1. Introduction
Group A streptococci (GAS) are responsible for a wide array of human illnesses that range from uncomplicated pharyngitis and skin infections to serious, invasive infections such as necrotizing fasciitis and toxic shock syndrome. Worldwide, morbidity and mortality are largely due to acute rheumatic fever and accompanying rheumatic heart disease (RHD), which are immune mediated complications of acute GAS infection [1]. Efforts to develop safe and effective vaccines to prevent these infections and their sequelae have been ongoing for decades. One of the leading vaccine targets is the surface M protein of the organism, which is a major virulence determinant that evokes serum opsonic antibodies that protect animals against challenge infections. In general, there are two approaches to the development of M protein-based vaccines, those containing type-specific, N-terminal epitopes contained in multivalent recombinant fusion proteins [2, 3] and peptides copying conserved C-repeat epitopes [4–6] that are shared by the majority of GAS serotypes.
The present studies were undertaken to compare the protective efficacy of a type-specific hexavalent M protein-based vaccine [2] and J14 [4, 7], which contains a conserved minimal B cell epitope from the C-repeat region of M protein that is constrained in conformation by the addition of flanking alpha-helical peptides. In addition, experiments were performed to determine if immunizing with combination vaccines resulted in levels of protection that were greater than that achieved with either vaccine alone.
2. Materials and Methods
2.1. Vaccines
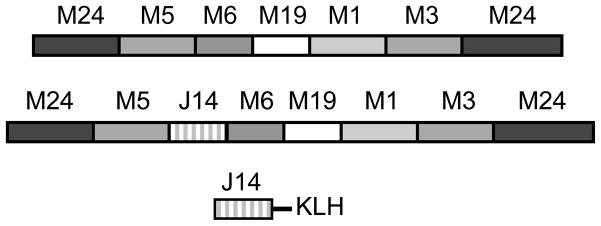
The recombinant hexavalent vaccine (Fig. 1) was constructed, expressed and purified as previously described [2]. The hexavalent-J14 vaccine was constructed by modifying the hexavalent gene by ligating complementary synthetic oligonucleotides (Integrated DNA Technologies, Inc., Coralville, IA) encoding the sequence of the J14 peptide [7] and which contained one half of a Sal1 restriction site on either end to facilitate insertion into the hexavalent gene between the M5 and M6 sequences (Fig. 1). The sequence of the top strand synthetic oligonucleotide was 5′TCGACAAACAGGCGGAAGACAAAGTTAAAGCGTCTCGTGAAGCGAAAAAACAGG TTGAAAAAGCGCTGGAACAGCTGGAAGACAAAGTTAAAG. The J14-KLH vaccine consisted of a synthetic peptide (Invitrogen, Carlsbad, CA) containing a C-terminal cysteine to facilitate coupling to KLH by methods previously reported [8]. The sequence of the J14 synthetic peptide was KQAEDKVKASREAKKQVEKALEQLEDKVKC.
Fig. 1.
Schematic drawing of the recombinant hexavalent, hexavalent-J14 and J14 synthetic peptide conjugated to KLH.
2.2 Immunization of rabbits and mice
All animal experiments were performed according to protocols approved by the University of Tennessee Health Science Center and Memphis VA Medical Center Institutional Animal Care and Use Committees. Groups of three New Zealand white rabbits (Myrtle’s Rabbitry, Thompsons Station, TN) were each immunized i.m. with 200μg of the hexavalent or hexavalent-J14 vaccines formulated on alum, as previously described [2]. Booster injections of the same dose were given at 4 and 8 weeks following the initial injection. 300μg of the J14-KLH vaccine was formulated in complete Freund’s adjuvant for the initial injection and then in incomplete adjuvant for booster injections 4 and 8 weeks after the initial injection. Serum was obtained from all animals prior to the first dose of vaccine and 2 weeks after the final dose.
ICR female mice, age 5–6 weeks (Harlan Sprague Dawley, Inc., Indianapolis, IN) were passively immunized by injecting into the peritoneal cavity 0.5 ml immune rabbit antisera against the hexavalent, hexavalent-J14 or J14-KLH vaccines 24 hrs prior to intraperitoneal (i.p.) challenge infections with virulent GAS. Normal rabbit serum (NRS) served as the control.
Groups of 5–6 week-old ICR or Balb/c mice (Harlan Sprague Dawley, Inc.) were actively immunized via the intramuscular (i.m.) route with 30μg of the three vaccines adsorbed on alum according to the dose schedules indicated for each experiment. In some experiments, mice received a combination vaccine containing 30μg of the hexavalent protein mixed with 30μg of J14-KLH conjugate on alum according to the schedule and dose indicated. Injections of alum alone served as the control in each experiment.
2.3 ELISA
Antibody levels in rabbit and mouse sera were determined by ELISA by methods previously described using either purified proteins [9] or whole streptococci [10] as antigens.
2.4 Opsonization and bactericidal assays
Opsonization assays and indirect bactericidal assays were performed as previously described [11, 12].
2.5 Challenge experiments
Immunized animals were challenged with virulent organisms using four different mouse models and three different serotypes of GAS, all of which are represented in the hexavalent vaccine. A serotype 6 strain was used to challenge mice that were passively or actively immunized. This serotype was used in previous studies showing the protective immunogenicity of conserved C-repeat epitopes [13]. Intranasal challenge infections were performed using type 24 streptococci, a serotype that has previously been shown to be virulent in mice when delivered via the mucosal route [14]. A strain of type 3 GAS, a serotype that has also been used in soft tissue models of infection [15], was mouse passed and used to challenge mice via subcutaneous (s.c.) injection.
Passive mouse protection tests were performed essentially according to the method described by Lancefield [16]. Briefly, female ICR mice (Harlan Sprague Dawley, Inc.) were given 0.5ml of normal rabbit serum (NRS) or immune rabbit serum via the i.p. route 24 hrs prior to i.p. challenge infections with 8.8×105 CFU of virulent type 6 GAS. Deaths were recorded daily for 5 days following the challenge.
For i.p. challenge infections of actively immunized mice, 5 groups of 5 female ICR mice each were injected with 10-fold increasing doses of ~103–107 CFU type 6 GAS. The number of surviving mice was recorded daily for 7 days. Moribund mice were sacrificed and recorded as dead. LD50 values were calculated using the method of Reed and Muench [17] with modifications [18].
For the upper respiratory tract infection model, immunized Balb/c mice were anesthetized with isoflurane, held vertically, and inoculated intranasally (i.n.) with 2.4×107 CFU of type 24 GAS in 10 μl of PBS. Deaths were recorded for seven days following the challenge infections.
Protection against soft tissue infections was assessed in ICR mice that were immunized i.m. in the thigh muscle. Following immunization, challenge infections with 1.2×107 CFU of type 3 GAS suspended in 50 μl saline were injected s.c. into the thigh that had been shaved and the mice were evaluated for lesion size and severity daily for 8 days. Lesion area was estimated by making two perpendicular measurements of the diameter and recorded daily as sq. mm. Lesion severity was assessed daily using the following criteria: 0= no lesion, 1=swelling only, 2=swelling and skin discoloration, 3=skin breakdown, ulcer formation, and 4=necrotic ulcer.
2.6 Statistical analyses
Fisher’s exact test (two-tailed) was used to evaluate differences in survival of immunized and control groups of mice following challenge infections. In the soft tissue infection model, differences in lesion area and severity were assessed by the method of Tukey-Kramer HSD based on a comparison of means (JMP version 4, SAS, Cary, NC)
3. Results
3.1 Immunogenicity of hexavalent and hexavalent-J14 vaccines
Initial experiments were designed to determine the immunogenicity of the hexavalent and hexavalent-J14 recombinant proteins and to compare the levels of opsonic antibodies evoked by each vaccine. In general the antibody titers of both sets of immune sera were comparable against the two vaccine proteins and the subunit peptides contained within the hexavalent protein (Table 1). As expected, only the hexavalent-J14 protein evoked antibodies against the J14 peptide. In vitro opsonization assays revealed that both vaccines evoked functional antibodies against the six vaccine serotypes of GAS (Table 2). The levels of opsonization promoted by the hexavalent-J14 antisera were either equivalent to those observed with the hexavalent antisera or in some cases they were lower, suggesting that the addition of J14 to the hexavalent construct did not augment the levels of opsonic antibodies evoked by the type-specific epitopes located in hexavalent-J14. In addition, hexavalent-J14 did not evoke opsonic antibodies against the two non-vaccine serotypes tested, types 12 and 18 (Table 2). All of the serotypes of GAS used in these experiments express M proteins containing the sequence found in the J14 peptide (unpublished data).
Table 1.
Type-specific and J14 antibodies evoked in rabbits by hexavalent and hexavalent-J14 vaccines
| Rabbit #2 | ELISA titer against1: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Hexa | Hexa-J14 | J14 | M24.2 | M5.2 | M6.6 | M19.2 | M1-0.2 | M3.2 | |
| Pre-pool | <200 | <200 | <200 | <200 | <200 | <200 | <200 | <200 | <200 |
| #1 | 51,200 | 51,200 | <200 | 6,400 | 6,400 | 25,600 | 6,400 | 3,200 | 1,600 |
| #2 | 102,400 | 102,400 | <200 | 25,600 | 12,800 | 51,200 | 6,400 | 12,800 | 6,400 |
| #3 | 204,800 | 204,800 | <200 | 25,600 | 25,600 | 51,200 | 6,400 | 12,800 | 12,800 |
| Pre-pool | <200 | <200 | <200 | <200 | <200 | <200 | <200 | <200 | <200 |
| #4 | 51,200 | 102,400 | 3,200 | 12,800 | 12,800 | 51,200 | 6,400 | 6,400 | 3,200 |
| #5 | 51,200 | 51,200 | 1,600 | 6,400 | 6,400 | 25,600 | 3,200 | 3,200 | 6,400 |
| #6 | 204,800 | 204,800 | 1,600 | 25,600 | 25,600 | 51,200 | 6,400 | 6,400 | 6,400 |
ELISA antigens were the purified recombinant vaccine proteins (hexavalent and hexavalent-J14), the unconjugated synthetic J14 peptide or recombinant dimeric peptides that copied the type-specific components of the hexavalent hybrid protein.
Rabbits #1–#3 were immunized with 200μg recombinant hexavalent protein and rabbits #4–#6 received i.m. injections of 200μg of recombinant hexavalent-J14 protein at times 0, 4 and 8 weeks. Serum was obtained 2 weeks after the final immunization.
Table 2.
Opsonic antibodies evoked in rabbits by hexavalent and hexavalent-J14 proteins against vaccine and non-vaccine serotypes of GAS1.
| Rabbit # | Percent opsonization of: |
|||||||
|---|---|---|---|---|---|---|---|---|
| M24 | M5 | M6 | M19 | M1 | M3 | M12 | M18 | |
| Pre-pool | 0 | 0 | 2 | 2 | 8 | 4 | 2 | 0 |
| Anti-hexavalent | ||||||||
| #1 | 98 | 98 | 84 | 96 | 44 | 66 | 0 | 4 |
| #2 | 100 | 96 | 98 | 92 | 78 | 80 | 0 | 0 |
| #3 | 98 | 98 | 98 | 100 | 76 | 80 | 0 | 0 |
| Anti-hexavalent-J14 | ||||||||
| #4 | 98 | 98 | 92 | 32 | 22 | 10 | 0 | 0 |
| #5 | 100 | 94 | 94 | 2 | 26 | 26 | 2 | 2 |
| #6 | 100 | 98 | 96 | 90 | 70 | 82 | 0 | 0 |
| Anti-pepM2 | 98 | 98 | 98 | 98 | 76 | 90 | 82 | 96 |
Rotation mixtures contained the test organism, 0.1 ml of indicated rabbit serum, and 0.4 ml non-immune human blood. The mixture was rotated at 37° and after 45 min. smears were made and stained. The percentage of PMN’s that had ingested or were associated with streptococci was estimated by microscopic counts [11, 12].
Anti-pepM antisera were raised in rabbits against purified pepsin extracted M proteins [12] derived form each of the serotypes indicated.
3.2 Bactericidal activity
Because the hexavalent-J14 vaccine may not have evoked levels of J14 antibodies sufficient to promote opsonization [19], we synthesized the J14 peptide and immunized three rabbits with the J14-KLH conjugate. The J14 antibody titers were 51,200, 25,600 and 25,600 and the KLH titers were 102,400, 51,200 and 51,200, respectively. We next tested the bactericidal activity of rabbit antisera raised against all three vaccine constructs. The hexavalent and hexavalent-J14 vaccines evoked bactericidal antibodies against all six vaccine serotypes of GAS, ranging from 48–100 percent killing of the test organisms (Table 3). J14-KLH antiserum exhibited minimal bactericidal activity, which ranged from 0–19 percent killing.
Table 3.
Bactericidal activity of rabbit antisera against hexavalent, hexavalent-J14 and J14-KLH vaccines
| Antiserum1 | Percent kill of: |
|||||
|---|---|---|---|---|---|---|
| M24 | M5 | M6 | M19 | M1 | M3 | |
| Anti-hexavalent | 100 | 77 | 94 | 50 | 100 | 100 |
| Anti-hexavalent-J14 | 95 | 82 | 89 | 48 | 100 | 94 |
| Anti-J14-KLH | 19 | 0 | 5 | 16 | 0 | 0 |
3.3 Binding of type-specific and J14 antibodies to M epitopes on the bacterial surface
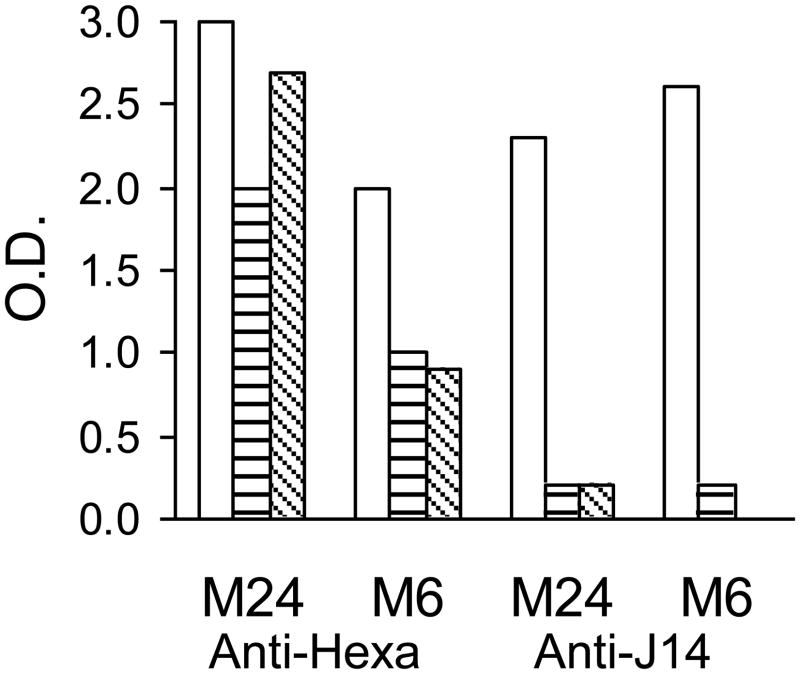
To determine whether hexavalent and J14 antibodies were able to bind to the respective regions of M protein on the bacterial surface, we performed whole-cell ELISAs with each antiserum in the presence of either PBS, human serum or human plasma. Since previous studies have shown that serum from adult humans may contain significant levels of antibodies to the M protein sequence contained in J14 [20] which could potentially block the binding of rabbit antibodies to the same epitopes, we the J14 peptide, as compared to the J14 rabbit antiserum which had a titer of 25,600. Serotypes M6 and M24 GAS were incubated in PBS, human serum or plasma and then reacted with either hexavalent or J14-KLH rabbit antisera (Fig. 2). Human plasma and serum almost completely inhibited the binding of J14 antibodies to the surface M protein of both serotypes of GAS, whereas the binding of hexavalent antibodies was reduced by 10–55 percent compared to PBS control values. As previous studies have indicated [21], our results suggested that J14 antibodies may not bind efficiently in the presence of human serum or plasma, thus limiting their ability to opsonize GAS in a mixture containing whole blood.
Fig. 2.
Human serum and plasma block the binding of J14 antibodies but not hexavalent antibodies to the surface of GAS. After blocking non-specific sites on the surface of GAS with PBS-tween plus 5% BSA, normal human serum (▤), human plasma (▧) or PBS (□) were then incubated with the bacteria. Hexavalent (serum #2, table 1) and J14 (titer=25,600 against J14) rabbit sera (1:200) were then added to the adherent bacteria, incubated, washed and followed with HRP-goat anti-rabbit IgG. After washing, ABTS (KPL, Gaithersburg, MD) was used to quantify detectable rabbit hexavalent and J14 antibodies.
3.4 Protection against challenge infections
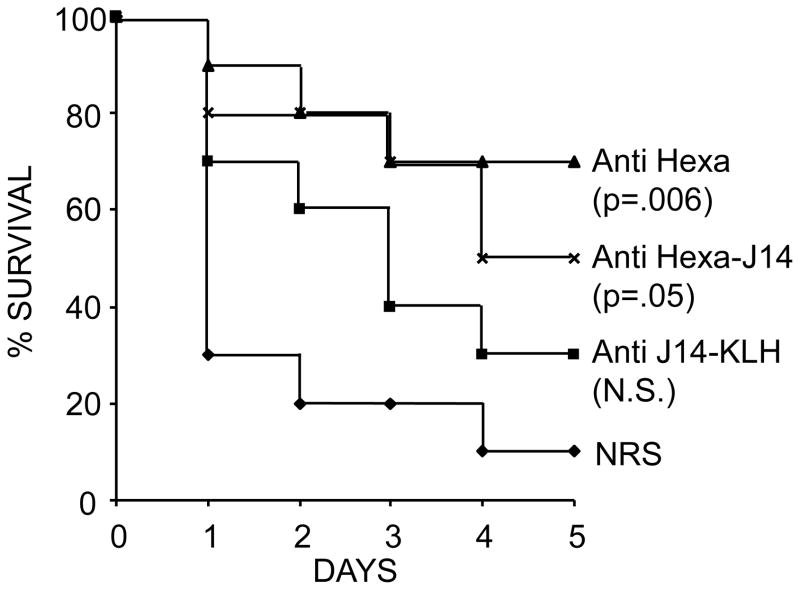
Passive mouse protection tests were performed by administering 0.5 ml immune serum or NRS i.p. 24 hours prior to i.p. challenge infections with type 6 GAS. For these experiments, we selected rabbit antisera #2 and #6 (Table 1) which had identical antibody titers against the M6 vaccine peptide and similar levels of opsonic activity against type 6 GAS (Table 2). The J14-KLH antiserum had a J14 antibody titer of 25,600. Mice that received hexavalent vaccine antiserum or hexavalent-J14 antiserum had significantly higher survival rates compared to mice that received NRS (Fig. 3). Although survival in the group that received J14-KLH antiserum was higher than the control group, it did not reach statistical significance. In addition, there was no significant difference in survival of mice that received hexavalent antiserum versus those that received hexavalent-J14 antiserum.
Fig. 3.
Survival of ICR mice passively immunized i.p. with 0.5 ml rabbit antisera against hexavalent (serum # 2 in Table 1), hexavalent-J14 (serum #6 in Table 1) or J14-KLH (J14 titer=25,600) and then challenged i.p. 24 hours later with 8.8 × 105 of type 6 GAS.
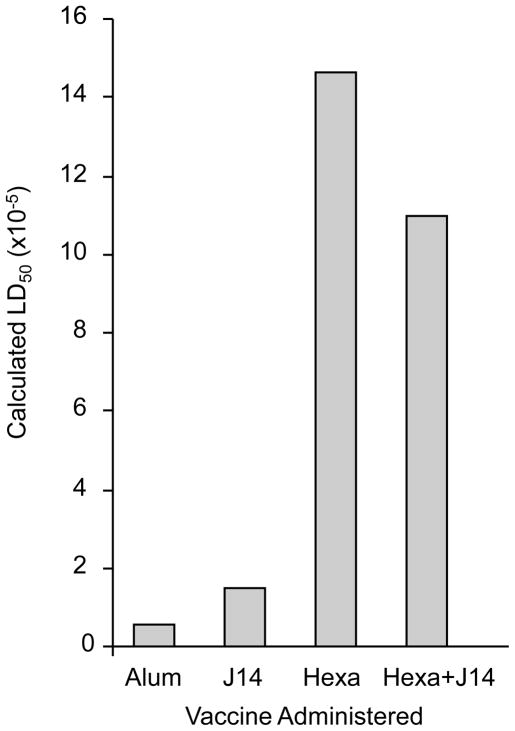
Groups of mice were actively immunized with the three vaccines via the i.m. route and then challenged i.p. with type 6 GAS (Fig. 4). The LD50 of the organism in mice that received the hexavalent and hexavalent-J14 vaccines was 1.46×106 and 1.10×106, respectively. The LD50 in mice that received the J14-KLH vaccine was 1.46×105 and in those that were sham immunized with alum alone the LD50 was 5.41×104 (Fig. 4).
Fig. 4.
Comparison of the protective immunogenicity of hexavalent, J14 and hexavalent-J14 vaccines. ICR mice were immunized i.m. with 30μg of each vaccine at time 0, 3, 6 and 9 weeks. Two weeks after the last immunization, five groups of five mice were challenged i.p. with 2.6 × 103 to 2.6 × 107 CFU of type 6 GAS and the LD50 was calculated.
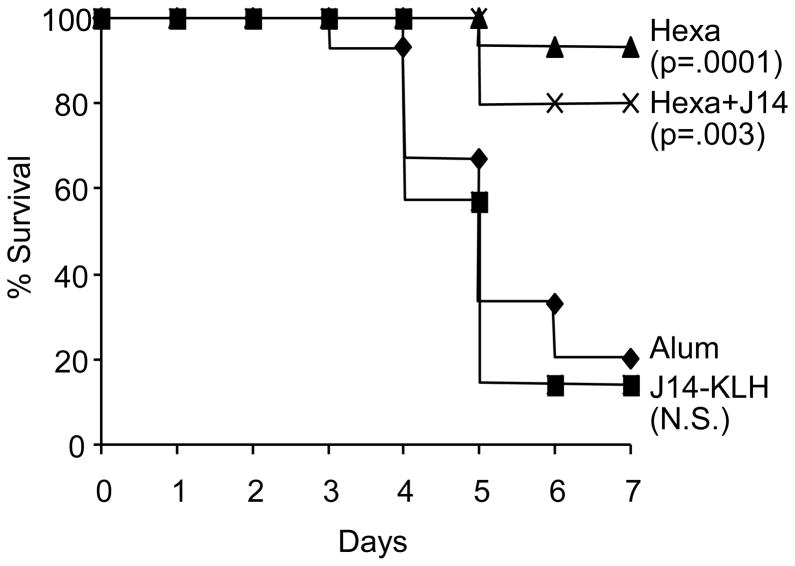
We next compared the protective immunogenicity of the hexavalent vaccine, the hexavalent vaccine plus J14-KLH and the J14-KLH vaccine administered to mice. All vaccines were formulated on alum and administered via the i.m. route. Prior to challenge infections, serum antibody levels were assessed against the hexavalent protein and the J14 peptide (Fig. 5). Immunized mice were then challenged with a single inoculum of type 24 GAS via the intranasal route (Fig. 6). Survival of mice that received the hexavalent vaccine or the hexavalent vaccine plus J14-KLH was significantly greater than in mice that received the J14-KLH vaccine or alum. In addition, there was no significant difference in survival between mice that received the hexavalent vaccine alone or the hexavalent vaccine plus J14-KLH (Fig. 6).
Fig. 5.
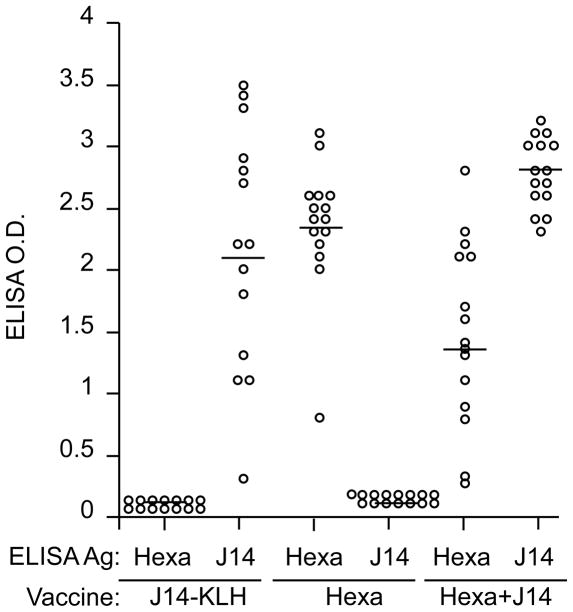
Serum immune responses in Balb/c mice (see also Fig. 6) determined 1 week after the last immunization with J14-KLH, hexavalent and hexavalent+J14. Mouse sera (1:500) were incubated with hexavalent protein or J14 peptide, which were the solid-phase ELISA antigens. Horizontal bars indicate mean O.D. values.
Fig. 6.
Protection of mice immunized i.m. with hexavalent, hexavalent+J14 and J14-KLH after i.n. challenge with type 24 GAS. Groups of 15 mice each were immunized i.m. with 30μg of the indicated vaccine at time 0, 3, 6 and 9 weeks. Three weeks after the final immunization, the mice were challenged via the i.n. route with 2.4 × 107 CFU of type 24 GAS.
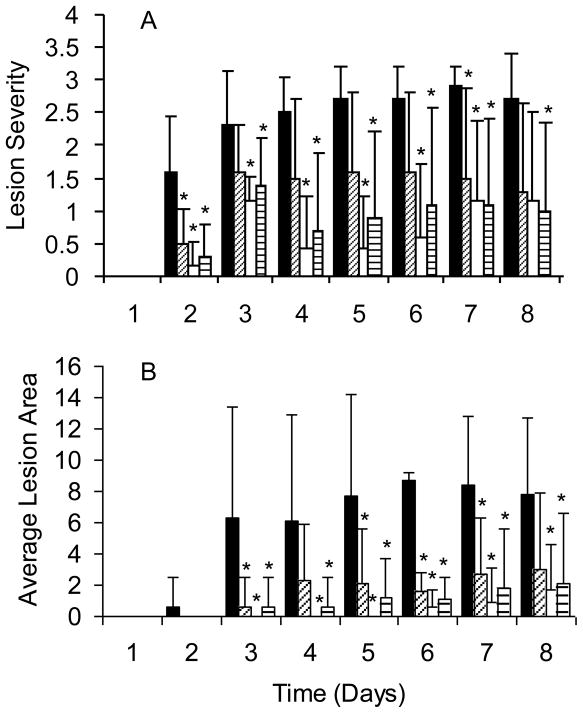
Finally, we assessed the protective efficacy of the hexavalent vaccine, J14-KLH and the combination vaccine in a mouse model of soft tissue infection. ICR mice were immunized i.m. in the thigh muscle and at the completion of the immunization schedule the animals were challenged subcutaneously with type 3 GAS. Lesion severity and lesion size were assessed daily for 8 days following the challenge infections (Fig. 7). All three groups of immunized mice developed lesions that were significantly less severe (Fig. 7A) and of smaller size (Fig. 7B) compared to mice that were sham immunized with alum. In general, mice immunized with the hexavalent vaccine developed smaller and less severe lesions than those immunized with J14-KLH or the combination vaccine but all three vaccines proved to be efficacious in reducing the clinical severity of the soft tissue infections.
Fig. 7.
Protection of ICR mice immunized i.m. at time 0, 3, 6 and 10 weeks with alum (■, N=10), 30μg J14-KLH (▨, N=10), 30μg hexavalent (□, N=7), or 30μg hexavalent+ 30μg J14 (▤, N=10). Four weeks after the final immunization, the mice were challenged s.c. with 1.2 × 107 CFU type 3 GAS. (A) Average lesion severity scores: 0=normal skin, 1=swelling, 2=swelling and discoloration, 3=skin breakdown, 4=necrotic ulcer and (B) average lesion size (sq. mm) were recorded daily post challenge. *Indicate p ≤ 0.05.
4. Discussion
In general there are two parallel approaches to the development of GAS vaccines: those based on the type-specific N-terminal peptides of M proteins and those based on common antigens that are shared by many or all serotypes of GAS [22, 23]. The prototype hexavalent M protein-based vaccine and a newer 26-valent vaccine have been evaluated in clinical trials and were shown to be well-tolerated, highly immunogenic and to evoke opsonic antibodies against the vaccine serotypes of GAS [24, 25]. The potential efficacy of M protein-based vaccines is supported by animal and human studies indicating that the presence of type-specific bactericidal antibodies is an immune correlate of serotype-specific protection [26, 27]. These vaccines could eventually meet the needs of economically developed countries where sufficient data from epidemiologic surveillance suggests that their impact on overall disease burden could be significant [28, 29]. However, the greatest burden of GAS disease is in the developing world where the epidemiology of these infections has been incompletely studied and the data that do exist suggest that multivalent vaccines alone may be insufficient to achieve a significant reduction in disease burden [30].
For this reason, we performed the present studies to assess the potential efficacy of combination vaccines containing both type-specific and conserved M protein epitopes. The J14 vaccine is the precursor to a similar peptide, J8, which is now being prepared for early stage clinical trials [23]. The M protein peptide sequence of the J14 vaccine is shared by most clinical isolates of GAS [31]. Previous studies have shown that J14 conjugated to carrier proteins is cross-opsonic and cross-protective against multiple serotypes of GAS [13]. Therefore, we reasoned that the combination of type-specific and shared epitopes would enhance the protective efficacy of either vaccine alone. In all but one animal model, we failed to show an added advantage of the J14 vaccine component. The J14-KLH vaccine alone showed some protective efficacy in the passive immunization model, but incorporation of the J14 peptide into the recombinant hexavalent protein or the addition of J14-KLH to the hexavalent vaccine in equal amounts by weight did not improve the survival of challenged animals above that observed by immunization with the hexavalent protein alone. The exception to this was observed in the soft tissue model of infection where both the J14-KLH vaccine alone and the hexavalent vaccine resulted in significantly smaller lesion sizes and lower lesion severity scores compared to sham immunized animals. We conclude that low levels of opsonic antibodies evoked by J14-KLH may be more efficacious in controlling the clinical severity of these localized infections as opposed to infections that result in dissemination of the organisms and death, as is observed in the other mouse models.
Previous studies have provided conflicting results related to the ability of antibodies against the C-repeat region of M proteins to opsonize GAS [5, 13, 32]. Some of the earliest reports showed that passive [14] or active immunization [5, 6] with conserved C-repeat epitopes via the intranasal route afforded protection from mucosal challenge infections, as indicated by a reduction in levels of colonization [6] and death [5, 14]. These studies also reported that the C-repeat antibodies did not promote opsonization in human blood. Later studies showed that human serum albumin binds specifically to the C-repeat region of type 5 M protein, blocks the binding of C-repeat antibodies [21] and thus the activation and deposition of complement necessary for efficient phagocytosis. One area of agreement is that C-repeat epitopes, whether delivered as recombinant proteins expressed by vaccinia virus [33] or as synthetic peptides coupled to carriers [5, 32, 34], evoke antibodies following mucosal immunization that protect animals from colonization and/or infection following mucosal challenge infections [5, 34]. Taken together with the results of the present study, one conclusion is that C-repeat M protein epitopes may be most efficacious when delivered via the mucosal route because antibodies present on the mucosal surface are able to bind to the C-repeat region of the M protein in the absence of serum inhibitors and effectively block attachment and subsequent infection.
The results of our current studies indicate the need to systematically assess potential GAS vaccine candidates for protective efficacy using the available in vitro and in vivo models prior to vaccine formulation and clinical trials in humans. For most of the GAS antigens described as potential vaccine components, there has not been established a human correlate of immunological protection against natural infection. There are concerns about the adequacy of the current animal models to predict clinical efficacy of GAS vaccines and the results of the current study may not adequately reflect the potential protective efficacy in humans of either vaccine approach. However, well-controlled and standardized assessments of combination vaccines using the existing models together with new epidemiologic and immunologic studies in humans may produce the most rational vaccine formulations for human testing. This approach will also consider the pressing needs of developing countries for an affordable and effective vaccine to reduce the significant burden of GAS disease around the world.
Acknowledgments
This work was supported by research funds from NIH/NIAID USPHS Grants AI10085 and AI60592 (J.B.D.) and research funds from the Department of Veterans Affairs (J.B.D). The authors acknowledge the expert technical assistance of Valerie Long. We thank Drs. David L. Hasty and Harry S. Courtney for their careful and critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5(11):685–94. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 2.Dale JB. Multivalent group A streptococcal vaccine designed to optimize the immunogenicity of six tandem M protein fragments. Vaccine. 1999;17:193–200. doi: 10.1016/s0264-410x(98)00150-9. [DOI] [PubMed] [Google Scholar]
- 3.Hu M, Walls M, Stroop S, Reddish M, Beall B, Dale J. Immunogenicity of a 26-Valent Group A Streptococcal Vaccine. Infect Immun. 2002;70:2171–7. doi: 10.1128/IAI.70.4.2171-2177.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayman WA, Brandt ER, Relf WA, Cooper J, Saul A, Good MF. Mapping the minimal murine T cell and B cell epitopes within a peptide vaccine candidate from the conserved region of the M protein of group A streptococcus. Int Immunol. 1997;9(11):1723–33. doi: 10.1093/intimm/9.11.1723. [DOI] [PubMed] [Google Scholar]
- 5.Bronze MS, Courtney HS, Dale JB. Epitopes of group A streptococcal M protein that evoke cross-protective local immune responses. J Immunol. 1992;148:888–93. [PubMed] [Google Scholar]
- 6.Bessen D, Fischetti VA. Influence of intranasal immunization with synthetic peptides corresponding to conserved epitopes of M protein on mucosal colonization by group A streptococci. Infect Immun. 1988;56:2666–72. doi: 10.1128/iai.56.10.2666-2672.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batzloff M, Yan H, Davies M, Hartas J, Good M. Preclinical evaluation of a vaccine based on conserved region of M protein that prevents group A streptococcal infection. Indian J Med Res. 2004;119 (Suppl):104–7. [PubMed] [Google Scholar]
- 8.Dale JB, Beachey EH. Sequence of myosin-cross-reactive epitopes of streptococcal M protein. J Exp Med. 1986;164:1785–90. doi: 10.1084/jem.164.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall MA, Stroop SD, Hu MC, Walls MA, Reddish MA, Burt DS, et al. Intranasal immunization with multivalent group A streptococcal vaccines protects mice against intranasal challenge infections. Infect Immun. 2004;72(5):2507–12. doi: 10.1128/IAI.72.5.2507-2512.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Courtney HS, Ofek I, Penfound T, Nizet V, Pence MA, Kreikemeyer B, et al. Relationship between expression of the family of M proteins and lipoteichoic acid to hydrophobicity and biofilm formation in Streptococcus pyogenes. PLoS ONE. 2009;4(1):e4166. doi: 10.1371/journal.pone.0004166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lancefield RC. Differentiation of group A streptococci with a common R antigen into three serologic types with special reference to the bactericidal test. J Exp Med. 1957;106:525–44. doi: 10.1084/jem.106.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beachey EH, Stollerman GH, Chiang EY, Chiang TM, Seyer JM, Kang AH. Purification and properties of M protein extracted from group A streptococci with pepsin: Covalent structure of the amino terminal region of the type 24 M antigen. J Exp Med. 1977;145:1469–83. doi: 10.1084/jem.145.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batzloff MR, Hayman WA, Davies MR, Zeng M, Pruksakorn S, Brandt ER, et al. Protection against group A streptococcus by immunization with J8-diphtheria toxoid: contribution of J8- and diphtheria toxoid-specific antibodies to protection. J Infect Dis. 2003;187(10):1598–608. doi: 10.1086/374800. [DOI] [PubMed] [Google Scholar]
- 14.Bronze M, McKinsey D, Corbett C, Beachey EH, Dale JB. Protective immunity evoked by locally administered group A streptococcal vaccines in mice. J Immunol. 1988;141:2767. [PubMed] [Google Scholar]
- 15.Ashbaugh CD, Wessels MR. Absence of a cysteine protease effect on bacterial virulence in two murine models of human invasive group A streptococcal infection. Infect Immun. 2001;69(11):6683–8. doi: 10.1128/IAI.69.11.6683-6688.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dochez AR, Avery OT, Lancefield RC. Studies on the Biology of Streptococcus. J Exp Med. 1919;30:179–213. doi: 10.1084/jem.30.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reed LJ, Muench H. A single method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493. [Google Scholar]
- 18.Kim CC, Monack D, Falkow S. Modulation of virulence by two acidified nitrite-responsive loci of Salmonella enterica serovar Typhimurium. Infect Immun. 2003;71(6):3196–205. doi: 10.1128/IAI.71.6.3196-3205.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandt ER, Sriprakash KS, Hobb RI, Hayman WA, Zeng W, Batzloff MR, et al. New multi-determinant strategy for a group A streptococcal vaccine designed for the Australian Aboriginal population. Nat Med. 2000;6(4):455–9. doi: 10.1038/74719. [DOI] [PubMed] [Google Scholar]
- 20.Brandt ER, Hayman WA, Currie B, Carapetis J, Wood Y, Jackson DC, et al. Opsonic human antibodies from an endemic population specific for a conserved epitope on the M protein of group A streptococci. Immunology. 1996;89(3):331–7. doi: 10.1046/j.1365-2567.1996.d01-754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandin C, Carlsson F, Lindahl G. Binding of human plasma proteins to Streptococcus pyogenes M protein determines the location of opsonic and non-opsonic epitopes. Mol Microbiol. 2006;59(1):20–30. doi: 10.1111/j.1365-2958.2005.04913.x. [DOI] [PubMed] [Google Scholar]
- 22.Dale JB. Current status of group A streptococcal vaccine development. Adv Exp Med Biol. 2008;609:53–63. doi: 10.1007/978-0-387-73960-1_5. [DOI] [PubMed] [Google Scholar]
- 23.Steer AC, Batzloff MR, Mulholland K, Carapetis JR. Group A streptococcal vaccines: facts versus fantasy. Curr Opin Infect Dis. 2009;22(6):544–52. doi: 10.1097/QCO.0b013e328332bbfe. [DOI] [PubMed] [Google Scholar]
- 24.Kotloff KL, Corretti M, Palmer K, Campbell JD, Reddish MA, Hu MC, et al. Safety and immunogenicity of a recombinant multivalent group a streptococcal vaccine in healthy adults: phase 1 trial. JAMA. 2004;292(6):709–15. doi: 10.1001/jama.292.6.709. [DOI] [PubMed] [Google Scholar]
- 25.McNeil SA, Halperin SA, Langley JM, Smith B, Warren A, Sharratt GP, et al. Safety and immunogenicity of 26-valent group A streptococcus vaccine in healthy adult volunteers. Clin Infect Dis. 2005;41(8):1114–22. doi: 10.1086/444458. [DOI] [PubMed] [Google Scholar]
- 26.Lancefield RC. Current knowledge of the type specific M antigens of group A streptococci. J Immunol. 1962;89:307–13. [PubMed] [Google Scholar]
- 27.Wannamaker LW, Denny FW, Perry WD, Siegel AC, Rammelkamp CH., Jr Studies on immunity to streptococcal infections in man. AMA Am J Dis Child. 1953;86(3):347–8. [PubMed] [Google Scholar]
- 28.Shulman ST, Tanz RR, Dale JB, Beall B, Kabat W, Kabat K, et al. Seven-year surveillance of north american pediatric group a streptococcal pharyngitis isolates. Clin Infect Dis. 2009;49(1):78–84. doi: 10.1086/599344. [DOI] [PubMed] [Google Scholar]
- 29.O’Loughlin RE, Roberson A, Cieslak PR, Lynfield R, Gershman K, Craig A, et al. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000–2004. Clin Infect Dis. 2007;45(7):853–62. doi: 10.1086/521264. [DOI] [PubMed] [Google Scholar]
- 30.Steer AC, Law I, Matatolu L, Beall BW, Carapetis JR. Global emm type distribution of group A streptococci: systematic review and implications for vaccine development. Lancet Infect Dis. 2009;9(10):611–6. doi: 10.1016/S1473-3099(09)70178-1. [DOI] [PubMed] [Google Scholar]
- 31.Steer AC, Magor G, Jenney AW, Kado J, Good MF, McMillan D, et al. emm and C-repeat region molecular typing of beta-hemolytic Streptococci in a tropical country: implications for vaccine development. J Clin Microbiol. 2009;47(8):2502–9. doi: 10.1128/JCM.00312-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bessen D, Fischetti VA. Synthetic peptide vaccine against mucosal colonization by group A streptococci. I. Protection against a heterologous M serotype with shared C repeat region epitopes. J Immunol. 1990;145(4):1251–6. [PubMed] [Google Scholar]
- 33.Fischetti VA, Hodges WM, Hruby DE. Protection against streptococcal pharyngeal colonization with a vaccinia: M protein recombinant. Science. 1989;244(4911):1487–90. doi: 10.1126/science.2660266. [DOI] [PubMed] [Google Scholar]
- 34.Batzloff MR, Hartas J, Zeng W, Jackson DC, Good MF. Intranasal vaccination with a lipopeptide containing a conformationally constrained conserved minimal Peptide, a universal T cell epitope, and a self-adjuvanting lipid protects mice from group a streptococcus challenge and reduces throat colonization. J Infect Dis. 2006;194(3):325–30. doi: 10.1086/505146. [DOI] [PubMed] [Google Scholar]