using diaminobenzidine/HRP histochemistry, as previously described (D’Ambrosio et al., 2005). Cases of electrode implantation damage, as indicated by ~0.5–1.5 mm diameter neocortical damage and focus of GFAP+ astroglial reactivity right under the electrodes were excluded from the study (~5% of implanted animals).
Video-ECoG in the rat
Video-ECoG acquisition, amplification and storage procedures were as previously described (D’Ambrosio et al., 2005). Briefly, electrical brain activity was amplified (x 5,000–10,000) and filtered (0.3 Hz high-pass, 100 Hz low-pass, 60Hz notch) using a Neurodata 12 or a M15 amplifier (Grass Instruments, Quincy, MA), acquired at 512 Hz per channel with DT3010 acquisition boards (DataTranslation Inc., Marlboro, MA), stored, and analyzed on Pentium-based computers equipped with SciWorks with Experimenter V3 software (Datawave Technologies Inc., Longmont, CO).
Identification of seizures
Video-ECoG data were manually analyzed offline as described previously (D’Ambrosio et al., 2004, 2005, 2009), and ictal events were designated as grade 1 (G1, detected by one electrode only), grade 2 (G2, spread to other electrodes after first detection by a perilesional electrode) or grade 3 (G3, bilateral at neocortical onset). Random samples of both video and ECoG of awake and sleep patterns were examined in drug-treated animals to assure drugs did not affect the typical ECoG patterns and our capability to recognize seizures. Consistent with the clinical definition of seizures, counts included stereotyped epileptiform ECoG events as short as 0.8 seconds with coincident behavioral change according to the behavioral scale of partial seizures previously described in the head injured rat (D’Ambrosio et al., 2004, 2005, 2009). To facilitate comparison with studies in which seizures are arbitrary defined based on duration, results considering only seizures lasting ≥ 5 seconds are also presented. CSRSs were then analyzed in terms of frequency (events/hr), duration (s) and proportion of time spent seizing (s/hr).
Statistical analysis
Simulations and bootstrap analyses were run using the Statistics101 resampling statistics program (http://www.statistics101.net/). Statistical tests were conducted using SPSS (v. 10, SPSS Inc., Chicago). Experimental effects of VPA and CBZ on rpFPI-induced CSRSs were evaluated using paired t-tests after logarithmic transformation of the data. Since the logarithm of zero is −∞ and the occurrence of no seizures in a finite observation period only suggests a seizure frequency of less than one per observation period, observations of zero seizure frequency in animals after drug treatment were assigned a floor value of 1/72hr. Statistical tests performed were always one-tailed to maximize the chance to detect an antiepileptic effect. Indeed, increases in seizure frequency or duration are not interesting outcome in the preclinical development of AEDs. Unless stated otherwise, all data are reported as mean±S.E.M.
RESULTS
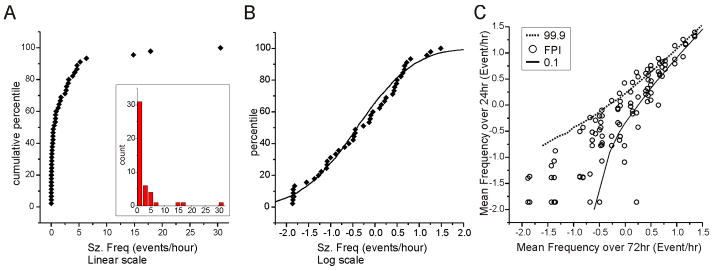
In accord with observations in other models of CSRSs (Arida et al., 1999; Bethmann et al., 2007; Grabbenstatter and Dudek, 2008), identically injured rpFPI rats vary widely in baseline seizure frequency. In 45 epileptic rpFPI animals, the frequency of seizure 4 weeks post-injury spanned 3 orders of magnitude (0.014–31 events/hr) with a distribution that was weighted toward lower values (Fig. 2A). Logarithmically transformed frequency data approximate the sigmoid cumulative histogram of a normal distribution (Fig. 2B), deviating from the sigmoid curve at low frequencies, where the measurement uncertainty is high in proportion to measured values. These data indicate the logarithm as a suitable function to transform the frequency of spontaneous seizure data for statistical analysis. Day-to-day variability in seizure frequency was examined in 35 identically injured rats from which three 24hr-long video-ECoG recordings were obtained during post-injury week 4 (Fig. 2C). For each rat, the three individually determined seizure frequencies were plotted against their mean. The 99.9th and 0.1th percentiles of seizure frequency expected if seizures were generated randomly by a Poisson process were determined in Monte-Carlo simulations. CSRSs occurred in clusters as they do in human PTE (Balish et al., 1991, Bauer and Burr, 2001; Haut et al., 2005). We found that twenty-eight percent of the 24-hr data points exceeded the bounds expected to enclose 99.8% of that data if CSRSs occurred randomly at a stationary rate.
Figure 2. Variability of epilepsy 1 month after injury.
A) Cumulative frequency histogram and frequency histogram (inset) of the distribution of seizure frequencies observed in 45 epileptic rats 4 weeks after rpFPI. B) Cumulative histogram of the data from (A) after logarithmic transformation (filled symbols). Solid line shows a cumulative histogram of 5000 random numbers drawn from a log-normal distribution (mean= −0.4; standard deviation= 1.0). Note the good correspondence of the experimental data to a log-normal distribution for all but the lowest frequency values. C) Comparison of the measurement-to-measurement variation in experimentally observed seizure frequencies (circles) with the computed variation expected for seizures occurring randomly with stationary mean frequencies ranging from 0.025–32 events/hr (lines). The lines delimit the 0.1th (solid) and 99.9th (dotted) percentiles of the distribution of 24-hr seizure frequencies determined in Monte-Carlo simulations (105 iterations). Experimental data are post-injury week 4 seizure frequencies determined in 3 sequential 24hr recordings each from 36 rats. For each rat, the 3 individual frequencies are plotted against their mean. Note that 28% of the experimentally determined values lie outside of the lines that would enclose 99.8% of the data if FPI-induced seizures occurred randomly with a stationary seizure frequency.
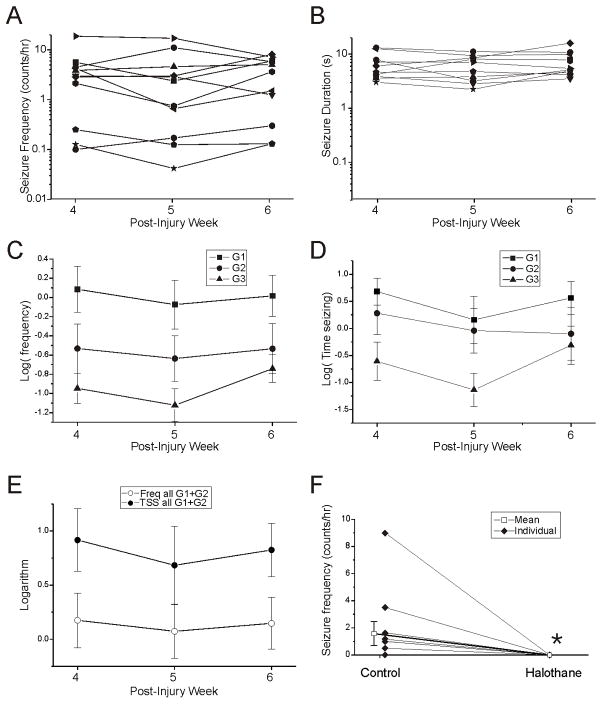
Nonetheless, controls for time-dependent changes in the properties of CSRSs during the testing period (wk 4–6 post-injury), showed no evidence of time trends in either the frequency of any seizure type (Fig. 3C), the duration of seizures (Fig. 3B), or the time spent in any type of seizure (Fig. 3D). These controls consisted of untreated, identically-injured rpFPI rats produced both before and after the tests for CBZ and VPA, and were recorded for 72-hr (N=4), 24-hr (N=1), or 8-hr (N=6) each week during post-injury weeks 4–6. The latter recordings were obtained at the same time of day for each rat. In these controls, the mean frequency of all seizures combined (G1, G2, G3) was 4.1±1.6 events/hr, 3.9±1.6 events/hr and 3.6±0.9 events/hr on post-injury weeks 4, 5 and 6, respectively. By design, and consistent with our previous reports (D’Ambrosio et al., 2004, 2005, 2009), CSRSs observed in this time period were predominantly frontal-lobe focal neocortical (G1 and G2), while G3 seizures, that are likely of limbic origin, were rare (Fig. 3C). The mean logarithms of the frequency of G1+G2 events during post-injury weeks 4, 5 and 6 were, respectively, 0.17±0.25, 0.07±0.25 and 0.15±0.24, while the mean logarithms of time spent in G1 and G2 seizures were 0.86±0.32, 0.72±0.40 and 0.82±0.39 (Fig. 3E). Linear regression analysis revealed no significant time- dependent changes in either the frequency (β=0.10, t=0.90, p=0.37) of or time spent in G1+G2 seizures (β=−0.20, t=0.84, p=0.41). The proportion of G2 seizures over total seizure activity ranged 0%–47% (mean, 15.5±0.05%, N=11).
Figure 3. Properties of untreated rpFPI-induced PTE 4–6 weeks after injury.
A) Plots of mean seizure frequencies in individual rats determined during post-injury weeks 4, 5 and 6. Note the rat-to-rat variability in seizure frequency is about two orders of magnitude larger than the measurement-to-measurement variability in each rat. B) Plots of mean seizure durations in individual rats determined at 4, 5 and 6 weeks post-injury. Note that the rat-to-rat variability in seizure duration is about two orders of magnitude smaller than the variability in seizure frequency. Data in A–B are on a logarithmic scale. C) Mean logarithms of frequencies of G1, G2 and G3 seizures (N=11). D) Mean logarithms of time spent seizing plotted for G1, G2 and G3 events (N=11). E) Mean frequencies (Freq) and times spent in neocortical (G1+G2) seizures (TSS) during post-injury weeks 4, 5 and 6 (N=11). A–E) Note the stability of PTE over the three weeks examined post-injury. F) Halothane completely abolished seizure activity in epileptic rats. While all animals were epileptic (N=11), 6 animals had seizures during a time-of-day matched reference period, and none seized on halothane. Filled symbols indicate individual rats. Open box indicates the mean seizure frequency determined in a time-of-day matched reference (Control) and halothane recording periods (* p=0.042, N=11, Wilcoxon).
Anesthesia was employed as a positive control for seizure suppression (Delgado-Escueta et al.,1982, 1983; Ropper et al., 1986; Murao et al., 2002). Eleven epileptic rpFPI rats were recorded for 72-hr six weeks after injury. Two to three days later, rats were ventilated through a nose cone with 1.5% halothane in 30% O2 and air. Each animal was recorded for 2hr under halothane, and data were compared to time-of-day matched segments of the longer pre-drug recordings. All animals had seizures during their 72hr control recording period and 6 rats seized during the 2hr control reference period (Fig. 3F). In contrast, no animal seized during halothane anesthesia (p=0.018; Fischer’s Exact Test). A comparison of seizure frequencies values before and during halothane was also statistically significant (N=11; p=0.042; Wilcoxon).
Power Analyses
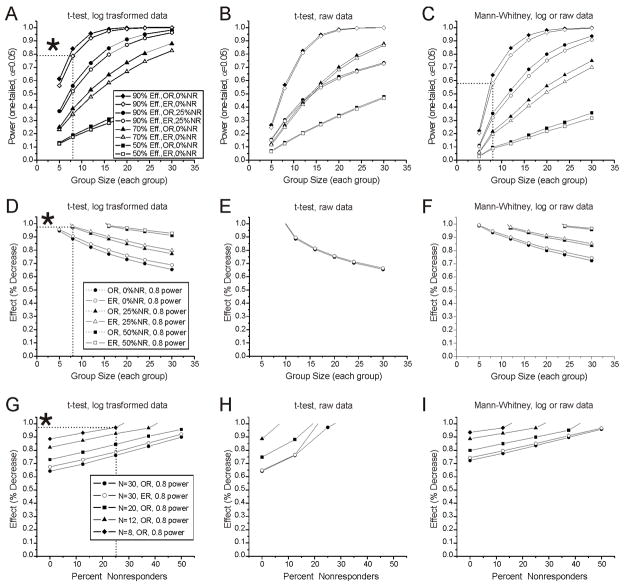
Bootstrap simulations were conducted to optimize the study design and data analysis to better detect changes in seizure frequency of preclinical interest. The variables investigated are listed in Table 1. We first examined the power to detect large treatment effects on raw or logarithmically transformed seizure frequency data in two independent groups randomly selected to receive either no treatment or an effective treatment (Table 1- I a ). Under the most favorable circumstances examined (variation within observed frequency ranges, 100% responders, comparison of log-transformed data with a t-test) group sizes of 8 and 24 (16 and 48 subjects, total) would be required to provide 0.8 power to detect decreases in seizure frequency of 90% and 70%, respectively, and a 50% decrease would be detected in ~50% of experiments with 30 subjects/group (Fig. 4A). This independent groups comparison was not markedly affected by increases in the measurement-to-measurement variability of the data, as modeled by a 50% increase in the ranges from which frequency values were selected, but was substantially degraded by the presence of non-responders in the treatment group (Fig. 4G,H,I). Under the most favorable circumstances examined, 32 subjects were required to achieve 0.8 power to detect a 90% decrease in seizure frequency in the presence of 25% non-responders (Fig. 4A,D). Logarithmic transformation of the frequency data greatly improved the performance of the t-test (compare Fig. 4A,D,G and 4B,E H), but had no effect on the rank-based Mann-Whitney test. Overall, use of a t-test with logarithmically transformed data provided the greatest power to detect anti-epileptic decreases in seizure frequency in independent-groups comparisons. However, this study design requires large numbers of animals to compensate for its sensitivity to non-responders and, thus, is impractical for preclinical development of AEDs.
Figure 4. Effect of measurement-to-measurement variability and non-responders on statistical power to detect decreases in seizure frequency in independent-groups comparisons (Table 1, Ia) conducted using Mann-Whitney U or unpaired t-test.
A–C) Power to detect 50%, 70% and 90% decreases in seizure frequencies drawn from observed (OR) and 50% extended (ER) frequency ranges, when non-responders (NR) comprise 0% or 25% (90% decrease, only) of the indicated experimental groups. A) Power as a function of group size for logarithmically transformed frequency data analyzed with t-test. B) Power as a function of group size for raw frequency data analyzed with t-test. C) Power as a function of group size for both logarithmically transformed and raw frequency data analyzed with Mann-Whitney. Note that the Mann-Whitney test returns identical results (overlapped) for raw and logarithmically transformed data. Legend in A refers to A–C. D–F) Group sizes required to provide 0.8 power to detect the indicated reductions in seizure frequency in groups containing 0%, 25% and 50% NR when logarithmically transformed (D) or raw (E) frequency data are analyzed using a t-test, or when either raw or transformed data are assessed with the Mann-Whitney test (F). Legend in D refers to D–F. G–I) Effect sizes detectable with 0.8 power as a function of the percentage of NR in independent groups of 8, 12, 20 and 30 subjects each when logarithmically transformed (G) or raw (H) frequency data are analyzed using t-test, or when either raw or transformed data are assessed with Mann-Whitney test (I). Legend in G refers to G–I. Throughout, asterisks in A, D, and G highlight the higher performance of the t-test and log transformed data over the Mann-Whitney U test. Note that the statistical power of independent groups analyses are minimally affected by a 50% increase in the range of data variation (ER), but is markedly diminished by non-responding subjects. Drug effects refer to responding animals, not to group averages. In panels D–I missing data points did not reach power of 0.8.
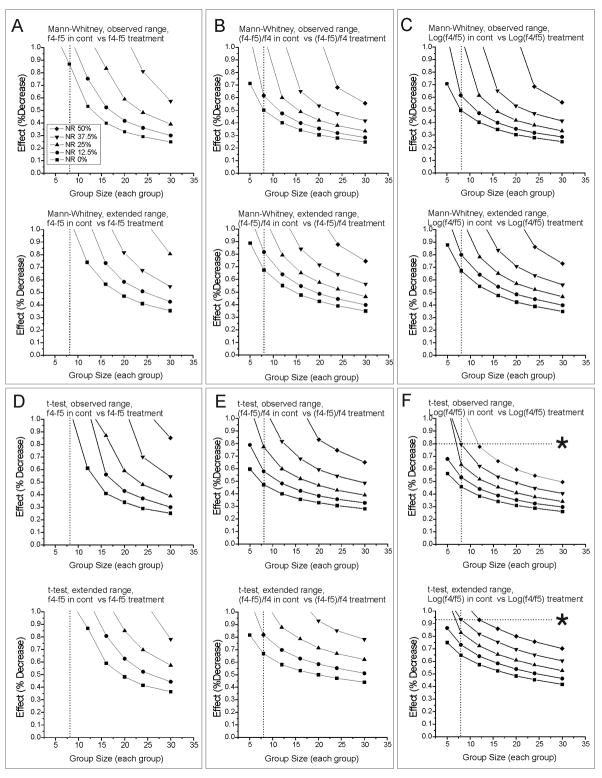
We then explored the performance of repeated-measure designs, which allow one to assess treatment effects as paired (pretreatment minus treatment) differences in seizure frequency, minimizing the effect of subject-to-subject variability on the analysis. We first examined the comparison of treatment effects determined in (paired) repeated measures of seizure frequency in independent groups of control or treated subjects (Table 1 – I b). Under the most favorable circumstances examined (variation within observed ranges, 100% responders, comparison of differences of log-transformed frequency data with a t-test), experimental groups of 8 subjects each (16, total) provide more than 0.8 power to detect a 45% decrease in seizure frequency. However, in the presence of up to 37.5% non-responders, detection of a much larger 80% decrease would require up to 32 animals (Fig. 5F). With this experimental design, the analysis of logarithmically transformed data improved the performance of the t-test (Fig. 5D–F) which surpasses that of the Mann-Whitney test (Fig. 5A–C) particularly in the presence of non-responders. The best performance was obtained using a t-test with logarithmically transformed data. Of note, the proportional effect (Table 1, III-d), commonly employed in preclinical drug development, could never yield adequate power to detect even large drug effects with either non-parametric (Fig. 5B) or parametric (Fig. 5E) statistical tests when non-responders represented more than 12% of the experimental group. While, this study design performs better than the previous unpaired independent-groups design, it is still impractical for preclinical development of AEDs when non-responders are expected.
Figure 5. Independent-groups comparison of paired pre-treatment minus treatment differences obtained from treated and matched control subjects in repeated-measures experiments (Table 1, Ib) conducted using Mann-Whitney U or unpaired t-test.
Each panel shows group sizes required to provide 0.8 power to detect the indicated reductions in seizure frequency in groups containing 0, 12.5%, 25%, 37.5% and 50% non-responders (NR). A) Mann-Whitney analysis of differences of raw frequencies (control f4 –control f5 vs treatment f4 – treatment f5) when seizure frequencies are obtained from observed ranges (top panel), and from ranges extended by 50% (bottom panel). B) Mann-Whitney analysis of proportional differences of raw frequencies [(control f4 –control f5)/control f4 vs (treatment f4 – treatment f5)/treatment f5] when seizure frequencies are obtained from observed ranges (top panel), and from ranges extended by 50% (bottom panel). C) Mann-Whitney analysis of differences of logarithmically transformed frequencies [log10(control f4)−log10(control f5) vs log10(treatment f4)−log10(treatment f5)] when seizure frequencies are obtained from observed ranges (top panel), and from ranges extended by 50% (bottom panel). D) t-test analysis of differences of raw frequencies (control f4 –control f5 vs treatment f4 – treatment f5) when seizure frequencies are obtained from observed ranges (top panel), and from ranges extended by 50% (bottom panel). E) t-test analysis of proportional differences of raw frequencies [(control f4 –control f5)/control f4 vs (treatment f4 – treatment f5)/treatment f5] when seizure frequencies are obtained from observed ranges (top panel), and from ranges extended by 50% (bottom panel). F) t-test analysis of differences of logarithmically transformed frequencies [log10(control f4)−log10(control f5) vs log10(treatment f4)−log10(treatment f5)] when seizure frequencies are obtained from observed ranges (top panel), and from ranges extended by 50% (bottom panel). Asterisks in F highlight the higher performance of the t-test and log transformed data in the presence of large proportion of NR and small group sizes. All drug effects refer to responding animals, not to group averages. Throughout, missing data points did not reach power of 0.8.
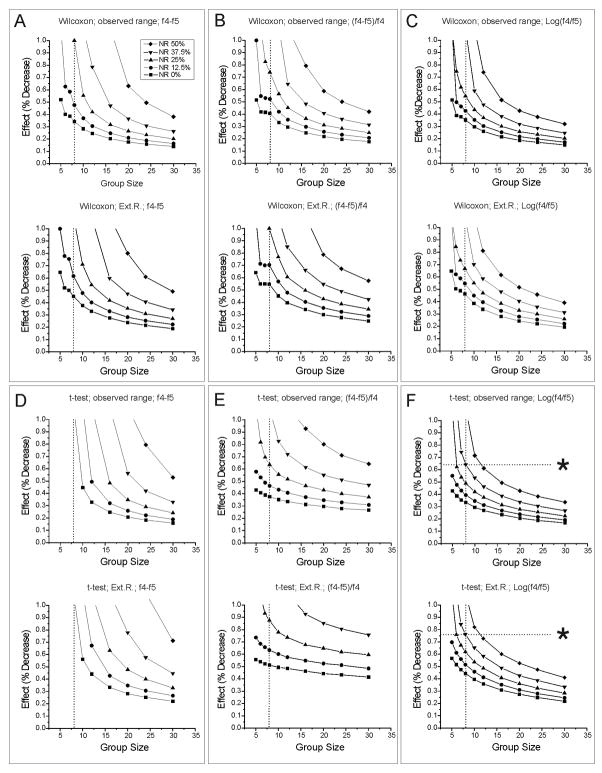
Because the frequency of neocortical seizure (G1+G2) in untreated animals is remarkably stable between the fourth and sixth weeks post-injury, and similar in different batches of animals (D’Ambrosio et al., 2004, 2005, and Fig. 3), anti-epileptic effects could also be evaluated in a paired analysis of pre-treatment minus treatment differences, with each subject serving as its own control (Table 1 – I c). To this end, we employed raw and logarithmically transformed seizure frequency data and proportional differences ((f4–f5)/f4) in seizure frequency using both the Wilcoxon matched-pairs rank sum test and a paired t-test to evaluate anti-epileptic effects (Fig. 6). In the absence of non-responders, these three measures performed similarly in Wilcoxon-based detection (Fig. 6A–C). Differences of log-transformed frequencies (Fig. 6C), however, provided superior performance when non-responders were present. As expected for a parametric test, the t-test displayed markedly greater sensitivity than the Wilcoxon test to the comparison statistic employed. Raw differences of seizure frequencies performed poorly under all circumstances examined (Fig. 6D). While proportional differences (Fig. 6E) and differences of logarithms (Fig. 6F) provided similar performance in the absence of non-responders, the performance of differences of log-transformed frequencies was much better when non-responders were present. With this approach, a single group of 8 subjects provided power in excess of 0.8 to detect anti-epileptic decreases in seizure frequency of preclinical interest (65–75%), even when up to 37.5% of subjects failed to respond to treatment (Fig. 6F). Additional simulations in which drug effects were set to zero confirmed the absence of significant bias in simulations of experiments with small group sizes. When evaluated using a t-test, the mean incidences of false positives were 4%, 5%, and 6% in simulations of groups of 5, 8, and 12 subjects, respectively. This group-size-dependent increase in false positives is attributable to the 3 event/72hr floor enforced on the simulated pre-treatment seizure frequency since the incidence of false positives varied between 4% and 5% when the floor was not enforced. Overall, parametric comparisons of paired differences of log-transformed frequencies represent the most practical strategy among those investigated for use of ECoG-monitored rpFPI-induced epilepsy in AED studies: robust detection of seizure-frequency decreases of preclinical interest can be achieved with small groups of just 8 subjects, total, even in the presence of non-responders.
Figure 6. Paired comparisons of pre-treatment minus treatment differences in seizure frequency (Table 1, Ic) conducted using paired t-test or Wilcoxon matched-pairs rank sum test.
Each panel shows group sizes required to provide 0.8 power to detect the indicated reductions in seizure frequency in groups containing 0, 12.5%, 25%, 37.5% and 50% non-responders (NR). A) Wilcoxon analysis of differences of raw frequencies (control f4 –control f5 vs treatment f4 – treatment f5) when seizure frequencies are obtained from observed ranges (top panel), and from ranges extended by 50% (bottom panel). B) Wilcoxon analysis of proportional differences of raw frequencies [(control f4 –control f5)/control f4 vs (treatment f4 – treatment f5)/treatment f5] when seizure frequencies are obtained from observed ranges (top panel), and from ranges extended by 50% (bottom panel). C) Wilcoxon analysis of differences of logarithmically transformed frequencies [log10(control f4)−log10(control f5) vs log10(treatment f4)−log10(treatment f5)] when seizure frequencies are obtained from observed ranges (top panel), and from ranges extended by 50% (bottom panel). D) Paired t-test analysis of differences of raw frequencies (control f4 –control f5 vs treatment f4 – treatment f5) when seizure frequencies are obtained from observed ranges (top panel), and from ranges extended by 50% (bottom panel). E) Paired t-test analysis of proportional differences of raw frequencies [(control f4 –control f5)/control f4 vs (treatment f4 – treatment f5)/treatment f5] when seizure frequencies are obtained from observed ranges (top panel), and from ranges extended by 50% (bottom panel). F) Paired t-test analysis of differences of logarithmically transformed frequencies [log10(control f4)−log10(control f5) vs log10(treatment f4)−log10(treatment f5)] when seizure frequencies are obtained from observed ranges (top panel), and from ranges extended by 50% (bottom panel). Asterisks in F highlight the higher performance of the paired t-test and log-transformed data in the presence of large proportion of NR and small group sizes. All drug effects refer to responding animals, not to group averages. Note that analysis of logarithmically transformed data permits the most sensitive detection of anti-epileptic effect in the presence of NR, and that analysis of log-transformed frequency data with a paired t-test provides superior performance under all circumstances examined. Throughout, missing data points did not reach power of 0.8.
Effects of carbamazepine and valproate on PTE induced by rpFPI
On the basis of our statistical power analyses, we examined the effects of CBZ and VPA on neocortical CSRSs using the optimized paired repeated-measures design. The frequency and duration of G1, G2 and G3 seizures was determined weekly on the basis of 48–72h of ECoG recording before, during and after drug treatment (post-injury weeks 4, 5 and 6). Seizure frequency data were logarithmically transformed, and pre-treatment minus treatment differences were computed and examined with paired t-tests. Based on the power analyses, we expected at least 0.8 power to detect an 65–75% decrease in seizure frequency in the presence of up to 37.5% unresponsive rats.
Carbamazepine
As in controls, individual seizure frequencies displayed high variability both between subjects and over time (Fig. 7A). The week 4 baseline frequency of all seizures in 8 epileptic rats injured for assessment of CBZ ranged from 0.04–5.2 events/hr (mean, 2.0±0.6 events/hr). The frequency of G1 and G2 seizures ranged 0.06–4.5 and 0–0.45 events/hour, respectively. The proportion of G2 seizures over total seizure activity ranged 0%–35.2% (mean, 9±0.04%).
Although plasma CBZ attained therapeutic levels of 7.8+1.2 μg/ml 4–5 days after pump implantation, as expected on the basis of a pharmacokinetic pilot study of 3-dose stepped dosing protocol (Fig. 7G), there was no detectable improvement in the FPI-induced epileptic syndrome during CBZ treatment. Treatment with CBZ resulted in no decrease in the mean logarithm of the frequency of any type of seizure (Fig. 7C), and the mean logarithm of the frequency of neocortical (G1+G2) seizures was −0.09±0.31 prior to treatment, −0.03 ±0.31 (p=0.33 vs pre-treatment, paired t-test) during treatment and −0.42±0.28 (p=0.07 vs pre-treatment, t-test) after washout of CBZ (Fig. 7E). The mean duration of neocortical seizures, 4.8±0.6 sec, 5.5±0.6 sec and 4.1±0.8 sec before, during and after treatment, was similarly undiminished by CBZ (Fig. 7B), and the time spent in neocortical seizures paralleled their frequency (Fig. 7E). The logarithms of time spent in neocortical seizures were 0.56±0.37 before treatment, 0.69±0.33 (p=0.24 vs pre-treatment, paired t-test) during treatment and 0.15±0.34 (p=0.07 vs pre-treatment, paired t-test) after washout of CBZ. To help put these results in context with studies in which short seizures are arbitrarily discounted, an equivalent analysis of neocortical seizures longer than 5s is displayed in figure 7F. Both analyses indicate no significant effect of CBZ on neocortical seizures.
Valproate
In 8 epileptic animals assigned to VPA treatment, the pre-drug baseline frequency of all seizures was 1.6+0.6 events/hr (range: 0.13–4.66 events/hr). The frequency of G1 and G2 seizures ranged 0.13–3.1 and 0–2.0 events/hour, respectively. The proportion of G2 seizures over total seizure activity ranged 0%–43% (mean, 14±0.06%).
In accord with pharmacokinetic pilot studies, VPA plasma levels were 37±5 μg/ml, two days after pump implantation and 30±3 μg/ml on treatment day 7 (Fig. 8G). VPA treatment resulted in a significant decrease in the logarithms of the frequencies of G1 events from −0.29±0.20 on post-injury week 4, versus −0.65±0.25 during VPA administration on week 5 (p=0.018, paired t-test) and −0.92 ±0.35 during week 6 (p=0.038, paired t-test; Fig. 8C). The effect of VPA developed slowly over 6 days of treatment (Fig. 8G), and persisted through the following week. No change was observed in the frequencies of G2 (p≥0.16, paired t-test) and G3 (p≥0.32, paired t-test) events either during or after VPA administration (Fig. 8C). The logarithm of time spent in G1 seizure decreased from 0.35±0.21 before treatment to −0.08±0.29 (p=0.020, paired t-test) during and −0.60±0.45 (p=0.17, paired t-test) after VPA (Fig. 8D). The mean logarithm of the frequency of focal neocortical seizures (G1+G2; Fig. 8E) was significantly decreased both during (−0.57±0.29, p=0.014, paired t-test) and after (−0.84±0.39; p=0.037, paired t-test) VPA treatment compared with post-injury week 4 (−0.20±0.22), despite confirmed washout of VPA prior to post-treatment recording. There was no appreciable change in seizure duration (Fig. 8B), and the time spent seizing paralleled seizure frequency (Fig. 8E). As was shown for CBZ, analyses of all detectable neocortical seizures (Fig. 8E), and of only those seizures lasting longer than 5s (Fig. 8F), lead to identical inferences about the effect of treatment. While treatment with VPA resulted in significant decreases in the group’s seizure frequency and time spent seizing, not all rats were similarly affected. Five animals displayed temporal patterns of seizure frequencies undistinguishable from untreated controls, while three trended downward to become seizure-free on week 6 (responders). Although the VPA plasma levels on the last day of treatment varied in individual rats (range 21–46ug/ml; mean 30±3 ug/ml), the anti-epileptic effect of VPA treatment did not correlate with its plasma levels (rho=0.02, p>0.96, Spearman, Fig. 9E,F).
Figure 8. Effect of valproate on rpFPI-induced PTE 5 weeks after injury.
A) Plots of mean seizure frequency (G1+G2+G3) in 8 individual rats before (week 4), during (week 5) and after (week 6) continuous subcutaneous infusion of VPA via osmotic minipump. Three animals became seizure free within 6 days of treatment at week 5, and were seizure free on post-injury week 6. B) Plots of mean duration of all seizures in the same rats before, during and after administration of VPA. Lines are truncated in those animals that did not seize during week 6 observation. Data in A–B are on a logarithmic scale. C) Mean logarithms of the frequencies of G1, G2 and G3 seizures before, during and after VPA administration (*p<0.05 versus week 4, paired t-test). D) Mean logarithms of time spent in G1, G2 and G3 seizures before, during and after VPA administration (*p<0.05 versus week 4, paired t-test). E) Mean logarithms of frequencies (Freq) and times spent in neocortical (G1+G2) seizure (TSS) before, during and after administration of VPA (*p<0.05 versus week 4, paired t-test). Displayed data include all detectable (longer than 0.8s) neocortical seizures. F) Effect of VPA on the activity of the neocortical focus demonstrated as in (E), but including only seizures longer than 5s. Note that the analyses displayed in (E) and (F) are similar but that the greater number of events included in (E) may result in enhanced sensitivity. G) Seizure frequency and [VPA]plasma in responders (filled squares) and non-responders (hollow circles) administered 480 mg*kg−1*day−1 VPA for 7 days. Mean frequencies are displayed for each 24h observation period (3 recordings per week) and refer to the scale to the left. Plasma levels refer to the scale to the right. The time scale is shared for both frequency and [VPA] data. Note that the responders exhibit a slow, steady decline in seizure frequency during VPA administration, and no seizures in the last recording during treatment and in the week after treatment. The value −1.45 represents the logarithm of the floor seizure-frequency value assigned to observations of no seizures in each 24h recordings.
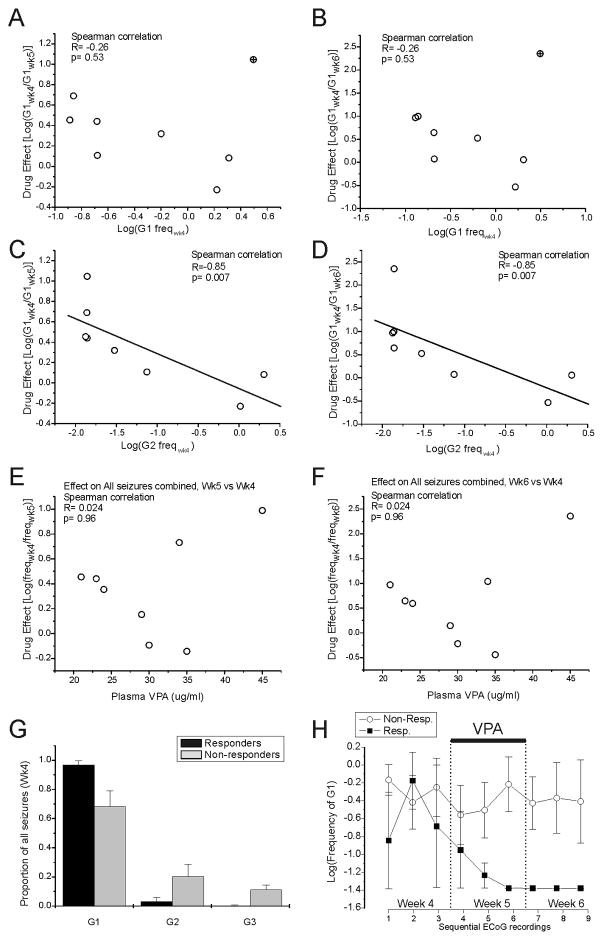
Figure 9. The effect of VPA correlates with the pre-treatment frequency of spreading seizures.
A–D) Correlations of the effect of VPA on the frequency of G1 seizures during week 5 or 6 vs week 4 with the pre-treatment frequency of G1 and G2 seizures. The pre-treatment frequency of non-spreading G1 seizures is not correlated with the effect of VPA at week 5 (A) and after cessation of VPA treatment at week 6 (B). The crossed circle indicates the one animal critical for the lack of correlation. C) The pre-treatment frequency of G2 events is significantly correlated with the effect of VPA at wk 5. D) The pre-treatment frequency of G2 events is significantly correlated with the effect of VPA at week 6. Straight lines in B and D represent linear least-square fits to the experimental points. E–F) Lack of correlation of the effect of VPA on the frequency of all seizure types during week 5 or 6 vs week 4 with plasma levels. Plasma [VPA] at week 5 is not significantly correlated with the effect of VPA assessed either during (E) or after cessation (F) of VPA administration. G) Proportions of G1 (non-spreading), and G2 and G3 (spreading) seizures assessed prior to treatment in rats that became seizure-free with VPA treatment (responders) and in those that did not (non-responders). The non-responders exhibited a larger proportion of spreading seizures than the responders. H) VPA does not affect the frequency of G1 events in non-responders (hollow circles), but abolishes G1 seizures in responders (filled squares). Data are displayed for each 24h observation period (3 recordings per week). The value −1.45 represents the logarithm of the floor seizure-frequency value assigned to observations of no seizures in each 24h recordings. Note that the responders exhibit a slow, steady decline in seizure frequency during VPA administration, and no seizures in the last recording during treatment and in the week after treatment.
Therefore, we sought features of the epilepsy evident prior to treatment that correlated with the efficacy of VPA. Responders exhibited just 0.01±0.01 spreading seizures (G2 and G3) per hour on post-injury week 4 (3±3% of total seizure count), while non-responders exhibited 0.68±0.11 such events per hour accounting for 32±11% of their total seizure counts (Fig. 9G). Thus we examined correlations of the pre-treatment (week 4) frequencies of the various seizure types with the week 5 and week 6 anti-epileptic effect as indicated by the pre-treatment minus week 5 or week 6 difference in the log-transformed frequencies of G1 events. The logarithm of the week 4 frequency of G2 (rho=0.85; p=0.007; Spearman, Fig. 9C,D), G3 (rho=0.78; p=0.022, Spearman, not shown), G2+G3 (rho=0.75; p=0.031; Spearman, not shown) events were all significantly correlated with anti-epileptic effect.
The robustness of this correlation was confirmed by systematically eliminating each of the data points, one at a time, from the analysis. In all 8 cases, the correlations remained statistically significant (p=0.015 at most). The logarithm of the frequency of G1 (rho=0.24; p=0.57, Spearman, Fig. 9A,B), G1+G2 (rho=0.40; p=0.32, Spearman, not shown) and of all events combined (rho=0.40; p=0.32, Spearman, not shown) did not significantly correlate with the effect of valproate. However, the absence of statistical significance depended in all cases on one single animal (top right symbol in Fig. 9A and 9B) with high frequency of G1 seizures before treatment, and large effect of the drug on week 5 and 6. Significant correlations between the effect of VPA and the frequency of G1 (R=−0.893; p=0.007; Spearman), G1+G2 (−0.893; 0.007; Spearman), and all events (−0.786; 0.036; Spearman) were obtained when this animal is removed from the dataset.
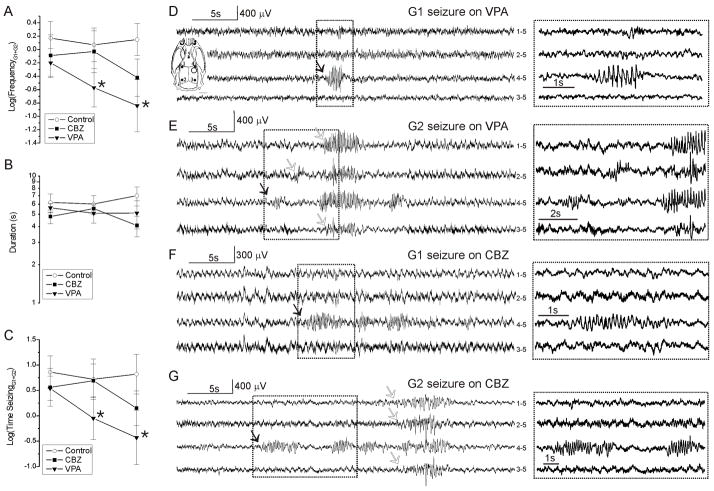
Neocortical seizure frequency, duration and time spent seizing are compared among untreated, CBZ-treated and VPA-treated epileptic rats in Fig. 10. The mean duration of G1+G2 seizures was similar in untreated controls and in drug-treated animals, and did not change appreciably over time or as a result of treatment (Fig. 10B). Accordingly, times spent seizing (Fig. 10C) paralleled frequencies of seizure (Fig. 10A). There was no significant change in seizure frequency or time spent seizing either in untreated controls or in rats treated with CBZ. VPA-treated rats, in contrast, exhibited a decrease in both seizure frequency and time spent seizing that persisted 1 week after cessation of treatment.
Figure 10. Comparison of frotal-lobe seizure frequency, duration and time spent seizing in untreated, CBZ-treated and VPA-treated rats.
A) The mean logarithms of the frequencies of neocortical (G1+G2) seizures decreased progressively in VPA treated rats (filled triangles; N=8 *p<0.05 compared to week 4, paired t-test) but not in untreated controls (hollow circles; N=11) or CBZ-treated (filled squares; N=8) rats. B) The mean logarithms of the duration of neocortical seizure in A is similar among treatments and stable over the course of the study. C) The times spent in neocortical seizure in A parallels seizure frequency, and decreases progressively in VPA treated rats (*p<0.05 compared to week 4, paired t-test) but not in untreated controls or CBZ-treated rats. D–G) Representative examples of G1 and G2 seizures coincident to behavioral arrest and detected by ECoG during week 5 treatment with CBZ or VPA. Filled arrows indicate seizure onset. Hollow arrows indicate seizure spread. Boxed regions are shown at higher temporal resolution at right. Inset in D represents the schematic of the locations of the five epidural ECoG electrodes (filled circles) and of the injury site (hollow circle) in respect to the rat skull for all ECoG recordings shown in D–G. Throughout the panels, numbers next to each ECoG trace indicate the electrodes by which the trace is recorded, and its reference.
DISCUSSION
This study is the first effort to adapt the FPI model of PTE to ECoG-based AED testing, and it departs from most previous pre-clinical AED studies in several respects. First, anti-epileptic effects are evaluated on CSRSs that arise as a consequence of an etiologically realistic model injury. Second, animals are chronically exposed to AEDs. Third, seizures are sensitively detected and quantitated by chronic ECoG recording. Fourth, all detectable seizures, rather than only those that exceed an arbitrary duration-based criterion, are measured. Finally, the drug testing protocol was optimized on the basis of extensive nonparametric power analyses. Because FPI-induced epilepsy displays high rat-to-rat variability in frequency of CSRSs and seizure clustering, as expected for a clinically relevant model of human PTE (Haut et al., 2005; Balish et al., 1991; Bauer and Burr, 2001), and because human PTE is often pharmacoresistant (Semah et al., 1998), detailed optimization of the study protocols and of the analytical methods was required before attempting AED studies. We demonstrate that, despite the variability of CSRS frequency and the existence of non-responders, an optimized testing protocol can reliably detect anti-epileptic effects of preclinical interest with small numbers of animals. We further demonstrate that halothane blocks all seizures, while CBZ is ineffective and VPA has a slow, progressive effect in animals suffering from neocortical non-spreading seizures.
Optimization of the drug-testing protocol by bootstrap power analyses
We employed a non-parametric bootstrap strategy to conduct a comprehensive analysis of the impact of several variables (Table 1) on the statistical power to detect antiepileptic effects by ECoG. In order to model both subject-to-subject and measurement-to-measurement variability closely on experimental data, bootstrap power analyses (Fig. 1A) were based on actual seizure frequency data from rpFPI rats recorded 4 weeks after injury (Fig. 2). To ensure that power analyses were conservative and reliable, we also examined how expansion of subjects’ seizure-frequency ranges (i.e., variability) affected the statistical power to detect antiepileptic effects, and observed a small and progressive deterioration in power, as expected. We found that independent-groups comparisons (Table 1, Ia) are not practical for AED testing mostly because of the detrimental effect of non-responders on study power. Thirty-two animals are required to provide 0.8 power to detect a large effect of 90% seizure frequency decrease in the presence of just 25% non-responders (Fig. 4A,G). Consistent with the dominance of subject-to-subject over measurement-to-measurement seizure frequency variance (Fig. 3A), the latter had little effect on the performance of any independent group comparison, as demonstrated by the small impact of the extended range on the power analyses (Fig. 4A–F). A decrease of subject-to-subject variability, and thus an increase in power, could be achieved with independent-groups comparisons by assessing anti-epileptic effects as pre-treatment minus treatment differences in seizure frequency in a repeated measures design (Table 1, Ib), but at a cost of doubling the amount of data collected and analyzed for each subject. Here, the logarithmic transformation of the seizure frequency data improves the performance of both the parametric and non-parametric analyses of the data (Fig. 5). When differences are compared between control and treatment groups, the optimal analysis (t-test performed with differences of log-transformed frequencies) requires just 5 subjects per group (10 animals total) to provide 0.8 power to detect a 55% reduction in seizure frequency in the absence of non-responders, while a 90% reduction in seizure frequency can be detected with two groups of 8 subjects including up to 40% non-responders (Fig. 5I–L). As expected for a repeated measure design, measurement-to-measurement variation has a larger effect on this comparison of differences (Fig. 5G–H) than on the independent-groups comparison described above (Fig. 4D). Overall, this experimental design represents an improvement over a simple independent-groups comparison when there is an expectation that some subjects may be refractory to treatment. However, when non-responders represent ~37% of the subjects, the optimal analysis (Fig. 5F) can only detect large effects (80–90% seizure frequency decrease) with a manageable number of animals (<16).
When there are no systematic changes in seizure frequency over time post-injury, as is the case for neocortical seizures at wk4–6 after rpFPI (D’Ambrosio et al, 2004, 2005; Fig. 3), the treatment effects can more powerfully be assessed by paired pre-treatment minus treatment differences in a repeated measures experiment in which each subject serves as its own control (Table 1, Ic). With this experimental design, the performance of both parametric and non-parametric analyses was improved by logarithmic transformation of the data, particularly in the presence of non-responders. The t-test applied after log transformation (Fig. 6F) provided greater power to detect AED effects in the presence of non-responders than the Wilcoxon test applied to any of the raw or transformed data (Fig. 6A–C). Indeed, simulations show a group of just 8 subjects to provide adequate (0.8) power to detect a 65%–75% decrease in seizure frequency in an experimental group composed of up to ~37% non-responders. Thus, for the purpose of exploring investigational drugs or treatments, this protocol is superior to the others presented because it allows detection of preclinically important drug effects with significantly fewer animals, ECoG data acquisition and analysis.
The advantage of log transforming seizure data
Our data support the use of a logarithmic transformation for analysis of rpFPI seizure frequency data on multiple grounds. First, we show that the frequencies of seizure in rpFPI rats approximate a log-normal distribution (Fig. 2B). This property, which is likely specific to the chronic model, seizure definition and diagnostic technique employed, suggests a logarithmic transformation as most appropriate for analysis because this distribution is normalized under logarithmic transformation, permitting use of efficient parametric statistical techniques. Indeed, parametric analysis of log-transformed data provided the best performance in all of our simulations (Figs. 4–6). Secondly, for the purpose of evaluating anti-epileptic treatments efficiently, it is important that all subjects responding to the drug contribute substantially to the determination of the effect regardless of their initial seizure frequency. The logarithmic transformation permits an equal weighting of changes in seizure frequency in a magnitude-independent manner because the difference in the logarithm of seizure frequency before and after a specified percentage decrease in frequency is identical regardless of the initial seizure frequency. A simple proportion (Table 1, IIId, IIIg) has this same property, but we found it is exquisitely sensitive to variability in seizure frequency and to the presence of non-responders, making it the lowest performing transformation among those tested (Figs. 5, 6). Conversely, we show that the use of the logarithm renders drug testing more robust to random assortments of non-responders and of subjects with different seizure frequencies (Figs. 5, 6). This is important because rats, like humans, show a wide variation in seizure frequency and sensitivity to drugs. The power analyses indicated that the data obtained with our study protocol (Fig. 1B), when analyzed with a paired t-test after logarithmic transformation of repeated measures (Fig. 6), provide adequate power (>0.8) to detect (with α=0.05) a 40–75% decrease in seizure frequency with a group size of 8 even if 25–40% of the subjects are pharmacoresistant (Fig. 6F). This performance is adequate for preclinical studies of AEDs.
Effects of halothane, carbamazepine and valproate
Control data obtained from animals injured before and after the studies of CBZ and VPA verified the temporal stability and reproducibility of the rpFPI-induced PTE model (Fig. 3A–E). These epileptic animals were also employed as positive controls for the antiepileptic effect. Because anesthetics are often employed to control otherwise pharmacoresistant status epilepticus (Minicucci et al., 2006), we employed halothane anesthesia to verify that a complete suppression of CSRSs is achieved by pharmacological coma (Fig. 3F). Thus, the observation that CBZ attained therapeutic plasma levels but exerted no detectable anti-epileptic effect on any type of rpFPI-induced CSRSs despite the adequate group size (Fig. 7), strongly suggests that it has no appreciable effect on the neocortical partial seizures in the early months after rpFPI. The mechanistic bases of this resistance, possibly including the blood-brain-barrier expression of membrane transporters (Luna-Tortos et al., 2008) and the lack of effect on voltage operated Na+-channels (Remy et al., 2003), remain to be determined. VPA, in contrast, induced significant decreases in seizure frequency and time spent seizing, but not in the mean duration of seizure, in a subset of epileptic rats (Figs. 8–9). In addition, VPA was found to be ineffective on neocortical spreading G2 seizures (Fig. 8C). Thus, VPA did not affect mechanisms of seizure maintenance and termination that determine seizure duration, nor those involved in seizure spread, inconsistent with the old hypothesis that its use-dependent action at the Na+-channel inactivation gate is an important mediator of its observed anti-ictogenic effect. Indeed, Loscher (2002) noted the existence of late effects of VPA, evident in concentration-independent time-dependent increases in efficacy during chronic treatment in both humans and animals, that were independent from its acute actions on Na+ channels. Our data support these observations showing that VPA’s effects on rpFPI-induced CSRSs are negligible within 2 days of administration and begin to be detectable after 4 days of administration (Fig. 8G). Thus, acute drug-administration protocols would have failed to identify this slow effect, further demonstrating the need to prolong the exposure of epileptic animals to investigational AEDs in preclinical studies.
The varied effects of VPA on individual epileptic animals cannot be explained by the animal’s plasma levels (Fig. 9E,F). However, VPA’s antiepileptic effect significantly inversely correlated with the pre-treatment frequency of G2 (Fig. 9C,D) and G3 spreading seizures, which signal a more mature and/or more severe PTE (D’Ambrosio et al., 2004, 2005). This correlation was robust, as it did not depend on any single datapoint, and was not due to seizure-type selectivity, since VPA had no effect on G2 seizures, and G1 seizures were insensitive to VPA in non-responding rats but sensitive to it in responding rats (Fig. 9H). Notably, there was no significant correlation between the effect of VPA and the frequency of either G1 seizures or all seizures, but this lack of significant correlation is entirely dependent on one animal (upper right data point, Fig. 9A,B). Thus, it is possible that VPA’s effects could be correlated, as well, with overall seizure frequency - though this is not supported by our data. In either case, the data indicate that the effect of VPA may depend on the severity of the epileptic condition of the animal, quantified either by the overall frequency of seizures, or by the frequency of the spreading seizures (G2+G3). These data are consistent with the recently articulated hypothesis that refractoriness may reflect the severity of the underlying epilepsy (Rogawski and Johnson, 2008; Schmidt and Loscher, 2009). If this hypothesis is correct, FPI-epilepsy is expected to be progressively less sensitive to VPA, and other AEDs, since both frequency of all seizures and the frequency of spreading seizures increase with time post-injury (D’Ambrosio et al., 2004, 2005). Further work is needed to address the issue.
Also, the data indicate that a subpopulation of epileptic rpFPI rats is resistant to VPA, and that drug responsiveness may be predictable prior to treatment. VPA exhibited strong anti-epileptic effect on ~40% of the animals, while CBZ had no detectable effect on any animals despite their similarity in frequency and proportion of spreading seizures. Thus, a significant subset of FPI rats (~60%) are resistant to two AEDs and are therefore pharmacoresistant by the most common definition (French, 2007). A notable finding was the persistence of a significant anti-epileptic effect of VPA after cessation of administration and confirmed washout of the drug (Fig. 8). VPA acted slowly during the week of administration, resulting in the apparent remission of seizures in 3 out of 8 treated rats within 6 days of treatment (Fig. 8G). This rate of apparent remission is significant when compared to the 0 apparent remissions out of 19 animals (11 untreated and 8 CBZ-treated; Figs. 3A, 7A) observed at week 6 (p=0.019, Fischer’s exact test).
While these data might seem to be at odds with clinical data reporting no effect of VPA treatment after head injury on the subsequent development of PTE (Temkin 2001, 2009), differences in population, types of head injuries, treatment protocols, and diagnostics of epilepsy hamper meaningful comparison. The clinical trial included adult patients of both genders suffering from several different types and severities of head injury. Even when broken down by severity based on the Glasgow Coma Scale very diverse types of injuries (closed vs. acceleration injury; contusive vs. diffuse; single blunt-injury vs. motor-vehicle accidents), with likely different epileptogenic action, were grouped together. The prophylactic VPA administration was initiated within 24hr of injury, well within the acute phase of the injury and before the onset of chronic seizures, and continued for up to 6 months, but more recent work has shown that AEDs given acutely during the phase of epileptogenesis may produce both tolerance and cross-tolerance to similar AEDs (Zhang et al., 2003; Krupp et al., 2000). In addition, epilepsy was only identified in patients who later suffered seizures sufficiently severe to be noticed. Patients experiencing seizures that remained subclinical or that manifested subtly were likely not identified (D’Ambrosio et al., 2009). Conversely, in the present preclinical study, we utilized a single, carefully calibrated, contusive closed head injury delivered to a relatively homogeneous population of out-bred male juvenile rats. VPA was then administered to epileptic rats during week 5 post-injury, well after the acute phase had resolved, and after the full development of the neocortical epileptic focus (D’Ambrosio et al., 2005). In addition, we evaluated epilepsy prospectively by video-ECoG in all injured animals regardless of the severity of their PTE. Further experimental work will be required to determine the exact mechanisms of action of VPA, and therefore the population of patients that would benefit from it, the duration of its effect, and the time window within which intervention is effective.
Relationship of rpFPI-induced epilepsy to other models of CSRSs
Most previous work has measured antiepileptic effects on evoked motor seizures at peak blood levels after bolus injection of AED. While, this approach permits fast screening, recent evidence indicates that antiepileptic effects can develop on a much slower temporal scale (Loscher, 2007). Thus, our dosing protocol was designed to provide a constant exposure to AEDs for a week. However, this approach, and our use of ECoG instead of behavioral observations, prevents a direct comparison with most previous pharmacological work. CBZ has been reported to be effective on motor CSRSs induced by pilocarpine-induced status epilepticus (Leite and Cavalheiro, 1995), but blood levels achieved during behavioral observations were much higher (50–90μg/ml) than those used in humans (4–12μg/ml; Loscher, 2007) and those attained in this study (Fig. 7F). A fixed dose of CBZ (40mg/kg i.p. every 8hr) was reported to be ineffective on CSRS observed by ECoG three months after status epilepticus induced by electrical stimulation (Nissinen et al., 2007), but blood levels were not reported and a fixed dosing protocol failed to maintain stable blood levels in the younger animals (2 vs 6 months old) used in this study (Fig. 7F). The effect of VPA on neocortical seizures we observed 1 month after rpFPI is also different from that observed 3 months after status induced by electrical stimulation. We observed absence of seizures in 3/8 animals, while the effect on the remainder was minimal. Conversely, after status no seizure-free animals were reported and all animals observed responded to the drug (Nissinen et al., 2007). Further work will be required to determine whether these differences are due to intrinsic properties of the rpFPI model, predominance of neocortical partial seizures, the age of subjects at time of injury, the time post-injury investigated, or to the dosing protocol employed. However, the fact that rpFPI-induced frontal-lobe neocortical partial seizures are resistant to CBZ, and only partially respond to VPA, suggest a pharmacological profile that should be advantageous to identify novel AEDs more effective on human pharmacoresistant partial epilepsy.
Future directions for methodological improvements
Economic considerations constitute the most important impediment to the adoption of video/ECoG-based studies of etiologically relevant epilepsy models for use in AED development. Costs arise from the surgical preparation of experimental animals, their care throughout the course of chronic studies, and the laborious and time-consuming analysis of many hours of video/ECoG recording. Technical improvements that increase the yield of acceptable injuries or the durability of recording headset placements will reduce the fixed costs of surgical preparations. Further work will be required to determine optimal data acquisition times. Here we use three 1-day ECoG recordings a week (~43% of total study time) as a compromise to keep costs down while still sampling the week-to week variability in seizure frequency. While this strategy was successful, both study duration and data-analysis costs could be further reduced if shorter ECoG monitoring were proven to be adequate. Decreasing the large variability in seizure frequency data could also decrease workload and costs. Higher seizure frequencies can be achieved with higher mechanical severities at the cost of a higher acute mortality, and the optimization of the two variables is needed. The variability of the data, and group size required to detect an anti-epileptic effect, could be reduced by a seizure-frequency based selection of subjects (vanVliet et al., 2008), but at the cost of more animal surgeries and unproductive recordings. Moreover, a wide variation in the frequency of seizure is seen also in human epilepsy and frequency-based exclusion criteria could prevent proper translation of the experimental results to the human condition. Improvements in automation of data analysis offer the greatest hope for substantial labor and cost savings. Currently, reliable evaluation of these large ECoG datasets can only be achieved by manually scanning the data. Such ECoG analysis requires extensive training and takes qualified investigators ~1.5 hours to produce the primary analysis of 24hr of continuous rat ECoG. Thus, a complete ECoG-based study using just 8 rats to examine a single compound with the protocol used in this study requires about 3 weeks just for the primary analysis of the data. Thus, the development of an automated system capable of reliable analysis of ECoG data could dramatically reduce the times and costs of chronic ECoG-based studies.
Implications for human PTE
While clinical trials have been conducted to examine possible prophylactic treatments for PTE, antiepileptic treatments have never been evaluated specifically for PTE (Temkin, 2009), much less for PTE that may arise from different types of head injuries. Indeed, treatments are presently determined by seizure type (Langendorf, 2008), not etiology. In North America, CBZ is often chosen as first monotherapy for symptomatic epilepsy (Karceski et al., 2005), likely because it performs better than VPA for the treatment of CPS and possibly because of its different side effect profile (Mattson et al., 1992). These human data, however, do not allow one to determine whether the better control of CPS derives from an intrinsically superior efficacy of CBZ on the mechanisms of ictogenesis in acquired epilepsy or to its different adverse effects. The data reported here suggest that VPA may actually have a superior efficacy than CBZ on CSRSs in the early weeks-to-months after contusive closed head injury, and highlight the need for heightened attention to etiology in clinical studies of AED. Further work may elucidate whether VPA is similarly efficacious on a more chronic form of PTE, months to years after head injury.
Conclusions
ECoG monitoring and chronic epilepsy models based on etiologically realistic epileptogenic insults are expected to provide superior prediction of the anti-epileptic effects of investigational therapies, but they are costly in comparison to conventional modes of drug testing. We show that rpFPI-induced PTE can provide adequate statistical power to detect clinically meaningful anti-epileptic effects with modest numbers of experimental animals. Thus, CSRSs induced by head injury in the rat may be a useful model to find AEDs more effective on partial seizures. With further optimization of study design, and the development of reliable automated systems for data analysis, economic barriers to the routine use of ECoG and chronic epilepsy models in drug discovery should be reduced further. We also show that while therapeutic doses of CBZ had no detectable effect on rpFPI-induced CRSPSs five weeks post-injury, VPA was effective in animals suffering from focal non-spreading seizures. These data suggest that, consistent with clinical studies demonstrating epilepsy due to head injury as a risk factor for pharmacoresistance, a subset of rpFPI epileptic animals are resistant to two classic AEDs. The data also raises the possibility that VPA could have greater efficacy than CBZ on some forms of neocortical PTE, and highlight the need for chronic exposures to experimental drugs in preclinical studies, and a greater attention to etiology in clinical studies of AEDs.
Acknowledgments
This work was supported by National Institutes of Health [NS053928 to RD]. We thank Drs. Shahin Hakimian, John W. Miller and Emilio Perucca for helpful discussion and review of the manuscript.
Non-standard abbreviations
- AED
antiepileptic drug
- VPA
valproaic acid
- CBZ
carbamazepine
- CSRSs
chronic spontaneous recurrent seizures
- CPS
complex partial seizure
- TD50
dose producing toxicity in 50% of subjects
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arida RM, Scorza FA, Peres CA, Cavalheiro EA. The course of untreated seizures in the pilocarpine model of epilepsy. Epilepsy Res. 1999;34:99–107. doi: 10.1016/s0920-1211(98)00092-8. [DOI] [PubMed] [Google Scholar]
- 2.Balish M, Albert PS, Theodore WH. Seizure frequency in intractable partial epilepsy: a statistical analysis. Epilepsia. 1991;32:642–649. doi: 10.1111/j.1528-1157.1991.tb04703.x. [DOI] [PubMed] [Google Scholar]
- 3.Bauer J, Burr W. Course of chronic focal epilepsy resistant to anticonvulsant treatment. Seizure. 2001;10:239–246. doi: 10.1053/seiz.2000.0499. [DOI] [PubMed] [Google Scholar]
- 4.Berg AT, Shinnar S, Levy SR, Testa FM, Smith-Rapaport S, Beckerman B. Early development of intractable epilepsy in children: a prospective study. Neurology. 2001;56:1445–1452. doi: 10.1212/wnl.56.11.1445. [DOI] [PubMed] [Google Scholar]
- 5.Bancaud J, Talairach J. Clinical semiology of frontal lobe seizures. In: Chauvel P, Delgado-Escueta AV, Halgren E, Bancaud J, editors. Advances in Neurology. Vol. 57. Raven Press; NY: 1992. pp. 3–58. [PubMed] [Google Scholar]
- 6.Bethmann K, Brandt C, Löscher W. Resistance to phenobarbital extends to phenytoin in a rat model of temporal lobe epilepsy. Epilepsia. 2007;48:816–826. doi: 10.1111/j.1528-1167.2007.00980.x. [DOI] [PubMed] [Google Scholar]
- 7.Blum DE, Eskola J, Bortz JJ, Fisher RS. Patient awareness of seizures. Neurology. 1996;47:260–4. doi: 10.1212/wnl.47.1.260. [DOI] [PubMed] [Google Scholar]
- 8.Callaghan BC, Anand K, Hesdorffer D, Hauser WA, French JA. Likelihood of seizure remission in an adult population with refractory epilepsy. Ann Neurol. 2007;62:382–389. doi: 10.1002/ana.21166. [DOI] [PubMed] [Google Scholar]
- 9.Cavalheiro EA, Leite JP, Bortolotto ZA, Turski WA, Ikonomidou C, Turski L. Long-term effects of pilocarpine in rats: structural damage of the brain triggers kindling and spontaneous recurrent seizures. Epilepsia. 1991;32:778–782. doi: 10.1111/j.1528-1157.1991.tb05533.x. [DOI] [PubMed] [Google Scholar]
- 10.Collaborative Group for the Study of Epilepsy. Prognosis of epilepsy in newly referred patients: a multicenter prospective study of the effects of monotherapy on the long-term course of epilepsy. Epilepsia. 1992;33:45–51. doi: 10.1111/j.1528-1157.1992.tb02281.x. [DOI] [PubMed] [Google Scholar]
- 11.Dudek FE, Clark S, Williams PA, Grabenstatter H. Kainate-induced status epilepticus: a chronic model of acquired epilepsy. In: Pitkanen A, Schwartzkroin PA, Moshe SL, editors. Models of seizures and epilepsy. Elsevier; New York: 2006. pp. 415–432. [Google Scholar]
- 12.D’Ambrosio R, Fairbanks JP, Fender JS, Born DE, Doyle DL, Miller JW. Post-traumatic epilepsy following fluid percussion injury in the rat. Brain. 2004;127(Pt 2):304–14. doi: 10.1093/brain/awh038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Ambrosio R, Fender JS, Fairbanks JP, Simon EA, Born DE, Doyle DL, Miller JW. Progression from frontal-parietal to mesial-temporal epilepsy after fluid percussion injury in the rat. Brain. 2005;128(Pt 1):174–88. doi: 10.1093/brain/awh337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Ambrosio R, Hakimian S, Stewart T, Verley DR, Fender JS, Eastman CL, Sheerin AH, Gupta P, Diaz-Arrastia R, Ojemann J, Miller JW. Functional definition of seizures provides new insights into posttraumatic epileptogenesis. Brain. 2009;132(10):2805–21. doi: 10.1093/brain/awp217. Epub 2009 Sep 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Ambrosio R, Miller JW. What is an Epileptic Seizure? Unifying Definitions in Clinical Practice and Animal Research to Develop Novel Treatments. Epilepsy Currents. doi: 10.1111/j.1535-7511.2010.01358.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delgado-Escueta AV, Wasterlain C, Treiman DM, Porter RJ. Current concepts in neurology: management of status epilepticus. N Engl J Med. 1982;306:1337–1340. doi: 10.1056/NEJM198206033062205. [DOI] [PubMed] [Google Scholar]
- 17.Delgado-Escueta AV, Treiman DM, Walsh GO. The treatable epilepsies. N Engl J Med. 1983;308:1576–1584. doi: 10.1056/NEJM198306303082607. [DOI] [PubMed] [Google Scholar]
- 18.Dubé C, Richichi C, Bender RA, Chung G, Litt B, Baram TZ. Temporal lobe epilepsy after experimental prolonged febrile seizures: prospective analysis. Brain. 2006;129(Pt 4):911–922. doi: 10.1093/brain/awl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult Epilepsy. Lancet. 2006;367(9516):1087–1100. doi: 10.1016/S0140-6736(06)68477-8. [DOI] [PubMed] [Google Scholar]
- 20.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Chapman & Hall/CRC; New York: 1998. [Google Scholar]
- 21.French JA. Refractory epilepsy: clinical overview. Epilepsia. 2007;48 (Suppl 1):3–7. doi: 10.1111/j.1528-1167.2007.00992.x. [DOI] [PubMed] [Google Scholar]
- 22.Gilliam, Carter J, Vahle V. Tolerability of antiseizure medications: implications for health outcomes. Neurology. 2004;63(10 Suppl 4):S9–S12. doi: 10.1212/wnl.63.10_suppl_4.s9. [DOI] [PubMed] [Google Scholar]
- 23.Grabenstatter HL, Ferraro DJ, Williams PA, Chapman PL, Dudek FE. Use of chronic epilepsy models in antiepileptic drug discovery: the effect of topiramate on spontaneous motor seizures in rats with kainate-induced epilepsy. Epilepsia. 2005;46:8–14. doi: 10.1111/j.0013-9580.2005.13404.x. [DOI] [PubMed] [Google Scholar]
- 24.Haut SR, Shinnar S, Moshé SL. Seizure clustering: risks and outcomes. Epilepsia. 2005;46:146–149. doi: 10.1111/j.0013-9580.2005.29004.x. [DOI] [PubMed] [Google Scholar]
- 25.Hitiris N, Mohanraj R, Norrie J, Sills GJ, Brodie MJ. Predictors of pharmacoresistant epilepsy. Epilepsy Res. 2007;75:192–196. doi: 10.1016/j.eplepsyres.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Juul-Jensen P. Epidemiology of intractable epilepsy. In: Schmidt D, Morselli P, editors. Intractable Epilepsy. Raven Press; New York: 1986. pp. 5–11. [Google Scholar]
- 27.Karceski S, Morrell MJ, Carpenter D. Treatment of Epilepsy in Adults: Expert Opinion. Epilepsy & Behavior. 2005;7 (Suppl 1):S1–S64. doi: 10.1016/j.yebeh.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Kelly KM, Kharlamov A, Hentosz TM, Kharlamova EA, Williamson JM, Bertram EH, 3rd, Kapur J, Armstrong DM. Photothrombotic brain infarction results in seizure activity in aging Fischer 344 and Sprague Dawley rats. Epilepsy Res. 2001;47(3):189–203. doi: 10.1016/s0920-1211(01)00294-7. [DOI] [PubMed] [Google Scholar]
- 29.Kharlamov EA, Jukkola PI, Schmitt KL, Kelly KM. Electrobehavioral characteristics of epileptic rats following photothrombotic brain infarction. Epilepsy Res. 2003 Oct;56(2–3):185–203. doi: 10.1016/j.eplepsyres.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Klein PM, Rakhade S, Jensen FE. Hippocampal depth recordings from long evans rats subjected to neonatal seizures reveal development of spontaneous limbic seizures. Proceedings of the American Epilepsy Society meeting; Epilepsia. 2009. p. 385. Abstract. [Google Scholar]
- 31.Kraus JF, McArthur DL. Epidemiology of brain injury. In: Cooper PR, Golfinos JG, editors. Head Injury. 4. McGraw-Hill; New York: 2000. pp. 1–26. [Google Scholar]
- 32.Krupp E, Heynen T, Li XL, Post RM, Weiss SR. Tolerance to the anticonvulsant effects of lamotrigine on amygdala kindled seizures: cross-tolerance to carbamazepine but not valproate or diazepam. Exp Neurol. 2000;162:278–89. doi: 10.1006/exnr.1999.7343. [DOI] [PubMed] [Google Scholar]
- 33.Kwan P, Brodie MJ. Clinical trials of antiepileptic medications in newly diagnosed patients with epilepsy. Neurology. 2003;60(11 Suppl 4):S2–12. doi: 10.1212/wnl.60.11_suppl_4.s2. [DOI] [PubMed] [Google Scholar]
- 34.Langendorf FG, Pedley TA, Temkin NR. Posttraumatic seizures. In: Engel J Jr, Pedley TA, editors. Epilepsy: a comprehensive textbook. Lippincott Williams & Wilkins; Philadelphia: 2008. pp. 2537–2542. [Google Scholar]
- 35.Leite JP, Cavalheiro EA. Effects of conventional antiepileptic drugs in a model of spontaneous recurrent seizures in rats. Epilepsy Res. 1995;20:93–104. doi: 10.1016/0920-1211(94)00070-d. [DOI] [PubMed] [Google Scholar]
- 36.Löscher W. Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs. 2002;16:669–694. doi: 10.2165/00023210-200216100-00003. [DOI] [PubMed] [Google Scholar]
- 37.Löscher W. The pharmacokinetics of antiepileptic drugs in rats: consequences for maintaining effective drug levels during prolonged drug administration in rat models of epilepsy. Epilepsia. 2007;48:1245–1258. doi: 10.1111/j.1528-1167.2007.01093.x. [DOI] [PubMed] [Google Scholar]
- 38.Löscher W, Hönack D. Comparison of anticonvulsant efficacy of valproate during prolonged treatment with one and three daily doses or continuous (“controlled release”) administration in a model of generalized seizures in rats. Epilepsia. 1995;36:929–37. doi: 10.1111/j.1528-1157.1995.tb01637.x. [DOI] [PubMed] [Google Scholar]
- 39.Löscher W, Schmidt D. New horizons in the development of antiepileptic drugs: the search for new targets. Epilepsy Res. 2004;60:77–159. doi: 10.1016/j.eplepsyres.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Lüders HO, Lesser RP, Dinner DS, Morris HH, Wyllie E, Godoy J, Hahn JH. A negative motor response elicited by electrical stimulation of the human frontal neocortex. In: Chauvel P, Delgado-Escueta AV, Halgren E, Bancaud J, editors. Advances in Neurology. Vol. 57. Raven Press; NY: 1992. pp. 149–157. [PubMed] [Google Scholar]
- 41.Luna-Tortós C, Fedrowitz M, Löscher W. Several major antiepileptic drugs are substrates for human P-glycoprotein. Neuropharmacology. 2008;55:1364–1375. doi: 10.1016/j.neuropharm.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 42.Mattson RH, Cramer JA, Collins JF. Prognosis for total control of complex partial and secondarily generalized tonic clonic seizures. Department of Veterans Affairs Epilepsy Cooperative Studies No 118 and No 264 Group. Neurology. 1996;47:68–76. doi: 10.1212/wnl.47.1.68. [DOI] [PubMed] [Google Scholar]
- 43.Mattson RH, Cramer JA, Collins JF. A comparison of valproate with carbamazepine for the treatment of complex partial seizures and secondarily generalized tonic-clonic seizures in adults. The Department of Veterans Affairs Epilepsy Cooperative Study No 264 Group. N Engl J Med. 1992;327:765–771. doi: 10.1056/NEJM199209103271104. [DOI] [PubMed] [Google Scholar]
- 44.Meldrum B. Do preclinical seizure models preselect certain adverse effects of antiepileptic drugs? Epilepsy Res. 2002;50:33–40. doi: 10.1016/s0920-1211(02)00066-9. [DOI] [PubMed] [Google Scholar]
- 45.Minicucci F, Muscas G, Perucca E, Capovilla G, Vigevano F, Tinuper P. Treatment of status epilepticus in adults: guidelines of the Italian League against Epilepsy. Epilepsia. 2006;47(S5):9–15. doi: 10.1111/j.1528-1167.2006.00870.x. [DOI] [PubMed] [Google Scholar]
- 46.Murao K, Shingu K, Miyamoto E, Ikeda S, Nakao S, Masuzawa M, Yamada M. Anticonvulsant effects of sevoflurane on amygdaloid kindling and bicuculline-induced seizures in cats: comparison with isoflurane and halothane. J Anesth. 2002;16:34–43. doi: 10.1007/s540-002-8092-0. [DOI] [PubMed] [Google Scholar]
- 47.Nissinen J, Pitkänen A. Effect of antiepileptic drugs on spontaneous seizures in epileptic rats. Epilepsy Res. 2007;73:181–191. doi: 10.1016/j.eplepsyres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Oakley JC, Kalume F, Yu FH, Scheuer T, Catterall WA. Temperature- and age-dependent seizures in a mouse model of severe myoclonic epilepsy in infancy. Proc Natl Acad Sci U S A. 2009;106(10):3994–9. doi: 10.1073/pnas.0813330106. Epub 2009 Feb 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Remy S, Gabriel S, Urban BW, Dietrich D, Lehmann TN, Elger CE, Heinemann U, Beck H. A novel mechanism underlying drug resistance in chronic epilepsy. Ann Neurol. 2003;53:469–479. doi: 10.1002/ana.10473. [DOI] [PubMed] [Google Scholar]
- 50.Rogawski MA, Johnson MR. Intrinsic severity as a determinant of antiepileptic drug refractoriness. Epilepsy Curr. 2008;8:127–130. doi: 10.1111/j.1535-7511.2008.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ropper AH, Kofke WA, Bromfield EB, Kennedy SK. Comparison of isoflurane, halothane, and nitrous oxide in status epilepticus. Ann Neurol. 1986;19:98–99. doi: 10.1002/ana.410190124. [DOI] [PubMed] [Google Scholar]
- 52.Rüegg SJ, Dichter MA. Diagnosis and Treatment of Nonconvulsive Status Epilepticus in an Intensive Care Unit Setting. Curr Treat Options Neurol. 2003;5(2):93–110. doi: 10.1007/s11940-003-0001-4. [DOI] [PubMed] [Google Scholar]
- 53.Schmidt D, Löscher W. New developments in antiepileptic drug resistance: an integrative view. Epilepsy Curr. 2009;9:47–52. doi: 10.1111/j.1535-7511.2008.01289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmidt D, Rogawski M. New strategies for the identification of drugs to prevent the development or progression of epilepsy. Epilepsy Research. 2002;50:71–78. doi: 10.1016/s0920-1211(02)00070-0. [DOI] [PubMed] [Google Scholar]
- 55.Semah F, Picot MC, Adam C, Broglin D, Arzimanoglou A, Bazin B, Cavalcanti D, Baulac M. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology. 1998;51:1256–1262. doi: 10.1212/wnl.51.5.1256. [DOI] [PubMed] [Google Scholar]
- 56.Sloviter RS. The neurobiology of temporal lobe epilepsy: too much information, not enough knowledge. C R Biol. 2005;328:143–153. doi: 10.1016/j.crvi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 57.Stables JP, Bertram EH, White HS, Coulter DA, Dichter MA, Jacobs MP, Loscher W, Lowenstein DH, Moshe SL, Noebels JL, Davis M. Models for epilepsy and epileptogenesis: report from the NIH workshop; Bethesda, Maryland Epilepsia. 2002. pp. 1410–1420. [DOI] [PubMed] [Google Scholar]
- 58.Tatum WO, 4th, Winters L, Gieron M, Passaro EA, Benbadis S, Ferreira J, Liporace J. Outpatient seizure identification: results of 502 patients using computer-assisted ambulatory EEG. J Clin Neurophysiol. 2001;18:14–9. doi: 10.1097/00004691-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 59.Temkin N. Preventing and treating posttraumatic epilepsy: the human experience. Epilepsia. 2009;50(suppl 2):10–13. doi: 10.1111/j.1528-1167.2008.02005.x. [DOI] [PubMed] [Google Scholar]
- 60.Temkin N. Antiepileptogenesis and seizure prevention trials with antiepileptic drugs: meta-analysis of controlled trials. Epilepsia. 2001;42:515–524. doi: 10.1046/j.1528-1157.2001.28900.x. [DOI] [PubMed] [Google Scholar]
- 61.Thompson HJ, Lifshitz J, Marklund N, Grady MS, Graham DI, Hovda DA, McIntosh TK. Lateral fluid percussion brain injury: a 15-year review and evaluation. Neurotrauma. 2005;22:42–75. doi: 10.1089/neu.2005.22.42. [DOI] [PubMed] [Google Scholar]
- 62.van Vliet EA, van Schaik R, Edelbroek PM, da Silva FH, Wadman WJ, Gorter JA. Development of tolerance to levetiracetam in rats with chronic epilepsy. Epilepsia. 2008;49:1151–1159. doi: 10.1111/j.1528-1167.2007.01516.x. [DOI] [PubMed] [Google Scholar]
- 63.Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia. 2005;46:1724–1743. doi: 10.1111/j.1528-1167.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 64.White HS. Chemoconvulsants. In: Peterson SL, Albertson TE, editors. Neuropharmacology methods in epilepsy research. CRC Press; Boca Raton, FL: 1998. pp. 27–40. [Google Scholar]
- 65.White HS. Animal models of epileptogenesis. Neurology. 2002;59(9 Suppl 5):S7–S14. doi: 10.1212/wnl.59.9_suppl_5.s7. [DOI] [PubMed] [Google Scholar]
- 66.White HS. Preclinical development of antiepileptic drugs: past, present, and future directions. Epilepsia. 2003;44 (Suppl 7):2–8. doi: 10.1046/j.1528-1157.44.s7.10.x. [DOI] [PubMed] [Google Scholar]
- 67.Williams PA, Dudek FE. A chronic histopathological and electrophysiological analysis of a rodent hypoxic-ischemic brain injury model and its use as a model of epilepsy. Neuroscience. 2007;149:943–961. doi: 10.1016/j.neuroscience.2007.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Williamson PD, Spencer DD, Spencer SS, Novelly RA, Mattson RH. Complex partial seizures of frontal lobe origin. Ann Neurol. 1985;18(4):497–504. doi: 10.1002/ana.410180413. [DOI] [PubMed] [Google Scholar]
- 69.Williamson PD, Spencer SS. Clinical and EEG features of complex partial seizures of extratemporal origin. Epilepsia. 1986;27(Suppl 2):S46–63. doi: 10.1111/j.1528-1157.1986.tb05740.x. [DOI] [PubMed] [Google Scholar]
- 70.Zhang ZJ, Xing GQ, Russell S, Obeng K, Post RM. Unidirectional cross-tolerance from levetiracetam to carbamazepine in amygdala-kindled seizures. Epilepsia. 2003;44:1487–93. doi: 10.1111/j.0013-9580.2003.34803.x. [DOI] [PubMed] [Google Scholar]