Abstract
Background
Despite increasing prevalence, the economic implications of celiac disease (CD) are just emerging. We assessed the impact of CD diagnosis on healthcare costs and the incremental costs associated with CD.
Methods
Administrative data for a population-based cohort of CD cases and matched controls from Olmsted County, Minnesota was used to compare 1) direct medical costs 1 year pre- and post-CD diagnosis for 133 index cases and 2) 4-year cumulative direct medical costs incurred by 153 index cases versus 153 controls. Analyses exclude diagnostic-related and outpatient pharmaceutical costs.
Results
Average total costs were reduced by $1,764 in the year following diagnosis (pre-diagnosis cost of $5,023 vs. $3,259; 95% CI of difference: $688 to $2,993). Over a 4-year period, CD cases experienced higher outpatient costs (mean difference of $1,457; P = 0.016) and higher total costs than controls (mean difference of $3,964; P = 0.053). Excess average total costs were concentrated among males with CD ($14,191 vs. $4,019 for male controls; 95% CI of difference: $2,334 to $20,309).
Conclusions
CD-associated costs indicate a significant economic burden of disease, particularly for diseased males. Diagnosis and treatment of CD reduces medical costs of care suggesting an economic advantage to earlier detection and treatment.
Keywords: Coeliac disease, Small intestine, Diarrhoea, Health economics
Introduction
Celiac disease (CD) is defined as a permanent intolerance to ingested gluten that damages the small intestine and resolves with the removal of gluten from the diet (1, 2). Classically, CD causes diarrhea and malabsorption with resulting malnutrition and deficiency of micronutrients (iron, folate, and the fat-soluble vitamins). However, the disorder frequently presents with only the vaguest gastrointestinal symptoms and a variety of extraintestinal manifestations (so-called “atypical’ CD). CD may remain entirely silent for many years despite much damage to the intestine (1). Although the wide spectrum of clinical manifestations of CD is now better recognized in the United States than decades ago, patients with CD may still have a long duration of symptoms and often undergo extensive and expensive medical investigations prior to an accurate diagnosis (3).
Once thought to be a rare disease, CD is now recognized as a common chronic disorder affecting ~1% of the general population in Western countries (1,2,4,5). Thus, the condition is of increasing public health concern in the United States, given the increasing prevalence and a belief that early diagnosis and treatment may prevent complications and reduce the economic burden of the disease (6). Much of the economic literature in this area has, in fact, focused on estimating costs associated with CD screening or diagnosis and patient dietary costs (7–14). However, research on the effect that detecting and treating CD has on health care resource use and the overall economic burden of illness is scarce and just emerging (15). Taking advantage of unique resources linking medical record and administrative data for a geographically defined population, we assessed the impact of CD diagnosis on costs of care and also provide longitudinal estimates of the incremental costs associated with CD prior to diagnosis.
Methods
Study Setting
A unique set of circumstances exists in Olmsted County, Minnesota, that facilitates population-based epidemiologic and health services research. The county is relatively geographically isolated with few medical providers in the area. Under the auspices of the Rochester Epidemiology Project (REP), medical records for care received at Mayo Clinic and Olmsted Medical Center since 1966 have been successfully linked to provide medical record data from essentially all sources of medical care utilized by members of this local population (16). More recently, an Electronic Data Sharing Agreement was signed by these providers to also provide research access to patient level administrative data which are archived within the Olmsted County Health Care Expenditure and Utilization Database (OCHEUD). OCHEUD currently provides billing information on all hospital and ambulatory care delivered to Olmsted County residents locally from 1987 through 2007.
Because of well-known discrepancies between billed charges and true resource use, utilization in OCHEUD is valued using standard methods by grouping services into the Medicare Part A and B classifications; Part A billed charges (hospital-billed services and procedures) are adjusted using hospital cost-to-charge ratios at the departmental level and wage indexes. Medicare Part B items (primarily physician-billed services) are valued using national average Medicare reimbursement rates. Costs are adjusted for inflation to express costs in constant dollars. The combination of OCHEUD and REP resources make population-based health economic research feasible for numerous medical conditions (17, 18).
Identification of CD Cases and Controls
Cases of CD were initially identified by searching the REP diagnostic index for Olmsted County residents who were assigned a diagnosis of CD (ICD-9 code 579) or dermatitis herpetiformis (ICD-9 codes: 694, 694.2) within calendar years 1989 to 2006. The complete inpatient and outpatient medical record for each candidate case was then thoroughly reviewed by a physician investigator to confirm that individuals met conventional diagnostic criteria for CD (2, 19). CD cases included those with a positive biopsy (jejunal or duodenal biopsies with partial or total villous atrophy associated with crypt hyperplasia and an increased number of intraepithelial lymphocytes) and either clinical or histological improvement after the introduction of a gluten-free diet or a positive endomysial or tissue transglutaminase antibody IgA. Note that serological testing was not widely performed in Olmsted County until 1995 (20). The diagnostic approach for CD at Mayo Clinic follows conventional recommendations and does not routinely include additional testing such as HLA genotyping (2, 20). The date of diagnosis and year of symptom onset were recorded and residency at the time of diagnosis was confirmed via record review.
Each incident CD case was matched to a general population control residing in Olmsted County, identified by the REP, who was free of CD as indicated by diagnostic coding. Controls were matched on gender, age (birth year +/− 1 year), a service date within 1 year of the CD index case diagnosis, and a Mayo registration date within 2 years of the index case initial visit (this matches for the deviation of prior medical record documentation). The complete medical records of potential controls were reviewed by a physician investigator to exclude the presence of CD.
Residency Status Verification
Complete capture of health care utilization and associated cost data likely hinged on residency status. Thus, we further verified residency in Olmsted County for both CD cases and controls throughout the observation duration for economic analysis. Initially, this verification was conducted electronically by searching REP databases for health care encounters with county zip codes. If at least one provider visit as a county resident was found during a year, then residency was assumed throughout that year. Cases of uncertain residency underwent further review by a REP residency specialist who reviewed the detailed provider-linked medical records (including correspondence), serial telephone books, maps and plat books, city directories, and public record databases to validate residency throughout the period of interest.
Ethical Issues
The study was approved by Mayo Clinic and Olmsted Medical Center Institutional Review Boards. All patients who did not grant authorization to use their medical records for research were excluded from analyses as per Minnesota law (21).
Economic Outcomes and Statistical Analyses
Impact of CD Diagnosis on Direct Medical Costs
Health care utilization and associated costs were tracked in OCHEUD for the identified CD cases to estimate the impact of diagnosis on total direct medical costs of care. The impact of diagnosis on costs was determined by comparing the costs incurred by cases in the year prior to and the year following their diagnosis. Thus, only cases with verified local residency during this observation period were included in this analysis. Analyses were conducted including and excluding diagnostic-related costs as well as stratified by cost categories of interest (inpatient, outpatient, total costs of care). Services and costs we identified as related to the diagnosis of CD included serologic testing (endomysial, gliadin, and anti-reticulin antibody tests, tissue transglutaminase, HLA typing), endoscopy (small intestinal and upper gastrointestinal with biopsy), surgical pathology and consultation, and bone densitometry. These services were considered related to the CD diagnosis if they occurred within 90 days of the recorded diagnosis date. Similarly, we considered any Gastroenterology office visit within 15 days of diagnosis to be a diagnosis-related cost of care.
Incremental Costs Associated with CD
We estimated the direct medical costs attributable to CD by comparing the cumulative costs incurred by CD cases with those incurred by the matched controls over equivalent periods of observation in the OCHEUD database. In an attempt to estimate the economic burden of undiagnosed and untreated CD, we focused our analysis on 4 years of observation prior to the year before CD diagnosis for cases and the 4 years prior to that index year for control subjects. For a patient diagnosed with CD in 2003, for instance, we estimated and compared his/her estimated cumulative costs incurred between 1999 and 2002 with the costs incurred by the matched control over the same time frame. We conducted analyses stratified by cost categories of interest (inpatient episodes, total outpatient care and subcategories [surgery, radiology, laboratory services, office visits], total costs) and by sex and age at index (i.e. ≤ 40 years and > 40 years). As preexisting disease may confound assessments of incremental costs, we compared CD cases and matched controls using a validated systematic method of classifying comorbidity when diagnostic codes from administrative bases are utilized (Deyo adaption of Charlson comorbidity index) based on diagnoses assigned in the 4-year observation period of interest (22).
In a post hoc analysis, CD cases were categorized by severity and clinical presentation to assess the impact of these variables on incremental costs associated with CD. Severe CD was defined by the presence of both diarrhea and weight loss. “Classical” CD include patients with a diarrhea-predominant syndrome and “atypical” CD was used for patients with nonspecific gastrointestinal symptoms (including bloating or abdominal pain) and/or extraintestinal manifestations (e.g., iron-deficiency anemia, abnormal liver function tests, osteoporosis, etc.). (2) Patients with refractory celiac disease were identified by conventional criteria (23, 24).
Statistical analysis
Continuous data are summarized as mean ± standard deviation. Discrete data are presented as frequency (percentage). Characteristics of CD cases and non-CD controls as of index were compared using Student’s t-test for continuous data and Pearson’s chi-squared test for categorical data, as appropriate. In all economic analyses, observed costs were compared using paired Student’s t-tests and nonparametric bootstrapped confidence intervals (25, 26). All statistical tests were two-sided, and p values less than 0.05 were considered significant. SAS version 9.1 (SAS Institute Inc., Cary, NC) was used in the analyses.
Results
Patients
We initially identified 168 CD patients who were Olmsted County residents at the time of CD diagnosis. Six patients denied research authorization and were excluded. Nine additional patients were not local residents during the entire 4 years prior to the CD diagnosis year. Thus, the final analytic cohort for analysis of incremental cost associated with CD consisted of 153 cases and 153 controls (153 matched pairs). Twenty-nine additional patients may not have been local residents during the year prior to and the year following diagnosis and thus were excluded from the cohort used to assess the impact of CD diagnosis on direct medical costs (final cohort of 133 CD cases).
The mean age for the 133 CD patients included to assess the impact of CD diagnosis on direct medical cost was 41 years, with the majority (66%) of female gender. Sixty (45%) patients had one or more Charlson comorbidities in the years since first electronically available diagnosis at Mayo clinic to CD diagnosis date (16.6 ± 8.7 years). Table 1 provides a summary of clinical characteristics of CD patients and between-group comparisons for the 153 CD patients and non-CD matched controls. Sex and age distributions did not differ between cases and controls (by virtue of the study design) nor did the estimated summary measures of comorbidity during the 4-year observation period of interest.
Table 1.
Characteristics of Olmsted County, Minnesota, residents with celiac disease diagnosed in 1989–2006 and controls without celiac disease as of indexa
| Variable | CD Cases (n = 153) | Controls (n = 153) | P-value |
|---|---|---|---|
| Age, years | 40.2 ± 20.5 | 40.2 ± 20.5 | --- |
| Male gender (No./%) | 51 (33) | 51 (33) | --- |
| Charlson comorbidity count | 0.46 ± 0.92 | 0.33 ± 0.68 | 0.16 |
| Value > 1 (No./%) | 45 (29) | 38 (25) | 0.37 |
| Charlson comorbidity indexb | 0.60 ± 1.41 | 0.73 ± 0.91 | 0.18 |
| Value > 1 (No./%) | 15 (10) | 17 (11) | 0.71 |
| Clinical characteristics of CD | |||
| Villous atrophy in diagnostic biopsy | 153/153 (100%) | - | |
| Positive tTGA or EMA at diagnosis | 100/114 (88%) | - | |
| Classical presentation | 58/151 (38%) | - | |
| Atypical presentation | 93/151 (62%) | - | |
| Severe CD | 22/151 (15%) | - | |
| Treatment with a gluten-free diet | 142/147 (98%) | - | |
| Clinical response to gluten-free diet | 109/109 (100%) | - | |
| HLA-DQ2 or DQ8 | 69/69 (100%) | - | |
Index was defined as date of CD diagnosis for cases and the same year for matched controls.
Charlson comorbidity index was calculated using the Deyo adaption based on diagnoses in the 4 years of observation prior to the year before diagnosis for cases and the 4 years prior to that same year for controls.
CD = celiac disease; tTGA, tissue transglutaminase antibodies; EMA, endomysial antibodies; HLA, human leukocyte antigens
Sufficient information for assessment of severity and clinical presentation was available in 151 (99%) patients. Severe CD was present in 22 (15%) patients and CD was non-severe in 129 (85%) patients. Classical CD was observed in 58 (38%) patients and atypical CD in 93 (62%) patients. Two patients met criteria for refractory celiac disease. The diagnosis of the refractory state was made after 4 years and 5 years of CD diagnosis, respectively, because of recurrence of a severe malabsorptive syndrome despite strict adherence to GFD.
Impact of Celiac Disease Diagnosis on Direct Medical Costs
Significant cost differences in inpatient, outpatient, and total direct medical costs were observed when comparing costs in the year prior to and the year following CD diagnosis (Table 2). Total annual direct medical costs were reduced by $2,118, on average, following CD diagnosis ($5,457 vs. $3,339; bootstrapped 95% CI of difference: $922 to $3,417). This difference was driven by both reduced inpatient costs and outpatient (professional service) costs in the year following diagnosis (mean reductions: $1338 and $742, respectively). Observed reductions in inpatient and total costs of care following diagnosis persisted even with the exclusion of services deemed to be related to the diagnostic process itself (Table 2). Mean total direct medical costs were reduced by $1,764, on average, in the year following diagnosis with exclusion of CD-diagnostic costs of care ($5,023 vs. $3,259; bootstrapped 95% CI of difference: $688 to $2,993).
Table 2.
Annual direct medical costs in the year prior to and following the diagnosis of celiac diseasea
| Before diagnosis (n=133) | After diagnosis (n=133) | |||
|---|---|---|---|---|
| Endpoint | Mean difference (95% CI) | P-value | ||
|
Diagnosis costs included | ||||
| Total costs | $5457 ($4659, $7395) | $3339 ($2551, $4895) | $2118 ($922, $3417) | <0.001 |
| Inpatient costsc | $2851 ($2079, $4138) | $1512 ($955, $2543) | $1339 ($400, $2260) | 0.003 |
| Outpatient costsd | $2598 ($2412, $3266) | $1856 ($1551, $2415) | $742 ($389, $1274) | <0.001 |
| Diagnosis costs excludede | ||||
| Total costs | $5023 ($4037, $6296) | $3259 ($2377, $4538) | $1764 ($688, $2993) | 0.003 |
| Inpatient costs | $2851 ($2079, $4138) | $1512 ($955, $2543) | $1339 ($400, $2260) | 0.003 |
| Outpatient costs | $2154 ($1816, $2533) | $1773 ($1428, $2200) | $381 ($36, $796) | 0.054 |
Mean costs per patient in 2007 constant dollars.
Bootstrap 95% CI using the percentile method.
Hospital (Medicare Part A) costs
Professional (Medicare Part B) costs
Diagnostic services of interest included serologic testing, endoscopy with biopsy and pathology, bone density radiography, and gastroenterology office visits.
Incremental Cost Associated with Celiac Disease
Table 3 compares mean costs incurred by CD patients and non-CD matched control subjects over the 4-year observation period (before CD diagnosis for cases and prior to corresponding index year for controls). Over the 4 years, CD cases experienced significantly higher outpatient costs of care and borderline significantly higher total costs of nearly $4,000, on average, compared with control subjects (mean total costs: $11,037 vs. $7,073; bootstrapped 95% CI of difference: $65 to $8,020). The higher outpatient costs observed among CD patients relative to non-CD controls (mean difference: $1,457; p=0.02) was driven by increased radiologic and laboratory service use, as well as increased costs related to office visits (office visit mean cost difference: $466; p=0.002).
Table 3.
Direct medical costs incurred by Olmsted County, Minnesota, residents with celiac disease diagnosed in 1989–2006 and controls without celiac disease.a
| CD Cases (n = 153) | Controls (n = 153) | |||
|---|---|---|---|---|
| Endpoint | Mean difference (95% CI)b | P-value | ||
| Total costs | $11 037 ($7866, $14793) | $7073 ($5211, $9483) | $3964 ($65, $8020) | 0.053 |
| Inpatient costsc | $5976 ($3625, $8762) | $3497 ($2206, $5311) | $2478 (−$402, $5532) | 0.106 |
| Outpatient costsd | $4937 ($3921, $6082) | $3480 ($2816, $4253) | $1457 ($293, $2689) | 0.016 |
| Surgery | $795 ($615, $1114) | $685 ($503, $991) | $110 (−$208, $453) | 0.469 |
| Radiology | $910 ($671, $1351) | $546 ($406, $770) | $364 ($101, $702) | 0.009 |
| Laboratory | $546 ($476, $723) | $377 ($304, $481) | $169 ($73, $351) | 0.006 |
| Office visits | $1472 ($1215, $1969) | $1006 ($847, $1253) | $466 ($157, $945) | 0.002 |
| Other medical | $981 ($709, $1465) | $724 ($464, $1090) | $256 (−$178, $781) | 0.234 |
Mean 4-year cumulative costs per patient in 2007 constant dollars.
Bootstrap 95% CI using the percentile method.
Hospital (Medicare Part A) costs
Professional (Medicare Part B) costs
CD = celiac disease.
Total cumulative 4-year costs were observed to be higher both for the 76 CD patients aged ≤ 40 compared with their controls (mean cost difference for case minus control of $1,856, p=0.40) as well as for the 77 CD patients and matched controls over 40 years of age at index (mean cost difference for case minus control of $6,044, p = 0.08). These differences, however, did not reach statistical significance. Cumulative costs were similar for the 102 female CD patients and matched controls ($9,460 vs. $8,600; p=0.66). By contrast, cumulative 4-year costs for the 51 male CD patients were significantly higher then those incurred by their non-CD controls ($14,191 vs. $4,018; p=0.03).
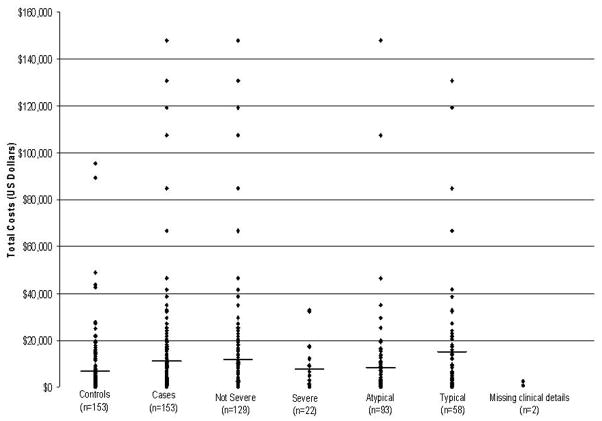
The distribution of 4-year costs for CD cases stratified by severity and clinical presentation and for matched controls are presented in Figure 1. Post hoc analyses comparing 4-year costs in these CD subgroups with matched controls find patients with classical CD presentation to have higher outpatient costs as compared to controls (p=0.05) but individuals with atypical CD presentation incurred similar costs of care. Severe CD patient cumulative costs were similar to their matched controls however patients with non-severe CD had significantly higher outpatient costs ($5,181 vs. $3,475; p=0.01).
FIGURE 1.
The distribution of 4- year costs for CD cases, stratified by severity and clinical presentation, and for matched controls. Black lines represent the mean 4-year cost for that group or subgroup.
Discussion
This population-based study estimates the impact of CD diagnosis on direct medical costs of care as well as the incremental costs associated with CD prior to diagnosis. Even with the exclusion of costs related to the diagnostic process, we find that direct medical outpatient and total costs were significantly reduced for patients in the year following CD diagnosis. Furthermore, CD patients experienced significantly higher direct medical costs during the 4-year observation period (nearly $4,000, on average) compared with unaffected peers, suggesting a substantial economic burden of disease.
These results are consistent with those recently published by Green et al. (2008), who used outpatient administrative claims data to assess the economic implications of CD diagnosis (15). A particular strength of our study was our ability to link detailed medical records data (including laboratory data) to line item utilization and cost data regardless of payer type. In our population-based design, the diagnosis of CD was confirmed by re-review of the medical record and was not only based on diagnostic codes. Thus, this study design decreased bias induced by referral, missing data, and inaccurate coding. Our results suggest a 29% reduction in outpatient costs in the year following diagnosis as well as an overall 39% reduction in total medical cost of care. Our decision to limit costs that relate to the diagnosis of CD to 90 days for tests and 15 days for office visits is consistent with our experience in a major tertiary referral center with expertise in CD but may be too restrictive in other settings or populations.
To our knowledge, we are the first study to assess incremental costs attributable to CD despite the fact that cost-of-illness studies are increasingly needed for modeling the cost-effectiveness of alternative treatments and for informing clinical and public health policy (27, 28). In our sample, undiagnosed and untreated CD patients incurred nearly $4,000 in excess 4-year medical costs compared with non-CD matched peers. This significant economic burden was even more pronounced among males with CD, who incurred over $10,000 more, on average, in medical costs during the study period than their matched non-diseased controls. The reasons for this finding are not totally clear, but clinically-detected CD is twice as frequent among women as men and it is possible that male symptoms could more often be attributed to different causes other than CD (such as irritable bowl syndrome), increasing both the number of clinic visits to make the correct diagnosis and the cost of care (1, 29, 30). Sex-related differences in both clinical presentation of CD and comorbidity may also affect estimates of cost of care, including prior evidence of more severe disease in males (31, 32).
Our post hoc analyses of incremental costs between CD cases and controls stratified by severity and clinical presentation suggest that the excess total costs associated with CD we observed are largely driven by significant excess outpatient costs in patients with classical CD (characterized by a diarrhea-predominant syndrome) and in patients with non-severe CD (defined by the absence of a combination of diarrhea and weight loss). Reasons to explain these differences are unknown but we can speculate it may be related to a more extensive differential diagnosis in non-severe CD, expedited diagnosis of severe cases, cost inherent to the approach of undiagnosed chronic diarrhea, and serology-driven diagnosis of atypical cases. Further studies to address these hypotheses are not only necessary but are clinically relevant.
Although not statistically significant, we also found it curious that inpatient costs were observed to be over $2,400 higher (p=0.11), on average, for CD patients in our sample compared with controls since anecdotally CD does not seem to be a condition with an acute need for inpatient care. To investigate this further, we performed additional subgroup analyses stratifying by whether inpatient events were considered CD-related or not based on a blinded clinical review of primary discharge diagnoses. Examples of episodes considered CD-related include a diagnosis of anemia, unspecified constipation, generalized abdominal pain, and intussusception. Interestingly, inpatient costs for CD-related episodes were significantly higher, on average, for CD cases than controls ($392 vs. $33; p=0.05). Costs for non-CD inpatient episodes were also observed to be $1,565 higher, on average, for CD cases compared with controls, but this difference was not statistically significant. Thus, at least some of the higher inpatients cost in CD cases are explained by higher CD-related inpatient episodes.
Our study also has potential limitations to note. The present study was conducted in a single geographic setting, Olmsted County, where the population is predominately of white ethnicity and much of the care obtained by study participants occurred at a high volume referral center (16). Patients and results may differ in other practice settings and locales. We also acknowledge our relatively small sample sizes, particularly in stratified analyses, hindering cost comparisons given the high variability and skewed nature of this outcome. Furthermore, while we did restrict our sample to individuals with verified county residency to limit missing data concerns, the possibility still remains that some individuals may have obtained healthcare outside our local setting. Finally, a significant limitation of this study design is that our analysis was focused on costs from the health care provider perspective, and it does not include pharmaceutical costs, decreased work productivity, or the very significant out-of-pocket cost associated with gluten-free food that can be 2–4 times as expensive as its wheat-based foods (13, 14). It remains possible that our observed reduction in medical cost after CD diagnosis and treatment may have shifted the economic burden to patients as the excess cost associated with the gluten-free diet is not reimbursed by payers in the U.S unlike in other countries such as Finland where a monthly compensation (“dietary grant”) has been paid by a Social Insurance Institution since 2002 (33). Finally, quality of life implications associated with a gluten-free diet or CD that may be of significant concern from a patient perspective were not analyzed (34, 35, 36).
In conclusion, using a population-based study design, our estimates of CD-associated costs indicate a significant economic burden of disease, particularly for men with CD. Diagnosis and treatment of CD significantly reduces direct medical costs of care, suggesting an economic advantage to earlier detection and treatment.
Acknowledgments
This study was made possible by the Rochester Epidemiology Project (Grant #R01-AR30582) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Financial support: Funding for this study was provided by National Institutes of Health grant R01-DK57892 and the Mayo Foundation for Medical Education and Research. Additional National Institutes of Health support was obtained under the Ruth L. Kirschstein National Research Service Award/Training Grant in Gastrointestinal Allergy and Immunology T32 AI07047 (awarded to A.R.-T.).
Abbreviations
- CD
celiac disease
- REP
Rochester Epidemiology Project
- OCHEUD
Olmsted County Health Care Expenditure and Utilization Database
Footnotes
Conflict of Interest
Guarantor of the article: Kirsten Hall Long, Ph.D.
Specific author contributions: Kirsten Hall Long: design, collection and interpretation of data, statistical analysis, and manuscript preparation; Alberto Rubio-Tapia: conception, design, funding, collection and interpretation of data, and manuscript preparation; Amy E. Wagie: data collection and statistical analysis; L. Joseph Melton III: design, interpretation of data, and manuscript preparation; Brian D. Lahr: collection and interpretation of data, statistical analysis; Carol T. Van Dyke: data collection; Joseph A. Murray: conception, design, funding, collection and interpretation of data, and manuscript preparation. All authors have reviewed and approved the final draft submitted.
Potential competing interests: No
References
- 1.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–43. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 2.Rostom A, Murray JA, Kagnoff MF. American Gastroenterological Association (AGA) Institute technical review on the diagnosis and management of celiac disease. Gastroenterology. 2006;131:1981–2002. doi: 10.1053/j.gastro.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Green PHR, Stavropoulos SN, Panagi SG, Goldstein SL, McMahon DJ, Absan H, Neugut AI. Characteristics of adult celiac disease in the USA: results of a national survey. Am J Gastroenterol. 2001;96:126–31. doi: 10.1111/j.1572-0241.2001.03462.x. [DOI] [PubMed] [Google Scholar]
- 4.Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, Drago S, Elitsur Y, Green PH, Guandalini S, Hill ID, Pietzak M, Ventura A, Thorpe M, Kryszak D, Fornaroli F, Wasserman SS, Murray JA, Horvath K. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286–92. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 5.Catassi C. The world map of celiac disease. Acta Gastroenterol Latinoam. 2005;35:37–55. [PubMed] [Google Scholar]
- 6.Rubio-Tapia A, Kyle RA, Kaplan EL, Johnson DR, Page W, Erdtmann F, Brantner TL, Kim WR, Phelps TK, Lahr BD, Zinsmeister AR, Melton LJ, 3rd, Murray JA. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009;137:88–93. doi: 10.1053/j.gastro.2009.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorn SD, Matchar DB. Cost-effectiveness analysis of strategies for diagnosing celiac disease. Dig Dis Sci. 2008;53:680–8. doi: 10.1007/s10620-007-9939-5. [DOI] [PubMed] [Google Scholar]
- 8.Harewood GC, Murray JA. Diagnostic approach to a patient with suspected celiac disease: a cost analysis. Dig Dis Sci. 2001;46:2510–4. doi: 10.1023/a:1012396408370. [DOI] [PubMed] [Google Scholar]
- 9.Harwood GC. Economic comparison of current endoscopic practices: Barrett’s surveillance vs. ulcerative colitis surveillance vs. bipsoy for sprue vs. biopsy for microscopic colitis. Dig Dis Sc. 2004;49:1808–1814. doi: 10.1007/s10620-004-9575-2. [DOI] [PubMed] [Google Scholar]
- 10.Spiegel BM, DeRosa VP, Gralnek IM, Wang V, Dulai GS. Testing for celiac sprue in irritable bowel syndrome with predominant diarrhea: a cost-effectiveness analysis. Gastroenterology. 2004;126:1721–32. doi: 10.1053/j.gastro.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Mein SM, Ladabaum U. Serological testing for coeliac disease in patients with symptoms of irritable bowel syndrome: a cost-effectiveness analysis. Aliment Pharmacol Ther. 2004;19:1199–210. doi: 10.1111/j.1365-2036.2004.01958.x. [DOI] [PubMed] [Google Scholar]
- 12.Yagil Y, Goldenberg I, Arnon R, Ezra V, Ashkenazi I. Serologic testing for celiac disease in young adults--a cost-effect analysis. Dig Dis Sci. 2005;50:796–805. doi: 10.1007/s10620-005-2576-y. [DOI] [PubMed] [Google Scholar]
- 13.Stevens L, Rashid M. Gluten-free and regular foods: a cost comparison. Can J Diet Pract Res. 2008;69:147–50. doi: 10.3148/69.3.2008.147. [DOI] [PubMed] [Google Scholar]
- 14.Lee AR, Ng DL, Zivin J, Green PH. Economic burden of a gluten-free diet. J Hum Nutr Diet. 2007;20:423–30. doi: 10.1111/j.1365-277X.2007.00763.x. [DOI] [PubMed] [Google Scholar]
- 15.Green PH, Neugut AI, Naiyer AJ, Edwards ZC, Gabinelle S, Chinburapa V. Economic benefits of increased diagnosis of celiac disease in a national managed care population in the United States. J Insur Med. 2008;40:218–28. [PubMed] [Google Scholar]
- 16.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 17.Leibson CL, Katusic SK, Barbaresi WJ, Ransom J, O’Brien PC. Use and costs of medical care for children and adolescents with and without attention-deficit/hyperactivity disorder. Jama. 2001;285:60–6. doi: 10.1001/jama.285.1.60. [DOI] [PubMed] [Google Scholar]
- 18.Gabriel SE, Tosteson AN, Leibson CL, Crowson CS, Pond GR, Hammond CS, Melton LJ., 3rd Direct medical costs attributable to osteoporotic fractures. Osteoporos Int. 2002;13:323–30. doi: 10.1007/s001980200033. [DOI] [PubMed] [Google Scholar]
- 19.Revised criteria for diagnosis of coeliac disease. Report of Working Group of European Society of Paediatric Gastroenterology and Nutrition. Arch Dis Child. 1990;65:909–11. doi: 10.1136/adc.65.8.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray JA, Van Dyke C, Plevak MF, Dierkhising RA, Zinsmeister AR, Melton LJ., 3rd Trends in the identification and clinical features of celiac disease in a North American community, 1950–2001. Clin Gastroenterol Hepatol. 2003;1:19–27. doi: 10.1053/jcgh.2003.50004. [DOI] [PubMed] [Google Scholar]
- 21.Melton LJ., 3rd The threat to medical-records research. N Engl J Med. 1997;337:1466–70. doi: 10.1056/NEJM199711133372012. [DOI] [PubMed] [Google Scholar]
- 22.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 23.Cellier C, Delabesse E, Helmer C, Patey N, Matuchansky C, Jabri B, Macintyre E, Cerf-Bensussan N, Brousse N. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French Coeliac Disease Study Group Lancet. 2000;356:203–8. doi: 10.1016/s0140-6736(00)02481-8. [DOI] [PubMed] [Google Scholar]
- 24.Rubio-Tapia A, Kelly DG, Lahr BD, Dogan A, Wu TT, Murray JA. Clinical staging and survival in refractory celiac disease: a single center experience. Gastroenterology. 2009;136:99–107. doi: 10.1053/j.gastro.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Efron B, Tibshirani RJ. An introduction to the bootstrap. Boca Raton, FL: Chapman & Hall/CRC; 1998. [Google Scholar]
- 26.Briggs A, Gray A. The distribution of health care costs and their statistical analysis for economic evaluation. J Health Serv Res Policy. 1998;3:233–45. doi: 10.1177/135581969800300410. [DOI] [PubMed] [Google Scholar]
- 27.Duran-Zaleski I. Why cost-of-illness studies are important and inform policy. Vascular Medicine. 2008;13:251–253. doi: 10.1177/1358863X08091738. [DOI] [PubMed] [Google Scholar]
- 28.Hershcovici T, Leshno M, Goldin E, Shamir R, Isreli E. Cost effectiveness of mass screening for celiac disease is determined by time-delay to diagnosis and quality of life on a gluten free diet. Aliment Pharmacol Ther. 2010 doi: 10.1111/j.1365-2036.2010.04242.x. [DOI] [PubMed] [Google Scholar]
- 29.Dickey W, McConnell JB. How many hospital visits does it take before celiac sprue is diagnosed? J Clin Gastroenterol. 1996;23:21–3. doi: 10.1097/00004836-199607000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Kostopoulou O, Devereaux-Walsh C, Delaney BC. Missing celiac disease in family medicine: the importance of hypothesis generation. Med Decis Making. 2009;29:282–90. doi: 10.1177/0272989X08327493. [DOI] [PubMed] [Google Scholar]
- 31.Bardella MT, Fredella C, Saladino V, Trovato C, Cesana BM, Quatrini M, Prampolini L. Gluten intolerance: gender- and age-related differences in symptoms. Scand J Gastroenterol. 2005;40:15–9. doi: 10.1080/00365520410008169. [DOI] [PubMed] [Google Scholar]
- 32.Bai D, Brar P, Holleran S, Ramakrishnan R, Green PH. Effect of gender on the manifestations of celiac disease: evidence for greater malabsorption in men. Scand J Gastroenterol. 2005;40:183–7. doi: 10.1080/00365520510011498. [DOI] [PubMed] [Google Scholar]
- 33.Virta LJ, Kaukinen K, Collin P. Incidence and prevalence of diagnosed coeliac disease in Finland: results of effective case finding in adults. Scand J Gastroenterol. 2009;44:933–8. doi: 10.1080/00365520903030795. [DOI] [PubMed] [Google Scholar]
- 34.Usai P, Minerba L, Marini B, Cossu R, Spada S, Carpiniello B, Cuomo R, Boy MF. Case control study on health-related quality of life in adult coeliac disease. Dig Liver Dis. 2002;34:547–52. doi: 10.1016/s1590-8658(02)80087-1. [DOI] [PubMed] [Google Scholar]
- 35.Whitaker JK, West J, Holmes GK, Logan RF. Patient perceptions of the burden of coeliac disease and its treatment in the UK. Aliment Pharmacol Ther. 2009;29:1131–6. doi: 10.1111/j.1365-2036.2009.03983.x. [DOI] [PubMed] [Google Scholar]
- 36.Haines ML, Anderson RP, Gibson PR. Systematic review: The evidence base for long-term management of coeliac disease. Aliment Pharmacol Ther. 2008;28:1042–66. doi: 10.1111/j.1365-2036.2008.03820.x. [DOI] [PubMed] [Google Scholar]