Abstract
Background
Low-dose methotrexate is a widely used and efficacious therapy in chronic inflammatory disorders such as psoriasis and rheumatoid arthritis. Prospective randomized controlled trials have demonstrated the efficacy of parenteral methotrexate in Crohn’s disease (CD). We performed a systematic review of the efficacy of methotrexate in ulcerative colitis (UC) and discuss the results in the context of the known pharmacokinetics and adverse events of methotrexate therapy in inflammatory bowel diseases and other inflammatory conditions.
Materials and Methods
We performed a systematic review of the literature in Medline, Embase, and Web of Science. All publications describing patients with UC treated with methotrexate were included.
Results
We identified 12 studies or retrospective case series and 5 meeting abstracts that met the inclusion criteria. Only 1 study reported a prospective randomized placebo-controlled trial using methotrexate at a dose of 12.5 mg orally with no significant clinical benefit. However, the majority of uncontrolled retrospective analyses suggest a clinical response to methotrexate therapy in a range of 30%–80% when the drug is applied by parenteral route in doses between 20–25 mg.
Conclusions
The only randomized controlled trial of methotrexate in UC employed oral dosing and doses lower than those shown to be effective in CD and did not demonstrate efficacy, whereas uncontrolled, retrospective studies using doses and routes of administration similar to those employed in CD suggest benefit. Well-designed, prospective, placebo-controlled trials of methotrexate in UC are needed.
Keywords: methotrexate, ulcerative colitis, inflammatory bowel disease
Methotrexate, which was initially developed in 1948 for the treatment of leukemia, has been used clinically for nearly 60 years as low- and high-dose therapy in other diseases including certain lymphomas, Wegeners’ disease, psoriasis, and rheumatoid arthritis. In inflammatory bowel diseases (IBDs) methotrexate’s clinical efficacy has been established for steroid-dependent Crohn’s disease (CD) in adults and also in children refractory or intolerant to thiopurine therapy.1–5 The effectiveness of intramuscularly (i.m.) administered methotrexate 25 mg once weekly in steroid-dependent CD is well documented. In the North American Crohn’s Study Group’s landmark trial, 39.4% of the patients receiving methotrexate (25 mg, i.m./week) were in clinical remission as defined by the Crohn’s Disease Activity Index (CDAI) <150 points and the discontinuation of prednisone, compared with 19.1% of the patients in the placebo group, at 16 weeks.6 Similarly, Feagan et al7 demonstrated the efficacy of methotrexate treatment (15 mg/i.m./once weekly) in maintaining steroid-free remission in CD patients (after 40 weeks 65% remission in the methotrexate group versus 39% in the placebo group). Of note is that 2 smaller placebo-controlled studies in CD patients with orally administered methotrexate, given at lower doses than used in the trial of Feagan et al,6 did not demonstrate a statistically significant differences in response or remission rates compared to placebo, suggesting that the route of administration and the dose of methotrexate play important roles in the treatment of IBD.8,9
Proven effective medications for patients with steroid-dependent CD patients include azathioprine or 6-mercaptopurine (6-MP), methotrexate, infliximab, adalimumab, certolizumab, and natalizumab. In comparison, therapeutic choices for steroid-dependent ulcerative colitis (UC) patients are currently limited only to azathioprine or 6-MP and infliximab. In addition, proctocolectomy with or without construction of an ileal pouch anal anastomosis (IPAA) is an option for patients who do not tolerate azathioprine and fail infliximab; however, UC patients with mildly to moderately active steroid dependency might not experience an improved quality of life after IPAA. IPAA is associated with a number of complications that may adversely affect the long-term outcome of these patients, such as antibiotic-dependent or antibiotic-refractory pouchitis, cuffitis, CD of the pouch, and infertility of female patients.10–12 Therefore, there is a clear need for further exploration of new therapies with a calculable risk profile for this patient group.
We performed a systematic review to explore the efficacy of methotrexate in patients with UC and discuss the data in the context of current knowledge of effects, adverse events, and pharmacokinetics of methotrexate therapy in patients with other inflammatory conditions.
MATERIALS AND METHODS
To identify relevant articles and abstracts describing the efficacy of methotrexate in patients with UC a systematic literature search was performed using both medical subject headings (MESH) and keywords. The following terms were used: “Ulcerative colitis,” “Inflammatory bowel disease,” “IBD,” and “Methotrexate.” The search was restricted to English-language publications involving humans and was performed in the following databases: MEDLINE (1966 to November 2009), EMBASE (keyword search in abstracts; 1988 to November 2009), Web of Science (for meeting abstracts), and Clinical trials database (Clinical-Trials.gov). The keywords were linked by the Boolean operators (and, or) to refine the search. Data from full articles and meeting abstracts were extracted. Additional efforts to identify relevant trials were made through review of reference lists of included articles. Meeting abstracts, which were later published as a full article, were not included.
THERAPEUTIC EFFICACY OF METHOTREXATE IN UC
A systematic literature research in Medline and Embase yielded 494 and 598 reports, respectively, of which 12 studies were relevant to our topic. The search in Web of Science yielded additionally 5 meeting abstracts, all of which are currently not yet published as full articles.
The first case series by Kozarek et al13 described a successful induction of remission in 5 of 7 patients with UC given methotrexate 25 mg i.m./week. The therapy, which was applied for 12 weeks and then tapered down to an oral dose as low as 7.5 mg/week if improvement was evident by week 12, achieved a significant reduction of steroid use and improvement of mucosal inflammation as judged by sigmoidoscopy. The same group later described a total of 30 patients with refractory UC and a 70% response rate after 12 weeks of 25 mg i.m./week and a long-term response of 40% on oral methotrexate at a dose 7.5–15 mg.14 Another open-label prospective study of oral methotrexate 15 mg/week in 8 patients with steroid-dependent UC reported a steroid-sparing effect with a mean dose reduction from 26.3 ± 3.2 mg/day to 12.7 ± 2.0 mg/day and a partial response in 3/8 patients.15 Of note, in this study methotrexate was started at a dose of 2.5 mg/week and then increased to 5 mg, 10 mg, and finally 15 mg at weeks 2, 3, and 4–18, respectively.
These initial observations, along with the significant therapeutic effectiveness of methotrexate in patients with CD, led to the first and only prospective, placebo-controlled trial investigating the efficacy of oral methotrexate therapy in patients with active UC.16 Currently, there are no published, prospectively collected, placebo-controlled data regarding the clinical value of parenterally administered methotrexate in patients with UC.17,18
Oren et al16 conducted a prospective, randomized, placebo-controlled trial investigating the efficacy of oral methotrexate at a dose of 12.5 mg orally/week in patients with at least moderately active UC (Mayo score ≥7). The treatment duration was 9 months; 5-aminosalicylates (5-ASA) and steroids were allowed to continue during the study at the discretion of the treating physician and the primary outcome measures were the proportion of patients entering first remission, time to reach that remission, and maintenance of remission. A total of 67 patients were enrolled (37 placebo, 30 methotrexate). There were no significant differences among the groups with regard to the primary outcomes (Table 1), monthly steroid use or clinical Mayo scores (evaluated every 4 weeks), or the sigmoidoscopy scores for inflammation. Interestingly, dropout rates were 4 times higher among placebo-treated patients than in the methotrexate-treated patients. This could reflect partial improvement benefit with methotrexate.
TABLE 1.
Outcome of Oral Methotrexate Therapy (12.5 mg orally/week) in a Prospective, Randomized Trial (Ref. 16)
| Placebo n=37 |
Methotrexate n=30 |
|
|---|---|---|
| Remission (n) | 48.6% (18) | 46.7% (14) |
| Time to remission (months) | 3.4 ± 1.7 | 4.1 ± 1.9 |
| Relapse after achieving remission (n) |
44.4% (8) | 46.7% (9) |
| Drop outs (n) | 24.3% (9) | 6.7% (2) |
| Treatment failure | 5.4% (2) | 10% (3) |
| Withdrawal because of side effect | 2.7% (1) | 6.7% (2) |
Three prospective open-label studies have investigated the effectiveness of methotrexate compared to 6-MP or 5-ASA. Mate-Jimenez et al54 randomized patients with steroid-dependent UC and CD in an open-label study to either 6-MP 1.5 mg/kg bodyweight (bw)/day, 15 mg/week oral methotrexate, or 3 g/day 5-ASA in a 2:2:1 design. In addition to being treated with at least 20 mg prednisone/ day, the UC patients had to have a Mayo clinical score of ≥7. UC patients were randomized to either 1 of the abovedescribed therapy regimens (14 received 6-MP, 12 received methotrexate, 8 received 5-ASA). Two weeks after randomization prednisone was reduced by 8 mg each week and discontinued if clinical remission was achieved. Additionally, the methotrexate dose was reduced to 10 mg orally once weekly and the 6-MP dose to 1 mg/kg bw/day if remission was achieved. The remission rates after 30 weeks of therapy were 78.6% (11/14) for 6-MP, 58.3% (7/12) for methotrexate, and 25% for 5-ASA (2/8). In the second part of this study patients in remission were maintained on their respective drug regimen until week 106. At this timepoint only 1/7 patients on methotrexate remained in remission compared to 7/11 in the 6-MP group and none in the 5-ASA group. The study has been criticized for its small sample size and poor methodological quality, including vague definitions of inclusion criteria. Moreover, the authors failed to describe the mode of randomization. In the trial CD and UC patients were randomized together for treatments, but later separated in groups postrandomization for evaluation. In addition, patients and physicians were not blinded to their treatment assignment.17,18 Perhaps most important, in the maintenance phase the dose of methotrexate was reduced below even that demonstrated to be effective for CD.
Egan et al32 prospectively investigated the usefulness of 2 different doses of parenterally administered methotrexate in a cohort of steroid-dependent patients (at least ≥10 mg prednisone/day) with IBD. Thirty patients (16 with CD and 14 with UC) were included and randomized (patients blinded) to receive either 15 or 25 mg methotrexate/week. The authors report the overall results for the whole cohort, including both the UC and CD patients, but did not provide a breakdown of results by diagnosis. After 16 weeks, 17% (3/18 in the 15 mg group and 2/12 in the 25 mg group) in both groups were in remission and 39% in the 15 mg and 33% in the 25 mg group had improved. In a second phase, 11 of the study patients who were not responding (n = 9) or partially responding (n = 2) to 15 mg methotrexate/ week were dose-escalated to 25 mg methotrexate/week. Three patients had a partial response and 2 were in remission after another 16 weeks of therapy.
Paoluzi et al19 report the results of short- and long-term methotrexate therapy (12.5 mg i.m./week) in 10 steroid-dependent UC patients who were either intolerant (n = 8) or resistant to azathioprine therapy. After 6 months all 10 patients were in clinical remission, 6/10 had also complete endoscopic and histological remission, whereas 4/10 demonstrated endoscopic and histologic improvement of rectosigmoid mucosal inflammation. In the median follow-up of 2 years all patients in clinical and endoscopic remission remained stable and 2 of the 4 patients with incomplete endoscopic/histologic remission had relapsed.
Retrospective Studies of Methotrexate Therapy for UC
In addition to the limited number of prospective trials described above, several retrospective single-center observations have been published (see also Table 2). In these reports methotrexate is typically administered to UC patients in doses similar to those used in CD patients.
TABLE 2.
Studies on the Efficacy of Methotrexate in UC Patients
| Author | Type of Study | Clinical Type of UC | n | Methotrexate mg/week |
Mode of Application |
Study Length or Period |
Outcome |
|---|---|---|---|---|---|---|---|
| Kozarek 1989 (13) |
Case series | Refractory* | 7 | 25 | IM | 12 weeks | Remission 71% |
| Kozarek 1992 (14) (A) |
Case series | Refractory* | 30 | 25 for 12 weeks then 7.5–15 |
IM (12 weeks) then O |
Median 59 weeks | After 12 weeks 70% response, after 59 weeks 40% remained in remission, 50% colectomy. |
| Baron 1993(15) | Open label study | Steroid dependent | 8 | 15 | O | 18 weeks | Steroid sparing and partial response in 38% |
| Oren 1996(16) | Prospective, placebo controlled |
Moderate acitive; Mayo score ≥ 7 |
30 | 12.5 | O | 9 months | Remission placebo 49%, methotrexate 47%. In this study response not defined as primary or secondary outcome |
| Egan 1999(32) | Randomized, single blind, parallel group comparison (include- ing CD) |
Steroid dependent | 14 | 25 or 15 | P | 16 weeks | Only pooled results for UC (n=14) and CD (n=16) reported: 39% improvement in 15 mg group, 33% improvement in 25 mg group, 17% remission both dose groups. |
| Mate-Jiminez 2000 (54) |
Prospective, open- label either 5-ASA, 6-MP or methotrexate |
Steroid dependent | 12 | 15 | O | 30 weeks | 25% remission on 5-ASA (3 g) 79% remission on 6-MP (1.5 mg/kg.bw) 58% remission on methotrexate |
| Paoluzi 2002 (19) |
Steroid and 6-MP/ azathioprine intolerant or refractory |
10 | 12.5 | IM | 6 months | 100% remission, 60% endoscopic and histologic remission |
|
| Fraser 2002 (20) |
Retrospective, single center (including CD) |
Thiopurine refractory or intolerant |
22 | Mean 20 (range 10–25) |
IM, O | Mean 17.1 months (range 0.5–71) |
Only pooled results for UC (n=22) and CD (n=48) reported: 62% remission after 3 months |
| Fraser 2003 (A) (55) |
Prospective, single center |
Steroid dependent and 6-MP/ azathioprine refractory |
8 | 25 | P | 16 weeks | 25% stopped therapy due to adverse event, 75% completed study without achieving response or remission |
| Sieveke 2003 (56) |
Case series | Not mentioned | 4 | 25 | IM | Not mentioned | 100% remission |
| Soon 2004 (57) | Retrospective, single center (including CD) |
Steroid dependent | 6 | Mean 18 (range 5–30) |
O, IM | 6 months | 100 % steroid sparing, 50% clinical response |
| Cummings 2005 (21) |
Retrospective, single center |
Thiopurine refractory or intolerant or concurrent RA |
50 | 20–25 | O | Median 30 weeks (range: 7–395) |
45% remission, 58% response in thiopurine intolerant 0% remission, 27% response in thiopurine failure 62% remission, 80% response in concomitant RA |
| Rook 2005 (A)(58) |
Retrospective, single center |
Thiopurine intolerant | 8 | 25 for 12 weeks then 15 |
SC for 12 weeks then O |
Mean 10 months (range 4–18) |
75% clinical remission |
| Naik 2007 (A) (59) |
Retrospective, single center |
Thiopurine intolerant or steroid dependent |
54 | 15±6 (O) 18±8 (SC) |
O, SC | > 6 months | 31 patient long term therapy with 84% of these patients benefiting from methotrexate therapy |
| Nathan 2008 (22) |
Retrospective, single center (including CD) |
Thiopurine refractory or intolerant |
23 | Mean 23 (range 15–25) |
SC (90%) O (10%) |
Median 15 months (range 3–37) |
48% remission, 67% response |
| Gonzalez-Lama 2009 (A) (60) |
Retrospective several centers (including CD) |
steroid dependent or steroid resistance |
15 | Mean 15 (range 83–25) |
O, IM, SC | Mean 17 months (range 1–108) |
Only pooled results for UC (n=15) and CD (n=62) reported:82% response, 28% remission. |
| Wahed 2009 (23) |
Retrospective, single center (including CD) |
Thiopurine refractory or intolerant |
32 | Induction Median 25 (range 10–25) Maintenance Median 20 mg (range 10–25) |
O, IM | 6 months | 65% response in thiopurine intolerant 78% response in thiopurine refractory 35% steroid withdrawal |
(A) published as meeting abstract only;
no exact definition provided; P, parenteral; O, oral; SC, subcutaneous; IM, intramuscular; bw, bodyweight; RA, rheumatoid arthritis.
Fraser et al20 included 22 patients with UC and 48 patients with CD in a retrospective analysis of methotrexate efficacy in a tertiary referral hospital in Oxford. Most of the included patients had been previously treated with azathioprine or were intolerant to that agent. The outcome of the UC patients was not separately analyzed. The mean dose of methotrexate was 20 mg/week, with most patients receiving the therapy by oral administration and a minority group by i.m. application. Overall, remission was achieved in 34 of 55 patients (62%) who completed more than 3 months of therapy. Interestingly, the only factor significantly associated with achieving remission was the dose of methotrexate. Other factors used in the logistic regression model to predict remission were diagnosis (CD versus UC), disease site, presence of joint symptoms or other extraintestinal manifestations, age at time of treatment and age at diagnosis. A life-table analysis demonstrated that the rates of remission on methotrexate were 90%, 73%, and 51% for 12, 24, and 36 months, respectively.
Cummings et al21 analyzed the efficacy of orally administered methotrexate in the range of 20–25 mg once weekly in 42 patients with either lack of benefit (n = 11) or intolerance (n = 31) to azathioprine. An additional 8 UC patients were primarily treated with methotrexate because of their rheumatoid arthritis. The response and remission rates correspondingly were 58% and 45% in azathioprine-intolerant patients, 27% and 0% in previous azathioprine failure patients, and 80% and 62% in patients with concurrent rheumatoid arthritis.
As both the analyses by Cummings et al21 and by Fraser et al20 were performed at the same Oxford tertiary hospital, the patients in these reports may be overlapping.
Nathan et al22 reported a retrospective analysis of patients treated in a single hospital in Victoria, Australia. They observed remission rates of 48% (11/23 patients) following administration of 15–25 mg/week subcutaneous (s.c.) methotrexate (90% of the patients were treated s.c., 10% received methotrexate orally). Three patients (13%) achieved improvement and could decrease the dose of steroids. Wahed et al23 described a clinical response in 22/32 (68%) of UC patients in a retrospective analysis at a single university hospital in London. Response in this retrospective study was defined as steroid withdrawal by 6 months, normalization of previously raised C-reactive protein or physician’s clinical assessment of improvement as assessed in the patient record. The induction dose of methotrexate was administered orally in 20 patients and i.m. in 12 patients, with a median dose of 25 mg/week (10–25) in the induction period and 20 mg/week (10–25) in the maintenance period. The analyzed group consisted mainly of patients who were either previously intolerant (efficacy 15/23; 65%) or failed (efficacy 7/9; 78%) to achieve adequate response with purine analogs. Steroid withdrawal was possible in 7/19 patients (37%) who were on steroids at baseline.
DISCUSSION
This systematic review of the published literature regarding the effectiveness of methotrexate in patients with UC illustrates the lack of prospectively collected efficacy data, especially in a dose range demonstrated to be effective in the treatment of CD. When viewed in its totality, these data indicate that if methotrexate is given in a similar dose and manner (i.m. or s.c.) as in CD it might be a clinically useful therapy for steroid-dependent UC patients, including patients who did not tolerate or did not respond to thiopurine therapy. The studies that used low-dose orally administered methotrexate demonstrated very low response rates among patients with UC, similar to the experience in CD.1 The available therapeutic experience with methotrexate in other inflammatory diseases and especially in patients with rheumatoid arthritis indicate that 2 factors, the dose and the mode of application, are associated with the efficacy of a methotrexate treatment.
Clinical Pharmacology of Methotrexate
Methotrexate is an analog of folic acid and of aminopterin, which is also a folic acid antagonist. One of the main mechanisms of its action is the inhibition of dihydrofolate reductase, the enzyme involved in the de novo synthetic pathway for purines and pyrimidines. The rationale for the use of high-dose methotrexate in the treatment of cancer is that the rapidly proliferating malignant cells become starved of purine and pyrimidine precursors and therefore are unable to sufficiently maintain DNA and RNA synthesis, leading to decreased proliferation. The underlying antiinflammatory effect of low-dose methotrexate in inflammatory diseases such as rheumatoid arthritis, psoriasis, and IBD is less clear, as the antiproliferative activity of low-dose methotrexate is minimal. Multiple mechanisms of actions are proposed, including promotion of adenosine release, inhibition of production of proinflammatory cytokines, suppression of lymphocyte proliferation, reduction of neutrophil chemotaxis, and adherence and decrease of serum immunoglobulins.24,25
Methotrexate can be administered by oral, s.c., i.m., or (i.v.) routes. With oral administration, methotrexate is absorbed in the jejunum with good bioavailability at doses below 15 mg. Above this threshold absorption rates can be decreased by 30%–70% compared to s.c. or i.m. applications.26–29 The s.c. or i.m. administration results in similar bioavailability of the drug, but s.c. administration is normally better-tolerated.29,30 The serum half-life of methotrexate is ≈7–10 hours, although some patients demonstrate prolonged serum half-life for up to 26 hours. After methotrexate is taken up by tissues it is converted to methotrexate polyglutamates, which are long-lived derivates that retain biochemical and biological activities. These polyglutamates migrate through cellular membranes poorly, leading to cellular entrapment, which is one of the mechanisms of the prolonged retention of the drug in tissues such as the liver. Between 65% and 80% of the drug is excreted by the kidneys (the major part in the first 12 hours after administration) and 20%–35% is secreted in bile. Studies in patients with rheumatoid arthritis and IBD found no meaningful correlation of methotrexate metabolites in serum or plasma and clinical efficacy, suggesting that there is little clinical value in monitoring these levels.31,32
Dose of Methotrexate and Clinical Effectiveness
There is a positive correlation between dose and response to methotrexate in patients with rheumatoid arthritis. This has led to the recommended step-up approach for methotrexate therapy in this patient group. Methotrexate is started at a dose of 10–15 mg once weekly and depending on disease activity the dose is then further increased by 5 mg every 2–4 weeks up to 20–30 mg methotrexate once weekly.33,34 Overall, higher doses (25 mg methotrexate once weekly) are more effective than lower doses (15 mg methotrexate once weekly) in patients with rheumatoid arthritis, but these doses are also associated with a higher likelihood of gastrointestinal toxicity.35
The dose response is also partially reflected in the IBD literature, but in contrast to patients with rheumatoid arthritis lower doses seem to be ineffective at least with regard to induction of remission. In several studies methotrexate doses between 10–15 mg/week orally were not effective in patients with active IBD.8,9,16 These negative results could have been partially confounded by the oral administration of methotrexate in these prospective studies (see below). However, after induction of remission with 25 mg methotrexate/week i.m., 15 mg methotrexate i.m. once weekly can successfully maintain remission in patients with CD.7 Notably, patients suffering a relapse while on this lower dose of methotrexate can be successfully brought back into remission and then maintained with the higher dose (25 mg methotrexate once weekly).7,20
The only study that did not show a dose response but rather equal efficacy of 15 and 25 mg methotrexate applied parenterally to patients with IBD is also characterized by very low remission rates of only 17%.32 Considering the higher remission rates reported in other blinded, randomized controlled trials of methotrexate in CD, one may speculate that this low remission rate may indicate that only a very refractory patient group was included in this trial.
Route of Administration of Methotrexate and Clinical Effectiveness
There is ongoing debate about possible differences in the bioavailability of methotrexate given parenterally or orally. Pharmacokinetic studies employing methotrexate suggest that in the dose range of 15–25 mg an oral application results in a significantly lower bioavailability of the drug, at least in a subgroup of patients.26,27,36,37 Interestingly, in a prospective, double-blind, controlled trial comparing an oral and an s.c. regimen of methotrexate in patients with rheumatoid arthritis, a significantly higher response rate in patients treated s.c. with methotrexate was observed compared to the orally treated group.38 Similarly, in a retrospective study of children with juvenile idiopathic arthritis who were treated with orally administered methotrexate in doses up to 20 mg weekly, an improved efficacy was reported when the oral administration of methotrexate was changed to a parenteral application.39
One of the factors associated with the lower bioavailability of higher doses of methotrexate might be interference in the absorption process of the drug. Interestingly, splitting a high oral methotrexate dose into 2 divided doses taken 8 hours apart can achieve bioavailability levels similar to s.c. administration.36 The current recommendation for methotrexate therapy in patients with rheumatoid arthritis is to start with oral administration with a stepwise increase of the dose as described above. In case of an insufficient response to the higher doses of methotrexate the patient should be switched to s.c. administration of methotrexate.33,34
In patients with IBD there are very limited data comparing clinical effectiveness in oral and parenteral modes of administration. In 1 retrospective study, patients on parenteral methotrexate had superior outcome in long-term remission rates compared to the patients taking oral methotrexate,40 whereas others did not find such a difference in their retrospective analyses.22,23 A small study evaluating the bioavailability of 12.5 mg oral methotrexate in 5 patients with CD, 4 patients with UC, and 6 patients with rheumatoid arthritis did not find a significant difference with regard to overall bioavailability of methotrexate in all 3 groups, but a significant difference in absorption rate between the UC and CD patients.41 However, the authors did not investigate the clinical efficacy in this study.
Overall, the CD data, demonstrating no significant effect in patients treated with oral doses of methotrexate between 12.5–22.5 mg/week8,9,16 and very good efficacy of the parenteral administration of doses of 15–25 mg/week for both induction or maintenance6,7,42 also indicate that in addition to the dose the route of administration may represent a crucial factor at least in patients with CD.
Side Effects of Low-dose Methotrexate Therapy
The most commonly observed adverse events (AEs) of low-dose methotrexate therapy are associated with the gastrointestinal tract, including nausea, anorexia, and less often stomatitis or diarrhea. More serious AEs include hepatotoxicity, bone marrow suppression,43 and rarely hyper-sensitivity pneumonitis and opportunistic infections.44 Due to these potentially severe side effects, a specific evaluation is recommended for patients before the start of methotrexate (Table 3).33,45 Methotrexate is a known teratogen and is contraindicated in pregnancy.46 Currently there are no prospective or retrospective studies in humans evaluating the effects of methotrexate on male or female fertility, on newborns during breast feeding, or on spermatogenesis with regard to later miscarriages or birth defects. Nevertheless, in a recent publication summarizing multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders, the expert opinion was to stop methotrexate at least 3 months before planned pregnancy in both men and women and not to use methotrexate during pregnancy or breast-feeding.33
TABLE 3.
Recommendations for Work-up of Patients Before the Start of Methotrexate
| Assess Clinical Risk Factors |
Laboratory Work-up | Radiology | Consideration of the Following Tests |
|---|---|---|---|
| Obesitya | AST, ALT Albuminb | Chest x-ray to rule out | Serology testing for: hepatitis B, C |
| Diabetes mellitusa | CBC | interstitial lung disease d | HIV |
| Alcohol intake | Creatininec | Pregnancy test Lipid profilea | |
| Blood fasting glucosea |
Adapted from a recently published multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders (33).
Associated with hepatotoxicity in the context of nonalcoholic fatty liver disease (NAFLD) and the risk of preexisting liver fibrosis.
Low albumin is associated with thrombocytopenia, liver and pulmonary toxicity.
Creatinine clearance <79 mL/min reduces methotrexate clearance and is associated with more severe toxicity.
Obtained within the previous year before start of methotrexate therapy.
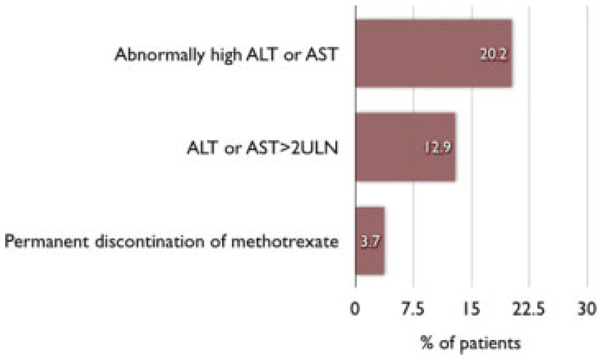
Elevations of liver function tests (LFTs; aspartate aminotransferase, AST; alanine aminotransferase, ALT) are frequently observed in patients on methotrexate therapy (Fig. 1), but the development of hepatic fibrosis or cirrhosis, believed to be due to the intrahepatic accumulation of methotrexate metabolites, is a rare event.47 According to a systematic review of the literature the pooled percentages of mild and severe fibrosis and cirrhosis in 1113 patients with rheumatoid arthritis after a mean of 4.1 years of methotrexate therapy were 15.3%, 1.3%, and 0.5%, respectively.48 Factors associated with hepatoxicity are alcohol consumption, obesity, diabetes mellitus, and viral hepatitis.49,50 A small case series (n = 20) reports little hepatotoxicity in patients with IBD treated with methotrexate in a cumulative dose of higher than 1500 mg.51 Fraser52 analyzed the toxicity of long-term administration of methotrexate in a pooled analysis including 565 IBD patients with an exposure to low-dose methotrexate between 12 weeks and 18 months. He found 7 cases of leukopenia (0.01%), 3 cases of pneumonitis (0.005%), and 28 cases of raised ALT levels (5%).
FIGURE 1.
Elevation of liver enzymes and discontinuation of methotrexate. Pooled results of 27 prospective studies in patients with rheumatoid arthritis. This analysis included 3808 patients with a mean dose of 10.5 mg methotrexate/ week (range 4.6–18) over a mean duration of 55.8 months (range 27–180).49
Severe Side Effects of Methotrexate Therapy in Prospective Placebo-controlled Trials in IBD
In the only placebo-controlled prospective clinical trial of methotrexate therapy in patients with UC by Oren et al,16 3 patients were withdrawn from the study for suspected side effects: transient leukopenia, migraine, and a severe rash. The first 2 patients were administered methotrexate and the third patient was administered placebo. In the largest prospective trials of induction and maintenance therapy with methotrexate in patients with CD, a dose of 25 mg i.m./week, 16 patients (17%) receiving methotrexate had to be withdrawn from the study compared to 1 patient (2%) in the placebo group. The reasons for withdrawal were: asymptomatic elevation of LFTs (7 patients), nausea (6 patients), skins rash (1 patient), pneumonia probably due to mycoplasma (1 patient), and optic neuritis (1 patient). Interestingly, whereas the elevated LFTs leading to withdrawal from this trial in 7 patients (7.4% of all patients treated with methotrexate), no patient experienced elevations of LFTs warranting withdrawal with 15 mg methotrexate i.m./week.6,7 No significant toxicities of methotrexate 25 mg/s.c./weekly applied over 50 weeks were reported in the meeting abstract of a recently completed study comparing the combination of methotrexate and infliximab to infliximab alone in patients with CD.53
CONCLUSION
Limited therapeutic options are available for induction and maintenance of remission for patients with active UC. Given the high costs of biologics, there is a need for affordable, easy to administer therapies such as methotrexate. Additionally, use of small molecules is favorable over biologics as the risk of antibody formation to the administered proteins and the associated risks of an allergic reaction or loss of response is believed to be less likely. Methotrexate has been shown to be effective in both induction and maintenance of remission in patients with CD. Given the lack of large, placebo-controlled, prospective studies investigating methotrexate in patients with active UC at doses that were clinically effective in inducing steroid-free remission in CD patients, no strong conclusions can be drawn from the published data. As there is an obvious dose–response relationship observed for methotrexate in the treatment of rheumatoid arthritis and in the several retrospective analyses suggesting a good clinical effect of methotrexate in patients with UC, there seems to be an unmet need for investigating methotrexate prospectively in studies using 1) higher doses and 2) employing parenteral administration (s.c. or i.m.) in this patient group. Unfortunately, it is highly unlikely that this drug will be investigated prospectively in the setting of an industry-sponsored study, as methotrexate is an old drug and long off patent. Therefore, clinical trials employing methotrexate will require sponsorship either by national funding agencies or patient interest groups.
Two clinical trials to assess the efficacy of methotrexate in UC are under way or in the planning phase. An investigator-initiated trial in France, “Comparison of Methotrexate vs Placebo in Steroid-Refractory Ulcerative Colitis (METEOR)” is currently investigating the efficacy of 25 mg methotrexate/week administered s.c. compared to placebo in inducing clinical remission (www.Clinical-Trials.gov NCT00498589). The second clinical trial “Randomized, double blind, prospective trial investigating the efficacy of methotrexate in induction and maintenance of steroid free remission in ulcerative colitis (MERIT-UC)” is in preparation and the prestudy administrative work is currently funded by the National Institutes of Health (NIH) and supported by the Crohn’s and Colitis Foundation of America (CCFA). These trials should provide an accurate appraisal of the efficacy of methotrexate in UC for both induction and maintenance therapy.
Acknowledgments
Drs. Herfarth, Isaacs, Lewis, Osterman, and Sands are supported by a National Institutes of Health grant 1U34DK084511-01 and the Crohn’s and Colitis Foundation of America (CCFA).
Footnotes
Published online in Wiley InterScience www.interscience.wiley.com).
REFERENCES
- 1.Alfadhli AA, McDonald JW, Feagan BG. Methotrexate for induction of remission in refractory Crohn’s disease. Cochrane Database Syst Rev. 2005:CD003459. doi: 10.1002/14651858.CD003459.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Patel V, Macdonald JK, McDonald JW, et al. Methotrexate for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2009:CD006884. doi: 10.1002/14651858.CD006884.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Weiss B, Lerner A, Shapiro R, et al. Methotrexate treatment in pediatric Crohn disease patients intolerant or resistant to purine analogues. J Pediatr Gastroenterol Nutr. 2009;48:526–530. doi: 10.1097/MPG.0b013e318196df3e. [DOI] [PubMed] [Google Scholar]
- 4.Uhlen S, Belbouab R, Narebski K, et al. Efficacy of methotrexate in pediatric Crohn’s disease: a French multicenter study. Inflamm Bowel Dis. 2006;12:1053–1057. doi: 10.1097/01.mib.0000235103.47280.bb. [DOI] [PubMed] [Google Scholar]
- 5.Turner D, Grossman AB, Rosh J, et al. Methotrexate following unsuccessful thiopurine therapy in pediatric Crohn’s disease. Am J Gastroenterol. 2007;102:2804–2812. doi: 10.1111/j.1572-0241.2007.01474.x. [DOI] [PubMed] [Google Scholar]
- 6.Feagan BG, Rochon J, Fedorak RN, et al. Methotrexate for the treatment of Crohn’s disease. The North American Crohn’s Study Group Investigators. N Engl J Med. 1995;332:292–297. doi: 10.1056/NEJM199502023320503. [DOI] [PubMed] [Google Scholar]
- 7.Feagan BG, Fedorak RN, Irvine EJ, et al. A comparison of methotrexate with placebo for the maintenance of remission in Crohn’s disease. North American Crohn’s Study Group Investigators. N Engl J Med. 2000;342:1627–1632. doi: 10.1056/NEJM200006013422202. [DOI] [PubMed] [Google Scholar]
- 8.Oren R, Moshkowitz M, Odes S, et al. Methotrexate in chronic active Crohn’s disease: a double-blind, randomized, Israeli multicenter trial. Am J Gastroenterol. 1997;92:2203–2209. [PubMed] [Google Scholar]
- 9.Arora S, Katkov W, Cooley J, et al. Methotrexate in Crohn’s disease: results of a randomized, double-blind, placebo-controlled trial. Hepatogastroenterology. 1999;46:1724–1729. [PubMed] [Google Scholar]
- 10.Mahadevan U, Sandborn WJ. Diagnosis and management of pouchitis. Gastroenterology. 2003;124:1636–1650. doi: 10.1016/s0016-5085(03)00325-1. [DOI] [PubMed] [Google Scholar]
- 11.Johnson P, Richard C, Ravid A, et al. Female infertility after ileal pouch-anal anastomosis for ulcerative colitis. Dis Colon Rectum. 2004;47:1119–1126. doi: 10.1007/s10350-004-0570-7. [DOI] [PubMed] [Google Scholar]
- 12.Ording Olsen K, Juul S, Berndtsson I, et al. Ulcerative colitis: female fecundity before diagnosis, during disease, and after surgery compared with a population sample. Gastroenterology. 2002;122:15–19. doi: 10.1053/gast.2002.30345. [DOI] [PubMed] [Google Scholar]
- 13.Kozarek RA, Patterson DJ, Gelfand MD, et al. Methotrexate induces clinical and histologic remission in patients with refractory inflammatory bowel disease. Ann Intern Med. 1989;110:353–356. doi: 10.7326/0003-4819-110-5-353. [DOI] [PubMed] [Google Scholar]
- 14.Kozarek RA, Patterson DJ, Gelfand JM, et al. Long-term use of methotrexate in inflammatory bowel disease. Gastroenterology. 1992;102:A 648. (abstract) [Google Scholar]
- 15.Baron TH, Truss CD, Elson CO. Low-dose oral methotrexate in refractory inflammatory bowel disease. Dig Dis Sci. 1993;38:1851–1856. doi: 10.1007/BF01296109. [DOI] [PubMed] [Google Scholar]
- 16.Oren R, Arber N, Odes S, et al. Methotrexate in chronic active ulcerative colitis: a double-blind, randomized, Israeli multicenter trial. Gastroenterology. 1996;110:1416–1421. doi: 10.1053/gast.1996.v110.pm8613046. [DOI] [PubMed] [Google Scholar]
- 17.Chande N, MacDonald JK, McDonald JW. Methotrexate for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007:CD006618. doi: 10.1002/14651858.CD006618.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Ei-Matary W, Vandermeer B, Griffiths AM. Methotrexate for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2009:CD007560. doi: 10.1002/14651858.CD007560.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Paoluzi OA, Pica R, Marcheggiano A, et al. Azathioprine or methotrexate in the treatment of patients with steroid-dependent or steroid-resistant ulcerative colitis: results of an open-label study on efficacy and tolerability in inducing and maintaining remission. Aliment Pharmacol Ther. 2002;16:1751–1759. doi: 10.1046/j.1365-2036.2002.01340.x. [DOI] [PubMed] [Google Scholar]
- 20.Fraser AG, Morton D, McGovern D, et al. The efficacy of methotrexate for maintaining remission in inflammatory bowel disease. Aliment Pharmacol Ther. 2002;16:693–697. doi: 10.1046/j.1365-2036.2002.01227.x. [DOI] [PubMed] [Google Scholar]
- 21.Cummings JR, Herrlinger KR, Travis SP, et al. Oral methotrexate in ulcerative colitis. Aliment Pharmacol Ther. 2005;21:385–389. doi: 10.1111/j.1365-2036.2005.02331.x. [DOI] [PubMed] [Google Scholar]
- 22.Nathan DM, Iser JH, Gibson PR. A single center experience of methotrexate in the treatment of Crohn’s disease and ulcerative colitis: a case for subcutaneous administration. J Gastroenterol Hepatol. 2008;23:954–958. doi: 10.1111/j.1440-1746.2007.05006.x. [DOI] [PubMed] [Google Scholar]
- 23.Wahed M, Louis-Auguste JR, Baxter LM, et al. Efficacy of methotrexate in Crohn’s disease and ulcerative colitis patients unresponsive or intolerant to azathioprine/mercaptopurine. Aliment Pharmacol Ther. 2009;30:614–620. doi: 10.1111/j.1365-2036.2009.04073.x. [DOI] [PubMed] [Google Scholar]
- 24.Swierkot J, Szechinski J. Methotrexate in rheumatoid arthritis. Pharmacol Rep. 2006;58:473–492. [PubMed] [Google Scholar]
- 25.Wessels JA, Huizinga TW, Guchelaar HJ. Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatology (Oxford) 2008;47:249–255. doi: 10.1093/rheumatology/kem279. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton RA, Kremer JM. Why intramuscular methotrexate may be more efficacious than oral dosing in patients with rheumatoid arthritis. Br J Rheumatol. 1997;36:86–90. doi: 10.1093/rheumatology/36.1.86. [DOI] [PubMed] [Google Scholar]
- 27.Kurnik D, Loebstein R, Fishbein E, et al. Bioavailability of oral vs. subcutaneous low-dose methotrexate in patients with Crohn’s disease. Aliment Pharmacol Ther. 2003;18:57–63. doi: 10.1046/j.1365-2036.2003.01614.x. [DOI] [PubMed] [Google Scholar]
- 28.Balis FM, Mirro J, Jr, Reaman GH, et al. Pharmacokinetics of subcutaneous methotrexate. J Clin Oncol. 1988;6:1882–1886. doi: 10.1200/JCO.1988.6.12.1882. [DOI] [PubMed] [Google Scholar]
- 29.Jundt JW, Browne BA, Fiocco GP, et al. A comparison of low dose methotrexate bioavailability: oral solution, oral tablet, subcutaneous and intramuscular dosing. J Rheumatol. 1993;20:1845–1849. [PubMed] [Google Scholar]
- 30.Brooks PJ, Spruill WJ, Parish RC, et al. Pharmacokinetics of methotrexate administered by intramuscular and subcutaneous injections in patients with rheumatoid arthritis. Arthritis Rheum. 1990;33:91–94. doi: 10.1002/art.1780330112. [DOI] [PubMed] [Google Scholar]
- 31.Lafforgue P, Monjanel-Mouterde S, Durand A, et al. Lack of correlation between pharmacokinetics and efficacy of low dose methotrexate in patients with rheumatoid arthritis. J Rheumatol. 1995;22:844–849. [PubMed] [Google Scholar]
- 32.Egan LJ, Sandborn WJ, Tremaine WJ, et al. A randomized dose-response and pharmacokinetic study of methotrexate for refractory inflammatory Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther. 1999;13:1597–1604. doi: 10.1046/j.1365-2036.1999.00667.x. [DOI] [PubMed] [Google Scholar]
- 33.Visser K, Katchamart W, Loza E, et al. Multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders with a focus on rheumatoid arthritis: integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E Initiative. Ann Rheum Dis. 2009;68:1086–1093. doi: 10.1136/ard.2008.094474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Visser K, van der Heijde D. Optimal dosage and route of administration of methotrexate in rheumatoid arthritis: a systematic review of the literature. Ann Rheum Dis. 2009;68:1094–1099. doi: 10.1136/ard.2008.092668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furst DE, Koehnke R, Burmeister LF, et al. Increasing methotrexate effect with increasing dose in the treatment of resistant rheumatoid arthritis. J Rheumatol. 1989;16:313–320. [PubMed] [Google Scholar]
- 36.Hoekstra M, Haagsma C, Neef C, et al. Splitting high-dose oral methotrexate improves bioavailability: a pharmacokinetic study in patients with rheumatoid arthritis. J Rheumatol. 2006;33:481–485. [PubMed] [Google Scholar]
- 37.Teresi ME, Crom WR, Choi KE, et al. Methotrexate bioavailability after oral and intramuscular administration in children. J Pediatr. 1987;110:788–792. doi: 10.1016/s0022-3476(87)80025-2. [DOI] [PubMed] [Google Scholar]
- 38.Braun J, Kastner P, Flaxenberg P, et al. Comparison of the clinical efficacy and safety of subcutaneous versus oral administration of methotrexate in patients with active rheumatoid arthritis: results of a six-month, multicenter, randomized, double-blind, controlled, phase IV trial. Arthritis Rheum. 2008;58:73–81. doi: 10.1002/art.23144. [DOI] [PubMed] [Google Scholar]
- 39.Alsufyani K, Ortiz-Alvarez O, Cabral DA, et al. The role of subcutaneous administration of methotrexate in children with juvenile idiopathic arthritis who have failed oral methotrexate. J Rheumatol. 2004;31:179–182. [PubMed] [Google Scholar]
- 40.Chong RY, Hanauer SB, Cohen RD. Efficacy of parenteral methotrexate in refractory Crohn’s disease. Aliment Pharmacol Ther. 2001;15:35–44. doi: 10.1046/j.1365-2036.2001.00908.x. [DOI] [PubMed] [Google Scholar]
- 41.Moshkowitz M, Oren R, Tishler M, et al. The absorption of low-dose methotrexate in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 1997;11:569–573. doi: 10.1046/j.1365-2036.1997.00175.x. [DOI] [PubMed] [Google Scholar]
- 42.Ardizzone S, Bollani S, Manzionna G, et al. Comparison between methotrexate and azathioprine in the treatment of chronic active Crohn’s disease: a randomised, investigator-blind study. Dig Liver Dis. 2003;35:619–627. doi: 10.1016/s1590-8658(03)00372-4. [DOI] [PubMed] [Google Scholar]
- 43.Stein RB, Hanauer SB. Comparative tolerability of treatments for inflammatory bowel disease. Drug Saf. 2000;23:429–448. doi: 10.2165/00002018-200023050-00006. [DOI] [PubMed] [Google Scholar]
- 44.Searles G, McKendry RJ. Methotrexate pneumonitis in rheumatoid arthritis: potential risk factors. Four case reports and a review of the literature. J Rheumatol. 1987;14:1164–1171. [PubMed] [Google Scholar]
- 45.Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762–784. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 46.Lewden B, Vial T, Elefant E, et al. Low dose methotrexate in the first trimester of pregnancy: results of a French collaborative study. J Rheumatol. 2004;31:2360–2365. [PubMed] [Google Scholar]
- 47.Kremer JM, Alarcon GS, Lightfoot RW, Jr, et al. Methotrexate for rheumatoid arthritis. Suggested guidelines for monitoring liver toxicity. American College of Rheumatology. Arthritis Rheum. 1994;37:316–328. doi: 10.1002/art.1780370304. [DOI] [PubMed] [Google Scholar]
- 48.Visser K, van der Heijde D. Incidence of liver enzyme elevations and liver biopsy abnormalities during methotrexate treatment in rheumatoid arthritis: A systematic review of the literature. Arthritis Rheum. 2008;58 suppl:S557. [Google Scholar]
- 49.Salliot C, van der Heijde D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis. 2009;68:1100–1104. doi: 10.1136/ard.2008.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas JA, Aithal GP. Monitoring liver function during methotrexate therapy for psoriasis: are routine biopsies really necessary? Am J Clin Dermatol. 2005;6:357–363. doi: 10.2165/00128071-200506060-00003. [DOI] [PubMed] [Google Scholar]
- 51.Te HS, Schiano TD, Kuan SF, et al. Hepatic effects of long-term methotrexate use in the treatment of inflammatory bowel disease. Am J Gastroenterol. 2000;95:3150–3156. doi: 10.1111/j.1572-0241.2000.03287.x. [DOI] [PubMed] [Google Scholar]
- 52.Fraser AG. Methotrexate: first-line or second-line immunomodulator? Eur J Gastroenterol Hepatol. 2003;15:225–231. doi: 10.1097/01.meg.0000049994.68425.dd. [DOI] [PubMed] [Google Scholar]
- 53.Feagan B, McDonald J, Panaccione R, et al. A randomized trial of methotrexate in combination with infliximab for the treatment of Crohn’s Disease. Gastroenterology. 2008;135:294–295. (abstract) [Google Scholar]
- 54.Mate-Jimenez J, Hermida C, Cantero-Perona J, et al. 6-Mercaptopurine or methotrexate added to prednisone induces and maintains remission in steroid-dependent inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2000;12:1227–1233. doi: 10.1097/00042737-200012110-00010. [DOI] [PubMed] [Google Scholar]
- 55.Fraser GM, Ben-Bassat O, Segal N, et al. Parenteral methotrexate is not effective treatment for refractory ulcerative colitis. Gastroenterology. 2003;124:A525. (abstract) [Google Scholar]
- 56.Siveke JT, Folwaczny C. Methotrexate in ulcerative colitis. Aliment Pharmacol Ther. 2003;17:479–480. doi: 10.1046/j.1365-2036.2003.01480.x. [DOI] [PubMed] [Google Scholar]
- 57.Soon SY, Ansari A, Yaneza M, et al. Experience with the use of low-dose methotrexate for inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2004;16:921–926. doi: 10.1097/00042737-200409000-00018. [DOI] [PubMed] [Google Scholar]
- 58.Rook L, Kelly SM. Rescue methotrexate therapy in chronic active ulcerative colitis patients intolerant of thiopurines. Gut. abstract;54:A92. [Google Scholar]
- 59.Naik A, Weber LR, Knox JF, et al. Longterm methotrexate maintenance immunomodulator therapy in ulcerative colitis. Gastroenterology. 2007;132:A185. (abstract) [Google Scholar]
- 60.Gonzalez-Lama Y. Efficacy and safety of methotrexate therapy in inflammatory bowel disease. The Madrid experience. Gastroenterology. 2009;136:A662. (abstract) [Google Scholar]